Abstract
Biostimulants’ application to plants can reduce the damage caused by abiotic factors such as drought or salinity and improve crop yield under these stressful conditions. In this work, several biostimulants, namely Terrabion Aminovit® (a commercial product based on amino acids), potassium fulvate, humic acids, and a seaweed extract, were applied to cherry tomato plants using fertigation at two doses of 0.2 and 1.0 g L−1. The plants were then subjected to a water stress treatment by completely withholding irrigation for 12 days. After the treatments, all plants were harvested to determine several growth and biochemical parameters. Pre-treatment with all biostimulants protected the tomato plants against dehydration, as indicated by a significant increase in leaf water content compared to the non-irrigated controls. Leaf fresh weight and root water content also increased, except in the plants treated with humic acids, by about 2 fold in plants pre-treated with Terrabion Aminovit® and 1.5 fold in the presence of potassium fulvate and the seaweed extract. The water stress treatment caused a significant increase in leaf proline content, up to 113.6 μmol g−1 DW, approximately 18 fold higher than in well-irrigated control plants; this value was significantly lower in Terrabion Aminovit® pre-treated plants but even higher, ca. 180 μmol g−1 DW, in those treated previously with the seaweed extract. These results indicate that proline is a suitable water stress biomarker in tomatoes and that the biostimulants probably differ in their mode of action, suggesting that the effect of the seaweed extract is mediated by proline accumulation. A significant activation of antioxidant enzymes, namely superoxide dismutase, ascorbate peroxidase, and glutathione reductase, was also observed in water-stressed plants; application of the biostimulants resulted in all cases, in a significant reduction in the specific activities of the three enzymes, indicating reduced levels of drought-induced oxidative stress in the plants. We conclude that applying these biostimulants, particularly Terrabion Aminovit®, may help minimise the adverse effects of water stress on tomatoes by maintaining turgor and improving growth through mechanisms still unknown but which appear to involve, at least in part, enhancing the plants’ antioxidant defence responses.
1. Introduction
Plants respond to different abiotic stress conditions by activating a series of common mechanisms, although there are also specific responses for each particular type of stress. Many reviews describe these mechanisms in detail [1,2,3,4]. Summarising, the most relevant stress responses are based on: (a) the control of water, ion transport and ion homeostasis [5,6,7]; (b) the synthesis of protective molecules, such as compatible osmolytes for osmotic adjustment (sugars, polyalcohols, or some amino acids and derivatives) and specific proteins (heat shock proteins, cryoprotectants, LEA proteins, osmotin) [5,8,9,10]; and (c) the activation of enzymatic and non-enzymatic antioxidant systems to counteract the oxidative stress generated by increased levels of Reactive Oxygen Species (ROS) to prevent damage to proteins, membranes, and DNA [11,12]. ROS are produced by different metabolic reactions in the mitochondria, leaf peroxisomes, or chloroplasts [13,14]. Some examples of antioxidant compounds synthesised by plants to eliminate ROS are reduced glutathione (GSH), carotenoids, or phenolic compounds, especially the subclass of flavonoids. Amongst antioxidant enzymes, some of the most relevant are superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APx), glutathione reductase (GR), glutathione-S-transferase (GST), and glutathione peroxidase (GPx) [15,16,17].
Biostimulants have been used for several decades to strengthen crop plants against stress by promoting improved yield and crop quality under adverse conditions [18,19]. Currently, there is a wide variety of biostimulant products, such as those based on mixtures of amino acids, seaweed and microalgae extracts, enzymes, humic substances, polysaccharides, phytohormones, and/or vitamins, sometimes supplemented with micro and/or macronutrients and microorganisms (e.g., fungi and bacteria) [19,20,21,22]. These products are also used to help the crop during periods of high metabolic demand, such as rooting, sprouting, flowering, fruit set, and/or fruit formation [23]. Generally, biostimulants are rapidly absorbed and metabolised, resulting in a very low energy cost to the plant. They are specifically recommended against abiotic stresses (e.g., phytotoxicity, high temperatures, salinity, drought, or UV radiation) [20,21]. Sometimes, they have also been used against biotic stresses (e.g., pests and diseases) [24]. However, despite the potential positive effects of biostimulants in improving crop performance, these effects are not always observed [25,26] as they usually depend on multiple factors, such as the dose applied, the biostimulant source, the environmental conditions in which they are applied, the species and variety, and the type and formulation of the product [19,27,28].
The objective of the present study was to compare the efficacy of different biostimulant formulations applied to cherry tomato (Solanum lycopersicum L., var. cerasiforme) plants before they were subjected to severe water stress treatments by the complete withholding of irrigation. The tomato is probably the most important horticultural crop worldwide from an economic point of view, and water deficit during tomato plants’ growth is known to negatively affect the yield and quality of the crop [29,30]. There are many publications on the mechanisms used by plants, including tomatoes, to counteract drought stress (e.g., [31,32,33,34]). However, although the beneficial effects of natural biostimulants on water-stressed tomato plants are known, there is little evidence in the literature on the underlying mechanisms, particularly those involving antioxidant responses (e.g., [35,36,37]). Therefore, the present work can contribute to increasing our knowledge of those mechanisms. From a practical point of view, it should also allow for selecting, amongst those tested, the most effective biostimulant at counteracting drought effects in tomatoes. To achieve these aims, we have determined biostimulant-induced changes in the growth and biochemical parameters of water-stressed plants, focusing primarily on the plants’ antioxidant responses. The initial hypothesis was that pre-treatment with the biostimulants could protect the tomato plants against the subsequent water stress treatment by stimulating their antioxidant machinery.
2. Materials and Methods
2.1. Plant Material and Growth Conditions
Cherry tomato (Solanum Lycopersicon L. var. cerasiforme) seeds (Spicegarden-Bernd Wildt, Barcelona, Spain) were germinated in a phytotron under long-day conditions (16 h light photoperiod), 130 µmol m−2 s−1 photosynthetic active radiation (PAR), 80% relative humidity, and 25 °C average temperature. Sowing was carried out on seedlings trays (2–3 seeds per socket, ca. 300 seeds in total) using a mixture of peat and vermiculite (1:1). Two weeks after sowing, plants of similar size with 3–4 true leaves were transplanted individually to 12 cm Ø pots in plastic trays, five pots per tray, until the end of the experiments. Each treatment consisted of five repetitions of individual plants placed in a tray. Plants were always watered with a standard nutrient solution [38], supplemented with biostimulants where indicated. Irrigation was applied directly to the trays, 0.5 L per plant every 3–4 days, so that the solution was absorbed by capillarity.
2.2. Biostimulants
Different biostimulants produced by Fertinagro Biotech (Teruel, Spain) were tested: (1) Terrabion Aminovit®, a commercialised liquid biostimulant rich in organic matter and free amino acids (hereafter referred to as ‘TB’). The exact composition is protected (patent number #011050499), and only the main active compounds are listed (Table 1). (2) Potassium fulvate (KF). (3) Humic acids 7–9 (reference number provided by the company) (HA). (4) A seaweed extract (SE). The chemical composition of the last three biostimulants is proprietary to the company and has not been disclosed.

Table 1.
Aminogram of Terrabion Aminovit® expressed as a percentage for each free amino acid.
2.3. Biostimulant and Water Stress Treatments
Biostimulant pre-treatments were started on day 34 after sowing, when the cherry tomato plants had 5–6 true leaves. The pots, distributed in different trays (one tray per treatment with five individuals), were watered (0.5 L per pot) with each biostimulant in nutrient solution at two doses of 0.2 or 1.0 g L−1. The biostimulant solutions were applied four times (every two to three days) until day 43 after sowing, when the water stress treatments were initiated by complete withholding of irrigation. The experiments were concluded on day 55 after sowing (i.e., after 12 days without irrigation), when plant material was sampled to determine several growth and biochemical parameters. Plants continuously watered with nutrient solution and not subjected to water stress were grown in parallel as a positive ‘irrigated’ control (IC). Plants subjected to the same water deficit treatment, but not pre-treated with biostimulants constituted a negative, ‘non-irrigated’ control (NIC). Considering the four biostimulants, applied at two different doses, the two control treatments (IC and NIC), and the five biological replicas (individual plants) per treatment, a total of 50 plants were used in the experiments.
2.4. Growth Parameters
The effect of the biostimulant pre-treatments on plant growth was determined 12 days after irrigation suppression. Plant roots and aerial parts were harvested separately, and the roots were thoroughly cleaned with a brush. The measured parameters were root and leaf fresh weight (FW), stem length (cm), leaf and flower numbers, and leaf area (mm2)—the latter determined using an LI-3100C area meter (LI-COR®, Lincoln, NE, USA). Part of the root and leaf material was weighed, dried in an oven at 65 °C for 72 h, and weighed again (dry weight, DW) to calculate the water content percentage (WC%) of both organs. The dried fraction of the leaf material was stored at room temperature in sealed tubes, and the remainder was flash frozen in liquid N2 and stored at −75 °C.
2.5. Proline Quantification
Proline (Pro) was extracted with 3% (w/v) sulphosalicylic acid from 0.1 g of the leaf material stored at −75 °C and quantified according to the acid-ninhydrin method [39] with minor modifications [40]. Briefly, the extracts were mixed with ninhydrin (dissolved in acetic acid) and phosphoric acid, incubated in a water bath at 95 °C for 60 min, cooled to room temperature, and extracted with toluene; the absorbance of the organic phase was determined at 520 nm using toluene as the blank. Samples containing known Pro concentrations were assayed in parallel to obtain a standard curve. Pro contents were finally expressed as µmol g−1 DW.
2.6. Antioxidant Enzymes
Specific enzyme activities were determined in crude protein extracts prepared from approximately 2 g of plant leaf material stored frozen at −75 °C for less than two months, as previously described [41]. The protein concentration in the extracts was estimated by Bradford’s assay [42], using a commercial reagent (Bio-Rad, Alcobendas, Spain) and bovine serum albumin (BSA) as the standard.
2.6.1. Superoxide Dismutase (SOD)
SOD specific activity in the extracts was estimated from the inhibition of nitroblue tetrazolium (NBT) photoreduction in the presence of riboflavin, used as the source of superoxide radicals [43]. The reaction mixtures were exposed to three 23 W Osram DULUX PRO compact fluorescent lamps for 10 min at 25 °C, generating 300 µmol m−2 s−1 radiation. The absorbance of the samples was then measured at 560 nm against the corresponding non-irradiated reaction mixtures used as a blank. One SOD unit was defined as the amount of enzyme that causes 50% inhibition of NBT photoreduction under the assay conditions.
2.6.2. Ascorbate Peroxidase (APx)
APx activity was calculated following the oxidation of ascorbate with H2O2, both added to the protein extracts, by the decrease in the absorbance at 290 nm [44], using the H2O2 extinction coefficient (ε290 = 2.8 mM−1 cm−1). No correction for ascorbate oxidation in the absence of protein extract was necessary as no activity was observed in the controls. One APx unit was defined as the amount of enzyme required to oxidise one µmol of ascorbate per min at 25 °C.
2.6.3. Glutathione Reductase (GR)
GR activity was calculated following the oxidation of NADPH, which is coupled to the GR-catalysed reduction of oxidised glutathione (GSSG). The reaction was initiated by adding the NADPH cofactor to samples containing the protein extract supplemented with GSSG under the conditions previously described [45]. After 25 min of incubation at room temperature, the decrease in absorbance at 340 nm was determined to calculate the amount of oxidised NADPH from its extinction coefficient (ε340 = 6.22 mM−1 cm−1). Control reactions without protein extract were performed simultaneously to correct for non-enzymatic NADPH oxidation. One GR unit was defined as the amount of enzyme that oxidises one µmol of NADPH per min at 25 °C.
2.7. Antioxidant Compounds Determination
2.7.1. Total Phenolic Compounds (TPC) and Flavonoids (TF)
Dry leaf material (approximately 0.1 g) was ground to a fine powder in a mortar and extracted with 80% methanol. Samples were gently shaken overnight at room temperature. Supernatants were collected by centrifugation and stored at −20 °C until used in the assays. TPCs contents were determined by their reaction with the Folin–Ciocalteu reagent [46]. The methanol extracts were incubated with the reagent and Na2CO3 for 90 min at room temperature in the dark, and the absorbance was then measured at 765 nm. TPC concentrations were expressed as equivalents of gallic acid (GA), used as the standard (mg eq. GA g−1 DW). TFs contents were quantified in the same extracts by their reaction with AlCl3 at a basic pH after nitration of the catechol groups with NaNO2, as described by Zhishen et al. [47]. TF contents were expressed as equivalents of the standard catechin (mg eq.C g−1 DW).
2.7.2. Ascorbic Acid (AsA)
Ascorbic acid (AsA) was quantified by a previously described procedure [48] based on the reduction of Fe3+ to Fe2+ by AsA at acidic pH, followed by the formation of a complex of Fe2+ with bipyridyl. Briefly, tomato leaf material was ground with 5% (w/v) TCA, the extract was centrifuged, and the supernatant was incubated with phosphoric acid, bipyridyl, and FeCl3 for one hour at 37 °C in the dark. The absorbance of the samples was read at 525 nm and the AsA concentrations were calculated from a calibration curve established by parallel reactions with known AsA amounts. Ascorbic acid contents were expressed as mg g−1 DW.
2.8. Statistical Analysis
Analysis of variance (ANOVA) at the 95% confidence level was used to compare the effects of the different treatments on all determined growth and biochemical parameters. Prior to the analysis of variance, the data requirements of the normality and homogeneity of variances were checked using Levene’s and Shapiro–Wilk’s tests. When the null ANOVA hypothesis was rejected, post hoc comparisons were performed to detect possible statistical differences between the applied treatments using Tukey’s test. Data are shown as means and standard deviations. Statgraphics Centurion v.15 statistical software (Statgraphics Technologies, Inc., The Plains, VA, USA) was used to perform the analyses.
3. Results
3.1. Effects of Water Stress and Biostimulant Pre-Treatments on Tomato Plants
The water stress treatment, applied by altogether withholding irrigation, had a strong negative effect on tomato plants (Figure 1). Compared to normally watered plants (IC, irrigated control, Figure 1a), the plants showed extreme wilting symptoms after 12 days without any irrigation (NIC, non-irrigated control, Figure 1b). Pre-treatment of the plants with the biostimulants for ten days before stopping irrigation seemed to protect the tomato plants against dehydration, although to different extents (Figure 1c–j), especially when the higher biostimulant dose, 1.0 g L−1, was used (Figure 1c,e,g,i). This effect was most evident with Terrabion Aminovit® (TB, Figure 1c,d), followed by the seaweed extract (SE, Figure 1i,j). Plants pre-treated with potassium fulvate (KF, Figure 1e,f) still showed some improvement over the non-irrigated controls, at least at the 1.0 g L−1 dose, whereas the effect of humic acids was barely visible (HA, Figure 1g,h).
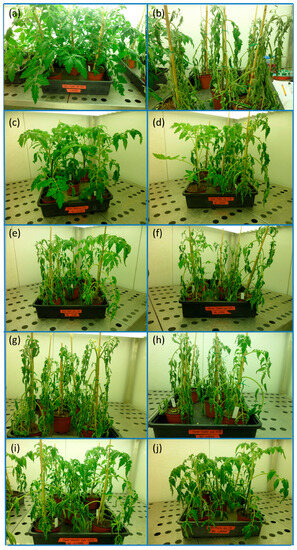
Figure 1.
Effect of biostimulant pre-treatments on cherry tomato plants 12 days after irrigation suppression. (a) irrigated control (IC); (b) non-irrigated control (NIC); (c–j) biostimulant applied at 1.0 g L−1 (left panels) or 0.2 g L−1 (right panels) of irrigation solution: (c,d) Terrabion Aminovit® (TB); (e,f) potassium fulvate (KF); (g,h) humic acids (HA); and (i,j) seaweed extract (SE).
3.2. Growth Parameters
Several growth parameters were determined after harvesting the plants (Table 2, Figure 2) in an attempt to quantify the biostimulants’ effects shown in Figure 1.

Table 2.
Effect of biostimulant pre-treatments on growth parameters of cherry tomato plants after 12 days of irrigation suppression. Terrabion Aminovit® (TB), potassium fulvate (KF), humic acids (HA), and seaweed extract (SE) applied at 0.2 and 1.0 g L−1 of irrigation solution. Controls: non-stressed, normally watered plants (irrigated control, IC) and plants subjected to the water stress treatment without biostimulant pre-treatment (non-irrigated control, NIC). Values are mean ± SD (n = 5). Different letters within each column indicate statistically significant differences between treatments (ANOVA, p ≤ 0.05).
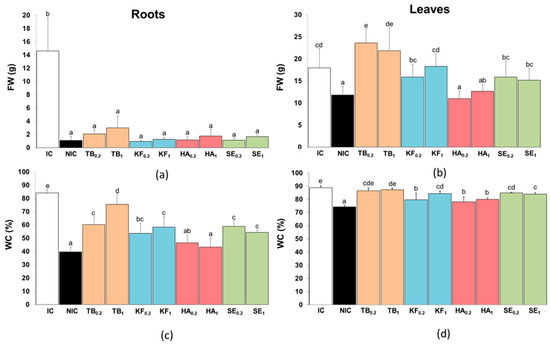
Figure 2.
Effect of biostimulant pre-treatments on fresh weight (FW) (a,b) and water content (WC%), (c,d) of roots (a,c) and leaves (b,d) of cherry tomato plants after 12 days of irrigation suppression. Biostimulants: Terrabion Aminovit® (TB), potassium fulvate (KF), humic acids (HA), and seaweed extract (SE) applied at 0.2 and 1 g L−1 of irrigation solution. Controls: non-stressed, normally watered plants (irrigated control, IC) and plants subjected to the water stress treatment without biostimulant pre-treatment (non-irrigated control, NIC). Values are mean ± SD (n = 5). In each panel, different letters above the columns indicate statistically significant differences between treatments (ANOVA, p ≤ 0.05).
The growth inhibition of tomato plants, induced by the water stress treatment, was reflected in significant reductions in stem length, leaf and flower numbers, and leaf area (comparing IC and NIC values in Table 2). Pre-treatment with the biostimulants did not improve the growth of tomato plants, considering these parameters, which showed very small and in most cases, non-significant differences with NIC plants (Table 2). The only significant differences were observed in stem length for TB and KF applied at 0.2 g L−1. Interestingly, humic acids appeared to significantly increase the number of flowers compared to NIC plants, although they reached only about 50% of the irrigated controls, an effect not observed for any of the other tested biostimulants (Table 2).
The effect of biostimulant pre-treatments was more clearly reflected in the differences observed in root and leaf fresh weight after the water stress period (Figure 2a,b). Indeed, the water deficit caused a strong reduction in root FW, down to less than 8% of that measured in the irrigated controls. None of the tested biostimulants significantly affected the root FW reduction (Figure 2a). The stress-induced reduction in leaf FW was not as pronounced as in roots, amounting to approximately 35% of the corresponding IC. Contrary to the roots, all biostimulants tested, except HA, showed a protective effect against stress-induced growth inhibition, with significant increases in leaf FW compared to NIC plants and no differences between the two applied doses, 0.2 and 1.0 g L−1, for any biostimulant (Figure 2b). In TB-treated plants, leaf FW was even higher than in IC plants.
The stress-induced FW reduction was partly due to the dehydration of roots and leaves (Figure 2c,d). The roots of the irrigated controls (IC plants) contained 84% water, a value that was reduced to 40% after 12 days of a lack of irrigation (NIC plants). The pre-treatment with all biostimulants, except HA, showed some protective effect against water-stress-induced root dehydration. Here again, TB had the most substantial effect on increasing the root water content of the tomato plants compared to the non-irrigated control; TB also showed a dose-dependent effect, as its application at 1.0 g L−1 was more effective than at 0.2 g L−1 (Figure 2c). Leaf dehydration was also observed in non-irrigated plants, although to a lesser extent than in the roots, with a decrease in WC from 89% to 74%. In any case, the effect of the biostimulant pre-treatments, in this case also including HA, was similar to that observed in the roots: a significant increase in leaf WC with respect to NIC plants, which for TB reached the same value as in the irrigated controls (Figure 2d).
3.3. Proline Content
Low levels of proline (Pro), 6.4 μmol g−1 DW, were measured in the leaves of the non-stressed tomato plants (IC), consistent with the known role of Pro as an abiotic stress biomarker [14]. After 12 days of irrigation suppression (NIC plants), the leaf Pro concentrations increased approximately 18-fold to 113.6 μmol g−1 DW (Figure 3). The pre-treatment with TB reduced the mean Pro contents compared to NIC plants, although the difference was significant only at the higher dose of 1.0 g L−1. KF and HA had no significant effect, and SE even increased the leaf Pro levels compared to stressed plants not treated with biostimulants to about 180 μmol g−1 DW (Figure 3).
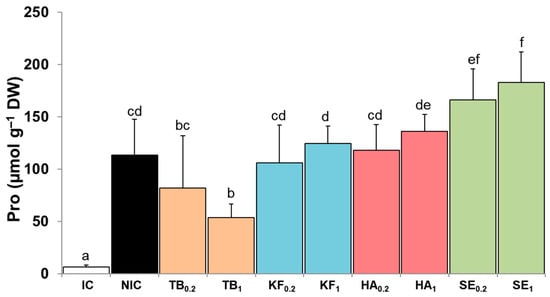
Figure 3.
Effect of biostimulant pre-treatments on leaf proline (Pro) contents in cherry tomato plants after 12 days of irrigation suppression. Biostimulants: Terrabion Aminovit® (TB), potassium fulvate (KF), humic acids (HA), and seaweed extract (SE) applied at 0.2 and 1.0 g L−1 of irrigation solution. Controls: non-stressed, normally watered plants (irrigated control, IC) and plants subjected to the water stress treatment without biostimulant pre-treatment (non-irrigated control, NIC). Values are mean ± SD (n = 5). Different letters above the columns indicate statistically significant differences between treatments (ANOVA, p ≤ 0.05).
3.4. Antioxidant Enzymes
The specific activity of three relevant antioxidant enzymes, namely superoxide dismutase (SOD), ascorbate peroxidase (APx), and glutathione reductase (GR), was determined in the leaf protein extracts of all plants harvested after the water stress treatment (Figure 4). Under non-stress conditions (IC plants), the calculated activities of SOD, APx, and GR were relatively low but increased significantly (4.7-, 19-, and 12.2-fold, respectively) upon application of the water stress treatment (NIC plants), suggesting that these antioxidant enzymes are activated to counteract the oxidative stress generated by subjecting the plants to a water deficit. The pre-treatment with all the biostimulants resulted in a significant decrease in the specific activities of the three enzymes in the water-stressed plants, with some quantitative differences between them. For example, all tested biostimulants reduced SOD activity to the control (IC) levels at the two applied doses (Figure 4a). In the case of APx activity (Figure 4b), pre-treatment with SE at both doses, HA at 0.2 g L−1 and KF at 1.0 g L−1, also significantly reduced the enzyme activity compared to NIC plants, although not reaching values as low as those of IC plants, as was observed with all the other biostimulant pre-treatments. Finally, GR activities showed the same qualitative pattern as APx, although pre-treatment with HA and SE seemed to be more effective in reducing the activity of this enzyme (Figure 4c). Interestingly, the HA and SE effects of reducing APx and GR activities in stressed plants were dose-dependent, whereas KF had a stronger effect on the three enzymatic activities at the lower dose, 0.2 g L−1 (Figure 4).
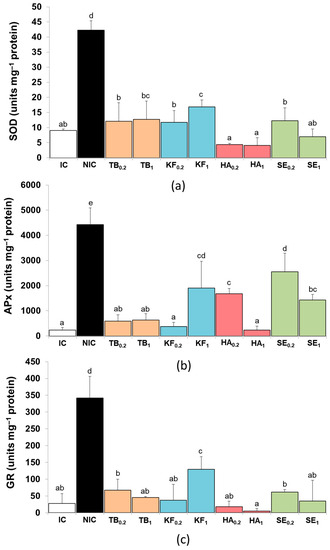
Figure 4.
Effect of biostimulant pre-treatments on the specific activity of antioxidant enzymes, superoxide dismutase (SOD) (a), ascorbate peroxidase (Apx) (b), and glutathione reductase (GR) (c), in cherry tomato plants after 12 days of irrigation suppression. Biostimulants: Terrabion Aminovit® (TB), potassium fulvate (KF), humic acids (HA), and seaweed extract (SE) applied at 0.2 and 1 g L−1 of irrigation solution. Controls: non-stressed, normally watered plants (irrigated control, IC) and plants subjected to the water stress treatment without biostimulant pre-treatment (non-irrigated control, NIC). Values are mean ± SD (n = 5). Different letters above the columns indicate statistically significant differences between treatments (ANOVA, p ≤ 0.05).
3.5. Antioxidant Compounds
The effects of the water stress treatment and the pre-treatment with the tested biostimulants on the leaf contents of antioxidant compounds (total phenolics, flavonoids, and ascorbic acid) are shown in Table 3. Even though it could be expected that the oxidative stress generated under water deficit conditions would activate the synthesis of antioxidant compounds, only the ascorbic acid contents increased significantly after the water stress period. No significant differences were found for total phenolic compounds (TFC), and flavonoid contents (TF) even decreased in NCI plants compared to the irrigated controls (Table 3). No clear patterns were observed regarding the effect of the biostimulants. Differences in TFC contents between treatments were small and generally not significant, and TF concentrations in biostimulant pre-treated plants did not differ or were even lower than those measured in NCI plants. Some significant differences were observed only in the case of ascorbic acid, which decreased with respect to NCI values upon pre-treatment with TB and KF, whereas HA and SE had no effect (Table 3).

Table 3.
Effect of biostimulant pre-treatments on antioxidant compounds, total phenolic compounds (TPC), total flavonoids (TF), ascorbic acid contents in leaves of cherry tomato plants after 12 days of irrigation suppression. Biostimulants: Terrabion Aminovit® (TB), potassium fulvate (KF), humic acids (HA), and seaweed extract (SE) applied at 0.2 and 1.0 g L−1 of irrigation solution. Controls: non-stressed, normally watered plants (irrigated control, IC) and plants subjected to the water stress treatment without biostimulant pre-treatment (non-irrigated control, NIC). Values are mean ± SD (n = 5). Different letters within each column indicate statistically significant differences between treatments (ANOVA, p ≤ 0.05). GA: gallic acid; C: catechin.
4. Discussion
Drought has negative effects on plant metabolic processes, slowing development and reducing crop yield [29,49]. The phenological stage, duration, and intensity of stress, as well as the tissue or organ involved in the response mechanism, may all influence the severity of these detrimental processes [50]. Under conditions of irreversible wilting, like those applied in this study, the cell’s ability to retain water is mainly determined by morphological adaptations, such as lignin depositions, and by antioxidant response mechanisms, which influence the rate at which plants wilt [51]. In this respect, tomatoes are particularly sensitive to drought [30], as shown in the present experiments, where the tomato plants showed irreversible wilting symptoms 12 days after irrigation was stopped. Proper irrigation management in fields or greenhouses is essential to avoid triggering these degenerative water-deficit processes [52]. The practical importance of studying the response mechanisms to abiotic stresses lies in the possibility of reducing their deleterious effects and increasing crop yields [53,54]. For example, the expression of stress-tolerance genes in transgenic plants or the direct application of certain osmolytes (e.g., sugars, proline, or glycine-betaine) in irrigation water can help reduce water stress effects [8,55,56,57].
Using biostimulants in the form of protein hydrolysates, algae extracts, or humic acids is a useful strategy to improve growth and increase crop productivity under various abiotic stresses [20,21,22]. Recent studies on tomato plants have shown the positive effects of applying naturally derived biostimulants such as those used here. For example, plants treated with a protein hydrolysate showed improved hydration status and enhanced antioxidant content, leading to higher yields under drought stress conditions [58]. Furthermore, an animal protein-based biostimulant obtained by enzymatic hydrolysis boosted antioxidant protection under water stress [59]. Extracts of the seaweed Ascophyllum nodosum combined with silicon applied to tomato plants under moderate drought (75% field capacity) improved fruit yield to the level of the optimally irrigated controls [60]. In another study, treatments with a seaweed biostimulant (Ascophyllum nodosum and Laminaria digitata) and yeast extracts reduced abscisic acid, malondialdehyde, and proline contents and the activity of ROS scavenging enzymes compared to untreated plants [36]. In addition, plants treated with Chondrus crispus, a common red seaweed, increased shoot height and biomass, as well as abscisic acid and proline levels under water stress conditions [61]. There is a paucity of literature on the effects of humic substances on tomato plants under water stress. Nevertheless, an increase in root and leaf biomass has been observed by applying humic acids to water-stressed horticultural crops, including tomato plants [62]. Under other stress conditions, such as salinity, a decrease in antioxidant activity, and H2O2, MDA, and proline contents was observed in cherry tomato plants after the application of humic-based substances as seed coatings or drench solutions [63]. On the other hand, humic substances also have beneficial properties under non-stress conditions; for example, foliar applications of potassium fulvate in greenhouse tomatoes stimulated potassium and nitrogen uptake with increased yields [64].
In the present study, cherry tomato plants were treated with different biostimulants (i.e., natural products based on amino acids, seaweed extracts, humic acids, and potassium fulvate) for 10 days before being subjected to water stress to evaluate their efficacy in reducing the water deficit effects on the plants. Although no clear differences were found in the determined primary growth parameters (stem length, number of leaves, number of flowers, and leaf area), all biostimulants exerted significant protection against drought-induced dehydration in the roots (except HA) and leaves, with the strongest effect observed for TB. Similarly, Top et al. [65] found that using a biostimulant derived from seaweed (Ascophyllum nodosum and Saccharina latissimi) extracts enabled plants to maintain water uptake levels during drought, similar to well-watered controls. Several studies have reported beneficial effects on tomato plants’ growth after applying biostimulants similar to those used here under different stress conditions, including water shortage [66,67,68] or nutritional stress [69], and also in non-stressed plants [70,71,72]. Controversial responses to the application of different biostimulants to tomato plants, including a protein-based biostimulant and a seaweed extract, under water stress were also reported by Kalozoumis et al. [73]. Additional reports on biostimulant effects improving plant growth and yield under abiotic stresses in tomato and other Solanaceae have recently been reviewed by Cristofano et al. [74].
Proline is considered an excellent biomarker of abiotic stress in many plant species, including tomatoes, as its concentration increases in response to drought, high salinity, and other environmental stressors [75,76,77]. When comparing related taxa, Pro generally accumulates to higher concentrations in the more tolerant genotypes [78,79], suggesting a direct involvement of this compound in the mechanisms of stress tolerance, acting in osmotic adjustment and/or through its additional functions as a low-molecular-weight chaperon, ROS scavenger, and signalling molecule [14]. However, in other cases, there is a negative correlation between Pro concentrations and the degree of tolerance; for instance, comparing a large number of bean cultivars subjected to drought and salt stress treatments, higher Pro levels were measured in the more sensitive genotypes [80]. In the present study, as expected, Pro contents increased under water stress (NIC plants), but a relative, dose-dependent decrease was observed in plants pre-treated with TB. This result indicated that this biostimulant reduced the level of stress caused by the water deficit treatment and that Pro was not directly involved in the TB mechanism of action. The KF and HA pre-treatments did not have any significant effect on Pro levels compared to the non-irrigated plants, whereas SE showed the opposite effect to TB; i.e., a significant additional increase in Pro contents compared to the NIC plants. Therefore, unlike the other biostimulants tested, seaweed extracts may act by accumulating Pro to even higher levels than those induced by water stress alone. Similar results have been previously reported by Goñi et al. [81], who showed that the application of a biostimulant based on the seaweed Ascophyllum nodosum to tomato plants before withholding irrigation for seven days increased Pro levels between 8- and 15-fold. Indeed, seaweed extracts have been reported as elicitors that protect cells under abiotic stress conditions through Pro accumulation [82].
The oxidative stress generated by the water deficit treatment resulted in significant increases in the specific activities of the antioxidant enzymes SOD, APx, and GR, which were very low in the well-watered controls (IC plants). This was expected since the activation of enzymatic antioxidant systems is a general response of plants to abiotic stress and has been reported in many different species, although with large quantitative and qualitative differences between species and even varieties or cultivars within a species (e.g., [79,80,81,82,83]). All the biostimulants tested significantly reduced the enzymes’ specific activities, in most cases to the same levels as the irrigated controls, strongly supporting the notion that the biostimulant pre-treatments protected the plants against oxidative stress. Similarly, the application of a commercial biostimulant based on polyphenols and glycine betaine significantly reduced SOD, catalase (CAT), and GR activities in experiments with tomato plants subjected to salt stress treatments [84]. There are few reports on the effects of biostimulants reducing the activity of ROS scavenging enzymes in water-stressed tomato plants (e.g., [36]). In fact, biostimulants’ application usually leads to an increase in antioxidant enzymes activities; for example, SOD activity increased in tomato plants under drought conditions by applying a biostimulant based on Ascophyllum nodosum mixed with macro- and micronutrients [37], or alginate (i.e., a polysaccharide extracted from algae) [85]. An increase in SOD and APx activities was also observed after applying an animal-derived protein hydrolysate to drought-stressed tomato plants [35]. Similar results have been reported in other crops, such as bentgrass, where SOD activity increased significantly after the application of a seaweed extract and humic acids [86]. SOD and APx activities also increased by the application of a biostimulant based on humic and fulvic acids in maize subjected to water stress [26]. These differences can be explained by the heterogeneity of the mechanisms used by different plants to counteract ROS and the nature and mechanism of action, generally unknown, of each biostimulant applied [31,34,87].
The antioxidant response of the tomato plants under our experimental conditions was based primarily on the enzymatic systems mentioned above and did not require the accumulation of common antioxidant metabolites such as phenolic compounds, especially the subgroup of flavonoids. Only ascorbic acid appeared to contribute to this response, as its concentrations increased upon the water stress treatment (NIC plants). The protective effect of TB and, to a lesser extent, KF pre-treatments was demonstrated by the reduction of AsA concentrations to those of the irrigated control (IC) plants in the case of TB. Similar results were obtained by Francesca et al. [58] when a protein hydrolysate was applied to tomato plants under non-stress conditions.
5. Conclusions
In this work, several biostimulants—Terrabion Aminovit® (a commercial product based on amino acids), potassium fulvate, humic acids, and a seaweed extract—were tested for their effectiveness in reducing the damage caused by water stress in cherry tomato plants. The pre-treatment with all biostimulants protected the plants against dehydration caused by the subsequent water deficit treatment, as shown by a significant increase in leaf WC compared to non-irrigated plants (and also increased leaf FW and root WC, except for the humic acids). Terrabion Aminovit® was the most effective in reducing wilting, followed by the seaweed extract, although these two biostimulants appear to have different modes of action, involving, in the case of the seaweed extract, the accumulation of proline in leaves at levels significantly higher than in the non-irrigated controls. The water deficit treatment caused oxidative stress in the tomato plants, which responded by activating antioxidant enzymes (SOD, APx, GR) and accumulating ascorbic acid. The pre-treatments with all biostimulants protected the plants against drought-induced oxidative stress, as shown by the significant decrease in SOD, APx, and GR activities and, in the case of Terrabion Aminovit® and potassium fulvate, also in ascorbic acid contents, with respect to NIC plants. Therefore, the application of these biostimulants, particularly Terrabion Aminovit® and the seaweed extract, may protect tomato plants against the adverse effects of drought, at least in part, by enhancing the plants’ antioxidant responses. However, further research is needed to elucidate the precise mechanisms underlying the biostimulants’ effects.
Author Contributions
Conceptualization, R.G.-O. and O.V.; methodology, R.G.-O. and O.V.; validation, M.Á.N.; formal analysis, R.G.-O.; investigation, R.G.-O. and O.V.; resources, R.G.-O.; data curation, R.G.-O.; writing—original draft preparation, R.G.-O. and O.V.; writing—review and editing, R.G.-O. and O.V.; visualization, M.Á.N. and S.A.; supervision, O.V.; project administration, M.Á.N; funding acquisition, M.Á.N. and S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Centre for Development of Industrial Technology (CDTI), Spanish Government. Project: Prevention of abiotic stress by potassium biostimulant fertilisers.
Data Availability Statement
All data are included within the article.
Acknowledgments
We would like to thank Laura Molina for her help with the tomato plants in the early stages of this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Vicente, O.; Boscaiu, M. Mechanisms of drought and salt stress tolerance in plants. Sci. Bull. Ser. F Biotechnol. 2019, 23, 29–36. [Google Scholar]
- Kaur, G.; Asthir, B. Molecular responses to drought stress in plants. Biol. Plant. 2017, 61, 201–209. [Google Scholar] [CrossRef]
- Moradi, P. Key plant products and common mechanisms utilized by plants in water deficit stress responses. Bot. Sci. 2016, 94, 657–671. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef]
- Houmani, H.; Corpas, F.J. Differential responses to salt-induced oxidative stress in three phylogenetically related plant species: Arabidopsis thaliana (glycophyte), Thellungiella salsuginea and Cakile maritima (halophytes). Involvement of ROS and NO in the control of K+/Na+ homeostasis. AIMS Biophys. 2016, 3, 380–397. [Google Scholar] [CrossRef]
- Liu, N.; Jin, Z.; Wang, S.; Gong, B.; Wen, D.; Wang, X.; Wei, M.; Shi, Q. Sodic alkaline stress mitigation with exogenous melatonin involves reactive oxygen metabolism and ion homeostasis in tomato. Sci. Hortic. 2015, 181, 18–25. [Google Scholar] [CrossRef]
- Gil, R.; Lull, C.; Boscaiu, M.; Bautista, I.; Lidón, A.L.; Vicente, O. Soluble carbohydrates as osmolytes in several halophytes from a Mediterranean salt marsh. Not. Bot. Horti Agrobot. 2011, 39, 9–17. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Kumari, P.H.; Sunita, M.S.; Sreenivasulu, N. Role of proline in cell wall synthesis and plant development and its implications in plant ontogeny. Front. Plant. Sci. 2015, 6, 544. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Biotechnol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008, 133, 481–489. [Google Scholar] [CrossRef]
- Podgórska, A.; Burian, M.; Szal, B. Extra-cellular but extra-ordinarily important for cells: Apoplastic reactive oxygen species metabolism. Front. Plant Sci. 2017, 8, 1353. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R.M. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.J. Thiol-based peroxidases and ascorbate peroxidases: Why plants rely on multiple peroxidase systems in the photosynthesizing chloroplast? Mol. Cells 2016, 39, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Nianiou-Obeidat, I.; Madesis, P.; Kissoudis, C.; Voulgari, G.; Chronopoulou, E.; Tsaftaris, A.; Labrou, N.E. Plant glutathione transferase-mediated stress tolerance: Functions and biotechnological applications. Plant Cell Rep. 2017, 36, 791–805. [Google Scholar] [CrossRef]
- Parađiković, N.; Vinković, T.; Vinković Vrček, I.; Žuntar, I.; Bojić, M.; Medić-Šarić, M. Effect of natural biostimulants on yield and nutritional quality: An example of sweet yellow pepper (Capsicum annuum L.) plants. J. Sci. Food Agric. 2011, 91, 2146–2152. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant. Sci. 2016, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Escaich, J.; Soler, F.; Juncosa, R.; Gomis, P. Fructificación en cultivos tratados con aminoácidos de hidrólisis enzimática. Horticultura 1991, 67, 47–53. [Google Scholar]
- Mire, G.L.; Nguyen, M.L.; Fassotte, B.; Jardin, P.D.; Verheggen, F.J.; Delaplace, P.; Jijakli, M.H. Implementing plant biostimulants and biocontrol strategies in the agroecological management of cultivated ecosystems. A review. Biotechnol. Agron. Soc. Environ. 2016, 20, 299–313. [Google Scholar] [CrossRef]
- Aslam, M.; Travis, R.L.; Rains, D.W. Differential effect of amino acids on nitrate uptake and reduction systems in barley roots. Plant Sci. 2001, 160, 219–228. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Zhang, X.; Ervin, E.; Kiehl, J. Enzymatic antioxidant responses to biostimulants in maize and soybean subjected to drought. Sci. Agric. 2009, 66, 395–402. [Google Scholar] [CrossRef]
- Torres-Rodríguez, J.A.; Reyes-Pérez, J.J.; González-Rodríguez, J.C. Effect of a natural biostimulant on some quality parameters of tomato seedlings (Solanum lycopersicum L.) under saline conditions. Biotecnia 2016, 18, 11–15. [Google Scholar] [CrossRef]
- Siwik-Ziomek, A.; Szczepanek, M. Soil enzyme activity and sulphur uptake by oilseed rape depending on fertilization and biostimulant application. Acta Agric. Scand. B Soil Plant Sci. 2018, 68, 50–56. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Maroto-Borrego, J.V. Horticultura Herbácea Especial, 5th ed.; Mundi-Prensa: Madrid, Spain, 2002; pp. 1–704. [Google Scholar]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Giannakoula, A.; Ilias, I. The effect of water stress and salinity on growth and physiology of tomato (Lycopersicon esculentum Mil.). Arch. Biol. Sci. 2013, 65, 611–620. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Moreno, D.A.; Ferreres, F.; Rubio-Wilhelmi, M.d.M.; Ruiz, J.M. Differential responses of five cherry tomato varieties to water stress: Changes on phenolic metabolites and related enzymes. Phytochemistry 2011, 72, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Zushi, K.; Matsuzoe, N. Seasonal and cultivar differences in salt-induced changes in antioxidant system in tomato. Sci. Hortic. 2009, 120, 181–187. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, W.; Lv, H.; Liang, B.; Jin, S.; Li, J.; Zhou, W. Animal-derived plant biostimulant alleviates drought stress by regulating photosynthesis, osmotic adjustment, and antioxidant systems in tomato plants. Sci. Hortic. 2022, 305, 111365. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Agliassa, C.; Mannino, G.; Vigliante, I.; Contartese, V.; Secchi, F.; Bertea, C.M. A biostimulant based on seaweed (Ascophyllum Nodosum and Laminaria digitata) and yeast extracts mitigates water stress effects on tomato (Solanum lycopersicum L.). Agriculture 2021, 11, 557. [Google Scholar] [CrossRef]
- Peripolli, M.; Dornelles, S.; Lopes, S.; Tabaldi, L.; Trivisiol, V.; Rubert, J. Application of biostimulants in tomato subjected to water deficit: Physiological, enzymatic and production responses. Rev. Bras. Eng. Agrícola Ambient. 2021, 25, 90–95. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil, 2nd ed.; Circular 347; University of California Agricultural Experiment Station: Berkeley, CA, USA, 1950; pp. 1–39. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Vicente, O.; Boscaiu, M.; Naranjo, M.Á.; Estrelles, E.; Bellés, J.M.; Soriano, P. Responses to salt stress in the halophyte Plantago crassifolia (Plantaginaceae). J. Arid Environ. 2004, 58, 463–481. [Google Scholar] [CrossRef]
- Gil, R.; Bautista, I.; Boscaiu, M.; Lidón, A.; Wankhade, S.; Sánchez, H.; Llinares, J.; Vicente, O. Responses of five Mediterranean halophytes to seasonal changes in environmental conditions. AoB Plants 2014, 6, plu049. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell. Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Connell, J.P.; Mullet, J.E. Pea chloroplast glutathione reductase: Purification and characterization. Plant Physiol. 1986, 82, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Law, M.Y.; Charles, S.A.; Halliwell, B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of Paraquat. Biochem. J. 1983, 210, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Giordano, M.; Petropoulos, S.A.; Rouphael, Y. Response and defence mechanisms of vegetable crops against drought, heat and salinity stress. Agriculture 2021, 11, 463. [Google Scholar] [CrossRef]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Bodner, G.; Nakhforoosh, A.; Kaul, H.-P. Management of crop water under drought: A review. Agron. Sustain. Dev. 2015, 35, 401–442. [Google Scholar] [CrossRef]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef]
- Petrozza, A.; Santaniello, A.; Summerer, S.; Di Tommaso, G.; Di Tommaso, D.; Paparelli, E.; Piaggesi, A.; Perata, P.; Cellini, F. Physiological responses to Megafol® treatments in tomato plants under drought stress: A phenomic and molecular approach. Sci. Hortic. 2014, 174, 185–192. [Google Scholar] [CrossRef]
- Chen, S.; Heuer, B. Effect of genotype and exogenous application of glycinebetaine on antioxidant enzyme activity in native gels of 7-day-old salt-stressed tomato (Solanum lycopersicum) seedlings. Sci. Hortic. 2013, 162, 106–116. [Google Scholar] [CrossRef]
- Claussen, W. Proline as a measure of stress in tomato plants. Plant Sci. 2005, 168, 241–248. [Google Scholar] [CrossRef]
- Goel, D.; Singh, A.K.; Yadav, V.; Babbar, S.B.; Murata, N.; Bansal, K.C. Transformation of tomato with a bacterial codA gene enhances tolerance to salt and water stresses. J. Plant Physiol. 2011, 168, 1286–1294. [Google Scholar] [CrossRef]
- Francesca, S.; Cirillo, V.; Raimondi, G.; Maggio, A.; Barone, A.; Rigano, M.M. A novel protein hydrolysate-based biostimulant improves tomato performances under drought stress. Plants 2021, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- Casadesús, A.; Polo, J.; Munné-Bosch, S. Hormonal effects of an enzymatically hydrolyzed animal protein-based biostimulant (pepton) in water-stressed tomato plants. Front. Plant Sci. 2019, 10, 758. [Google Scholar] [CrossRef]
- Ahmed, M.; Ullah, H.; Attia, A.; Tisarum, R.; Cha-um, S.; Datta, A. Interactive effects of Ascophyllum nodosum seaweed extract and silicon on growth, fruit yield and quality, and water productivity of tomato under water stress. Silicon 2023, 15, 2263–2278. [Google Scholar] [CrossRef]
- Domingo, G.; Marsoni, M.; Álvarez-Viñas, M.; Torres, M.D.; Domínguez, H.; Vannini, C. The role of protein-rich extracts from Chondrus crispus as biostimulant and in enhancing tolerance to drought stress in tomato plants. Plants 2023, 12, 845. [Google Scholar] [CrossRef]
- Qin, K.; Leskovar, D.I. Humic substances improve vegetable seedling quality and post-transplant yield performance under stress conditions. Agriculture 2020, 10, 254. [Google Scholar] [CrossRef]
- Turan, M.; Yildirim, E.; Ekinci, M.; Argin, S. Effect of biostimulants on yield and quality of cherry tomatoes grown in fertile and stressed soils. HortScience 2021, 56, 414–423. [Google Scholar] [CrossRef]
- Xu, X.; Lei, X.; Liao, S.; Li, Y.; Sun, Y. Foliar application of potassium silicate, potassium fulvate and betaine improve summer-time tomato yield by promoting plant nitrogen and potassium uptake. Folia Hortic. 2022, 34, 125–138. [Google Scholar] [CrossRef]
- Top, S.; Vandoorne, B.; Pauwels, E.; Perneel, M.; Van Labeke, M.C.; Steppe, K. Plant sensors untangle the water-use and growth effects of selected seaweed-derived biostimulants on drought-stressed tomato plants (Solanum lycopersicum). J. Plant Growth Regul. 2023, 42, 5615–5627. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Vaghela, P.; Trivedi, K.; Anand, K.G.V.; Brahmbhatt, H.; Nayak, J.; Khandhediya, K.; Prasad, K.; Moradiya, K.; Kubavat, D.; Konwar, L.J.; et al. Scientific basis for the use of minimally processed homogenates of Kappaphycus alvarezii (red) and Sargassum wightii (brown) seaweeds as crop biostimulants. Algal Res. 2023, 70, 102969. [Google Scholar] [CrossRef]
- Villa e Vila, V.; Marques, P.A.A.; Rezende, R.; Wenneck, G.S.; Terassi, D.D.S.; Andrean, A.F.B.A.; Nocchi, R.C.D.F.; Matumoto-Pintro, P.T. Deficit Irrigation with Ascophyllum nodosum Extract Application as a Strategy to Increase Tomato Yield and Quality. Agronomy 2023, 13, 1853. [Google Scholar] [CrossRef]
- Koleška, I.; Hasanagić, D.; Todorović, V.; Murtić, S.; Klokić, I.; Parađiković, N.; Kukavica, B. Biostimulant prevents yield loss and reduces oxidative damage in tomato plants grown on reduced NPK nutrition. J. Plant Interact. 2017, 12, 209–218. [Google Scholar] [CrossRef]
- Rady, M.M.; Rehman, H.u. Supplementing organic biostimulants into growing media enhances growth and nutrient uptake of tomato transplants. Sci. Hortic. 2016, 203, 192–198. [Google Scholar] [CrossRef]
- Zodape, S.T.; Gupta, A.R.; Bhandari, S.; Rawat, U.S.; Chaudhary, D.; Eswaran, K.; Chikara, J. Foliar application of seaweed sap as biostimulant for enhancement of yield and quality of tomato (Lycopersicon esculentum Mill.). J. Sci. Ind. Res. 2011, 70, 215–219. [Google Scholar]
- Vinoth, S.; Gurusaravanan, P.; Jayabalan, N. Effect of seaweed extracts and plant growth regulators on high-frequency in vitro mass propagation of Lycopersicon esculentum L (tomato) through double cotyledonary nodal explant. J. Appl. Phycol. 2012, 24, 1329–1337. [Google Scholar] [CrossRef]
- Kalozoumis, P.; Vourdas, C.; Ntatsi, G.; Savvas, D. Can biostimulants increase resilience of hydroponically-grown tomato to combined water and nutrient stress? Horticulturae 2021, 7, 297. [Google Scholar] [CrossRef]
- Cristofano, F.; El-Nakhel, C.; Rouphael, Y. Biostimulant substances for sustainable agriculture: Origin, operating mechanisms and effects on cucurbits, leafy greens, and nightshade vegetables species. Biomolecules 2021, 11, 1103. [Google Scholar] [CrossRef]
- Ali, S.; Rab, A.; Khan, D.N.; Nawab, K. Enhanced proline synthesis may determine resistance to salt stress in tomato cultivars. Pak. J. Bot. 2011, 43, 2707–2710. [Google Scholar]
- Amini, F.; Ehsanpour, A. Soluble Proteins, Proline, Carbohydrates and Na+/K+ Changes in two tomato (Lycopersicon esculentum Mill.) cultivars under in vitro salt stress. Am. J. Biochem. Biotechnol. 2005, 1, 212–216. [Google Scholar] [CrossRef]
- Machado, J.; Vasconcelos, M.W.; Soares, C.; Fidalgo, F.; Heuvelink, E.; Carvalho, S.M.P. Young tomato plants respond differently under single or combined mild nitrogen and water deficit: An insight into morphophysiological responses and primary metabolism. Plants 2023, 12, 1181. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, S.; Al Hassan, M.; Bandara, W.M.C.; Yabor, L.; Llinares, J.V.; Boscaiu, M.; Vicente, O. Screening for salt tolerance in four local varieties of Phaseolus lunatus from Spain. Agriculture 2018, 8, 201. [Google Scholar] [CrossRef]
- Plazas, M.; Nguyen, H.T.; González-Orenga, S.; Fita, A.; Vicente, O.; Prohens, J.; Boscaiu, M. Comparative analysis of the responses to water stress in eggplant (Solanum melongena) cultivars. Plant Physiol. Biochem. 2019, 143, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, S.; Yabor, L.; Díez, M.J.; Prohens, J.; Boscaiu, M.; Vicente, O. The use of proline in screening for tolerance to drought and salinity in common bean (Phaseolus vulgaris L.) genotypes. Agronomy 2020, 10, 817. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O'Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- González-Orenga, S.; Al Hassan, M.; Llinares, J.V.; Lisón, P.; López-Gresa, M.P.; Verdeguer, M.; Vicente, O.; Boscaiu, M. Qualitative and quantitative differences in osmolytes accumulation and antioxidant activities in response to water deficit in four Mediterranean Limonium species. Plants 2019, 8, 506. [Google Scholar] [CrossRef] [PubMed]
- Zuzunaga-Rosas, J.; González-Orenga, S.; Calone, R.; Rodríguez-Heredia, R.; Asaff-Torres, A.; Boscaiu, M.; Ibáñez-Asensio, S.; Moreno-Ramón, H.; Vicente, O. Use of a biostimulant to mitigate the effects of excess salinity in soil and irrigation water in tomato plants. Plants 2023, 12, 1190. [Google Scholar] [CrossRef]
- Liu, R.; Jiang, X.; Guan, H.; Li, X.; Du, Y.; Wang, P.; Mou, H. Promotive effects of alginate-derived oligosaccharides on the inducing drought resistance of tomato. J. Ocean. Univ. China 2009, 8, 303–311. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.; Schmidt, R. Physiological effects of liquid applications of a seaweed extract and a humic acid on creeping bentgrass. J. Am. Soc. Hortic. Sci. 2003, 128, 492–496. [Google Scholar] [CrossRef]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).