A New Strategy of Cross-Protection Based on Attenuated Vaccines: RNA Viruses Are Used as Vectors to Control DNA Viruses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasmid Construction
2.3. Preparation and Inoculation of Agrobacterium Solution
2.4. Analysis of Pathogenicity and Stability of Mutants
3. Results and Discussion
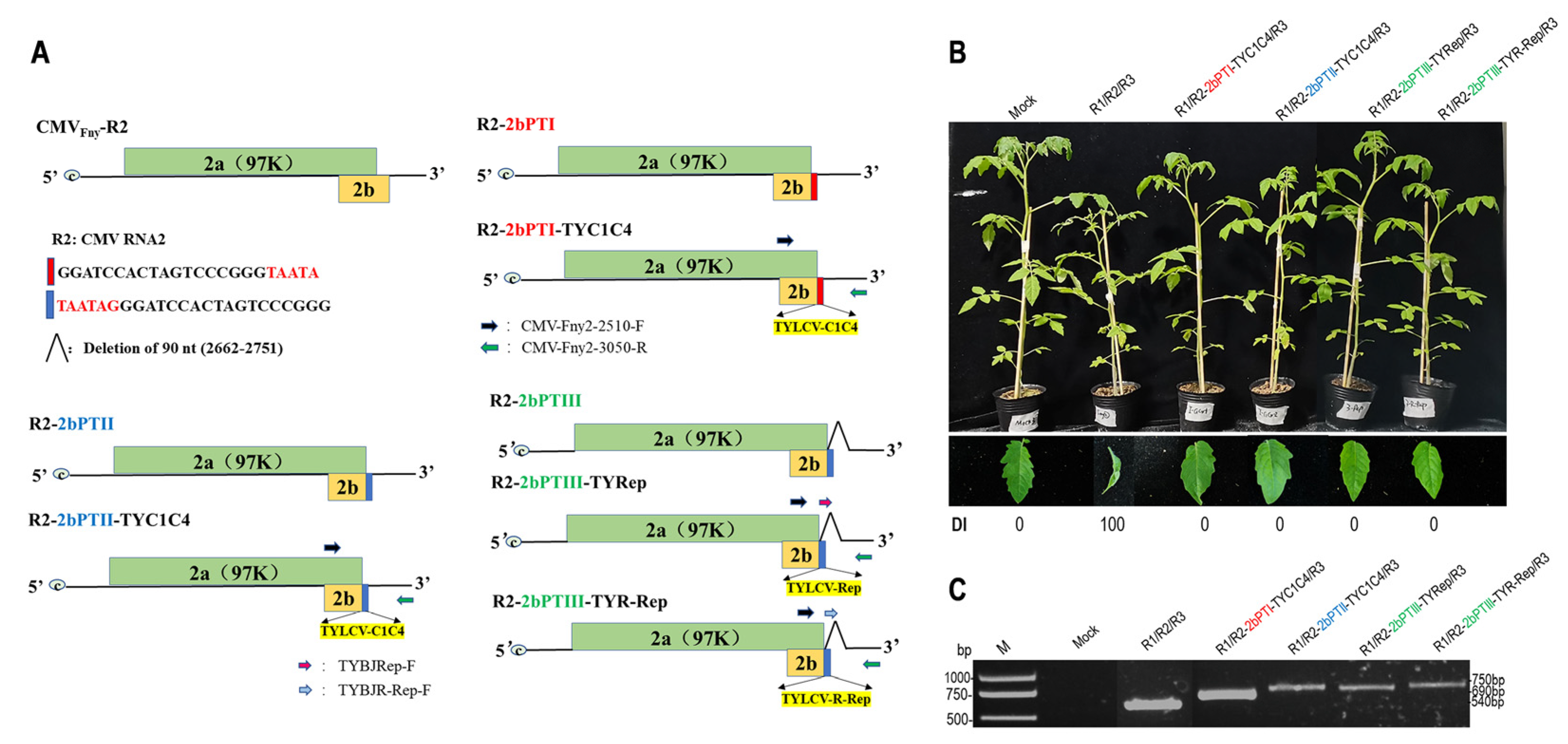
3.1. Construction and Virulence Analysis of Attenuated CMV Mutants Containing TYLCV Specific Fragments
3.2. Analysis of the Cross-Protection Effect of Tomato Plants Pre-Inoculated with Attenuated Mutants against TYLCV Infection
3.3. Analysis of the Cross-Protection Effect of Tomato Plants Pre-Inoculated with Attenuated Mutants against Co-Infection of TYLCV and CMV
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ziebell, H.; Carr, J.P. Cross-protection: A century of mystery. Adv. Virus Res. 2010, 76, 211–264. [Google Scholar] [CrossRef] [PubMed]
- Grant, T.J. Evidence of tristeza, or quick decline, virus in Florida. Proc. Fla. State Hort. Soc. 1952, 65, 28–31. Available online: https://eurekamagcom/research/013/704/013704162php (accessed on 4 September 2023).
- Lecoq, H.; Lemaire, J.M.; Wipf-Scheibel, C. Control of zucchini yellow mosaic virus in squash by cross-protection. Plant Dis. 1991, 75, 208–211. [Google Scholar] [CrossRef]
- Rast, A.T.B. M II-16, an artificial symptomless mutant of tobacco mosaic virus for seedling inoculation of tomato crops. J. Plant Pathol. 1972, 78, 110–112. [Google Scholar] [CrossRef]
- Tomlinson, J.A.; Shepherd, R.J. Studies on mutagenesis and cross-protection of cauliflower mosaic virus. Ann. Appl. Biol. 1978, 90, 223–231. [Google Scholar] [CrossRef]
- Fondong, V.N.; Pita, J.S.; Rey, M.E.; Kochko, A.; Beachy, R.N.; Fauquet, C.M. Evidence of synergism between African cassava mosaic virus and a new double-recombinant geminivirus infecting cassava in Cameroon. J. Gen. Virol. 2000, 81, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Scholthof, K.B.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; et al. Top10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Sharma, N.; Hari-Gowthem, G.; Muthamilarasan, M.; Prasad, M. Tomato yellow leaf curl virus: Impact, challenges, and management. Trends Plant Sci. 2020, 25, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Navas-Castillo, J.; Sánchez-Campos, S.; Díaz, J.A.; Sáez-Alonso, E.; Moriones, E. First report of tomato yellow leaf curl virus in Spain: Coexistence of two different geminiviruses in the same epidemic outbreak. Plant Dis. 1997, 81, 1461. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.H.; Guo, H.S. RNA silencing: From discovery and elucidation to application and perspectives. J. Integr. Plant Biol. 2022, 64, 476–498. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. Construction of the Attenuated Vaccine Vector from Cucumber Mosaic Virus and Research on the Mechanism of Mutation Repair. Ph.D. Dissertation, Shandong Agricultural University, Tai’an, China, 2021. [Google Scholar] [CrossRef]
- Weigel, D.; Glazebrook, J. Transformation of agrobacterium using the freeze-thaw method. CSH Protoc 2006, 2006, pdb.prot4666. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hao, K.; Yu, F.; Shen, L.; Wang, F.; Yang, J.; Su, C. Field application of nanoliposomes delivered quercetin by inhibiting specific hsp70 gene expression against plant virus disease. J. Nanobiotechnol. 2022, 20, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Lewsey, M.; Surette, M.; Robertson, F.C.; Ziebell, H.; Choi, S.H.; Ryu, K.H.; Canto, T.; Palukaitis, P.; Payne, T.; Walsh, J.A.; et al. The role of the cucumber mosaic virus 2b protein in viral movement and symptom induction. Mol Plant Microbe Interact. 2009, 22, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Lewsey, M.G.; González, I.; Kalinina, N.O.; Palukaitis, P.; Canto, T.; Carr, J.P. Symptom induction and RNA silencing suppression by the cucumber mosaic virus 2b protein. Plant Signal Behav. 2010, 5, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Pechinger, K.; Chooi, K.M.; MacDiarmid, R.M.; Harper, S.J.; Ziebell, H. A new era for mild strain cross-protection. Viruses 2019, 11, 670. [Google Scholar] [CrossRef] [PubMed]
- Krstic, B.; Ford, R.E.; Shukla, D.D.; Tosic, M. Cross-protection studies between strains of sugarcane mosaic, maize dwarf mosaic, Johnsongrass mosaic, and sorghum mosaic potyviruses. Plant Dis. 1995, 79, 135–138. [Google Scholar] [CrossRef]
- Cong, Q.Q.; Wang, Y.; Liu, J.; Lan, Y.F.; Guo, Z.K.; Yang, J.G.; Li, X.D.; Tian, Y.P. Evaluation of Potato virus X mild mutants for cross protection against severe infection in China. Virol. J. 2019, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Tian, Y.; Geng, C.; Guo, Y.; Jia, M.; Li, X. Development and application of a full-length infectious clone of potato virus Y isolate belonging to SYR-I strain. Virus Res. 2020, 276, 197827. [Google Scholar] [CrossRef] [PubMed]


| Primer Name | Primer Sequence (5′-3′) | Product Size | Purpose |
|---|---|---|---|
| TYBJ-C1C4-F | ATGGATCCTGACATCTGTTGAGCTCTTAGCT | 150 bp | Amplification of TYLCV-C1C4 |
| TYBJ-C1C4-R | ATCCCGGGACGAGAATGGGGAACCACATCT | ||
| TYBJRep-F | ATGGATCCTTACGCCTTATTGGTTTCTTCTTGG | 150 bp | Amplification of TYLCV-Rep |
| TYBJRep-R | ATCCCGGGGATGACGTAGACCCGCATTATTTAA | ||
| TYBJR-Rep-F | ATCCCGGGTTACGCCTTATTGGTTTCTTCTTGG | 150 bp | Amplification of TYLCV-R-Rep |
| TYBJR-Rep-R | ATGGATCCGATGACGTAGACCCGCATTATTTAA | ||
| CMV-Fny2-2510-F | AGAATCGACGGGAACGAGGT | 540 + 150 bp or 540 + 210 bp | Detection of mutant stability |
| CMV-Fny2-3050-R | TGGTCTCCTTTTGGAGGCC |
| Vaccine Name | Disease Index | Relative Control Efficacy |
|---|---|---|
| TYLCV | 100 | 0% |
| R1/R2-2bPTI-TYC1C4/R3 | 11 | 89% |
| R1/R2-2bPTII-TYC1C4/R3 | 19 | 81% |
| R1/R2-2bPTIII-TYRep/R3 | 26 | 74% |
| R1/R2-2bPTIII-TYR-Rep/R3 | 33 | 67% |
| R1/R2-2bPTIII-TYRep + R-Rep/R3 | 11 | 89% |
| Vaccine Name | Disease Index | Relative Control Efficacy |
|---|---|---|
| TYLCV + CMV | 100 | 0% |
| R1/R2-2bPTI-TYC1C4/R3 | 56 | 44% |
| R1/R2-2bPTII-TYC1C4/R3 | 56 | 44% |
| R1/R2-2bPTIII-TYRep/R3 | 63 | 37% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Liu, S.; Wang, Z.; Yu, C.; Yuan, X. A New Strategy of Cross-Protection Based on Attenuated Vaccines: RNA Viruses Are Used as Vectors to Control DNA Viruses. Agronomy 2023, 13, 2334. https://doi.org/10.3390/agronomy13092334
Zhu M, Liu S, Wang Z, Yu C, Yuan X. A New Strategy of Cross-Protection Based on Attenuated Vaccines: RNA Viruses Are Used as Vectors to Control DNA Viruses. Agronomy. 2023; 13(9):2334. https://doi.org/10.3390/agronomy13092334
Chicago/Turabian StyleZhu, Mingjing, Shanshan Liu, Zhao Wang, Chengming Yu, and Xuefeng Yuan. 2023. "A New Strategy of Cross-Protection Based on Attenuated Vaccines: RNA Viruses Are Used as Vectors to Control DNA Viruses" Agronomy 13, no. 9: 2334. https://doi.org/10.3390/agronomy13092334
APA StyleZhu, M., Liu, S., Wang, Z., Yu, C., & Yuan, X. (2023). A New Strategy of Cross-Protection Based on Attenuated Vaccines: RNA Viruses Are Used as Vectors to Control DNA Viruses. Agronomy, 13(9), 2334. https://doi.org/10.3390/agronomy13092334






