Abstract
Melatonin (MT), a naturally occurring compound, is found in various species worldwide. In 1958, it was first identified in the pineal gland of dairy cows. MT is an “old friend” but a “new compound” for plant biology. It brings experts and research minds from the broad field of plant sciences due to its considerable influence on plant systems. The MT production process in plants and animals is distinct, where it has been expressed explicitly in chloroplasts and mitochondria in plants. Tryptophan acts as the precursor for the formation of phyto-melatonin, along with intermediates including tryptamine, serotonin, N-acetyl serotonin, and 5-methoxy tryptamine. It plays a vital role in growth phases such as the seed germination and seedling growth of crop plants. MT significantly impacts the gas exchange, thereby improving physio-chemical functions in plant systems. During stress, the excessive generation and accumulation of reactive oxygen species (ROS) causes protein oxidation, lipid peroxidation, nucleic acid damage, and enzyme inhibition. Because it directly acts as an antioxidant compound, it awakens the plant antioxidant defense system during stress and reduces the production of ROS, which results in decreasing cellular oxidative damage. MT can enhance plant growth and development in response to various abiotic stresses such as drought, salinity, high temperature, flooding, and heavy metals by regulating the antioxidant mechanism of plants. However, these reactions differ significantly from crop to crop and are based on the level and kind of stress. The role of MT in the physiological functions of plants towards plant growth and development, tolerance towards various abiotic stresses, and approaches for enhancing the endogenous MT in plant systems are broadly reviewed and it is suggested that MT is a steering compound in directing major physiological functions of plants under the changing climate in future.
1. Introduction
MT (N-acetyl-5-methoxy-tryptamine) is a naturally occurring compound in various species. In 1958, it was identified in the pineal gland of dairy cows [1]. MT is an “old friend” but a “new compound” for plant biology. MT was first identified in higher plants as reported by Dubbels et al. [2], Van Tassel et al. [3], and Hattori et al. [4]. MT has drawn a lot of study interest since it was discovered and found in plants in 1995. It has been detected and measured in more than 140 plant species in recent years [5,6]. It is a versatile compound that is extensively distributed in a variety of plant organs, including the roots, stems, leaves, fruits, and seeds. Different plant tissues contain significantly varied amounts of MT. Blask and his co-workers proposed the term “phytomelatonin” in 2004, referring to its plant-based source [7]. Tryptophan, a type of indoleamine, is the starting molecule for MT like that of auxin and ought to be involved in the control of growth and development. It has physiological impacts on plants, which include stimulating seedling growth, formation of primary roots, lateral and adventitious roots, and modifying the branching and growth cycles of leaves and stems, and also resists against leaf senescence by enhancing photosynthesis, stimulating flowering and seed development [8]. MT also takes part in several cellular processes in the name of antioxidant and free radical scavenging [9]. Additionally, MT has been linked to improved seed sprouting, maturation, photosynthesis, biomass production, circadian rhythm, redox network, membrane integrity, root development, leaf senescence, osmoregulation, and resistance to environmental stresses like salt, drought, heat, oxidative stress, and heavy metals. MT levels in plants are noticeably higher when exposed to a various stressors, including salt, drought, temperatures, UV radiation, metal pollution, and pathogenic infections, implicating that MT plays a role in plant stress tolerance [10]. It functions as an antioxidant and contributes to controlling ROS and nitrogen species (RNS) in plants because of its pleiotropic qualities. It is more efficient than glutathione and vitamin E at regulating a number of antioxidant enzymes, including glutathione reductase, catalase (CAT), peroxidase (POX), and superoxide dismutase (SOD). It boosts the mitochondria’s electron transport chain’s effectiveness, thereby reducing electron leakage. Because MT functions as a signaling molecule connected to defense systems against diverse biotic and abiotic threats, it is regarded as a master plant regulator that supports plant development and growth [11]. The signaling molecules in MT biosynthesis in plants under stress are yet to be clearly identified [12]. Employing MT as a bio-stimulator for the sustained production of crops without damaging the surrounding environment could, therefore, be of utmost relevance.
MT was found to increase the secondary metabolites like fatty acid and alkaloid content in different crops like coffee and soybean under various abiotic stresses [13], but the mechanism behind this has to be investigated. MT helps in stomatal closure at night to avoid water loss in arid regions by regulating ROS signaling through its receptor PMTR1 and maintaining homeostasis [14]. External application of a low concentration of MT was found to enhance seed germination, lateral root growth, and photosynthesis under various abiotic stresses [13]. Application of MT increased salt stress tolerance in rice, melon, and grapevine [15,16]; drought stress tolerance in corn and apple [17,18]; heat stress tolerance in Arabidopsis [19]; cold stress tolerance in corn and cucumber [20,21]; and heavy metal stress tolerance in wheat, tomato, Arabidopsis, and rice [22,23,24,25].
The study of MT action in plants is quickly expanding due to its phenotypic hormone effect on plant growth systems. It examines the crucial function of MT in regulating plant growth and development as well as its potential physiological mechanism for reducing abiotic stressors on plants. This review will contribute to a detailed knowledge of the current state of plant MT research and help us to understand MT’s role in directing plant physiology more meticulously, and we may speculate that plant MT research will go on a new path in the future.
2. Biosynthesis of Melatonin in Plants
The MT production process in plants and animals is distinct. Many elements, including light, have a vital role in controlling its production in plants. MT is specifically expressed in chloroplasts and mitochondria in plants. Tryptophan acts as the precursor for the formation of phyto-melatonin, along with intermediates including tryptamine, serotonin, N-acetyl serotonin, and 5-methoxy tryptamine (Figure 1). According to the report from Tan and Reiter [26], the intermediates of MT production are found in several sub-cellular compartments including the cytoplasm, mitochondria, endoplasmic reticulum and chloroplasts. Tryptophan decarboxylase (TDC) first decarboxylates tryptophan to produce tryptamine in the cytoplasm, tryptamine-5-hydroxylase (T5H), and then performs an enzymatic hydroxylation to produce serotonin in the endoplasmic reticulum. N-acetyltransferase (SNAT) and acetyl serotonin methyl transferase (ASMT) convert serotonin through acetylation and methylation reactions into N-acetyl serotonin in chloroplasts and 5-methoxytryptamine in the cytoplasm. N-acetyl serotonin produced in chloroplast reacts with the ASMT in the cytoplasm and transforms into MT; meanwhile, 5-methoxytryptamine produced in cytoplasm moves into the chloroplast and reacts with SNAT to synthesize MT [27]. Alternatively, an enzyme known as caffeic acid O-methyltransferase (COMT), which regulates several substrates, can also convert N-acetyl serotonin into MT in a different route that has been studied through plants. COMT can also transform serotonin into 5-methoxytryptamine and produce MT through SNAT catalyzation [28].
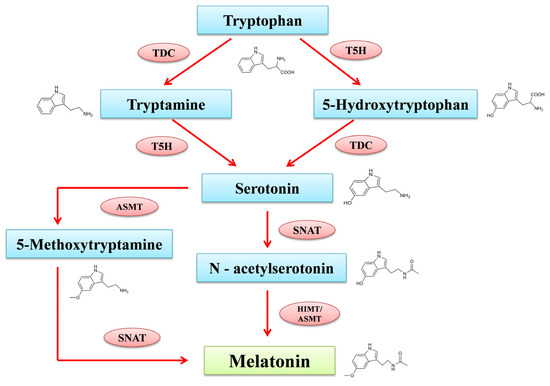
Figure 1.
MT biosynthesis pathway in plant system. TDC: Tryptophan decarboxylase; T5H: tryptamine 5-hydroxylase; SNAT: serotonin-N-acetyltransferase; ASMT: N-aceylserotonin methyltransferase; COMT: caffeic acid O-methyltransferase.
3. Melatonin’s Role in Plant Growth and Physiology
3.1. Germination
In the life cycle of higher plants, seed germination is a complicated process governed by several coordinated metabolic, cellular, and molecular activities. It is also a crucial time for the establishment of crop populations. Germination involves a number of metabolic and physical processes. This stage, which is similarly susceptible to stress and critical for determining whether plants will survive under adverse conditions, is greatly influenced by the external environment. According to Li et al. [29], MT functions as a signaling molecule and positively controls the germination process in Cucumis melo by upregulating the genes for gibberellin (GA) biosynthesis (CsGA20ox and CsGA3ox) and abscisic acid (ABA) catabolism (CsCYP707A1 and CsCYP707A2). A similar finding was also reported by Chen et al. [30] in cotton. Abiotic stress, such as stress like elevated temperature, lowers the cotton seeds’ ability to germinate, which leads to poor germination and crop stand which lends support to the findings of Snider et al. [31]. According to Lei et al. [32], application of MT improved seed germination and reserve mobilization in wheat under chromium stress. Raza et al. [33] revealed that exogenous MT promotes the activity of several antioxidant enzymes, which decreased the formation of ROS and enhanced the viability of seedlings under elevated temperatures. Application of MT in Lupinus albus potentially stimulated the vegetative growth in cotyledons and etiolated seedlings [34]. Similarly, in red cabbage (Brassica oleracea rubrum), exogenous MT promotes seed germination [35]. Previous studies have proven that pretreatment with MT can improve the seed germination of the various crops like green gram [36], rice [37], tomato [38], maize [39], Medicago sativa [40], Triticale hexaploide [41], and cotton [42]; also, it acts as a signaling molecule for the upregulation of genes involved in the biosynthesis of gibberellin (GA) that might be responsible for seed germination in cucumber [43]. Based on the result derived from Castañares and Bouzo [16], the germination percentage of the melon decreased drastically with increased Ec (electrical conductivity) of the water solution. However, the 6 h seed pretreatment with MT significantly increased the germination percentage. Findings of Rajora et al. [44] also revealed that under varied abiotic stress situations, priming seeds with MT enhances and speeds up the seed germination process. To speed up the germination process, seed priming changes the physiology of the embryo and activates hydrolytic enzymes [45].
3.2. Shoot and Root Growth
Due to the buildup of ABA, which further inactivates cell-wall-loosening enzymes under water stress in wheat, shifting the apoplastic pH from acidic to alkaline restricts the development of the plant’s shoots and roots [46]. The process of cell elongation involves an indoleamine molecule [47]. Pretreatment with MT results in a drop in intercellular pH to an acidic state and activates the enzymes responsible for loosening cell walls, which in turn triggers cell elongation like IAA [48]. As a consequence, seed priming with MT enhanced seed germination and seedling development through synthesizing stress-related proteins and activating signaling pathways in rice under stressful conditions [49]. Ahmad et al. [50] stated that MT along with the application of nitrogen significantly improved the shoot fresh and dry biomass in maize seedlings. Exogenous MT enhances the accumulation of soluble sugars and the protein level, which regulates osmotic adjustment under stressful conditions in cotton [51]. The application of MT stimulates the production of endogenous growth-inducing substances like metabolites, phytohormones, and increasing ROS and RNS scavenging systems in plants [52] which might lead to the production of higher shoots and denser roots. The fact that MT also causes the auxin-related genes to become active suggests that the auxin signal pathway is necessary for MT-mediated root development [53]. Ahmad et al. [54] described that increased shoot and root length, leaf area, and biomass accumulation after MT treatment improve the maize plant’s ability to withstand salt stress. Additionally, MT treatment boosted the amount of other endogenous growth induce factors as IAA, which led to the development of a denser root system [55]. A similar effect was found in other crops like rice [37], wheat [56], tomato [57], tobacco [58], and soybean [59] revealing that the growth and establishment of seedlings from seeds that had been pretreated with MT was favorable. Sultana and Barthakur [60] explored that seed priming with MT elicits positive effects on wheat root traits such as length, volume, and surface area of the seedling.
3.3. Gas Exchange
Photosynthesis is the most important physiological function found in all green plants that is severely affected by abiotic stresses [61]. Abdulbaki et al. [62] explained that abiotic stresses reduce the production of assimilatory powers (ATP and NADPH) and Rubisco activity by destroying the chloroplast grana structure and photosynthetic electron transport system. The reduced diffusion and concentration of intercellular CO2 in the carboxylation site of rubisco also decreases the photosynthetic rate under stress [63]. Chlorophyll is a key photosynthetic pigment found in all higher plants and plays a vital function in absorption of light energy. Fu et al. [64] reported that the metabolite concentrations of chlorophyll a, chlorophyll b, and carotenoids were decreased under heat stress in wheat. The enhanced activity of chlorophyll-degrading enzymes like chlorophyllase, pheophytinase, and chlorophyll-degrading peroxidase catalyze the breakdown of chlorophyll molecules in response to stress [65]. Wang et al. [66] suggested that a direct link was observed between MT and the concentration of photosynthetic pigment in soybean. MT reduces the rate of chlorophyll degradation by lowering the transcript levels of pheophorbide-a-oxygenase (PAO) which is involved in chlorophyll metabolism [67]. The expressions of genes such as Chlase, PPH, and Chl-PRX associated with degradation of chlorophyll biosynthesis were downregulated by MT in Agrostis stolonifera [68]. Shi et al. [69] stated that MT increases the Bermuda grass photosynthetic pathway by protecting the chlorophyll molecule from degradation and enhances the expression of photosynthetic proteins like LHCa and PsaG during oxidative stress. MT also protects the chloroplast ultrastructure from oxidative damage and recovers photosynthetic accessory pigments like carotenoids, chlorophyll b, xanthophyll, and anthocyanin from stress [70]. Liu et al. [67] suggested that application of MT decreases the expression level and its relative mRNA abundance of genes involved in senescence (SAG12) and the programmed cell death process. MT slows down the aging process of leaves by enhancing the ROS scavenging mechanism, which stabilizes the chloroplast structure and protects photosynthesis-related genes from deterioration [71] in the tomato plant.
Stomata play a vital role in the regulation of photosynthesis, transpiration rate, and water status of the plant [72]. MT regulates the opening of stomata through upregulation of the ABA catabolism process and simultaneously downregulates ABA anabolism that results in reduced accumulation of the endogenous ABA level. The decreased ABA level by MT reduces the production of H2O2 in guard cells of stomata that makes the stomata remain open and maintains the water status of the plant [73]. Leaf water status and leaf temperature are positively regulated by transpiration rate. The increased transpiration rate by MT enables the plant to maintain a lower leaf temperature, thereby improving photosynthetic efficiency [74]. The positive effect of MT on transpiration rate and stomatal conductance through the regulation of ABA level was also noticed in tomato [75], rice [76], and pepper [77].
Farooq et al. [78] observed the positive effect of MT on the photochemical efficiency (Fv/Fm) of the photosystem (PSII) in Brassica napus. Raza et al. [33] opined that stress induces excessive production of ROS which results in the peroxidation of lipid membranes and denaturation of proteins essential for chlorophyll biosynthesis that subsequently decreases the photosynthetic efficiency in plants. MT enhances the quantum yield of PSII by preventing photooxidative damage and assisting in the repair of photo-oxidatively damaged D1 protein [79]. The increase in the efficiency of PSII (Fv/Fm) is mainly attributed to the better functioning of PS II that has a higher number of reaction centers and improved photosynthetic electron transport rate (ETR) [57].
3.4. Antioxidant or ROS Scavenging
The crops are more vulnerable to the several abiotic stresses with changing climate during their growth phases. During stress conditions, plants convert 1–2% of the consumed oxygen into reactive oxygen species, specifically, hydroxyl radical (•OH), hydrogen peroxide (H2O2), superoxide radical (O2•−), and singlet oxygen (1O2). Stress enhances the production of ROS that results in cellular oxidative damage. The excessive generation and accumulation of ROS causes protein oxidation, lipid peroxidation, nucleic acid damage, enzyme inhibition, early leaf senescence, and necrosis [80]. Plants produced various enzymatic, such as CAT, POX, APX, SOD, GPX, and GR, and non-enzymatic antioxidants, like vitamins, carotenoids, stilbenes, and flavonoids, to capture the excess ROS in the plant system and thereby protect the plants from oxidative stress. Currently, MT is an inevitable compound present in the plant system and functions as a powerful antioxidant using both direct and indirect mechanisms during abiotic stress conditions. MT scavenges free radicals produced under stressful circumstances by increasing the endogenous antioxidants such as ascorbic acid and glutathione [58]. The expression level of genes related to antioxidant enzyme activity like SOD, CAT, APX, and GPX was also increased by MT in response to stress [81]. Kaur et al. [82] noticed that the Asada-Halliwell pathway, a crucial antioxidant enzymatic cycle, was regulated by MT in order to enhance the ROS scavenging mechanisms in stressed plants. Zhang et al. [83] suggested that MT stimulates the activity of H2O2 scavenging enzymes such as CAT, POD, and APX as well as ABA-degrading enzymes. Furthermore, MT controls the AsA-GSH cycle, which is essential for ROS detoxification, and enzymes like APX, MDHAR, DHAR, and GR were involved in the regulation of this cycle [84]. Rehman et al. [85] explained that MT effectively scavenges ROS by increasing the activity of the antioxidant enzyme glutathione peroxidase (GPX), which scavenges lipid peroxides, hydroperoxides, and H2O2 under stress.
MT possesses amphiphilic characteristics that enable it to diffuse and distribute readily across lipid membranes and the cytoplasm. The MT-bound hydrophilic side of the lipid bilayer prevented lipid peroxidation by directly neutralizing the damaging chemicals produced under stressful circumstances [86]. Lei et al. [87] opined that application of MT to rapeseed minimizes the free radical formation and generation of ROS like H2O2 and O2−. The integrity of the plant cell membrane was improved by MT through the increased activity of antioxidant enzymes like SOD, CAT, APX, and GPX [88]. MT reduces the effects of oxidative stress by directly scavenging ROS through enhanced antioxidant enzyme activity that ultimately reduces the MDA level in plants [89]. The increased antioxidant enzyme activity and defense system by the exogenous application of MT under stress conditions were also reported in wheat [90], tomato [91], cabbage [92], and rice [93]. The generation of superoxide anion radicals is inhibited by MT via limiting the level of O2 flux under stress conditions when ADP levels are higher [94]. MT functions through several methods as a mediator in many antioxidant pathways, such as the glutathione ascorbate cycle, peroxidases, superoxide dismutase, and CAT under abiotic stress responses in plants [95]. Talaat and Todorova et al. [96] also observed that the plants treated with MT have increased ascorbate (AsA) and reduced glutathione (GSH) content, thereby reducing the formation of H2O2 in plant cells. The increased non-enzymatic antioxidants like AsA and GSH production are thought to be crucial for maintaining the ROS balance in plants under stress. The positive role of MT on antioxidant enzyme activity was also reported by Ye et al. [97] and Yan et al. [98] in barley and tomato.
4. Melatonin’s Role in Secondary Metabolites’ Expression
Abiotic stress downregulates the accumulation and concentration of plant metabolites, whereas foliar application of MT positively upregulates the metabolites in the plant system. At the cellular level, the concentration of several metabolites was altered by the exogenous application of MT that was both directly or indirectly involved in plant tolerance against drought stress in green gram, and the expressed metabolites were involved in the intermediates of different metabolic pathways [99] (Figure 2). Xie et al. [100] reported that the metabolites involved in the carbon metabolic pathway which includes glycolysis, the oxidative pentose phosphate pathway and the tricarboxylic acid (TCA) cycle, were upregulated by MT and showed a direct link between the carbon metabolic pathway and MT in rice. Proline is one of the compatible solutes that accumulates in plant cells in response to cadmium stress and increases the osmotic adjustment in order to retain membrane integrity. In addition, the experiment found that exogenous application of MT could significantly improve the metabolite group such as amino acids, sugar, and sugar alcohols in tomato plant [91] and the compounds were assigned as intermediates for plant metabolic pathways. Sheikhalipour et al. [101] showed that increased proline concentration by MT also increases the stabilization of protein structures from denaturation under moisture stress. Saddhe et al. [102] described that metabolites like proline and some sugars such as glucose, fructose, sucrose, and trehalose were involved in the regulation of osmotic adjustment under osmotic stress. MT increased the transcription level of various sucrose-related enzymes like sucrose synthase, invertase, phosphatase, and fructokinase and sucrose transporters in plant cells [103]. Yang et al. [104] explained the importance of MT between MdFRK2 and plant growth and MdFRK2 was found to be involved in the MT-mediated accumulation of sugars like glucose, fructose, and sucrose in apple leaves. Jiang et al. [105] found that high levels of metabolite concentration related to amino acids were observed in MT treatment that results in enhanced physiological activities. The primary function of glycolysis in the plant metabolic pathway is to supply energy in the form of ATP and synthesize precursors essential for metabolism of fatty acids and amino acids [106]. Zhang et al. [107] stated that MT improves the metabolites engaged in carbohydrate and amino acid metabolism and upregulates the glycolysis pathway in plants. MT enhances plants’ tolerance to abiotic stresses through detoxification of ROS and osmotic adjustment by synthesizing and accumulating secondary metabolites such as phenols, ascorbic acid, and carbohydrates such as mannitol and ribose which play a major role in antioxidants and osmolytes [108]. Foliar application of MT during drought stress expressed multifaceted metabolites in Carya cathayensis which facilitates the upregulation of biosynthetic pathways such as ABC transporters, porphyrin and chlorophyll metabolism, carotenoid biosynthesis, carbon fixation and metabolism, sugar metabolism, and the phenylpropanoid pathway in MT-treated plants [109]. For plants to fight against various environmental stresses, MT regulates the stress signaling pathways through the accumulation of various flavonoids, polyamines, and phenolic compounds in the plant system [110].
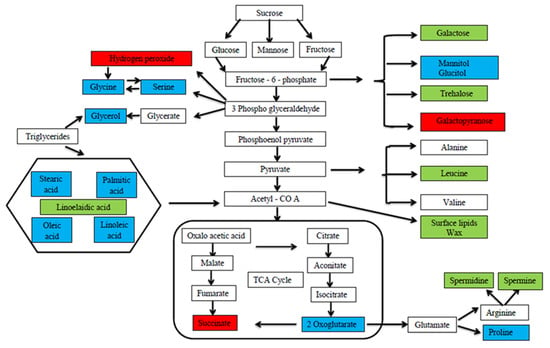
Figure 2.
Different metabolite expressions in control and MT-treated green gram plant under drought stress. In the figure, the blue color highlighted box shows the metabolite expressions in both control and MT-treated plants, the green color highlighted box shows the metabolite expression in MT-treated plants alone, and the red color highlighted box shows the metabolite expression in control plants [99].
The GC-MS metabolomic study of the investigation showed that more than 50 compounds were expressed and regulated by MT treatment in cassava plants [111]. These compounds include amino acids (glycine, arginine, and thymine), fatty acids (oleic acid, palmitic acid, streaic acid, linoleic acid, linolenic acid, and traumatic acid), antioxidants (coumarins, phenols, and flavonoids), aromatic compounds (piperidine), and digitoxin. Salt-stressed plants without MT treatment also expressed some compounds in minimum amounts such as gamolenic acid, gelsimine, burnamicine, oxalic acid, and melibiose, whereas traumatic acid, glycine, arginine, oleic acid, arginine, thymine, and phenols were some compounds found only in MT-treated plants and not in salt-stressed plants. MT application was responsible for the synthesis of spermidine, spermine, and putrescine bioactive compounds through activating precursors like arginine and ornithine [89]. The various abiotic stress studies found that MT endorses the secondary metabolites like spermidine, spermine, and putrescine in Cucumis sativus; flavones, flavanone, luteolin, and isoflavone in pigeon pea [95,112]; and rosmarinic acid, luteolin flavone, and apigenin flavone in Dracocephalum kotschyi Boiss [113].
5. Melatonin’s Role in Crop Yield and Quality
MT enhances the growth-related attributes as well as the photosynthetic pigments and thus maximizes the photoassimilate production and translocation efficiency from source to sink tissues and finally the yield [114]. Khan et al. [115] mentioned that in tomato plant, the number of fruits per plant, fruit yield, and quality characters (ascorbic acid, lycopene content, and β carotene) were increased in MT-treated plants. Hassan et al. [116] reported that exogenous MT significantly improves the weight of the bunch, hands per bunches, total weight of hands, and finger length in banana. In addition, Hu et al. [84] also stated that increased photosynthetic carbon metabolism and partitioning efficiency in the MT-treated plants enhanced the boll formation and seed yield in cotton. MT regulates a variety of physiological and biochemical processes in plants, thereby improving the net photosynthetic rate and productivity of the crop [117]. Medina-Santamarina et al. [118] explained that MT showed a positive effect on the improvement of sink strength that ultimately results in improved berry size, weight, and yield of pomegranate. MT enhances the seed filling rate, seed weight, and final yield of maize crop by regulating the hormonal balance [50]. Jiang et al. [105] also observed that MT delays the early leaf senescence process and improves the photosynthetic efficiency by minimizing the production of ROS, which shows a direct impact on the improvement of quality and yield of rice grains. Liu et al. [67] reported that the number of fruits per plant, per fruit weight, and yield per plant were significantly improved in MT-treated cucumber plants. Application of MT showed a positive correlation between photosynthetic rate, antioxidant enzymes, and seed yield in soybean [119] and maize [120].
Mohamed et al. [121] observed an improvement in oil quality of rapeseed cultivars due to the priming of Brassica napus L. seeds with MT, which increased the concentration of unsaturated fatty acids like linolenic and oleic acids with reduced glucosinolates and saturated fatty acids such as palmitic and arachidic acids under salinity stress. The application of MT improved yield-related characteristics such as seed yield per plant, 1000 seed weight, seed oil content, and seed yield in mustard [122]. Wang et al. [123] investigated the impact of MT on yield traits of soybean and reported an increased number of pods, seeds per pod, and grain yield under stress. In cucumber, the number of fruits, fruit weight, and total yield of the plant were increased under osmotic stress in response to MT treatment [124]. Pretreatment of MT improved the number of pods, seed number per pod, total seed weight, and seed yield of soybean under salt stress [125]. Debnath et al. (2018) found that exogenous application of MT improved the quality and yield of tomato fruits exposed to abiotic stress. Liu et al. [47] noticed that the priming of seeds with MT improved the fruit quality of tomato with the increased accumulation of lycopene, ascorbic acid, and mineral elements in fruits.
Ibrahim et al. [126] observed an enhanced fruit quality in tomato due to MT application which improved the antioxidant enzymes, lycopene, ascorbic acid, and total soluble solids. Gurjar et al. [127] found that exogenous MT increased the shelf life of fruits and vegetables. Medina-Santamarina et al. [118] described that the quality parameters of pomegranate fruits like fruit size, color, total acidity, total soluble solids, fruit number per tree, and fruit yield were improved by the application of MT. Nasser et al. [128] observed that the increase in transcriptome alterations during the ripening process in grape berries enhanced the quality of berries due to MT treatment. Under drought stress, foliar application of MT enhanced the yield and quality of Moringa oleifera L. in terms of amino acid composition, glutamic acid, and nutrition such as nitrogen, phosphorus, potassium, calcium, and magnesium [129]. In flax, total phenolic content, TSS, proline, and free amino acid contents of the seeds were increased by exogenous MT treatment [130]. Farouk and Al-Amri [131] reported that the application of MT in rosemary plants improved the essential oil content and yield under stress conditions. Foliar spray of MT in medicinal lemon verbena shrub (Lippia citriodora) enhanced the yield and essential oil content by 52% and 32%, respectively, under stress conditions [132].
6. Melatonin’s Role in Abiotic Stress Mitigation
Plants experience many adverse situations throughout their lifespan. In order to survive and reproduce successfully in adverse conditions such as drought, salinity, high temperature, flooding, and heavy metal stress, plants have evolved a variety of response mechanisms. MT is a universal compound participating in the nullification of the various abiotic stress responses as a pleiotropic signaling molecule. Furthermore, it is a proficient scavenger of RNS as well as ROS. Numerous research studies have been carried out to investigate the activities of MT in plants since its discovery, indicating its protective properties against abiotic stressors (Table 1).
Drought and high temperature stress reduce the permeability of water in the plants [133]. Stomata play a vital role in regulation of photosynthesis, transpiration rate, and plant water status in response to abiotic stresses [134]. Rao et al. [135] opined that ABA acts as a key mediator for the closure of stomata under stress conditions, which ultimately affects a cascade of physiological and molecular processes. Wang et al. [136] explained that exogenous MT ameliorates the oxidative stress and improves transpiration rate and stomatal conductance in sweet corn. The increase in transpiration rate and stomatal conductance might be due to the upregulation of the ABA catabolism process and the simultaneous downregulation of ABA anabolism that results in reduced accumulation of the endogenous ABA level; this fact was already reported by Hu et al. [137]. The decreased ABA level reduces the production of H2O2 in guard cells of stomata that makes the stomata remain open and maintains the water status of the plant under stress [29]. This might be the reason for the increased transpiration rate and stomatal conductance in green gram under water deficit and high temperature stress conditions. Jiang et al. [138] reported that MT improves the stomatal conductance by regulating the ROS-mediated stomatal closure that results in a higher transpiration rate in response to stress. Leaf water status and leaf temperature are positively regulated by transpiration rate. The increased transpiration rate by MT enables the plant to maintain lower leaf temperatures, thereby improving photosynthetic efficiency [139]. Supriya et al. [140] found that an increased stomatal conductance in MT-treated plants regulates the canopy temperature by enhancing the water loss which ultimately results in lower water use efficiency under stress. At the single-leaf level, the water use efficiency is governed by stomatal conductance and transpiration rate [136]. The response of water use efficiency is closely linked with physiological processes by regulating the concentration of CO2 and H2O in plant cells [27]. MT maintains better water use efficiency under stress through the control of stomatal movements; therefore, it improves the net photosynthetic rate as reported by Li et al. [141]. The positive effects of MT on transpiration rate and stomatal conductance through regulation of the ABA level were also noticed in tomato [142], rice [143], and barley [144].
6.1. Drought
Plants grown in water-stressed environments confront numerous biochemical and molecular challenges, resulting in reduced plant development [145]. Drought stress reduces photosynthesis by interfering with the mechanism of light harvesting and utilization, significantly altering the metabolism of photosynthetic pigments, resulting in a decrease in RuBisCo function and disruption of the photosynthetic apparatus [146] in finger millet [36]. MT helps plants to restore the photosynthetic efficiency by protecting the system from the harmful impacts of drought [147]. It reduces chlorophyll degradation during drought conditions and enhances photosynthesis, transpiration, and stomatal conductivity [148]. MT increases the photosynthetic rate by improving the photochemical efficiency (Fv/Fm) of photosystem II (PSII) and the rate of electron transport (ETR) [149]. After MT treatment, leaves have a higher relative water content, which favors the protection of chloroplast structures in maize [150]. It also helps to maintain cell turgor, which increases the capacity of stomatal openings and conductance [147]. This enhanced stomatal conductance promotes the passage of water and CO2, which in turn promotes photosynthesis in MT-treated plants [151].
Furthermore, it has been shown that MT upregulates the transcript levels of genes involved in ABA breakdown (MdCYP707A1 and MdCYP707A1) while it downregulates MdNCED3, a crucial gene in the ABA biosynthesis pathway. This cellular reaction was aided by an antioxidative mechanism and efficient H2O2 scavenging. Both these strategies are thought to work synergistically to improve stomatal function [152]. MT boosts the capacity of plants to scavenge ROS, protecting them from the damaging effects of drought-induced oxidative stress. This enhanced ROS scavenging is brought on by the MT-stimulated antioxidative defense system in plants developing under drought [153]. MT regulates the drought-induced synthesis of superoxide anions in plant cells, either by increasing scavenging or by limiting the creation of superoxide anions [154]. MT also improves H2O2 scavenging efficiency in plants growing in drought conditions [18]. This is followed by the increased detoxification of damaging hydroxyl radicals that contribute to oxidative stress induction [155]. MT also affects the ascorbate-glutathione cycle and causes ROS, such as H2O2, to be scavenged directly [156].
MT-mediated efficient ROS scavenging in drought-stressed plants protects plant cell walls. This is substantiated by lower MDA levels and less electrolyte leakage in MT-treated plants under water-stress circumstances (Figure 3). MT stimulates the activity of ABA-degrading enzymes as well as H2O2 scavenging enzymes such as CAT, APX, and POD in drought-stressed crops [18]. MT boosts cuticular wax formation and enhances deposition on the leaf’s surface, resulting in little water loss. This increased production is attributed to increased transcript levels of genes that encode enzymes implicated in wax biosynthetic pathways, like KCS1 (ketoacyl-CoA synthase 1) and LTP1 (lipid transfer protein 1) [157].
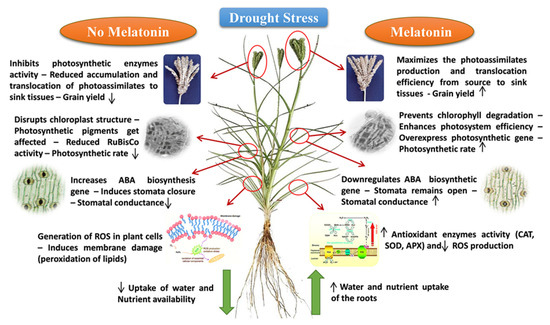
Figure 3.
Melatonin effects on finger millet under drought stress (source: Anitha [158]).
6.2. Salinity
According to Kesawat et al. [159], plants under salt stress are more likely to produce too many ROS, which can lead to membrane lipid or protein peroxidation and the death of normal plant cells. When salt concentrations are excessive, plant roots experience osmotic stress and have lower water potential. Additionally, the absorption of nutrients and water is impacted, which hinders plant growth and development and causes wilting and plant mortality [160]. Under stressful situations, the concentration of MT in the leaves and roots of grapevine seedlings is raised considerably, and the rise is amplified by the severity of stress [161]. To prevent water loss during salt stress, plants seal their stomata. This lowers stomatal conductance (GS), which in turn lowers photosynthesis [162]. However, when subjected to salt stress, employing the right amount of MT may improve stomatal function and enable plants to reopen their stomata [163]. Furthermore, under salt stress, MT increased photosynthesis-related gene transcription while preserving the photosynthetic apparatus [69].
By enhancing chlorophyll formation and reducing its breakdown during salt stress, MT treatment improved the total chlorophyll content and the maximum photochemical reaction efficiency of PSII (Fv/Fm). Under very salty conditions, plants transport extra salt ions from the cytoplasm inside the vacuole or compartmentalize them into separate tissues [164]. The salt-induced Na+/H+ antiporter in the tonoplast oversees compartmentalizing ions within the cytoplasm into vacuoles in order to reduce ion concentrations within the cytoplasm [165]. MT is essential for maintaining ion homeostasis; in order to maintain ion homeostasis under salt stress, MT specifically upregulates the transporter genes NHX1 and AKT1 [166]. The application of MT as a set treatment combined with foliar spray resulted in higher photosynthetic rate, stomatal conductance, transpiration rate, osmotic potential, osmatic adjustment, proline, and soluble protein content of cassava plants under salt stress [167].
6.3. Temperature
One of the main factors limiting plant growth is heat stress, which has a significant negative impact on agricultural production worldwide. In order to sustain numerous physiological, biochemical, and molecular mechanisms to deal with heat stress conditions, MT works as a plant growth regulator. In a recent study, scientists discovered that the ability of tomato to absorb CO2 and produce photosynthetic pigment increased when 100 M of MT was applied. MT lowers photoinhibition and defends the PSI and PSII reaction centers [168]. By enhancing antioxidant defense systems like the bate-glutathione cycle and rewiring the metabolic pathways for nitric oxide production and PAs, MT reduced the severity of heat stress damage [169]. MT enhances tea quality under heat stress by encouraging photosynthetic and biomass accumulation in tea plants [170]. In addition, MT-treated seedlings showed increased expression of anti-stress responsive genes like TaMYB80, TaWRKY26, and TaWRKY39 as well as ROS-related genes TaCAT, TaPOD, and TaSOD [90]. MT treatment increases the root length; leaf area; plant height; fresh and dry root weight; shoot weight; CAT, SOD, POD, and APX activities; soluble sugar content; and protein content of maize [120] and mung bean [73] (Figure 4) under stressful conditions.
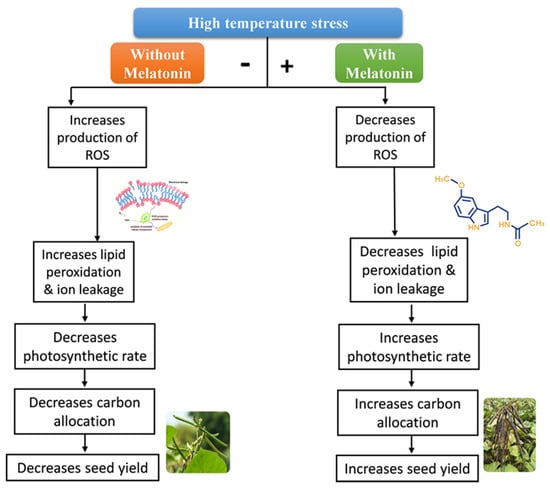
Figure 4.
Schematic representation of melatonin-mediated high−temperature stress tolerance in plants. (Source: Modified from Kuppusamy et al. [73].)
6.4. Flooding
MT has been shown to be an effective phytohormone for protecting apple plants against waterlogging stress as reported by [171]. A recent research study by [172] examined the effects of MT pretreatment on lucerne under waterlogging stress and found that it could mitigate the damage caused by the stress and improve chlorophyll content, plant growth, and PSII efficiency. Zheng et al. [171] originally suggested that MT facilitated the mechanism for tolerating waterlogging in apple seedlings by successfully preventing the ROS burst and subsequent mitochondrial breakdown; this mechanism preserves aerobic respiration and photosynthesis. Another concept in lucerne was proposed by [172] via interacting with or directly controlling the metabolic pathways of ethylene and polyamines (PAs). The scientists suggested that MT promotes waterlogging tolerance, at least in part, by regulating ethylene and polyamine’s production because ethylene is suppressed and polyamine is promoted. As a result, cell membranes are more stable, photosynthesis is improved, and there is less ethylene-responsive senescence [172].
6.5. Heavy Metals
Toxicity caused by heavy metals (HM) is one of the most harmful abiotic stressors. Plants do not need lead [31], cadmium [173], mercury (Hg), or arsenic (As), all of which are extremely detrimental to plants [174,175]. Authors including Chandrakar et al. [176], Chen et al. [177], and Umapathi et al. [91] reported that the majority of heavy metals continuously produce ROS which can lead to oxidative stress in plants and the unanticipated side effect of heavy metal toxicity (Figure 5). Lipid peroxidation, a harmful condition brought on by HM-induced ROS, impairs the integrity and functionality of cell membranes [178,179]. Numerous studies emphasized how heavy metals affect the accumulation of endogenous MT in plants. Studies revealed that HMs induced endogenous MT biosynthesis in the root tissue of Hordeum vulgare (barley), Solanum lycopersicum (tomato), and Lupinus albus (lupin) [34,180,181]; in the leaves of Nicotiana tabacum, Arabidopsis thaliana, and tomato [182,183]; and in the seedlings of Oryza sativa (rice) [184]. The structural integrity of cellular organelles such as chloroplasts, mitochondria, and the endoplasmic reticulum is dramatically compromised in HMs under Cd stress, for example. Endogenous serotonin N-acetyltransferase (SNAT) enzymes are subsequently released into the cytosol as a result [185,186] where they can easily come into contact with serotonin, resulting in N-acetyl serotonin synthesis and ultimately MT formation.
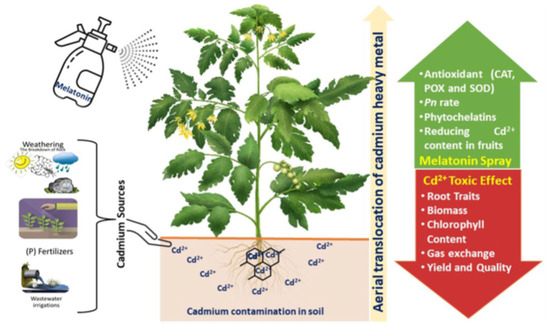
Figure 5.
Overview of melatonin mitigating Cd toxicity in tomato plant (source: Umapathi et al. [91]).
MT is useful for a variety of purposes, and because of its capacity to directly neutralize ROS, it aids in protecting plants from oxidative stress. Additionally, it possesses chelating properties, which may help to lessen the toxicity brought on by such metals. MT, an amphiphilic molecule, may easily diffuse through cell membranes, enter the cytoplasm, and go to subcellular compartments [187]. Through the activation of antioxidant defense mechanisms, exogenous MT application can reduce Cd and Zn damages and enhance tolerance in lemon balm plants as opined by Hodzic et al. [188].

Table 1.
Effect of melatonin on the physiological functions in crop plants under various abiotic stresses.
Table 1.
Effect of melatonin on the physiological functions in crop plants under various abiotic stresses.
| S.No. | Plant Species | Abiotic Stress | Melatonin Concentration (µM) | Plant Response | Reference |
|---|---|---|---|---|---|
| 1 | Rice | Salinity | 20 | Improve the root and shoot, dry weight, and K+ content | [15] |
| Drought | 200 | Improve the germination percentage and seedling characters | [37] | ||
| High temperature | 200 | Improved photosynthesis, stabilize starch synthesis, and reduce grain chalkiness | [189] | ||
| Low temperature | 150 | Improved seed germination and traits associated with germination | [190] | ||
| 2 | Wheat | Salinity | 200 | Seed germination and seedling characteristics | [56] |
| 3 | Maize | Drought | 100 | Effective increase in the antioxidant enzyme and photosystems activity Reduces the H2O2, superoxide anion, and MDA levels | [191] |
| 4 | Cotton | Drought | 100 | Delaying leaf senescence | [192] |
| 5 | Soybean | Nitrogen deficient | 100 | Better total nitrogen fixation capacity and upregulating the expression of genes related to nitrogen metabolism (NR2, NiR, GS1β, GOGAT, and GmAAP6a) | [66] |
| 6 | Green gram | High temperature | 100 | Improve the root and shoot length Reduced the MDA content and improve the antioxidant content | [193] |
| Drought | 100 | Increased seed germination and seedling vigor | [36] | ||
| 7 | Finger millet | Drought | 60 as nano formulation | Increased photosynthetic activity, effective antioxidant system, and improved carbohydrate assimilation and translocation | [194] |
| 40 and 60 | Improve the seed germination and seedling establishment | [195] | |||
| 8 | Tomato | Cadmium stress | 108 | Minimizing the Cd accumulation in fruit and increase the antioxidant enzyme activity | [91] |
| High temperature | 10 | Silencing the COMT1gene and increase the APX and CAT activity | [196] | ||
| 9 | Cassava | Salinity | 430 | Higher gas exchange and soluble protein content | [167] |
| 10 | Alfalfa | Salinity | 300 | Increase the antioxidant capacity, osmotic regulation, and photosynthesis | [72] |
| 11 | Apple | Salinity | 0.1 | Maintain ion homeostasis, enhance the level of antioxidant enzymes, and maintain photosynthesis | [166] |
| Drought | 100 | Improved nitrogen assimilation and endogenous MT content | [148] | ||
| 12 | Grapes | Drought | 100 | Prevent chloroplast damage and improve antioxidant activity | [197] |
| 13 | Coffee | Drought | 300 | Enhanced carboxylation efficiency and antioxidant activity | [153] |
| 14 | Tea | Chilling stress | 100 | Prevent oxidative damage and improved photosynthetic pigments | [198] |
| Drought | 100 | Reduce membrane damage and enhance the level of proline, total protein, and sugars | [199] | ||
| Cd Toxicity | 150 | Scavenge reactive oxygen species and enhance the level of antioxidants | [200] | ||
| 15 | Cucumber | Chilling stress | 200 | Reduce electrolyte leakage and improve photosynthesis | [201] |
| 16 | Melon | Chilling stress | 200 | Reduce ROS and increase proline and soluble protein content | [202] |
| 17 | Peach | Chilling stress | 200 | Prevent oxidative damage and improve the ascorbic acid content in fruits | [203] |
7. Approaches for Enhancing Endogenous Melatonin
MT is a pivotal compound present in the plant system. In that way, increasing endogenous MT is crucial to combat against abiotic stresses in the agricultural field. MT biosynthesis consists of four enzymatic processes, viz., TDC, T5H, SNAT, and ASMT.
The transgenic approach is a useful tool for improving the endogenous MT content. Nonetheless, notable studies were conducted concerning the overexpression of MT under various abiotic stresses in different crops. Previously, studies confirmed that abiotic stress significantly increases the MT level in plant systems [204,205]. In order to boost the synthesis of antioxidants like MT without impairing plant growth and development or having unintended side effects on other metabolic pathways, endogenous metabolic pathways must be modulated. Overexpression of genes and enzymes involved in MT biosynthesis through the transgenic approach might improve the endogenous MT. In plants, the major enzymes involved in the MT biosynthetic pathway such as serotonin N-acetyl transferase (SNAT) and N-acetyl serotonin methyl transferase (ASMT) were found to have maximum catalytic efficiency values at 55 °C (Byeon et al., 2014). N-acetyl serotonin O-methyltransferase (ASMT), one of the enzymes involved in the MT biosynthesis process, has been expressed in transgenic plants [206]. In transgenic tomato plants, the overexpression of MT biosynthetic genes such as arylalkyl amine N-acetyl transferase (AANAT) and hydroxyindole-O-methyl transferase (HIOMT) increases the endogenous MT level and enhances the tolerance capacity of plants against stresses [207]. The enzyme caffeine acid methyltransferase (COMT), which is involved in MT biosynthesis, may also help to control plant development, growth, and stress responses. CrCOMT from Carex iridescent overexpression alters MT production in Arabidopsis thaliana and causes an increase in salt stress [208]. In Brassica rapa, miR168a enhances the MT level through increased expression of the O-METHYLTRANSFERASE 1 (OMT1) gene, which is responsible for MT biosynthesis [209].
The serotonin N-acetyltransferase (SNAT) enzyme which is crucial for MT biosynthesis increases the accumulation of MT in plants in response to stress conditions [210]. Overexpression of the VvSNAT1 gene also increased MT synthesis in transgenic Arabidopsis [211]. Suppression of the OsSNAT gene reduced the levels of endogenous MT in transgenic rice (Oryza sativa L.) plants, which results in poor seedling growth and development [182]. In transgenic Arabidopsis plants, mutation of the apple MzSNAT5 gene leads to the reduced production of MT in the mitochondria, which results in enhanced ROS accumulation and susceptibility of plants to drought stress [186]. Increased MT synthesis was observed in transgenic Arabidopsis by overexpression of the TaCOMT gene [104]. The tolerance capacity of the tomato plant to salinity stress improved with the upregulation of MT biosynthetic gene SlCOMT1 [212]. Similarly, overexpressing the HIOMT gene in apple resulted in increased MT synthesis and reduced production of ROS [213].
Exogenous application is another approach to bring up the endogenous MT content inside the plant. In addition to being a natural bioregulator, exogenous compounds like benzothiadiazole (BTH) and chitosan (CHT) can be used to stimulate the production of MT in plants [214]. Numerous studies were conducted on the enhancement of endogenous MT through exogenous application in various plants such as tomato [183], Arabidopsis [215], groundnut [216], and hemp [217]. In response to various stresses in plants, the application of MT accumulates more endogenous MT by overexpressing the MT biosynthetic genes such as TDC, T5H, SNAT, and ASMT [27]. In addition, the foliar spray application of MT significantly enhanced the endogenous MT content in the tomato plant under cadmium-induced heavy metal stress [183].
8. Conclusions
Crop abiotic stress causes a significant yield decline, which has an impact on the safety of the world’s food supply. Therefore, it is more important to concentrate on raising agricultural plants’ resistance to stress. Globally, MT is evolving as a pioneer compound to mitigate the abiotic stresses in the agricultural field. We outlined the regulatory systems that underpin plants’ ability to withstand abiotic stress in this review. MT significantly improves the scavenging of ROS and RNS to enhance the antioxidant capacity. The biosynthetic pathway of MT has been identified in a number of plant species in which TDC, T5H, SNAT, ASMT, and COMT are the key enzymes for MT biosynthesis. Due to its positive impacts on plant tolerance to environmental stressors, the MT catabolic pathway and its metabolites have drawn more and more attention in recent years. The exogenous MT application in varied crops exhibited a better performance in physiological and biochemical traits associated with improved yield potential. Moreover, the exogenous application of MT is not specific to genotype and it is less time-consuming, more cost-effective, and is readily available for large-scale applications. The effect of MT on growth, physiology, yield, and biochemical parameters reveals that there might be a long-term effect of this compound in improving the abiotic stress tolerance. Hence, it is necessary to study how the pretreatment of MT could be effective to prepare crops for unpredicted sudden stress conditions. Some of the methods through which MT interacts with other phytohormones remain obscure, despite the fact that it can affect the manufacturing and signaling of other phytohormones. MT application studies have been extensively studied only at a laboratory level and the large-scale commercial application of MT has been rarely conducted. Hence, field-level examinations are required to assess the effects of MT on crop yield under open conditions.
Author Contributions
Conceptualization, K.M.K.; Bibliographic search and writing original draft, U.M., A.K. (Anitha Kuppusamy), M.R., and A.K. (Arunkumar Kathirvel); Review and editing, G.V., S.A., K.R., K.P.S., S.R., T.K. and S.K.; Visualization, U.M., A.K. (Anitha Kuppusamy) and A.K. (Arunkumar Kathirvel); All authors have read and agreed to the published version of the manuscript.
Funding
S.K. was supported by Formas—A Swedish Research Council for Sustainable Development (grant number 2018-01301) and C4F (Crops for the Future).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This study did not generate any new data or analyze any existing data. Sharing of data is not relevant to this subject.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocyteS1. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Dubbels, R.; Reiter, R.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Van Tassel, D.L.; Roberts, N.; Lewy, A.; O’Neill, S.D. Melatonin in plant organs. J. Pineal Res. 2001, 31, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Herbert, D.C.; Vaughan, M.K.; Yaga, K.; Reiter, R. Melatonin inhibits luteinizing hormone releasing hormone (LHRH) induction of LH release from fetal rat pituitary cells. Neurosci. Lett. 1995, 184, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Zohar, R.; Izhaki, I.; Koplovich, A.; Ben-Shlomo, R. Phytomelatonin in the leaves and fruits of wild perennial plants. Phytochem. Lett. 2011, 4, 222–226. [Google Scholar] [CrossRef]
- Murch, S.J.; Erland, L.A. A systematic review of melatonin in plants: An example of evolution of literature. Front. Plant Sci. 2021, 12, 683047. [Google Scholar] [CrossRef]
- Blask, D.E.; Dauchy, R.T.; Sauer, L.A.; Krause, J.A. Melatonin uptake and growth prevention in rat hepatoma 7288CTC in response to dietary melatonin: Melatonin receptor-mediated inhibition of tumor linoleic acid metabolism to the growth signaling molecule 13-hydroxyoctadecadienoic acid and the potential role of phytomelatonin. Carcinogenesis 2004, 25, 951–960. [Google Scholar] [PubMed]
- Debnath, B.; Islam, W.; Li, M.; Sun, Y.; Lu, X.; Mitra, S.; Hussain, M.; Liu, S.; Qiu, D. Melatonin mediates enhancement of stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 1040. [Google Scholar] [CrossRef]
- Mauriz, J.L.; Collado, P.S.; Veneroso, C.; Reiter, R.J.; González-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013, 54, 1–14. [Google Scholar] [CrossRef]
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A multifunctional factor in plants. Int. J. Mol. Sci. 2018, 19, 1528. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, M.; Guo, J.; Wang, Y.; Min, D.; Jiang, Q.; Ji, H.; Huang, C.; Wei, W.; Xu, H. Overexpression of soybean DREB1 enhances drought stress tolerance of transgenic wheat in the field. J. Exp. Bot. 2020, 71, 1842–1857. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Song, X.; Hussein Ibrahim, M.E.; Jamal, Y.; Younas, M.U.; Zhu, G.; Zhou, G.; Adam Ali, A.Y. The role of melatonin in plant growth and metabolism, and its interplay with nitric oxide and auxin in plants under different types of abiotic stress. Front. Plant Sci. 2023, 14, 1108507. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.; Cai, N.; Zhu, W.; Li, L.; Guan, M.; Pu, X.; Chen, Q. The role of phytomelatonin receptor 1-mediated signaling in plant growth and stress response. Front. Plant Sci. 2023, 14, 1142753. [Google Scholar] [CrossRef]
- Liu, J.; Shabala, S.; Zhang, J.; Ma, G.; Chen, D.; Shabala, L.; Zeng, F.; Chen, Z.H.; Zhou, M.; Venkataraman, G. Melatonin improves rice salinity stress tolerance by NADPH oxidase-dependent control of the plasma membrane K+ transporters and K+ homeostasis. Plant Cell Environ. 2020, 43, 2591–2605. [Google Scholar] [CrossRef]
- Castañares, J.L.; Bouzo, C.A. Effect of exogenous melatonin on seed germination and seedling growth in melon (Cucumis melo L.) under salt stress. Hortic. Plant J. 2019, 5, 79–87. [Google Scholar] [CrossRef]
- Li, Z.; Su, X.; Chen, Y.; Fan, X.; He, L.; Guo, J.; Wang, Y.; Yang, Q. Melatonin improves drought resistance in maize seedlings by enhancing the antioxidant system and regulating abscisic acid metabolism to maintain stomatal opening under PEG-induced drought. J. Plant Biol. 2021, 64, 299–312. [Google Scholar] [CrossRef]
- Li, C.; Tan, D.-X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef]
- Hernández, I.G.; Gomez, F.J.V.; Cerutti, S.; Arana, M.V.; Silva, M.F. Melatonin in Arabidopsis thaliana acts as plant growth regulator at low concentrations and preserves seed viability at high concentrations. Plant Physiol. Biochem. 2015, 94, 191–196. [Google Scholar] [CrossRef]
- Kołodziejczyk, I.; Dzitko, K.; Szewczyk, R.; Posmyk, M.M. Exogenous melatonin improves corn (Zea mays L.) embryo proteome in seeds subjected to chilling stress. J. Plant Physiol. 2016, 193, 47–56. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, Y.; Ji, Y.; Wu, X.; Xu, X.; Wu, P. Melatonin promotes seed germination via regulation of ABA signaling under low temperature stress in cucumber. J. Plant Growth Regul. 2023, 42, 2232–2245. [Google Scholar] [CrossRef]
- Dradrach, A.; Iqbal, M.; Lewińska, K.; Jędroszka, N.; Rana, M.A.K.; Tanzeem-ul-Haq, H.S. Effects of soil application of chitosan and foliar melatonin on growth, photosynthesis, and heavy metals accumulation in wheat growing on wastewater polluted soil. Sustainability 2022, 14, 8293. [Google Scholar] [CrossRef]
- Hasan, M.K.; Ahammed, G.J.; Sun, S.; Li, M.; Yin, H.; Zhou, J. Melatonin inhibits cadmium translocation and enhances plant tolerance by regulating sulfur uptake and assimilation in Solanum lycopersicum L. J. Agric. Food Chem. 2019, 67, 10563–10576. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Bai, Y.L.; Gong, C.; Song, W.; Wu, Y.; Ye, T.; Feng, Y.Q. The phytomelatonin receptor PMTR1 regulates seed development and germination by modulating abscisic acid homeostasis in Arabidopsis thaliana. J. Pineal Res. 2022, 72, e12797. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Asif, S.; Asaf, S.; Du, X.-X.; Park, J.-R.; Nari, K.; Bhatta, D.; Lee, I.-j.; Kim, K.-M. Melatonin alleviates arsenic (As) toxicity in rice plants via modulating antioxidant defense system and secondary metabolites and reducing oxidative stress. Environ. Pollut. 2023, 318, 120868. [Google Scholar] [CrossRef]
- Tan, D.-X.; Reiter, R.J. An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J. Exp. Bot. 2020, 71, 4677–4689. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef]
- Byeon, Y.; Back, K. Melatonin synthesis in rice seedlings in vivo is enhanced at high temperatures and under dark conditions due to increased serotonin N-acetyltransferase and N-acetylserotonin methyltransferase activities. J. Pineal Res. 2014, 56, 189–195. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Lan, Z.; Zhang, Z.; Ahammed, G.J.; Chang, J.; Zhang, Y.; Wei, C.; Zhang, X. Melatonin antagonizes ABA action to promote seed germination by regulating Ca2+ efflux and H2O2 accumulation. Plant Sci. 2021, 303, 110761. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Lu, B.; Ma, T.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Bai, Z. Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS ONE 2020, 15, e0228241. [Google Scholar] [CrossRef]
- Snider, J.; Virk, S.; Hand, L.C.; Porter, W.; Virk, G. Considerations for Stand Establishment and Early Seedling Growth in Cotton. Crops Soils 2022, 55, 48–55. [Google Scholar] [CrossRef]
- Lei, K.; Sun, S.; Zhong, K.; Li, S.; Hu, H.; Sun, C.; Zheng, Q.; Tian, Z.; Dai, T.; Sun, J. Seed soaking with melatonin promotes seed germination under chromium stress via enhancing reserve mobilization and antioxidant metabolism in wheat. Ecotoxicol. Environ. Saf. 2021, 220, 112241. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Charagh, S.; García-Caparrós, P.; Rahman, M.A.; Ogwugwa, V.H.; Saeed, F.; Jin, W. Melatonin-mediated temperature stress tolerance in plants. GM Crops Food 2022, 13, 196–217. [Google Scholar] [CrossRef]
- Hernández-Ruiz, J.; Arnao, M.B. Melatonin stimulates the expansion of etiolated lupin cotyledons. Plant Growth Regul. 2008, 55, 29–34. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Kuran, H.; Marciniak, K.; Janas, K.M. Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J. Pineal Res. 2008, 45, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Anitha, K.; Senthil, A.; Kalarani, M.; Senthil, N.; Marimuthu, S.; Djanaguiraman, M.; Umapathi, M. Exogenous melatonin improves seed germination and seedling growth in greengram under drought stress. J. Appl. Nat. Sci. 2022, 14, 1190–1197. [Google Scholar] [CrossRef]
- Megala, R.; Kalarani, M.; Jeyakumar, P.; Senthil, N.; Pushpam, R.; Umapathi, M. Standardization of optimum melatonin concentration for drought tolerance at germination and early development stage in rice (CO-54). J. Appl. Nat. Sci. 2022, 14, 1022–1030. [Google Scholar] [CrossRef]
- Umapathi, M.; Kalarani, M.; Srinivasan, S. Optimization of Melatonin to Mitigate Cadmium Stress at Seedling Level in Tomato. Madras Agric. J. 2018, 105. [Google Scholar] [CrossRef]
- Muhammad, I.; Yang, L.; Ahmad, S.; Farooq, S.; Khan, A.; Muhammad, N.; Ullah, S.; Adnan, M.; Ali, S.; Liang, Q.P. Melatonin-priming enhances maize seedling drought tolerance by regulating the antioxidant defense system. Plant Physiol. Biochem. 2023, 191, 2301–2315. [Google Scholar] [CrossRef]
- Yu, R.; Zuo, T.; Diao, P.; Fu, J.; Fan, Y.; Wang, Y.; Zhao, Q.; Ma, X.; Lu, W.; Li, A. Melatonin enhances seed germination and seedling growth of Medicago sativa under salinity via a putative melatonin receptor MsPMTR1. Front. Plant Sci. 2021, 12, 702875. [Google Scholar] [CrossRef]
- Guo, Y.; Li, D.; Liu, L.; Sun, H.; Zhu, L.; Zhang, K.; Zhao, H.; Zhang, Y.; Li, A.; Bai, Z. Seed priming with melatonin promotes seed germination and seedling growth of Triticale hexaploide L. under PEG-6000 induced drought stress. Front. Plant Sci. 2022, 13, 932912. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lu, B.; Liu, L.; Duan, W.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Li, C. Melatonin promotes seed germination under salt stress by regulating ABA and GA3 in cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2021, 162, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Zhang, N.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.Y.; Weeda, S.; Zhao, B.; Ren, S. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA 4 interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Rajora, N.; Vats, S.; Raturi, G.; Thakral, V.; Kaur, S.; Rachappanavar, V.; Kumar, M.; Kesarwani, A.K.; Sonah, H.; Sharma, T.R. Seed priming with melatonin: A promising approach to combat abiotic stress in plants. Plant Stress 2022, 4, 100071. [Google Scholar] [CrossRef]
- Farooq, M.; Romdhane, L.; Al Sulti, M.K.; Rehman, A.; Al-Busaidi, W.M.; Lee, D.J. Morphological, physiological and biochemical aspects of osmopriming-induced drought tolerance in lentil. J. Agron. Crop Sci. 2020, 206, 176–186. [Google Scholar] [CrossRef]
- Tatar, Ö.; Brück, H.; Asch, F. Atmospheric and soil water deficit induced changes in chemical and hydraulic signals in wheat (Triticum aestivum L.). J. Agron. Crop Sci. 2023, 209, 242–250. [Google Scholar] [CrossRef]
- Liu, G.; Hu, Q.; Zhang, X.; Jiang, J.; Zhang, Y.; Zhang, Z. Melatonin biosynthesis and signal transduction in plants in response to environmental conditions. J. Exp. Bot. 2022, 73, 5818–5827. [Google Scholar] [CrossRef]
- Yang, L.; You, J.; Li, J.; Wang, Y.; Chan, Z. Melatonin promotes Arabidopsis primary root growth in an IAA-dependent manner. J. Exp. Bot. 2021, 72, 5599–5611. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, M.; Wang, X.; Liu, L.; Wu, H.; Chen, X.; Wang, H.; Shen, Q.; Chen, G.; Wang, Y. Seed-soaking with melatonin for the improvement of seed germination, seedling growth, and the antioxidant defense system under flooding stress. Agronomy 2022, 12, 1918. [Google Scholar] [CrossRef]
- Ahmad, S.; Wang, G.-Y.; Muhammad, I.; Chi, Y.-X.; Zeeshan, M.; Nasar, J.; Zhou, X.-B. Interactive effects of melatonin and nitrogen improve drought tolerance of maize seedlings by regulating growth and physiochemical attributes. Antioxidants 2022, 11, 359. [Google Scholar] [CrossRef]
- Duan, W.; Lu, B.; Liu, L.; Meng, Y.; Ma, X.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H. Effects of exogenous melatonin on root physiology, transcriptome and metabolome of cotton seedlings under salt stress. Int. J. Mol. Sci. 2022, 23, 9456. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.; Singh, A.K.; Zahoor, I. Melatonin: Role in abiotic stress resistance and tolerance. In Plant Growth Regulators: Signalling under Stress Conditions; Springer: Berlin/Heidelberg, Germany, 2021; pp. 239–273. [Google Scholar]
- Wen, D.; Gong, B.; Sun, S.; Liu, S.; Wang, X.; Wei, M.; Yang, F.; Li, Y.; Shi, Q. Promoting roles of melatonin in adventitious root development of Solanum lycopersicum L. by regulating auxin and nitric oxide signaling. Front. Plant Sci. 2016, 7, 718. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Cui, W.; Kamran, M.; Ahmad, I.; Meng, X.; Wu, X.; Su, W.; Javed, T.; El-Serehy, H.A.; Jia, Z. Exogenous application of melatonin induces tolerance to salt stress by improving the photosynthetic efficiency and antioxidant defense system of maize seedling. J. Plant Growth Regul. 2021, 40, 1270–1283. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Mora-Poblete, F.; Arnao, M.B.; Naz, S.; Anwar, M.; Altaf, M.M.; Shahid, S.; Shakoor, A. Phytomelatonin: An overview of the importance and mediating functions of melatonin against environmental stresses. Physiol. Plant. 2021, 172, 820–846. [Google Scholar] [CrossRef]
- Wang, J.; Lv, P.; Yan, D.; Zhang, Z.; Xu, X.; Wang, T.; Wang, Y.; Peng, Z.; Yu, C.; Gao, Y. Exogenous melatonin improves seed germination of wheat (Triticum aestivum L.) under salt stress. Int. J. Mol. Sci. 2022, 23, 8436. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.-X.; Naz, S.; Altaf, M.M.; Khan, L.U.; Tiwari, R.K.; Lal, M.K.; Shahid, M.A.; Kumar, R. Melatonin improves drought stress tolerance of tomato by modulating plant growth, root architecture, photosynthesis, and antioxidant defense system. Antioxidants 2022, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, D.; Ma, Y.; Shen, H.; Zhao, S.; Wang, Y. Combined application of arbuscular mycorrhizal fungi and exogenous melatonin alleviates drought stress and improves plant growth in tobacco seedlings. J. Plant Growth Regul. 2021, 40, 1074–1087. [Google Scholar] [CrossRef]
- Imran, M.; Latif Khan, A.; Shahzad, R.; Aaqil Khan, M.; Bilal, S.; Khan, A.; Kang, S.-M.; Lee, I.-J. Exogenous melatonin induces drought stress tolerance by promoting plant growth and antioxidant defence system of soybean plants. AoB Plants 2021, 13, plab026. [Google Scholar] [CrossRef]
- Sultana, S.; Barthakur, S. Seed priming with melatonin improves seed germination and root system architecture in wheat (Triticum aestivum L.). Pharma Innov. J. 2023, 12, 574–578. [Google Scholar]
- Morales, F.; Ancín, M.; Fakhet, D.; González-Torralba, J.; Gámez, A.L.; Seminario, A.; Soba, D.; Ben Mariem, S.; Garriga, M.; Aranjuelo, I. Photosynthetic metabolism under stressful growth conditions as a bases for crop breeding and yield improvement. Plants 2020, 9, 88. [Google Scholar] [CrossRef]
- Abdulbaki, A.S.; Alsamadany, H.; Alzahrani, Y.; Olayinka, B.U. Rubisco and abiotic stresses in plants: Current assessment. Turk. J. Bot. 2022, 46, 541–552. [Google Scholar] [CrossRef]
- Ullah, A.; Al-Rajhi, R.S.; Al-Sadi, A.M.; Farooq, M. Wheat genotypes with higher intercellular CO2 concentration, rate of photosynthesis, and antioxidant potential can better tolerate drought stress. J. Soil Sci. Plant Nutr. 2021, 21, 2378–2391. [Google Scholar] [CrossRef]
- Fu, J.; Krishna Jagadish, S.; Bowden, R.L. Effects of post-flowering heat stress on chlorophyll content and yield components of a spring wheat diversity panel. Crop Sci. 2022, 62, 1926–1936. [Google Scholar] [CrossRef]
- Hundare, A.; Joshi, V.; Joshi, N. Salicylic acid attenuates salinity-induced growth inhibition in in vitro raised ginger (Zingiber officinale Roscoe) plantlets by regulating ionic balance and antioxidative system. Plant Stress 2022, 4, 100070. [Google Scholar] [CrossRef]
- Wang, H.; Ren, C.; Cao, L.; Zhao, Q.; Jin, X.; Wang, M.; Zhang, M.; Yu, G.; Zhang, Y. Exogenous melatonin modulates physiological response to nitrogen and improves yield in nitrogen-deficient soybean (Glycine max L. Merr.). Front. Plant Sci. 2022, 13, 865758. [Google Scholar] [CrossRef]
- Liu, K.; Jing, T.; Wang, Y.; Ai, X.; Bi, H. Melatonin delays leaf senescence and improves cucumber yield by modulating chlorophyll degradation and photoinhibition of PSII and PSI. Environ. Exp. Bot. 2022, 200, 104915. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, J.; Burgess, P.; Rossi, S.; Huang, B.J.E.; Botany, E. Interactive effects of melatonin and cytokinin on alleviating drought-induced leaf senescence in creeping bentgrass (Agrostis stolonifera). Environ. Exp. Bot. 2018, 145, 1–11. [Google Scholar] [CrossRef]
- Shi, H.; Wang, X.; Tan, D.X.; Reiter, R.J.; Chan, Z. Comparative physiological and proteomic analyses reveal the actions of melatonin in the reduction of oxidative stress in Bermuda grass (Cynodon dactylon (L). Pers.). J. Pineal Res. 2015, 59, 120–131. [Google Scholar] [CrossRef]
- Gholami, R.; Hoveizeh, N.F.; Zahedi, S.M.; Gholami, H.; Carillo, P. Melatonin alleviates the adverse effects of water stress in adult olive cultivars (Olea europea cv. Sevillana & Roughani) in field condition. Agric. Water Manag. 2022, 269, 107681. [Google Scholar]
- Yu, J.C.; Lu, J.Z.; Cui, X.Y.; Guo, L.; Wang, Z.J.; Liu, Y.D.; Wang, F.; Qi, M.F.; Liu, Y.F.; Li, T.L. Melatonin mediates reactive oxygen species homeostasis via SlCV to regulate leaf senescence in tomato plants. J. Pineal Res. 2022, 73, e12810. [Google Scholar] [CrossRef]
- Guo, X.; Shi, Y.; Zhu, G.; Zhou, G. Melatonin Mitigated Salinity Stress on Alfalfa by Improving Antioxidant Defense and Osmoregulation. Agronomy 2023, 13, 1727. [Google Scholar] [CrossRef]
- Kuppusamy, A.; Alagarswamy, S.; Karuppusami, K.M.; Maduraimuthu, D.; Natesan, S.; Ramalingam, K.; Muniyappan, U.; Subramanian, M.; Kanagarajan, S. Melatonin Enhances the Photosynthesis and Antioxidant Enzyme Activities of Mung Bean under Drought and High-Temperature Stress Conditions. Plants 2023, 12, 2535. [Google Scholar] [CrossRef]
- Jahan, M.S.; Zhao, C.J.; Shi, L.B.; Liang, X.R.; Jabborova, D.; Nasar, J.; Zhou, X.B. Physiological mechanism of melatonin attenuating to osmotic stress tolerance in soybean seedlings. Front. Plant Sci. 2023, 14, 1193666. [Google Scholar] [CrossRef]
- Jensen, N.B.; Ottosen, C.-O.; Zhou, R. Exogenous Melatonin Alters Stomatal Regulation in Tomato Seedlings Subjected to Combined Heat and Drought Stress through Mechanisms Distinct from ABA Signaling. Plants 2023, 12, 1156. [Google Scholar] [CrossRef]
- Barman, D.; Ghimire, O.; Chinnusamy, V.; Kumar, R.; Arora, A. Amelioration of heat stress during reproductive stage in rice by melatonin. Indian J. Agric. Sci. 2019, 89, 1151–1156. [Google Scholar] [CrossRef]
- Khosravi, S.; Haghighi, M.; Mottaghipisheh, J. Effects of melatonin foliar application on hot pepper growth and stress tolerance. Plant Stress 2023, 9, 100192. [Google Scholar] [CrossRef]
- Farooq, M.A.; Islam, F.; Ayyaz, A.; Chen, W.; Noor, Y.; Hu, W.; Hannan, F.; Zhou, W. Mitigation effects of exogenous melatonin-selenium nanoparticles on arsenic-induced stress in Brassica napus. Environ. Pollut. 2022, 292, 118473. [Google Scholar] [CrossRef]
- Lin, S.; Song, X.-F.; Mao, H.-T.; Li, S.-Q.; Gan, J.-Y.; Yuan, M.; Zhang, Z.-W.; Yuan, S.; Zhang, H.-Y.; Su, Y.-Q. Exogenous melatonin improved photosynthetic efficiency of photosystem II by reversible phosphorylation of thylakoid proteins in wheat under osmotic stress. Front. Plant Sci. 2022, 13, 966181. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Caparros, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative stress and antioxidant metabolism under adverse environmental conditions: A review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Gu, Q.; Xiao, Q.; Chen, Z.; Han, Y. Crosstalk between melatonin and reactive oxygen species in plant abiotic stress responses: An update. Int. J. Mol. Sci. 2022, 23, 5666. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Singh, D.; Rashid, F.; Kumar, A.; Kaur, H.; Kaur, K.; Singh, A.; Bedi, N.; Bedi, P.M.S.; Singh, B. Role of Melatonin-A Signaling Molecule in Modulation of Antioxidant Defense System in Plants: Amelioration of Drought and Salinity Stress. In Environmental Stress Physiology of Plants and Crop Productivity; Springer: Berlin/Heidelberg, Germany, 2021; Volume 124. [Google Scholar]
- Zhang, T.; Wang, J.; Sun, Y.; Zhang, L.; Zheng, S. Versatile roles of melatonin in growth and stress tolerance in plants. J. Plant Growth Regul. 2022, 41, 507–523. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, J.; Yan, K.; Zhou, Z.; Zhao, W.; Zhang, X.; Pu, Y.; Yu, R. Beneficial effects of abscisic acid and melatonin in overcoming drought stress in cotton (Gossypium hirsutum L.). Physiol. Plant. 2021, 173, 2041–2054. [Google Scholar] [CrossRef] [PubMed]
- Rehman, R.S.; Hussain, M.; Ali, M.; Zafar, S.A.; Pasha, A.N.; Bashir, H.; Ashraf, N.A.; Javed, A.; Shah, W.A. A Comprehensive Review on Melatonin Compound and Its Functions in Different Fungi and Plants. Int. J. Pathog. Res. 2022, 10, 9–21. [Google Scholar] [CrossRef]
- Manafi, H.; Baninasab, B.; Gholami, M.; Talebi, M.; Khanizadeh, S. Exogenous melatonin alleviates heat-induced oxidative damage in strawberry (Fragaria × ananassa Duch. cv. Ventana) Plant. J. Plant Growth Regul. 2022, 41, 52–64. [Google Scholar] [CrossRef]
- Lei, Y.; He, H.; Raza, A.; Liu, Z.; Xiaoyu, D.; Guijuan, W.; Yan, L.; Yong, C.; Xiling, Z. Exogenous melatonin confers cold tolerance in rapeseed (Brassica napus L.) seedlings by improving antioxidants and genes expression. Plant Signal. Behav. 2022, 17, 2129289. [Google Scholar] [CrossRef]
- Roy, R.; Sultana, S.; Begum, N.; Fornara, D.; Barmon, M.; Zhang, R.; Sarker, T.; Rabbany, M.G. Exogenous melatonin reduces water deficit-induced oxidative stress and improves growth performance of Althaea rosea grown on coal mine spoils. Environ. Sci. Pollut. Res. 2022, 29, 61550–61560. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xing, Q.; Ahammed, G.J.; Zhou, J. Functions and prospects of melatonin in plant growth, yield, and quality. J. Exp. Bot. 2022, 73, 5928–5946. [Google Scholar] [CrossRef]
- Buttar, Z.A.; Wu, S.N.; Arnao, M.B.; Wang, C.; Ullah, I.; Wang, C. Melatonin suppressed the heat stress-induced damage in wheat seedlings by modulating the antioxidant machinery. Plants 2020, 9, 809. [Google Scholar] [CrossRef]
- Umapathi, M.; Kalarani, M.; Srinivasan, S.; Kalaiselvi, P. Alleviation of cadmium phytotoxicity through melatonin modulated physiological functions, antioxidants, and metabolites in tomato (Solanum lycopersicum L.). BioMetals 2022, 35, 1113–1132. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.; Wi, S.; Yu, I.; Yeo, K.-H.; An, S.; Jang, Y.; Jang, S. Enhancement of growth and antioxidant enzyme activities on kimchi cabbage by melatonin foliar application under high temperature and drought stress conditions. Hortic. Sci. Technol. 2021, 39, 583–592. [Google Scholar]
- Yu, Y.; Deng, L.; Zhou, L.; Chen, G.; Wang, Y. Exogenous melatonin activates antioxidant systems to increase the ability of rice seeds to germinate under high temperature conditions. Plants 2022, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, S.; Wang, G.; Du, Y.; Zhang, Z.; Yu, G.; Ren, C.; Zhang, Y.; Du, J. Exogenous melatonin enhances soybean (Glycine max (L.) Merr.) seedling tolerance to saline-alkali stress by regulating antioxidant response and DNA damage repair. Physiol. Plant. 2022, 174, e13731. [Google Scholar] [CrossRef]
- Song, R.; Ritonga, F.N.; Yu, H.; Ding, C.; Zhao, X. Plant melatonin: Regulatory and protective role. Horticulturae 2022, 8, 810. [Google Scholar] [CrossRef]
- Talaat, N.B.; Todorova, D. Antioxidant machinery and glyoxalase system regulation confers salt stress tolerance to wheat (Triticum aestivum L.) plants treated with melatonin and salicylic Acid. J. Soil Sci. Plant Nutr. 2022, 22, 3527–3540. [Google Scholar] [CrossRef]
- Ye, F.; Jiang, M.; Zhang, P.; Liu, L.; Liu, S.; Zhao, C.; Li, X. Exogenous melatonin reprograms the rhizosphere microbial community to modulate the responses of barley to drought stress. Int. J. Mol. Sci. 2022, 23, 9665. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Li, S.; Cheng, Y.; Kebbeh, M.; Huan, C.; Zheng, X. Melatonin treatment maintains the quality of cherry tomato by regulating endogenous melatonin and ascorbate-glutathione cycle during room temperature. J. Food Biochem. 2022, 46, e14285. [Google Scholar] [CrossRef]
- Udhayabharathi, M. Physiological and Metabolomic Studies of Drought Tolerance in Greengram (Vigna radiata L.) by Exogenous Melatonin. Master’s Thesis, Tamil Nadu Agricultural University, Coimbatore, India, 2017. [Google Scholar]
- Xie, Z.; Wang, J.; Wang, W.; Wang, Y.; Xu, J.; Li, Z.; Zhao, X.; Fu, B. Integrated analysis of the transcriptome and metabolome revealed the molecular mechanisms underlying the enhanced salt tolerance of rice due to the application of exogenous melatonin. Front. Plant Sci. 2021, 11, 618680. [Google Scholar] [CrossRef]
- Sheikhalipour, M.; Mohammadi, S.A.; Esmaielpour, B.; Zareei, E.; Kulak, M.; Ali, S.; Nouraein, M.; Bahrami, M.K.; Gohari, G.; Fotopoulos, V. Exogenous melatonin increases salt tolerance in bitter melon by regulating ionic balance, antioxidant system and secondary metabolism-related genes. BMC Plant Biol. 2022, 22, 380. [Google Scholar] [CrossRef]
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol. Plant. 2021, 171, 739–755. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J.; Cano, A.; Reiter, R. Melatonin and carbohydrate metabolism in plant cells. Plants 2021, 10, 1917. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, C.; Wang, Z.; Sun, S.; Zhan, R.; Zhao, Y.; Ma, B.; Ma, F.; Li, M. Melatonin-mediated sugar accumulation and growth inhibition in apple plants involves down-regulation of fructokinase 2 expression and activity. Front. Plant Sci. 2019, 10, 150. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, S.; Ma, L.; Kong, L.; Pan, S.; Tang, X.; Tian, H.; Duan, M.; Mo, Z. Effect of exogenous melatonin application on the grain yield and antioxidant capacity in aromatic rice under combined lead–cadmium stress. Antioxidants 2022, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, B.; Baena-González, E. Management of plant central metabolism by SnRK1 protein kinases. J. Exp. Bot. 2022, 73, 7068–7082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yan, Y.; Zeng, X.; Wang, Y.; Zhang, Y. Quantitative proteomics analysis reveals proteins associated with high melatonin content in barley seeds under NaCl-induced salt stress. J. Agric. Food Chem. 2022, 70, 8492–8510. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Rehman, H.M.; Ahmed, N.; Nawaz, S.; Saleem, F.; Ahmad, S.; Uzair, M.; Rana, I.A.; Atif, R.M.; Zaman, Q.U. Using exogenous melatonin, glutathione, proline, and glycine betaine treatments to combat abiotic stresses in crops. Int. J. Mol. Sci. 2022, 23, 12913. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 136675. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, X.; Li, L.; Sun, Q.; Wang, Q.; Huang, H.; Tong, Z.; Zhang, J. Melatonin-mediated development and abiotic stress tolerance in plants. Front. Plant Sci. 2023, 14, 1100827. [Google Scholar] [CrossRef]
- Bhavithra, S. Mitigation of Salt Stress in Cassava (Manihot esculenta Crantz) by Exogenous Melatonin. Master’s Thesis, Tamil Nadu Agricultural University, Coimbatore, India, 2021. [Google Scholar]
- Shah, A.A.; Ahmed, S.; Ali, A.; Yasin, N.A. 2-Hydroxymelatonin mitigates cadmium stress in cucumis sativus seedlings: Modulation of antioxidant enzymes and polyamines. Chemosphere 2020, 243, 125308. [Google Scholar] [CrossRef] [PubMed]
- Vafadar, F.; Amooaghaie, R.; Ehsanzadeh, P.; Ghanadian, M.; Talebi, M.; Ghanati, F. Melatonin and calcium modulate the production of rosmarinic acid, luteolin, and apigenin in Dracocephalum kotschyi under salinity stress. Phytochemistry 2020, 177, 112422. [Google Scholar] [CrossRef]
- Behera, B.; Kancheti, M.; Raza, M.B.; Shiv, A.; Mangal, V.; Rathod, G.; Altaf, M.A.; Kumar, A.; Aftab, T.; Kumar, R. Mechanistic insight on boron-mediated toxicity in plant vis-a-vis its mitigation strategies: A review. Int. J. Phytoremediation 2023, 25, 9–26. [Google Scholar] [CrossRef]
- Khan, T.A.; Saleem, M.; Fariduddin, Q. Melatonin influences stomatal behavior, root morphology, cell viability, photosynthetic responses, fruit yield, and fruit quality of tomato plants exposed to salt stress. J. Plant Growth Regul. 2023, 42, 2408–2432. [Google Scholar] [CrossRef]
- Hassan, I.F.; Gaballah, M.S.; Ogbaga, C.C.; Murad, S.A.; Brysiewicz, A.; Bakr, B.M.; Mira, A.; Alam-Eldein, S.M. Does melatonin improve the yield attributes of field-droughted banana under Egyptian semi-arid conditions? J. Water Land Dev. 2022, 52, 221–231. [Google Scholar]
- Hu, C.-h.; Zheng, Y.; Tong, C.-l.; Zhang, D.-j. Effects of exogenous melatonin on plant growth, root hormones and photosynthetic characteristics of trifoliate orange subjected to salt stress. Plant Growth Regul. 2022, 97, 551–558. [Google Scholar] [CrossRef]
- Medina-Santamarina, J.; Serrano, M.; Lorente-Mento, J.M.; García-Pastor, M.E.; Zapata, P.J.; Valero, D.; Guillén, F. Melatonin treatment of pomegranate trees increases crop yield and quality parameters at harvest and during storage. Agronomy 2021, 11, 861. [Google Scholar] [CrossRef]
- Oliveira-Spolaor, B.; Chiari-Bertoli, S.; Silva-Sukert, D.; Sala, H.R.; Picoli de Oliveira, B.F.; de Freitas, Í.R.; Lima-Moro, A. Exogenous melatonin induces tolerance to drought stress damage in seedlings and soybean plants. Chil. J. Agric. Res. 2022, 82, 515–526. [Google Scholar] [CrossRef]
- Muhammad, I.; Yang, L.; Ahmad, S.; Mosaad, I.S.; Al-Ghamdi, A.A.; Abbasi, A.M.; Zhou, X.-B. Melatonin application alleviates stress-induced photosynthetic inhibition and oxidative damage by regulating antioxidant defense system of maize: A meta-analysis. Antioxidants 2022, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I.A.; Shalby, N.; MA El-Badri, A.; Saleem, M.H.; Khan, M.N.; Nawaz, M.A.; Qin, M.; Agami, R.A.; Kuai, J.; Wang, B. Stomata and xylem vessels traits improved by melatonin application contribute to enhancing salt tolerance and fatty acid composition of Brassica napus L. plants. Agronomy 2020, 10, 1186. [Google Scholar] [CrossRef]
- Chakraborty, S.; Tamili, D.; Bose, P.; Hazra, S.K.; Bhattacharjee, P. Melatonin-rich, erucic acid-lean nutraceutical supplements by microwave-assisted solvent extraction of brown and yellow mustard seeds. J. Food Process. Preserv. 2022, 46, e16875. [Google Scholar] [CrossRef]
- Wang, C.; Gu, Q.; Zhao, L.; Li, C.; Ren, J.; Zhang, J. Photochemical efficiency of photosystem II in inverted leaves of soybean [Glycine max (L.) Merr.] affected by elevated temperature and high light. Front. Plant Sci. 2022, 12, 772644. [Google Scholar] [CrossRef]
- Brengi, S.H.; Abd Allah, E.; Abouelsaad, I.A. Effect of melatonin or cobalt on growth, yield and physiological responses of cucumber (Cucumis sativus L.) plants under salt stress. J. Saudi Soc. Agric. Sci. 2022, 21, 51–60. [Google Scholar] [CrossRef]
- Wei, Y.; Zeng, H.; Hu, W.; Chen, L.; He, C.; Shi, H. Comparative transcriptional profiling of melatonin synthesis and catabolic genes indicates the possible role of melatonin in developmental and stress responses in rice. Front. Plant Sci. 2016, 7, 676. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.F.; Elbar, O.H.A.; Farag, R.; Hikal, M.; El-Kelish, A.; El-Yazied, A.A.; Alkahtani, J.; El-Gawad, H.G.A. Melatonin counteracts drought induced oxidative damage and stimulates growth, productivity and fruit quality properties of tomato plants. Plants 2020, 9, 1276. [Google Scholar] [CrossRef] [PubMed]
- Gurjar, P.; Killadi, B.; Pareek, P.K.; Hada, T. Application of melatonin in maintaining post harvest quality of fruits and vegetables: A review. Agric. Rev. 2022, 43, 193–198. [Google Scholar] [CrossRef]
- Nasser, M.A.; El-Mogy, M.M.; Samaan, M.S.; Hassan, K.M.; El-Sayed, S.M.; Alsubeie, M.S.; Darwish, D.B.E.; Mahmoud, S.F.; Al-Harbi, N.A.; Al-Qahtani, S.M. Postharvest exogenous melatonin treatment of table grape berry enhances quality and maintains bioactive compounds during refrigerated storage. Horticulturae 2022, 8, 860. [Google Scholar] [CrossRef]
- Sadak, M.S.; Abdalla, A.M.; Abd Elhamid, E.M.; Ezzo, M. Role of melatonin in improving growth, yield quantity and quality of Moringa oleifera L. plant under drought stress. Bull. Natl. Res. Cent. 2020, 44, 18. [Google Scholar] [CrossRef]
- Sadak, M.S.; Bakry, B.A. Alleviation of drought stress by melatonin foliar treatment on two flax varieties under sandy soil. Physiol. Mol. Biol. Plants 2020, 26, 907–919. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Amri, S. Ameliorative roles of melatonin and/or zeolite on chromium-induced leaf senescence in marjoram plants by activating antioxidant defense, osmolyte accumulation, and ultrastructural modification. Ind. Crops Prod. 2019, 142, 111823. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Samsampour, D.; Zahedi, S.M.; Zamanian, K.; Rahman, M.M.; Mostofa, M.G.; Tran, L.S.P. Melatonin alleviates drought impact on growth and essential oil yield of lemon verbena by enhancing antioxidant responses, mineral balance, and abscisic acid content. Physiol. Plant. 2021, 172, 1363–1375. [Google Scholar] [CrossRef]
- Prasad, V.R.; Govindaraj, M.; Djanaguiraman, M.; Djalovic, I.; Shailani, A.; Rawat, N.; Singla-Pareek, S.L.; Pareek, A.; Prasad, P.V. Drought and high temperature stress in sorghum: Physiological, genetic, and molecular insights and breeding approaches. Int. J. Mol. Sci. 2021, 22, 9826. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Li, R.; Chen, Z.-H.; Wang, Y. Stomata at the crossroad of molecular interaction between biotic and abiotic stress responses in plants. Front. Plant Sci. 2022, 13, 1031891. [Google Scholar] [CrossRef]
- Rao, S.; Tian, Y.; Zhang, C.; Qin, Y.; Liu, M.; Niu, S.; Li, Y.; Chen, J. The JASMONATE ZIM-domain–OPEN STOMATA1 cascade integrates jasmonic acid and abscisic acid signaling to regulate drought tolerance by mediating stomatal closure in poplar. J. Exp. Bot. 2023, 74, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, J.; Shi, S.; Huang, L.; Zhu, M.; Li, F. Exogenous melatonin ameliorates salinity-induced oxidative stress and improves photosynthetic capacity in sweet corn seedlings. Photosynthetica 2021, 59, 815769. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, J.; Wu, Z.; Loka, D.A.; Zhao, W.; Chen, B.; Wang, Y.; Meng, Y.; Zhou, Z.; Gao, L. Effects of single and combined exogenous application of abscisic acid and melatonin on cotton carbohydrate metabolism and yield under drought stress. Ind. Crops Prod. 2022, 176, 114302. [Google Scholar] [CrossRef]
- Jiang, D.; Lu, B.; Liu, L.; Duan, W.; Meng, Y.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Exogenous melatonin improves the salt tolerance of cotton by removing active oxygen and protecting photosynthetic organs. BMC Plant Biol. 2021, 21, 331. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Zheng, W.; Jiang, S.; Hong, S.-B.; Zhu, Z.; Zang, Y. Role of melatonin in promoting plant growth by regulating carbon assimilation and ATP accumulation. Plant Sci. 2022, 319, 111276. [Google Scholar] [CrossRef] [PubMed]
- Supriya, L.; Durgeshwar, P.; Muthamilarasan, M.; Padmaja, G. Melatonin mediated differential regulation of drought tolerance in sensitive and tolerant varieties of upland cotton (Gossypium hirsutum L.). Front. Plant Sci. 2022, 13, 821353. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Yu, Y.; Zeng, H.; Deng, L.; Zhu, L.; Chen, G.; Wang, Y. Melatonin-induced resilience strategies against the damaging impacts of drought stress in rice. Agronomy 2022, 12, 813. [Google Scholar] [CrossRef]
- Hu, E.; Liu, M.; Zhou, R.; Jiang, F.; Sun, M.; Wen, J.; Zhu, Z.; Wu, Z. Relationship between melatonin and abscisic acid in response to salt stress of tomato. Sci. Hortic. 2021, 285, 110176. [Google Scholar] [CrossRef]
- Li, R.; Yang, R.; Zheng, W.; Wu, L.; Zhang, C.; Zhang, H. Melatonin promotes SGT1-involved signals to ameliorate drought stress adaption in rice. Int. J. Mol. Sci. 2022, 23, 599. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J.; Ma, Y.; Huang, M.; Qiu, T.; Bian, H.; Han, N.; Wang, J. Function, mechanism, and application of plant melatonin: An update with a focus on the cereal crop, barley (Hordeum vulgare L.). Antioxidants 2022, 11, 634. [Google Scholar] [CrossRef]
- Hussain, M.B.; Mahmood, S.; Ahmed, N.; Nawaz, H. Rhizobial inoculation for improving growth physiology, nutrition and yield of maize under drought stress conditions. Pak. J. Bot. 2018, 50, 1681–1689. [Google Scholar]
- Foyer, C.H.; Noctor, G. Tansley Review No. 112 Oxygen processing in photosynthesis: Regulation and signalling. New Phytol. 2000, 146, 359–388. [Google Scholar] [CrossRef]
- Meng, J.F.; Xu, T.F.; Wang, Z.Z.; Fang, Y.L.; Xi, Z.M.; Zhang, Z.W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Ma, C.; Zhang, Z.; Wei, Z.; Gao, T.; Zhao, Q.; Ma, F.; Li, C. Long-term exogenous application of melatonin improves nutrient uptake fluxes in apple plants under moderate drought stress. Environ. Exp. Bot. 2018, 155, 650–661. [Google Scholar] [CrossRef]
- Liang, D.; Ni, Z.; Xia, H.; Xie, Y.; Lv, X.; Wang, J.; Lin, L.; Deng, Q.; Luo, X. Exogenous melatonin promotes biomass accumulation and photo148synthesis of kiwifruit seedlings under drought stress. Sci. Hortic. 2019, 246, 34–43. [Google Scholar] [CrossRef]
- Fleta-Soriano, E.; Díaz, L.; Bonet, E.; Munné-Bosch, S. Melatonin may exert a protective role against drought stress in maize. J. Agron. Crop Sci. 2017, 203, 286–294. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef]
- Li, L.; Li, M.; Yu, L.; Zhou, Z.; Liang, X.; Liu, Z.; Cai, G.; Gao, L.; Zhang, X.; Wang, Y. The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 2014, 15, 329–338. [Google Scholar] [CrossRef]
- Campos, C.N.; Ávila, R.G.; de Souza, K.R.D.; Azevedo, L.M.; Alves, J.D. Melatonin reduces oxidative stress and promotes drought tolerance in young Coffea arabica L. plants. Agric. Water Manag. 2019, 211, 37–47. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Wang, L.; Sun, Y. Exogenous melatonin improves seedling health index and drought tolerance in tomato. Plant Growth Regul. 2015, 77, 317–326. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H.J.; Weeda, S.; Yang, C.; Yang, Z.C.; Ren, S.; Guo, Y.D. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.; Liang, D.; Ma, F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2013, 54, 292–302. [Google Scholar] [CrossRef]
- Ding, F.; Wang, G.; Wang, M.; Zhang, S. Exogenous melatonin improves tolerance to water deficit by promoting cuticle formation in tomato plants. Molecules 2018, 23, 1605. [Google Scholar] [CrossRef] [PubMed]
- Anitha, K. Physiological Effect of Melatonin on Drought Alleviation in Finger Millet. Master’s Thesis, Tamil Nadu Agricultural University, Coimbatore, India, 2019. [Google Scholar]
- Kesawat, M.S.; Satheesh, N.; Kherawat, B.S.; Kumar, A.; Kim, H.-U.; Chung, S.-M.; Kumar, M. Regulation of reactive oxygen species during salt stress in plants and their crosstalk with other signaling molecules—Current perspectives and future directions. Plants 2023, 12, 864. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Z.; Gai, Z.; Wang, Y.; Wang, B.; Zhang, P.; Liu, X.; Chen, J.; Zhang, S.; Liu, D. Exogenous application of melatonin improves salt tolerance of sugar beet (Beta vulgaris L.) seedlings. Acta Physiol. Plant. 2022, 44, 57. [Google Scholar] [CrossRef]
- Yandi, W. Chinese Changes in Melatonin Content in Grape and Prokaryotic Expression Analysis of Its Synthetic Gene SNAT; Academy of Agricultural Sciences: Beijing, China, 2018. [Google Scholar]
- Meloni, D.A.; Oliva, M.A.; Martinez, C.A.; Cambraia, J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 2003, 49, 69–76. [Google Scholar] [CrossRef]
- Ye, J.; Wang, S.; Deng, X.; Yin, L.; Xiong, B.; Wang, X. Melatonin increased maize (Zea mays L.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiol. Plant. 2016, 38, 48. [Google Scholar] [CrossRef]
- Zhu, J.-K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Yang, M.F.; Song, J.; Wang, B.S. Organ-specific responses of vacuolar H+-ATPase in the shoots and roots of C3 halophyte Suaeda salsa to NaCl. J. Integr. Plant Biol. 2010, 52, 308–314. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Wei, Z.; Liang, D.; Liu, C.; Yin, L.; Jia, D.; Fu, M.; Ma, F. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J. Pineal Res. 2012, 53, 298–306. [Google Scholar] [CrossRef]
- Bhavithra, S.; Kalarani, M.; Kavitha, A.S.P. Exogenous melatonin on Physiological and Yield Traits of Cassava (Manihot esculenta Crantz) under Salt Stress. Madras Agric. J. 2021, 108, 1–4. [Google Scholar]
- Jahan, M.S.; Guo, S.; Sun, J.; Shu, S.; Wang, Y.; Abou El-Yazied, A.; Alabdallah, N.M.; Hikal, M.; Mohamed, M.H.; Ibrahim, M.F. Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Biochem. 2021, 167, 309–320. [Google Scholar] [CrossRef]
- Jahan, M.S.; Shu, S.; Wang, Y.; Chen, Z.; He, M.; Tao, M.; Sun, J.; Guo, S. Melatonin alleviates heat-induced damage of tomato seedlings by balancing redox homeostasis and modulating polyamine and nitric oxide biosynthesis. BMC Plant Biol. 2019, 19, 414. [Google Scholar] [CrossRef]
- Li, X.; Li, M.-H.; Deng, W.-W.; Ahammed, G.J.; Wei, J.-P.; Yan, P.; Zhang, L.-P.; Fu, J.-Y.; Han, W.-Y. Exogenous melatonin improves tea quality under moderate high temperatures by increasing epigallocatechin-3-gallate and theanine biosynthesis in Camellia sinensis L. J. Plant Physiol. 2020, 253, 153273. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhou, J.; Tan, D.-X.; Wang, N.; Wang, L.; Shan, D.; Kong, J. Melatonin improves waterlogging tolerance of Malus baccata (Linn.) Borkh. seedlings by maintaining aerobic respiration, photosynthesis and ROS migration. Front. Plant Sci. 2017, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, X.; Zhang, Z.; Liu, N.; Li, D.; Hu, L. Melatonin improved waterlogging tolerance in alfalfa (Medicago sativa) by reprogramming polyamine and ethylene metabolism. Front. Plant Sci. 2019, 10, 44. [Google Scholar] [CrossRef]
- McDonald, M.F.; Copeland, L.O. Seed Production: Principles and Practices; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 2012, 872875. [Google Scholar] [CrossRef]
- Chibuike, G.U.; Obiora, S.C. Heavy metal polluted soils: Effect on plants and bioremediation methods. Appl. Environ. Soil Sci. 2014, 2014, 752708. [Google Scholar] [CrossRef]
- Chandrakar, V.; Naithani, S.C.; Keshavkant, S. Arsenic-induced metabolic disturbances and their mitigation mechanisms in crop plants: A review. Biologia 2016, 71, 367–377. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Li, Y.; Wu, Y.; Chen, Y.; Zeng, G.; Zhang, J.; Li, H. Influence of biochar on heavy metals and microbial community during composting of river sediment with agricultural wastes. Bioresour. Technol. 2017, 243, 347–355. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Li, M.Q.; Hasan, M.K.; Li, C.X.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Reiter, R.J.; Yu, J.Q.; Xu, M.X. Melatonin mediates selenium-induced tolerance to cadmium stress in tomato plants. J. Pineal Res. 2016, 61, 291–302. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Growth conditions determine different melatonin levels in Lupinus albus L. J. Pineal Res. 2013, 55, 149–155. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.J.; Lee, H.Y.; Back, K. Cloning and functional characterization of the Arabidopsis N-acetylserotonin O-methyltransferase responsible for melatonin synthesis. J. Pineal Res. 2016, 60, 65–73. [Google Scholar] [CrossRef]
- Umapathi, M.; Kalarani, M.; Udhaya Bharathi, M.; Kalaiselvi, P. Cadmium induced stress mitigation in tomato by exogenous melatonin. Int. J. Pure App. Biosci. 2018, 6, 903–909. [Google Scholar] [CrossRef]
- Lee, K.; Choi, G.H.; Back, K. Cadmium-induced melatonin synthesis in rice requires light, hydrogen peroxide, and nitric oxide: Key regulatory roles for tryptophan decarboxylase and caffeic acid O-methyltransferase. J. Pineal Res. 2017, 63, e12441. [Google Scholar] [CrossRef]
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin biosynthesis in plants: Multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef]
- Wang, L.; Feng, C.; Zheng, X.; Guo, Y.; Zhou, F.; Shan, D.; Liu, X.; Kong, J. Plant mitochondria synthesize melatonin and enhance the tolerance of plants to drought stress. J. Pineal Res. 2017, 63, e12429. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef]
- Hodzic, E.; Galijasevic, S.; Balaban, M.; Rekanovic, S.; Makic, H.; Kukavica, B.; Mihajlovic, D. The protective role of melatonin under heavy metal-induced stress in Melissa officinalis L. Turk. J. Chem. 2021, 45, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhao, J.; Sun, X.; Zhu, Y.; Li, Q.; Zhang, L.; Zhao, D.; Huang, L.; Zhang, C.; Liu, Q. Exogenous melatonin improves the quality performance of rice under high temperature during grain filling. Agronomy 2022, 12, 949. [Google Scholar] [CrossRef]
- Li, R.; Jiang, M.; Song, Y.; Zhang, H. Melatonin alleviates low-temperature stress via ABI5-mediated signals during seed germination in rice (Oryza sativa L.). Front. Plant Sci. 2021, 12, 727596. [Google Scholar] [CrossRef]
- Guo, Y.; Li, H.; Zhao, C.; Xue, J.; Zhang, R. Exogenous melatonin improves drought tolerance in maize seedlings by regulating photosynthesis and the ascorbate–glutathione cycle. Russ. J. Plant Physiol. 2020, 67, 809–821. [Google Scholar] [CrossRef]
- Yang, K.; Sun, H.; Liu, M.; Zhu, L.; Zhang, K.; Zhang, Y.; Li, A.; Zhang, H.; Zhu, J.; Liu, X. Morphological and Physiological Mechanisms of Melatonin on Delaying Drought-Induced Leaf Senescence in Cotton. Int. J. Mol. Sci. 2023, 24, 7269. [Google Scholar] [CrossRef]
- Anitha, K.; Senthil, A.; Kalarani, M.; Senthil, N.; Marimuthu, S.; Umapathi, M. Melatonin mediated high-temperature tolerance at seedling stage in green gram (Vigna radiata L.). J. Appl. Nat. Sci. 2023, 15, 85–93. [Google Scholar] [CrossRef]
- Dheerkadharshini, K.; Kalarani, M.; Djanaguiraman, M.; Haripriya, S.; Umapathi, M. Exogenous Application of Melatonin-loaded Nanoformulation for Yield Enhancement in Finger Millet (Eleusine coracana) under Drought Condition. Int. J. Environ. Clim. Chang. 2022, 12, 817–826. [Google Scholar] [CrossRef]
- Anitha, K.; Senthil, A.; Sritharan, N.; Ravikesavan, R. Melatonin improves germination and seedling growth under drought stress in finger millet. Multi-Dimens. Approaches Transform. Agric. 2020, 248. [Google Scholar]
- Ahammed, G.J.; Xu, W.; Liu, A.; Chen, S. Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ. Exp. Bot. 2019, 161, 303–311. [Google Scholar] [CrossRef]
- Meng, J.-F.; Yu, Y.; Shi, T.-C.; Fu, Y.-S.; Zhao, T.; Zhang, Z.-W. Melatonin treatment of pre-veraison grape berries modifies phenolic components and antioxidant activity of grapes and wine. Food Sci. Technol. 2018, 39, 35–42. [Google Scholar] [CrossRef]
- Mao, K.; Li, J.; Wu, S.; Qian, J.; Liao, Y.; Zeng, L.; Gu, D. Melatonin treatment promotes cold adaptation and spring growth of tea plants. Ind. Crops Prod. 2023, 200, 116834. [Google Scholar] [CrossRef]
- Langaroudi, I.K.; Piri, S.; Chaeikar, S.S.; Salehi, B. Evaluating drought stress tolerance in different Camellia sinensis L. cultivars and effect of melatonin on strengthening antioxidant system. Sci. Hortic. 2023, 307, 111517. [Google Scholar] [CrossRef]
- Tan, X.; Huang, J.; Lin, L.; Tang, Q. Exogenous Melatonin Attenuates Cd Toxicity in Tea (Camellia sinensis). Agronomy 2022, 12, 2485. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, K.; Zhou, X.; Xi, L.; Wang, Y.; Xu, H.; Pan, T.; Zou, Z. Melatonin alleviates chilling stress in cucumber seedlings by up-regulation of CsZat12 and modulation of polyamine and abscisic acid metabolism. Sci. Rep. 2017, 7, 4998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, S.; Yang, S.; Chen, Y. Melatonin alleviates cold-induced oxidative damage by regulation of ascorbate–glutathione and proline metabolism in melon seedlings (Cucumis melo L.). J. Hortic. Sci. Biotechnol. 2017, 92, 313–324. [Google Scholar] [CrossRef]
- Cao, S.; Shao, J.; Shi, L.; Xu, L.; Shen, Z.; Chen, W.; Yang, Z. Melatonin increases chilling tolerance in postharvest peach fruit by alleviating oxidative damage. Sci. Rep. 2018, 8, 806. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.-H.; Wang, Y.; Liu, J.-C.; Pan, Z.-N.; Zhang, H.-L.; Sun, S.-C.; Zhang, Y. Melatonin reverses mitochondria dysfunction and oxidative stress-induced apoptosis of Sudan I-exposed mouse oocytes. Ecotoxicol. Environ. Saf. 2021, 225, 112783. [Google Scholar] [CrossRef]
- Qari, S.H.; Hassan, M.U.; Chattha, M.U.; Mahmood, A.; Naqve, M.; Nawaz, M.; Barbanti, L.; Alahdal, M.A.; Aljabri, M. Melatonin induced cold tolerance in plants: Physiological and molecular responses. Front. Plant Sci. 2022, 13, 843071. [Google Scholar] [CrossRef]
- Okazaki, M.; Higuchi, K.; Hanawa, Y.; Shiraiwa, Y.; Ezura, H. Cloning and characterization of a Chlamydomonas reinhardtii cDNA arylalkylamine N-acetyltransferase and its use in the genetic engineering of melatonin content in the Micro-Tom tomato. J. Pineal Res. 2009, 46, 373–382. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Reiter, R.J.; He, C.; Liu, G.; Lei, Q.; Zuo, B.; Zheng, X.D.; Li, Q.; Kong, J. Changes in melatonin levels in transgenic ‘Micro-Tom’tomato overexpressing ovine AANAT and ovine HIOMT genes. J. Pineal Res. 2014, 56, 134–142. [Google Scholar] [CrossRef]
- Zhang, K.; Cui, H.; Cao, S.; Yan, L.; Li, M.; Sun, Y. Overexpression of CrCOMT from Carex rigescens increases salt stress and modulates melatonin synthesis in Arabidopsis thaliana. Plant Cell Rep. 2019, 38, 1501–1514. [Google Scholar] [CrossRef]
- Shamloo-Dashtpagerdi, R.; Lindlöf, A.; Tahmasebi, S. Evidence that miR168a contributes to salinity tolerance of Brassica rapa L. via mediating melatonin biosynthesis. Physiol. Plant. 2022, 174, e13790. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Back, K. Cadmium disrupts subcellular organelles, including chloroplasts, resulting in melatonin induction in plants. Molecules 2017, 22, 1791. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, X.; Zhang, Y.; Jiang, J.; Sun, L.; Rahman, F.U.; Liu, C. VvSNAT1 overexpression enhances melatonin production and salt tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2021, 166, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-D.; Sun, X.-S.; Liu, L.; Shi, H.-D.; Chen, S.-Y.; Zhao, D.-K. Overexpression of the melatonin synthesis-related gene SlCOMT1 improves the resistance of tomato to salt stress. Molecules 2019, 24, 1514. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Wei, Z.; Ma, C.; Yin, B.; Li, C.; Ma, F. Ectopic expression of HIOMT improves tolerance and nitrogen utilization efficiency in transgenic apple under drought stress. Tree Physiol. 2023, 43, 335–350. [Google Scholar] [CrossRef]
- Vitalini, S.; Gardana, C.; Zanzotto, A.; Fico, G.; Faoro, F.; Simonetti, P.; Iriti, M. From vineyard to glass: Agrochemicals enhance the melatonin and total polyphenol contents and antiradical activity of red wines. J. Pineal Res. 2011, 51, 278–285. [Google Scholar] [CrossRef]
- Yang, W.-J.; Du, Y.-T.; Zhou, Y.-B.; Chen, J.; Xu, Z.-S.; Ma, Y.-Z.; Chen, M.; Min, D.-H. Overexpression of TaCOMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis. Int. J. Mol. Sci. 2019, 20, 652. [Google Scholar] [CrossRef]
- Shreya, S.; Supriya, L.; Padmaja, G. Melatonin induces drought tolerance by modulating lipoxygenase expression, redox homeostasis and photosynthetic efficiency in Arachis hypogaea L. Front. Plant Sci. 2022, 13, 1069143. [Google Scholar] [CrossRef]
- Allegrone, G.; Razzano, F.; Pollastro, F.; Grassi, G. Determination of melatonin content of different varieties of hemp (Cannabis sativa L.) by liquid chromatography tandem mass spectrometry. SN Appl. Sci. 2019, 1, 720. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).