The Synergistic Effect of Biochar and Microorganisms Greatly Improves Vegetation and Microbial Structure of Degraded Alpine Grassland on Qinghai–Tibet Plateau
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Materials
2.2. Experimental Design and Sampling
2.3. Soil Physicochemical Properties
2.4. Soil Microorganisms
2.5. Statistical Analysis
3. Results
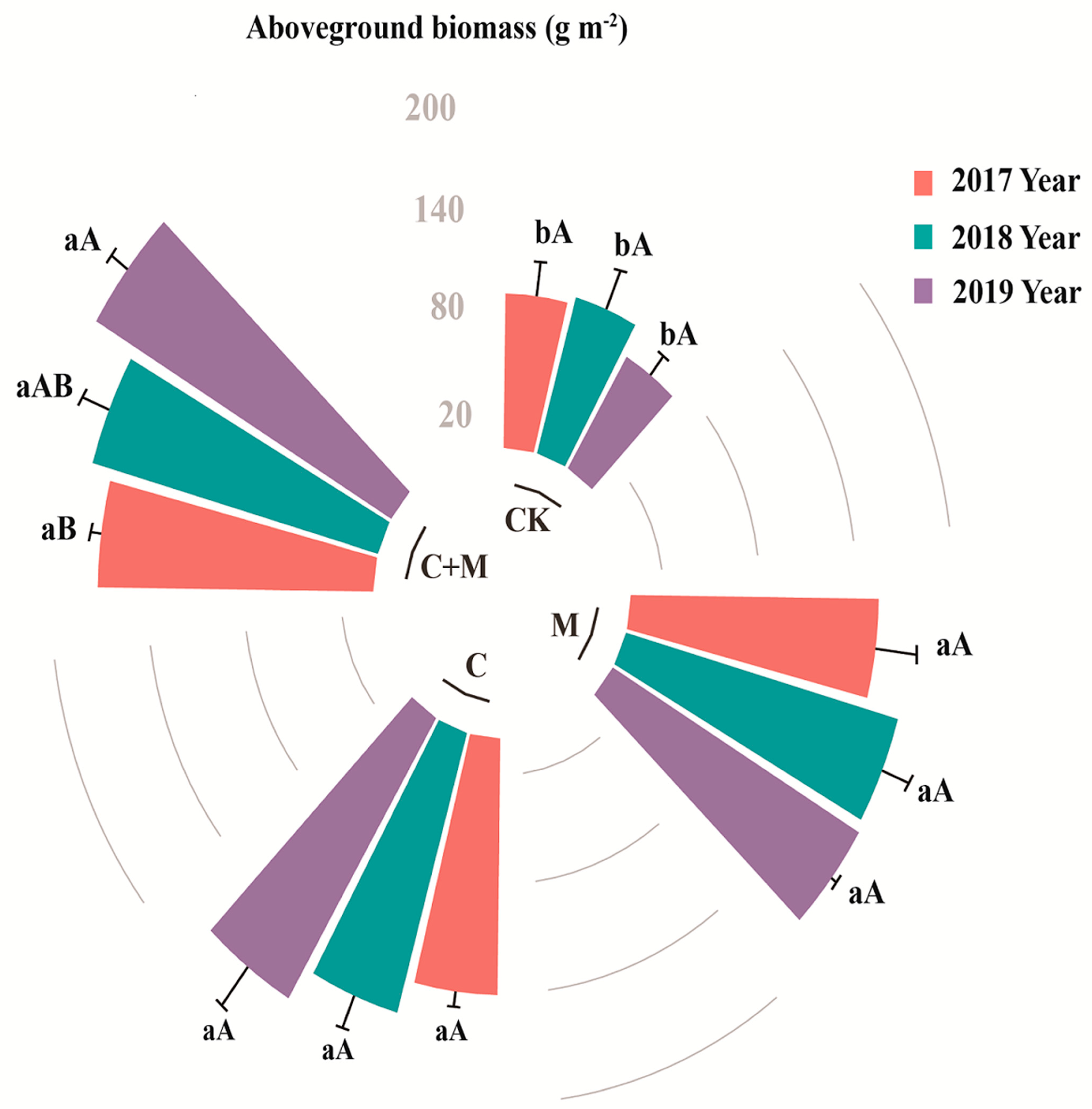
3.1. Changes in Aboveground Vegetation Biomass
3.2. Changes in Soil Physicochemical Properties
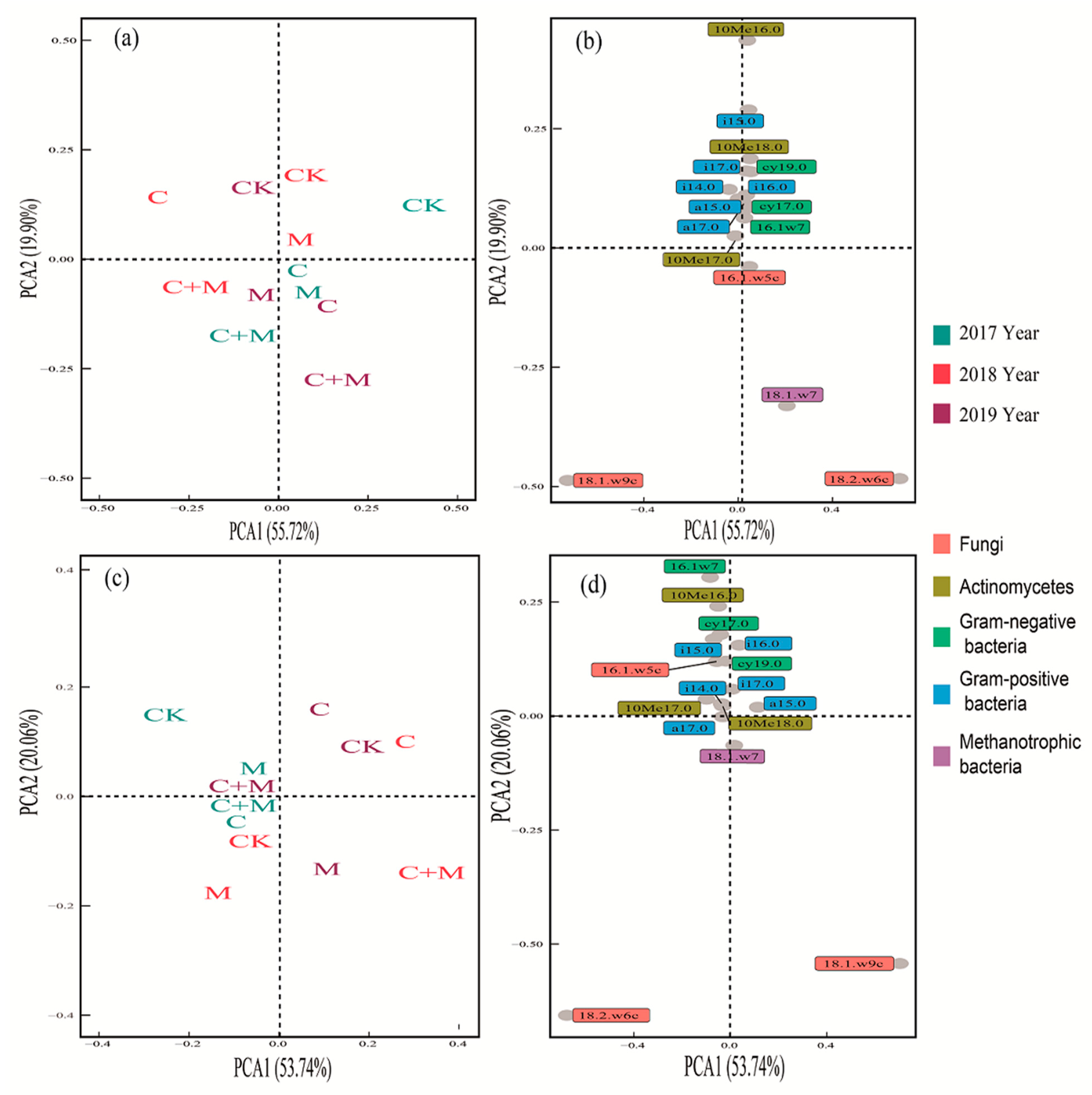
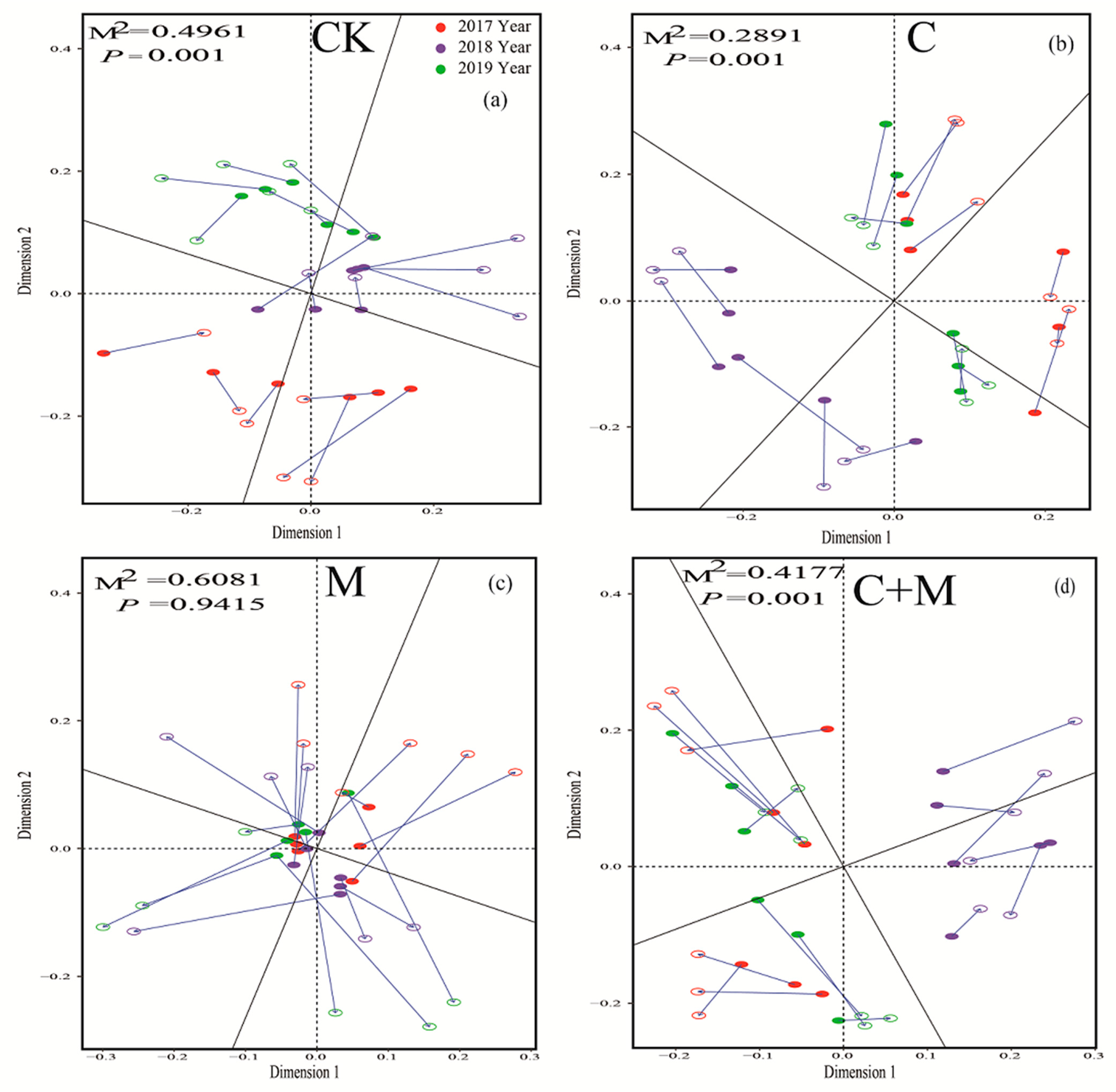
3.3. Changes in Soil Microorganisms
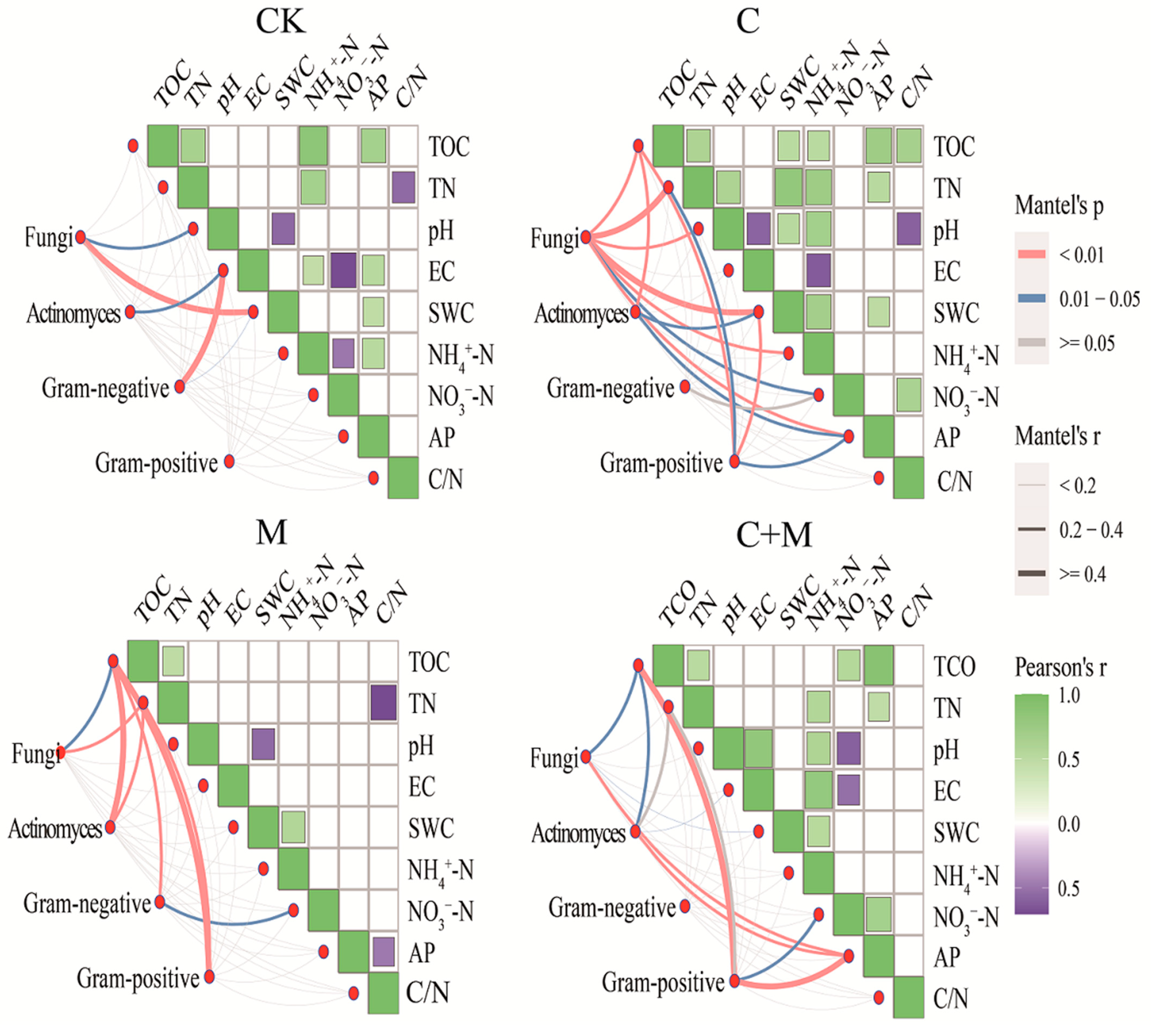
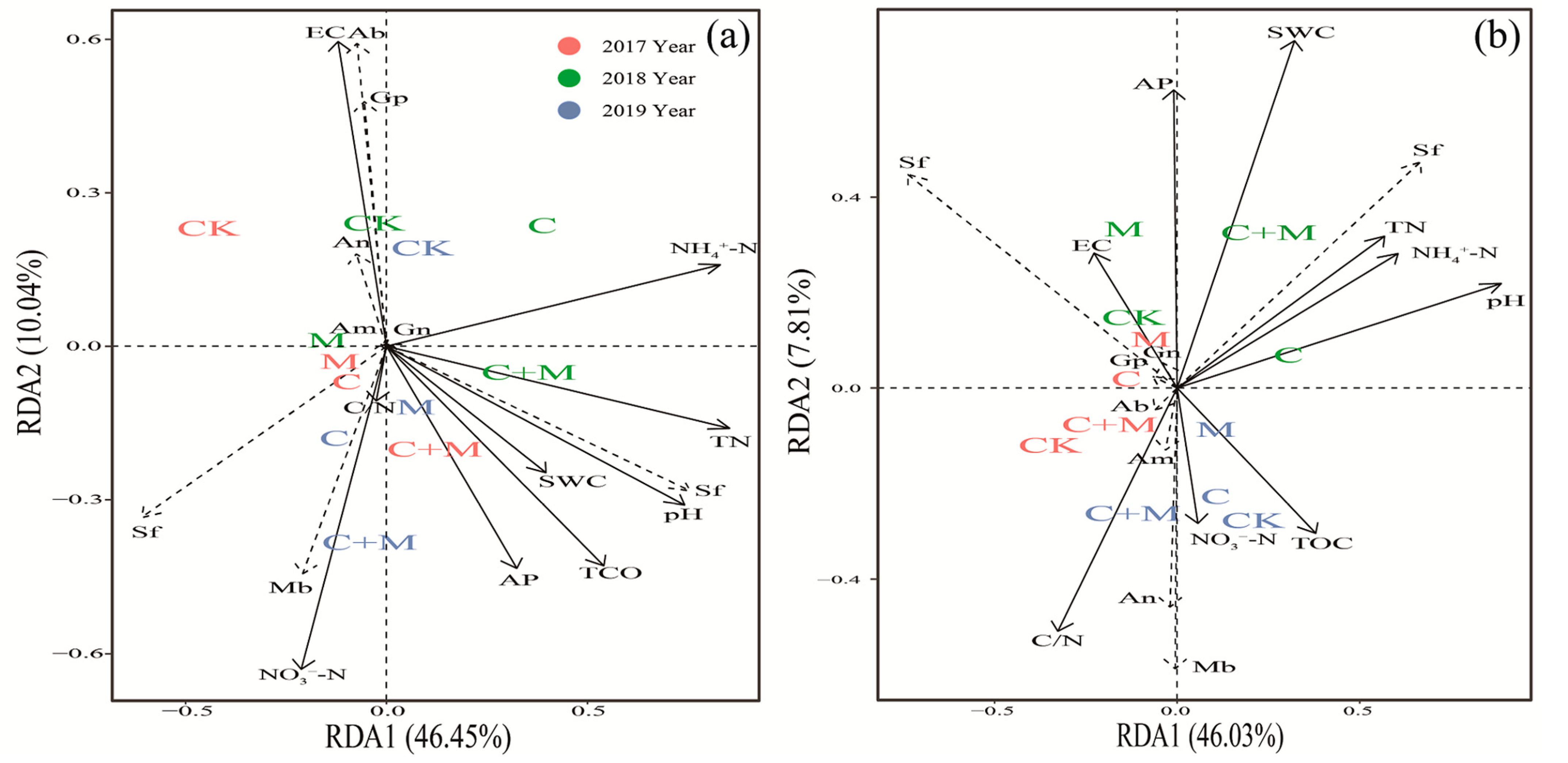
3.4. The Interaction between Microorganisms and Soil Properties
4. Discussion
4.1. Responses of Aboveground Vegetation Biomass to the Addition of Biochar or EM
4.2. Responses of Soil Physicochemical Properties to the Addition of Biochar or EM
4.3. Responses of Soil Microorganisms to the Addition of Biochar or EM
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, Y.; Lu, H.; Wang, J.; Wang, X.; Sun, J. Dual Influence of Climate Change and Anthropogenic Activities on the Spatiotemporal Vegetation Dynamics Over the Qinghai-Tibetan Plateau From 1981 to 2015. Earths Future 2022, 10, e2021EF002566. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, J.; Li, Y.; Liu, S.; Dong, Q.; Zhou, H.; Yeomans, J.; Li, Y.; Li, S.; Gao, X. Effect of grassland degradation on aggregate-associated soil organic carbon of alpine grassland ecosystems in Qinghai-Tibetan Plateau. Eur. J. Soil Sci. 2019, 71, 69–79. [Google Scholar] [CrossRef]
- Ran, Q.; Hao, Y.; Xia, A.; Liu, W.; Hu, R.; Cui, X.; Xue, K.; Song, X.; Xu, C.; Ding, B.; et al. Quantitative Assessment of the Impact of Physical and Anthropogenic Factors on Vegetation Spatial-Temporal Variation in Northern Tibet. Remote Sens. 2019, 11, 1183. [Google Scholar] [CrossRef]
- Su, X.; Han, W.; Liu, G.; Zhang, Y.; Lu, H. Substantial gaps between the protection of biodiversity hotspots in alpine grasslands and the effectiveness of protected areas on the Qinghai-Tibetan Plateau, China. Agric. Ecosyst. Environ. 2019, 278, 15–23. [Google Scholar] [CrossRef]
- Li, Y.; Dong, S.; Liu, S.; Zhou, H.; Gao, Q.; Cao, G.; Wang, X.; Su, X.; Zhang, Y.; Tang, L.; et al. Seasonal changes of CO2, CH4 and N2O fluxes in different types of alpine grassland in the Qinghai-Tibetan Plateau of China. Soil Biol. Biochem. 2015, 80, 306–314. [Google Scholar] [CrossRef]
- Han, W.; Lu, H.; Liu, G.; Wang, J.; Su, X. Quantifying Degradation Classifications on Alpine Grassland in the Lhasa River Basin, Qinghai-Tibetan Plateau. Sustainability 2019, 11, 7067. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Bullock, J.M.; Lavorel, S.; Manning, P.; Schaffner, U.; Ostle, N.; Chomel, M.; Durigan, G.; Fry, E.L.; Johnson, D.; et al. Combatting global grassland degradation. Nat. Rev. Earth Environ. 2021, 2, 720–735. [Google Scholar] [CrossRef]
- Du, C.; Gao, Y. Grazing exclusion alters ecological stoichiometry of plant and soil in degraded alpine grassland. Agric. Ecosyst. Environ. 2021, 308, 107256. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, Z.; Yin, X.A.; Zhu, Y. Impacts of biochars on bacterial community shifts and biodegradation of antibiotics in an agricultural soil during short-term incubation. Sci. Total Environ. 2021, 771, 144751. [Google Scholar] [CrossRef]
- Yan, Y.; Jarvie, S.; Liu, Q.; Zhang, Q. Effects of fragmentation on grassland plant diversity depend on the habitat specialization of species. Biol. Conserv. 2022, 275, 109773. [Google Scholar] [CrossRef]
- Che, R.; Wang, Y.; Lia, K.; Xu, Z.; Hua, J.; Wang, F.; Rui, Y.; Li, L.; Pang, Z.; Cui, X. Degraded patch formation significantly changed microbial community composition in alpine meadow soils. Soil Tillage Res. 2019, 195, 104426. [Google Scholar] [CrossRef]
- Raiesi, F. A minimum data set and soil quality index to quantify the effect of land use conversion on soil quality and degradation in native rangelands of upland arid and semiarid regions. Ecol. Indic. 2017, 75, 307–320. [Google Scholar] [CrossRef]
- Lei, L.; Xiao, W.; Zeng, L.; Frey, B.; Huang, Z.; Zhu, J.; Cheng, R.; Li, M.-H. Effects of thinning intensity and understory removal on soil microbial community in Pinus massoniana plantations of subtropical China. Appl. Soil Ecol. 2021, 167, 104055. [Google Scholar] [CrossRef]
- Shen, J.; Tao, Q.; Dong, Q.; Luo, Y.; Luo, J.; He, Y.; Li, B.; Li, Q.; Xu, Q.; Li, M.; et al. Long-term conversion from rice-wheat to rice-vegetable rotations drives variation in soil microbial communities and shifts in nitrogen-cycling through soil profiles. Geoderma 2021, 404, 115299. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Q.; Jin, X.; Li, D.; Zhu, Z.; Li, Q.X. Chiral enantiomers of the plant growth regulator paclobutrazol selectively affect community structure and diversity of soil microorganisms. Sci. Total Environ. 2021, 797, 148942. [Google Scholar] [CrossRef]
- Qiu, D.; Zhu, G.; Lin, X.; Jiao, Y.; Lu, S.; Liu, J.; Liu, J.; Zhang, W.; Ye, L.; Li, R.; et al. Dissipation and movement of soil water in artificial forest in arid oasis areas: Cognition based on stable isotopes. Catena 2023, 228, 107178. [Google Scholar] [CrossRef]
- Ding, M.Q.; Yang, S.S.; Ding, J.; Zhang, Z.R.; Zhao, Y.L.; Dai, W.; Sun, H.J.; Zhao, L.; Xing, D.; Ren, N.; et al. Gut Microbiome Associating with Carbon and Nitrogen Metabolism during Biodegradation of Polyethene in Tenebrio larvae with Crop Residues as Co-Diets. Sci. Total Environ. 2023, 57, 3031–3041. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Zhong, X.; Yao, T.; Qi, J.; Wang, Y.; Xue, Y. Quantifying the major drivers for the expanding lakes in the interior Tibetan Plateau. Sci. Bull. 2022, 67, 474–478. [Google Scholar] [CrossRef]
- Liang, J.-P.; Xue, Z.-Q.; Yang, Z.-Y.; Chai, Z.; Niu, J.-P.; Shi, Z.-Y. Effects of microbial organic fertilizers on Astragalus membranaceus growth and rhizosphere microbial community. Ann. Microbiol. 2021, 71, 11. [Google Scholar] [CrossRef]
- Mayer, J.; Scheid, S.; Widmer, F.; Fließbach, A.; Oberholzer, H.-R. How effective are ‘Effective microorganisms® (EM)’? Results from a field study in temperate climate. Appl. Soil Ecol. 2010, 46, 230–239. [Google Scholar] [CrossRef]
- Mise, K.; Koyama, Y.; Matsumoto, A.; Fujita, K.; Kunito, T.; Senoo, K.; Otsuka, S. Pectin drives microbial phosphorus solubilization in soil: Evidence from isolation-based and community-scale approaches. Eur. J. Soil Biol. 2020, 97, 103169. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Rady, M.M.; Taha, R.S.; Abd El Azeam, S.; Simpson, C.R.; Semida, W.M. Effects of integrated use of residual sulfur-enhanced biochar with effective microorganisms on soil properties, plant growth and short-term productivity of Capsicum annuum under salt stress. Sci. Hortic. 2020, 261, 108930. [Google Scholar] [CrossRef]
- Ortega, R.; Miralles, I.; Meca, D.E.; Gázquez, J.C.; Domene, M.Á. Effect of Organic and Synthetic Fertilizers on the Crop Yield and Macronutrients Contents in Soil and Pepper. Commun. Soil Sci. Plant Anal. 2016, 47, 1216–1226. [Google Scholar] [CrossRef]
- Cui, Q.; Xia, J.; Yang, H.; Liu, J.; Shao, P. Biochar and effective microorganisms promote Sesbania cannabina growth and soil quality in the coastal saline-alkali soil of the Yellow River Delta, China. Sci. Total Environ. 2021, 756, 143801. [Google Scholar] [CrossRef] [PubMed]
- Talaat, N.B. Effective microorganisms: An innovative tool for inducing common bean (Phaseolus vulgaris L.) salt-tolerance by regulating photosynthetic rate and endogenous phytohormones production. Sci. Hortic. 2019, 250, 254–265. [Google Scholar] [CrossRef]
- Mitchell, E.; Scheer, C.; Rowlings, D.; Cotrufo, F.; Conant, R.T.; Grace, P. Important constraints on soil organic carbon formation efficiency in subtropical and tropical grasslands. Glob. Change Biol. 2021, 27, 5383–5391. [Google Scholar] [CrossRef] [PubMed]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Gao, S.; DeLuca, T.H.; Cleveland, C.C. Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: A meta-analysis. Sci. Total Environ. 2019, 654, 463–472. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Gunarathne, V.; Senadeera, A.; Gunarathne, U.; Biswas, J.K.; Almaroai, Y.A.; Vithanage, M. Potential of biochar and organic amendments for reclamation of coastal acidic-salt affected soil. Biochar 2020, 2, 107–120. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, K.R.; Yang, J.E.; Ok, Y.S.; Owens, G.; Nehls, T.; Wessolek, G.; Kim, K.H. Effect of biochar on reclaimed tidal land soil properties and maize (Zea mays L.) response. Chemosphere 2016, 142, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Thangarajan, R.; Bolan, N.S.; Sarkar, B.; Khan, N.; Ok, Y.S.; Naidu, R. Biochar-induced concomitant decrease in ammonia volatilization and increase in nitrogen use efficiency by wheat. Chemosphere 2016, 142, 120–127. [Google Scholar] [CrossRef]
- Bhaduri, D.; Saha, A.; Desai, D.; Meena, H.N. Restoration of carbon and microbial activity in salt-induced soil by application of peanut shell biochar during short-term incubation study. Chemosphere 2016, 148, 86–98. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Awad, Y.M.; Yang, X.; Ryu, C.; Rizwan, M.; Rinklebe, J.; Tsang, D.C.W.; Ok, Y.S. Influence of soil properties and feedstocks on biochar potential for carbon mineralization and improvement of infertile soils. Geoderma 2018, 332, 100–108. [Google Scholar] [CrossRef]
- Elzobair, K.A.; Stromberger, M.E.; Ippolito, J.A.; Lentz, R.D. Contrasting effects of biochar versus manure on soil microbial communities and enzyme activities in an Aridisol. Chemosphere 2016, 142, 145–152. [Google Scholar] [CrossRef]
- Xu, W.; Whitman, W.B.; Gundale, M.J.; Chien, C.C.; Chiu, C.Y. Functional response of the soil microbial community to biochar applications. GCB Bioenergy 2020, 13, 269–281. [Google Scholar] [CrossRef]
- Dangi, S.; Gao, S.; Duan, Y.; Wang, D. Soil microbial community structure affected by biochar and fertilizer sources. Appl. Soil Ecol. 2020, 150, 103452. [Google Scholar] [CrossRef]
- Fernandez, J.M.; Nieto, M.A.; Lopez-de-Sa, E.G.; Gasco, G.; Mendez, A.; Plaza, C. Carbon dioxide emissions from semi-arid soils amended with biochar alone or combined with mineral and organic fertilizers. Sci. Total Environ. 2014, 482–483, 1–7. [Google Scholar] [CrossRef]
- Rafique, M.; Ortas, I.; Ahmed, I.A.M.; Rizwan, M.; Afridi, M.S.; Sultan, T.; Chaudhary, H.J. Potential impact of biochar types and microbial inoculants on growth of onion plant in differently textured and phosphorus limited soils. J. Environ. Manag. 2019, 247, 672–680. [Google Scholar] [CrossRef]
- Zhang, T.; Song, B.; Han, G.; Zhao, H.; Hu, Q.; Zhao, Y.; Liu, H. Effects of coastal wetland reclamation on soil organic carbon, total nitrogen, and total phosphorus in China: A meta-analysis. Land Degrad. Dev. 2023, 34, 3340–3349. [Google Scholar] [CrossRef]
- Li, W.; Shi, Y.; Zhu, D.; Wang, W.; Liu, H.; Li, J.; Shi, N.; Ma, L.; Fu, S. Fine root biomass and morphology in a temperate forest are influenced more by the nitrogen treatment approach than the rate. Ecol. Indic. 2021, 130, 108031. [Google Scholar] [CrossRef]
- GB 19733-2003 [S]; Classification Index of Natural Grassland Degradation, Desertification and Salinization. Ministry of Agriculture of the People’s Republic of China, Standards Press of China: Beijing, China, 2003.
- Li, J.; Zhao, Y.; Shao, X.; Huang, D.; Shang, J.; Li, H.; He, Y.; Liu, K. The Mixed Addition of Biochar and Nitrogen Improves Soil Properties and Microbial Structure of Moderate-Severe Degraded Alpine Grassland in Qinghai-Tibet Plateau. Front. Plant Sci. 2021, 12, 765041. [Google Scholar] [CrossRef] [PubMed]
- White, D.C.; Davis, W.M.; Nickels, J.S.; King, J.D.; Bobbie, R.J. Determination of the sedimentary microbial biomass by extractible lipid phosphate. Oecologia 1979, 40, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Laghari, M.; Mirjat, M.S.; Hu, Z.; Fazal, S.; Xiao, B.; Hu, M.; Chen, Z.; Guo, D. Effects of biochar application rate on sandy desert soil properties and sorghum growth. Catena 2015, 135, 313–320. [Google Scholar] [CrossRef]
- Zhang, H.; Ullah, F.; Ahmad, R.; Ali Shah, S.U.; Khan, A.; Adnan, M. Response of Soil Proteobacteria to Biochar Amendment in Sustainable Agriculture- A mini review. J. Soil Plant Environ. 2022, 1, 16–30. [Google Scholar] [CrossRef]
- Hu, C.; Qi, Y. Long-term effective microorganisms application promote growth and increase yields and nutrition of wheat in China. Eur. J. Agron. 2013, 46, 63–67. [Google Scholar] [CrossRef]
- Talaat, N.B.; Ghoniem, A.E.; Abdelhamid, M.T.; Shawky, B.T. Effective microorganisms improve growth performance, alter nutrients acquisition and induce compatible solutes accumulation in common bean (Phaseolus vulgaris L.) plants subjected to salinity stress. Plant Growth Regul. 2014, 75, 281–295. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Zhang, P.; Wu, F.; Wang, Y.; Xu, C.; Zhang, L.; An, S.; Kuzyakov, Y. Large-scale ecosystem carbon stocks and their driving factors across Loess Plateau. Carbon Neutrality 2023, 2, 5. [Google Scholar] [CrossRef]
- Cheng, M.; Cui, Y.; Yan, X.; Zhang, R.; Wang, J.; Wang, X. Effect of dual-modified cassava starches on intelligent packaging films containing red cabbage extracts. Food Hydrocoll. 2022, 124, 107225. [Google Scholar] [CrossRef]
- He, X.; Xie, H.; Gao, D.; Khashi, U.R.M.; Zhou, X.; Wu, F. Biochar and Intercropping with Potato-Onion Enhanced the Growth and Yield Advantages of Tomato by Regulating the Soil Properties, Nutrient Uptake, and Soil Microbial Community. Front. Microbiol. 2021, 12, 695447. [Google Scholar] [CrossRef] [PubMed]
- Schweinsberg-Mickan, M.S.Z.; Müller, T. Impact of effective microorganisms and other biofertilizers on soil microbial characteristics, organic-matter decomposition, and plant growth. J. Plant Nutr. Soil Sci. 2009, 172, 704–712. [Google Scholar] [CrossRef]
- Thomas, S.C.; Frye, S.; Gale, N.; Garmon, M.; Launchbury, R.; Machado, N.; Melamed, S.; Murray, J.; Petroff, A.; Winsborough, C. Biochar mitigates negative effects of salt additions on two herbaceous plant species. J. Environ. Manage. 2013, 129, 62–68. [Google Scholar] [CrossRef]
- Saifullah; Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar]
- Pan, S.-Y.; Dong, C.-D.; Su, J.-F.; Wang, P.-Y.; Chen, C.-W.; Chang, J.-S.; Kim, H.; Huang, C.-P.; Hung, C.-M. The Role of Biochar in Regulating the Carbon, Phosphorus, and Nitrogen Cycles Exemplified by Soil Systems. Sustainability 2021, 13, 5612. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, W.; Liang, G.; Song, D.; Zhang, X. Characteristics of maize biochar with different pyrolysis temperatures and its effects on organic carbon, nitrogen and enzymatic activities after addition to fluvo-aquic soil. Sci. Total Environ. 2015, 538, 137–144. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Jien, S.H.; Kuo, Y.L.; Liao, C.S.; Wu, Y.T.; Igalavithana, A.D.; Tsang, D.C.W.; Ok, Y.S. Effects of field scale in situ biochar incorporation on soil environment in a tropical highly weathered soil. Environ. Pollut. 2021, 272, 116009. [Google Scholar] [CrossRef]
- Hu, F.; Xu, C.; Ma, R.; Tu, K.; Yang, J.; Zhao, S.; Yang, M.; Zhang, F. Biochar application driven change in soil internal forces improves aggregate stability: Based on a two-year field study. Geoderma 2021, 403, 115276. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Yang, W.; Gong, T.; Wang, J.; Li, G.; Liu, Y.; Zhen, J.; Ning, M.; Yue, D.; Du, Z.; Chen, G. Effects of Compound Microbial Fertilizer on Soil Characteristics and Yield of Wheat (Triticum aestivum L.). J. Soil Sci. Plant Nutr. 2020, 20, 2740–2748. [Google Scholar] [CrossRef]
- Ren, H.; Qin, X.; Huang, B.; Fernandez-Garcia, V.; Lv, C. Responses of soil enzyme activities and plant growth in a eucalyptus seedling plantation amended with bacterial fertilizers. Arch. Microbiol. 2020, 202, 1381–1396. [Google Scholar] [CrossRef]
- Wang, X.; Lü, X.; Zhang, H.; Dijkstrac, F.A.; Jiang, Y.; Wang, X.; Lu, J.; Wu, Y.; Wang, Z.; Han, X. Changes in soil C:N:P stoichiometry along an aridity gradient in drylands of northern China. Geoderma 2019, 361, 114087. [Google Scholar] [CrossRef]
- Jenkins, J.R.; Viger, M.; Arnold, E.C.; Harris, Z.M.; Ventura, M.; Miglietta, F.; Girardin, C.; Edwards, R.J.; Rumpel, C.; Fornasier, F.; et al. Biochar alters the soil microbiome and soil function: Results of next-generation amplicon sequencing across Europe. GCB Bioenergy 2017, 9, 591–612. [Google Scholar] [CrossRef]
- Ye, Z.; Liu, L.; Tan, Z.; Zhang, L.; Huang, Q. Effects of pyrolysis conditions on migration and distribution of biochar nitrogen in the soil-plant-atmosphere system. Sci. Total Environ. 2020, 723, 138006. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, H.; Hu, W.; Wang, Y.; Zhang, H.; Zhou, X.; Fei, J.; Luo, G. Understanding how reed-biochar application mitigates nitrogen losses in paddy soil: Insight into microbially-driven nitrogen dynamics. Chemosphere 2022, 295, 133904. [Google Scholar] [CrossRef]
- Li, H.; Lu, X.; Xu, Y.; Liu, H. How close is artificial biochar aging to natural biochar aging in fields? A meta-analysis. Geoderma 2019, 352, 96–103. [Google Scholar] [CrossRef]
- Sun, H.; Lu, H.; Chu, L.; Shao, H.; Shi, W. Biochar applied with appropriate rates can reduce N leaching, keep N retention and not increase NH3 volatilization in a coastal saline soil. Sci. Total Environ. 2017, 575, 820–825. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Zhao, Q.; Jia, J.; Wang, W.; Wang, X. Bacterial Succession in Salt Marsh Soils Along a Short-term Invasion Chronosequence of Spartina alterniflora in the Yellow River Estuary, China. Microb. Ecol. 2019, 79, 644–661. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, J.; Pan, G.; Liu, X.; Zhang, X.; Li, L.; Bian, R.; Cheng, K.; Jinwei, Z. Biochar decreased microbial metabolic quotient and shifted community composition four years after a single incorporation in a slightly acid rice paddy from southwest China. Sci. Total Environ. 2016, 571, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Gomez, A.; Garcia-Estevez, I.; Garcia-Fraile, P.; Escribano-Bailon, M.T.; Rivas, R. Increase in phenolic compounds of Coriandrum sativum L. after the application of a Bacillus halotolerans biofertilizer. J. Sci. Food Agric. 2020, 100, 2742–2749. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, C.; Bao, J.; Zhu, H.; Chen, Y.; Luo, Y.; Zhang, L. Microbial diversity and physicochemical properties in farmland soils amended by effective microorganisms and fulvic acid for cropping Asian ginseng. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12563. [Google Scholar] [CrossRef]
- Cao, T.; Fang, Y.; Chen, Y.; Kong, X.; Yang, J.; Alharbi, H.; Kuzyakov, Y.; Tian, X. Synergy of saprotrophs with mycorrhiza for litter decomposition and hotspot formation depends on nutrient availability in the rhizosphere. Geoderma 2022, 410, 115662. [Google Scholar] [CrossRef]
- Djukic, I.; Zehetner, F.; Mentler, A.; Gerzabek, M.H. Microbial community composition and activity in different Alpine vegetation zones. Soil Biol. Biochem. 2010, 42, 155–161. [Google Scholar] [CrossRef]
- Evgrafova, S.Y.; Santruckova, H.; Shibistova, O.B.; Elhottova, D.; Cerna, B.; Zrazhevskaya, G.K.; Lloyd, D. Phospholipid fatty acid composition of microorganisms in pine forest soils of Central Siberia. Biol. Bull. 2008, 35, 452–458. [Google Scholar] [CrossRef]
- Frostegard, A.; Baath, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fert. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Li, J.; Shao, X.; Huang, D.; Shang, J.; Liu, K.; Zhang, Q.; Yang, X.; Li, H.; He, Y. The addition of organic carbon and nitrogen accelerates the restoration of soil system of degraded alpine grassland in Qinghai-Tibet Plateau. Ecol. Eng. 2020, 158, 106084. [Google Scholar] [CrossRef]
- Smith, A.P.; Marin-Spiotta, E.; Balser, T. Successional and seasonal variations in soil and litter microbial community structure and function during tropical postagricultural forest regeneration: A multiyear study. Glob. Chang. Biol. 2015, 21, 3532–3547. [Google Scholar] [CrossRef]
- Sundh, I.; Borjesson, G.; Tunlid, A. Methane oxidation and phospholipid fatty acid composition in a podzolic soil profile. Soil Biol. Biochem. 2000, 32, 1025–1028. [Google Scholar] [CrossRef]

| Parameter | Year | Layer (cm) | CK | M | C | C+M |
|---|---|---|---|---|---|---|
| pH | 2017 | 0–10 | 7.12 ± 0.05 bcB | 6.99 ± 0.08 cB | 7.36 ± 0.12 abC | 7.46 ± 0.18 aB |
| 2018 | 7.55 ± 0.03 bA | 7.63 ± 0.09 bA | 8.22 ± 0.22 aA | 8.32 ± 0.16 aA | ||
| 2019 | 7.28 ± 0.22 bAB | 7.77 ± 0.13 aA | 7.78 ± 0.05 aB | 8.05 ± 0.08 aA | ||
| 2017 | 10–20 | 7.24 ± 0.11 bB | 7.28 ± 0.12 bB | 7.31 ± 0.08 aC | 7.33 ± 0.11 aC | |
| 2018 | 7.48 ± 0.09 bA | 7.65 ± 0.07 bA | 8.44 ± 0.08 aA | 8.31 ± 0.08 aA | ||
| 2019 | 7.49 ± 0.05 cB | 7.78 ± 0.01 bA | 7.81 ± 0.11 bC | 7.99 ± 0.06 aB | ||
| EC | 2017 | 0–10 | 362.76 ± 46.31 aA | 163.87 ± 27.02 cA | 312.01 ± 20.85 bA | 121.77 ± 11.93 cC |
| 2018 | 274.09 ± 28.54 bB | 186.53 ± 16.66 bA | 302.58 ± 31.56 aA | 253.17 ± 14.39 bA | ||
| 2019 | 209.8 ± 21.20 aC | 208.83 ± 32.09 aA | 220.73 ± 26.97 aB | 172.53 ± 17.28 aB | ||
| 2017 | 10–20 | 324.91 ± 11.21 aA | 161.8 ± 28.45 bA | 319.01 ± 16.06 aA | 117.01 ± 11.6 cC | |
| 2018 | 305.27 ± 99.51 aA | 202.87 ± 26.05 cA | 282.62 ± 15.51 bB | 223.27 ± 13.27 cA | ||
| 2019 | 197.53 ± 14.41 aB | 171.83 ± 25.92 aA | 198.33 ± 23.1 aC | 161.10 ± 15.5 aB | ||
| SWC (%) | 2017 | 0–10 | 24.52 ± 0.93 bA | 24.48 ± 1.90 bA | 24.35 ± 0.83 bB | 30.41 ± 2.82 aA |
| 2018 | 18.82 ± 0.38 cB | 26.97 ± 2.41 bA | 30.69 ± 1.81 aA | 31.71 ± 1.42 aA | ||
| 2019 | 16.46 ± 1.01 cC | 24.18 ± 0.77 abA | 27.01 ± 2.66 aAB | 22.92 ± 0.87 bB | ||
| 2017 | 10–20 | 21.14 ± 0.71 bA | 21.71 ± 1.87 aA | 21.89 ± 0.83 bB | 25.68 ± 1.49 aB | |
| 2018 | 19.85 ± 0.51 bA | 25.43 ± 1.65 bB | 27.31 ± 0.75 aA | 29.70 ± 1.87 aA | ||
| 2019 | 16.08 ± 0.81 bA | 16.86 ± 0.94 bC | 20.55 ± 0.99 aB | 19.83 ± 1.06 aC | ||
| C/N | 2017 | 0–10 | 12.02 ± 0.49 bA | 10.78 ± 1.42 bA | 15.63 ± 1.35 aA | 15.81 ± 0.09 aA |
| 2018 | 12.02 ± 1.38 abA | 11.11 ± 0.31 bA | 13.13 ± 0.58 aB | 11.96 ± 1.37 aA | ||
| 2019 | 10.85 ± 0.57 bA | 11.43 ± 0.77 bA | 15.34 ± 0.58 aAB | 11.69 ± 1.28 bA | ||
| 2017 | 10–20 | 12.68 ± 1.38 aA | 12.33 ± 0.68 aA | 12.73 ± 0.89 aA | 11.77 ± 0.83 aA | |
| 2018 | 10.08 ± 0.47 aB | 9.70 ± 1.59 aA | 9.22 ± 0.82 aB | 10.33 ± 0.29 aA | ||
| 2019 | 11.28 ± 0.8 cA | 11.34 ± 0.92 cA | 13.67 ± 1.00 aA | 12.56 ± 1.10 abA |
| Parameter | Year | Layer (cm) | CK | M | C | C+M |
|---|---|---|---|---|---|---|
| TOC (g kg−1) | 2017 | 0–10 | 33.14 ± 1.63 bB | 39.56 ± 4.49 bA | 57.43 ± 5.43 aA | 58.88 ± 0.75 aA |
| 2018 | 40.92 ± 1.62 cA | 40.44 ± 1.05 cA | 63.54 ± 2.55 aA | 46.88 ± 0.46 bB | ||
| 2019 | 34.26 ± 1.04 dB | 43.73 ± 0.87 cA | 56.78 ± 0.43 aA | 49.85 ± 2.05 bB | ||
| 2017 | 10–20 | 30.16 ± 1.4 bA | 34.17 ± 1.91 aB | 36.60 ± 0.87 aB | 36.47 ± 0.73 aA | |
| 2018 | 30.45 ± 0.68 bA | 32.95 ± 0.55 bB | 36.61 ± 0.7 aB | 36.11 ± 2.26 aA | ||
| 2019 | 30.19 ± 0.21 cA | 37.49 ± 1.1 bA | 43.35 ± 4.12 aA | 39.90 ± 1.92 abA | ||
| TN (g kg−1) | 2017 | 0–10 | 2.76 ± 0.12 bB | 3.67 ± 0.27 aA | 3.67 ± 0.12 aB | 3.73 ± 0.10 aA |
| 2018 | 3.43 ± 0.26 bA | 3.64 ± 0.02 bA | 4.84 ± 0.17 aA | 3.97 ± 0.45 bA | ||
| 2019 | 3.17 ± 0.24 bAB | 3.84 ± 0.3 aA | 3.71 ± 0.17 abB | 3.80 ± 0.22 aA | ||
| 2017 | 10–20 | 2.40 ± 0.18 bB | 2.78 ± 0.19 abA | 2.89 ± 0.15 aB | 3.11 ± 0.17 aB | |
| 2018 | 3.03 ± 0.20 bA | 3.51 ± 0.71 abA | 4.00 ± 0.30 aA | 3.49 ± 0.13 abA | ||
| 2019 | 2.69 ± 0.20 bB | 3.33 ± 0.27 aA | 3.17 ± 0.12 aB | 3.31 ± 0.08 aAB | ||
| NH4+-N (mg kg−1) | 2017 | 0–10 | 18.33 ± 0.83 cC | 23.13 ± 2.22 bC | 11.74 ± 0.09 dC | 29.45 ± 1.61 aB |
| 2018 | 35.05 ± 3.67 cA | 43.15 ± 1.73 cA | 70.37 ± 6.75 aA | 58.74 ± 6.01 bA | ||
| 2019 | 26.13 ± 1.20 cB | 28.39 ± 2.31 bB | 48.35 ± 2.37 aB | 36.97 ± 3.07 abB | ||
| 2017 | 10–20 | 16.71 ± 1.50 bB | 23.96 ± 1.31 aA | 9.65 ± 0.43 cB | 19.36 ± 3.60 abB | |
| 2018 | 22.17 ± 1.31 cA | 16.42 ± 2.04 dB | 34.56 ± 2.70 bA | 40.44 ± 2.72 aA | ||
| 2019 | 14.64 ± 2.11 cB | 9.27 ± 0.93 cC | 36.06 ± 2.54 aB | 22.19 ± 3.78 bB | ||
| NO3—N (mg kg−1) | 2017 | 0–10 | 7.57 ± 1.56 cAB | 6.47 ± 0.47 cB | 21.62 ± 1.25 aB | 15.56 ± 0.33 bA |
| 2018 | 4.97 ± 0.70 bB | 7.51 ± 0.33 aB | 5.77 ± 1.08 bC | 6.40 ± 0.33 abB | ||
| 2019 | 8.96 ± 1.72 cA | 18.25 ± 4.49 bA | 31.70 ± 5.22 aA | 14.28 ± 2.08 bcA | ||
| 2017 | 10–20 | 7.18 ± 1.08 bAB | 7.95 ± 0.79 bA | 13.29 ± 1.11 aA | 13.30 ± 0.26 aA | |
| 2018 | 4.94 ± 0.79 bB | 9.09 ± 0.56 aA | 8.01 ± 1.31 aB | 6.99 ± 1.22 abC | ||
| 2019 | 11.09 ± 2.99 aAB | 9.90 ± 2.19 bA | 13.79 ± 1.11 aA | 10.83 ± 0.17 aB | ||
| AP (mg kg−1) | 2017 | 0–10 | 2.68 ± 0.24 bA | 2.91 ± 0.36 bA | 4.10 ± 0.41 aA | 4.35 ± 0.20 aA |
| 2018 | 3.02 ± 0.24 bA | 3.27 ± 0.95 abAB | 4.37 ± 0.07 aA | 3.43 ± 0.19 abB | ||
| 2019 | 1.57 ± 0.17 cB | 3.16 ± 0.10 bA | 3.31 ± 0.10 bB | 4.02 ± 0.15 aA | ||
| 2017 | 10–20 | 1.79 ± 0.13 bA | 2.83 ± 0.24 aAB | 3.12 ± 0.24 aA | 2.76 ± 0.10 aA | |
| 2018 | 1.89 ± 0.36 bA | 3.08 ± 0.35 aA | 2.72 ± 0.12 aAB | 2.79 ± 0.07 aA | ||
| 2019 | 1.49 ± 0.03 bA | 2.24 ± 0.19 aB | 2.37 ± 0.23 aA | 2.60 ± 0.22 aA |
| Parameter | Year | Layer (cm) | CK | M | C | C+M |
|---|---|---|---|---|---|---|
| F:B | 2017 | 0–10 | 0.18 ± 0.01 bB | 0.24 ± 0.01 aA | 0.22 ± 0.01 abB | 0.25 ± 0.04 aA |
| 2018 | 0.21 ± 0.01 bA | 0.25 ± 0.03 abA | 0.27 ± 0.01 aA | 0.29 ± 0.04 aA | ||
| 2019 | 0.20 ± 0.01 bA | 0.27 ± 0.04 aA | 0.19 ± 0.01 bC | 0.30 ± 0.04 aA | ||
| 2017 | 10–20 | 0.17 ± 0.01 aB | 0.19 ± 0.02 aB | 0.21 ± 0.06 aA | 0.19 ± 0.03 aB | |
| 2018 | 0.22 ± 0.03 bA | 0.27 ± 0.01 abA | 0.23 ± 0.05 bA | 0.32 ± 0.07 aA | ||
| 2019 | 0.19 ± 0.02 abC | 0.16 ± 0.01 bB | 0.24 ± 0.07 aA | 0.19 ± 0.03 abB | ||
| GP:GN | 2017 | 0–10 | 1.44 ± 0.11 aAB | 1.51 ± 0.07 aA | 1.37 ± 0.03 aB | 1.45 ± 0.04 aA |
| 2018 | 1.55 ± 0.05 aA | 1.47 ± 0.03 aA | 1.41 ± 0.17 aA | 1.41 ± 0.08 aA | ||
| 2019 | 1.29 ± 0.05 aB | 1.34 ± 0.01 aB | 1.13 ± 0.01 bB | 1.13 ± 0.04 bB | ||
| 2017 | 10–20 | 1.12 ± 0.19 aA | 1.24 ± 0.15 aA | 1.24 ± 0.08 aA | 1.00 ± 0.07 aB | |
| 2018 | 1.22 ± 0.04 aA | 1.14 ± 0.04 aA | 1.24 ± 0.17 aA | 1.22 ± 0.10 aA | ||
| 2019 | 1.11 ± 0.08 aA | 1.12 ± 0.05 aA | 1.21 ± 0.12 aA | 1.04 ± 0.05 aAB | ||
| MB:TOC | 2017 | 0–10 | 0.28 ± 0.01 aA | 0.30 ± 0.04 aAB | 0.21 ± 0.02 bA | 0.19 ± 0.01 bB |
| 2018 | 0.22 ± 0.01 bB | 0.25 ± 0.02 abB | 0.21 ± 0.01 bA | 0.27 ± 0.02 aA | ||
| 2019 | 0.30 ± 0.01 aA | 0.34 ± 0.01 aA | 0.23 ± 0.01 bA | 0.30 ± 0.03 aA | ||
| 2017 | 10–20 | 0.27 ± 0.04 aB | 0.24 ± 0.01 aB | 0.23 ± 0.01 aB | 0.23 ± 0.01 aB | |
| 2018 | 0.28 ± 0.04 abB | 0.23 ± 0.01 bB | 0.30 ± 0.01 aAB | 0.30 ± 0.04 aA | ||
| 2019 | 0.31 ± 0.01 aA | 0.26 ± 0.03 aA | 0.33 ± 0.05 aA | 0.24 ± 0.02 aB | ||
| MB:TN | 2017 | 0–10 | 3.36 ± 0.11 aA | 3.16 ± 0.34 aB | 3.20 ± 0.39 aAB | 3.04 ± 0.13 aB |
| 2018 | 2.65 ± 0.19 bB | 2.73 ± 0.10 bB | 2.82 ± 0.05 abB | 3.26 ± 0.35 aAB | ||
| 2019 | 3.27 ± 0.22 bA | 3.85 ± 0.29 abA | 3.57 ± 0.17 abA | 3.96 ± 0.44 aA | ||
| 2017 | 10–20 | 3.34 ± 0.16 aA | 2.95 ± 0.11 bAB | 2.94 ± 0.20 bB | 2.69 ± 0.08 bA | |
| 2018 | 2.84 ± 0.45 abAB | 2.21 ± 0.43 bB | 2.78 ± 0.24 abB | 3.14 ± 0.38 aA | ||
| 2019 | 3.50 ± 0.27 abA | 3.58 ± 0.20 aA | 3.74 ± 0.71 aA | 2.85 ± 0.09 bA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, H.; Shang, J.; Liu, K.; He, Y.; Shao, X. The Synergistic Effect of Biochar and Microorganisms Greatly Improves Vegetation and Microbial Structure of Degraded Alpine Grassland on Qinghai–Tibet Plateau. Agronomy 2023, 13, 2203. https://doi.org/10.3390/agronomy13092203
Li J, Li H, Shang J, Liu K, He Y, Shao X. The Synergistic Effect of Biochar and Microorganisms Greatly Improves Vegetation and Microbial Structure of Degraded Alpine Grassland on Qinghai–Tibet Plateau. Agronomy. 2023; 13(9):2203. https://doi.org/10.3390/agronomy13092203
Chicago/Turabian StyleLi, Jinsheng, Hui Li, Jianying Shang, Kesi Liu, Yixuan He, and Xinqing Shao. 2023. "The Synergistic Effect of Biochar and Microorganisms Greatly Improves Vegetation and Microbial Structure of Degraded Alpine Grassland on Qinghai–Tibet Plateau" Agronomy 13, no. 9: 2203. https://doi.org/10.3390/agronomy13092203
APA StyleLi, J., Li, H., Shang, J., Liu, K., He, Y., & Shao, X. (2023). The Synergistic Effect of Biochar and Microorganisms Greatly Improves Vegetation and Microbial Structure of Degraded Alpine Grassland on Qinghai–Tibet Plateau. Agronomy, 13(9), 2203. https://doi.org/10.3390/agronomy13092203








