Effect of the Exogenous Application of Different Concentrations of Indole-3-Acetic Acid as a Growth Regulator on Onion (Allium cepa L.) Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.1.1. Substrate for Cultivation
2.1.2. Preparation of Indole-3-Acetic Acid Solution
2.2. Agronomic Characteristics
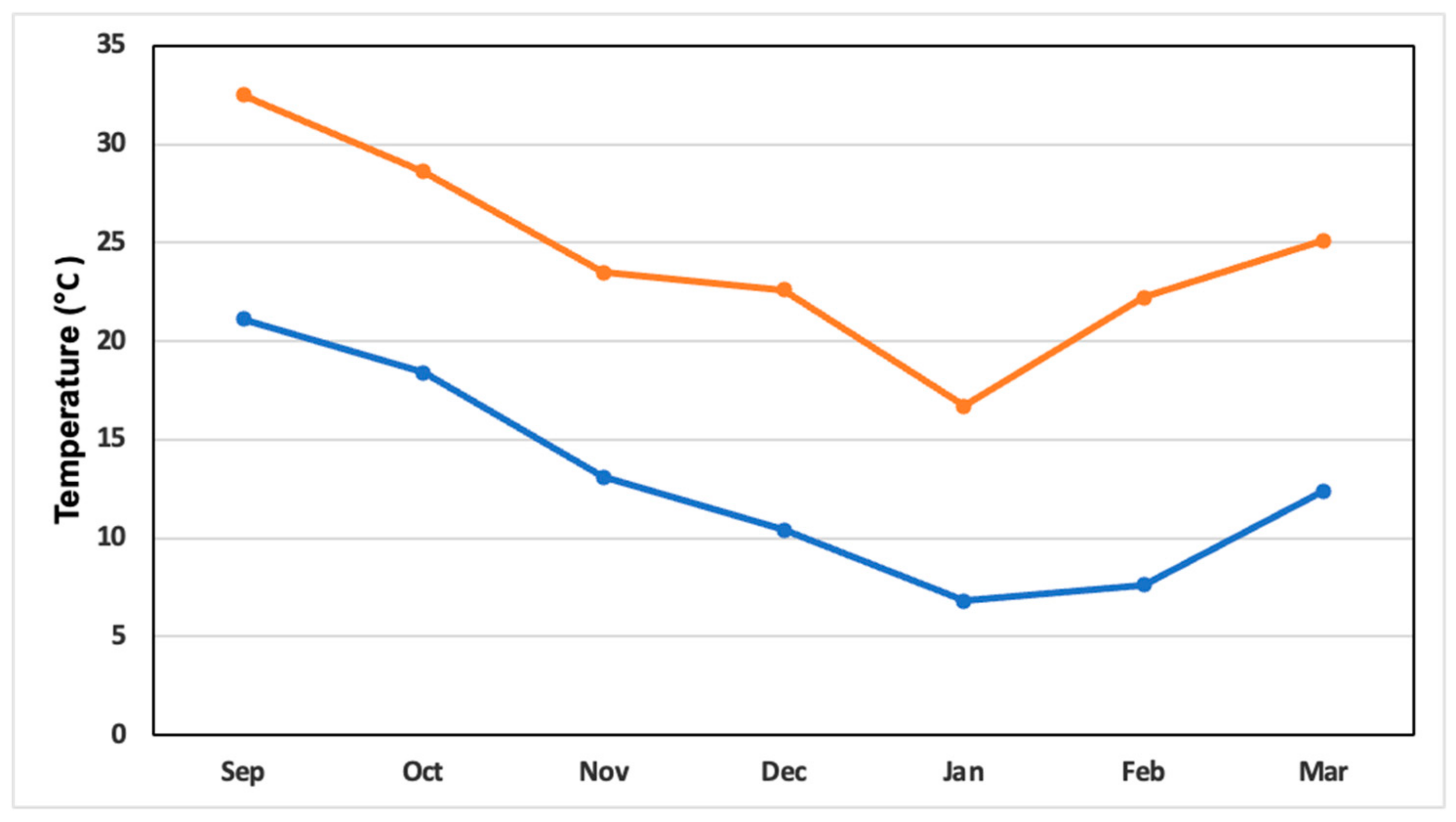
2.3. Greenhouse Climatic Variables
2.4. Determination of Growing Parameters
2.5. Chlorophyll, Carotenoids, and Nitrates
2.6. Total Phenolic Content and Antioxidant Activity (DPPH)
2.7. Statistical Analysis
2.7.1. InfoStat 2020
2.7.2. Minitab 2019
3. Results
3.1. Effect of the Concentrations of IAA on the Onion Growth Agronomic Properties Parameters
3.2. Effect of the Concentrations of IAA on Chlorophyll, Carotenoids, and Nitrates
3.3. Effect of the Concentrations of IAA on Phenolic Content and Antioxidant Activity (DPPH)
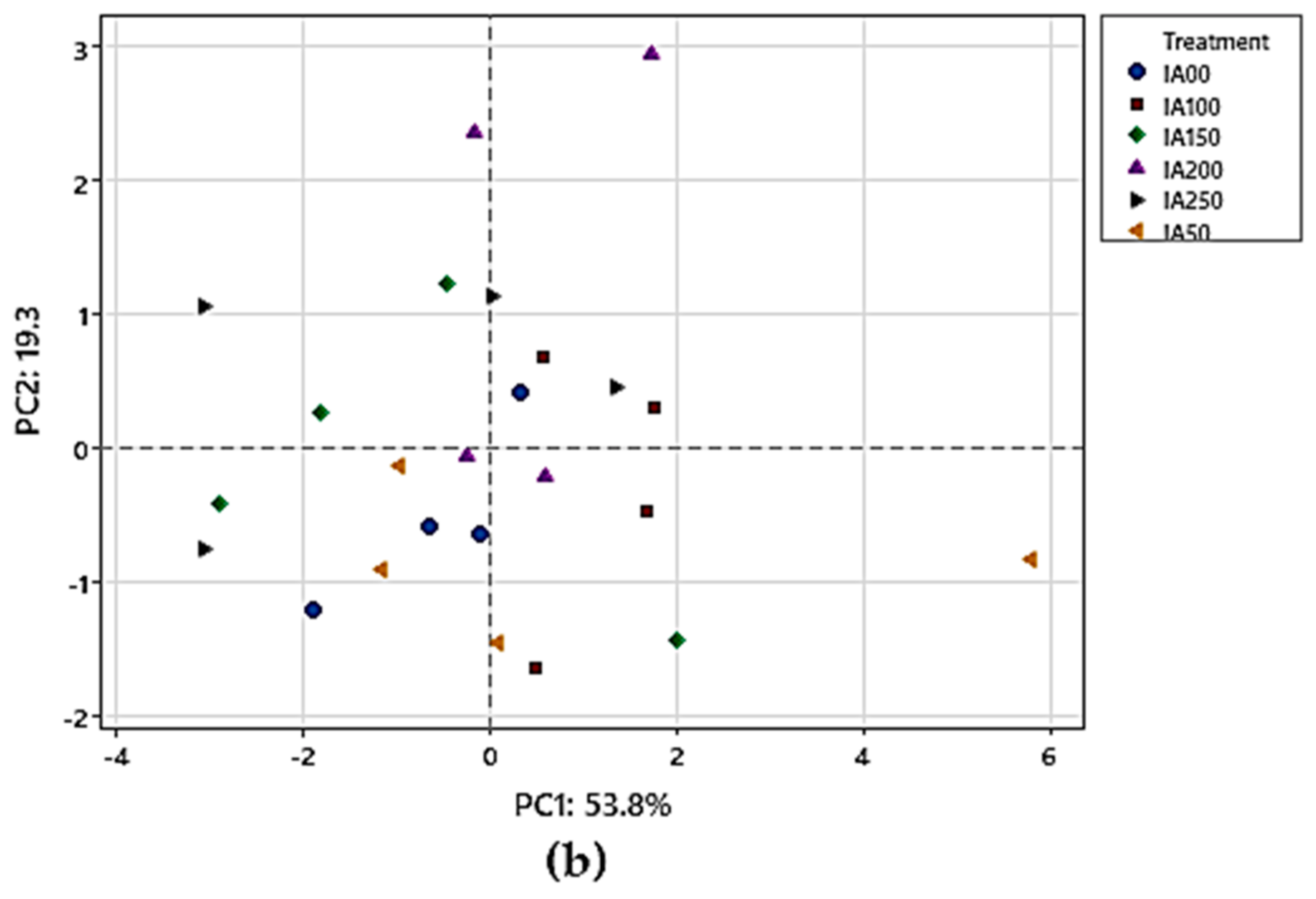
3.4. Classification Analysis
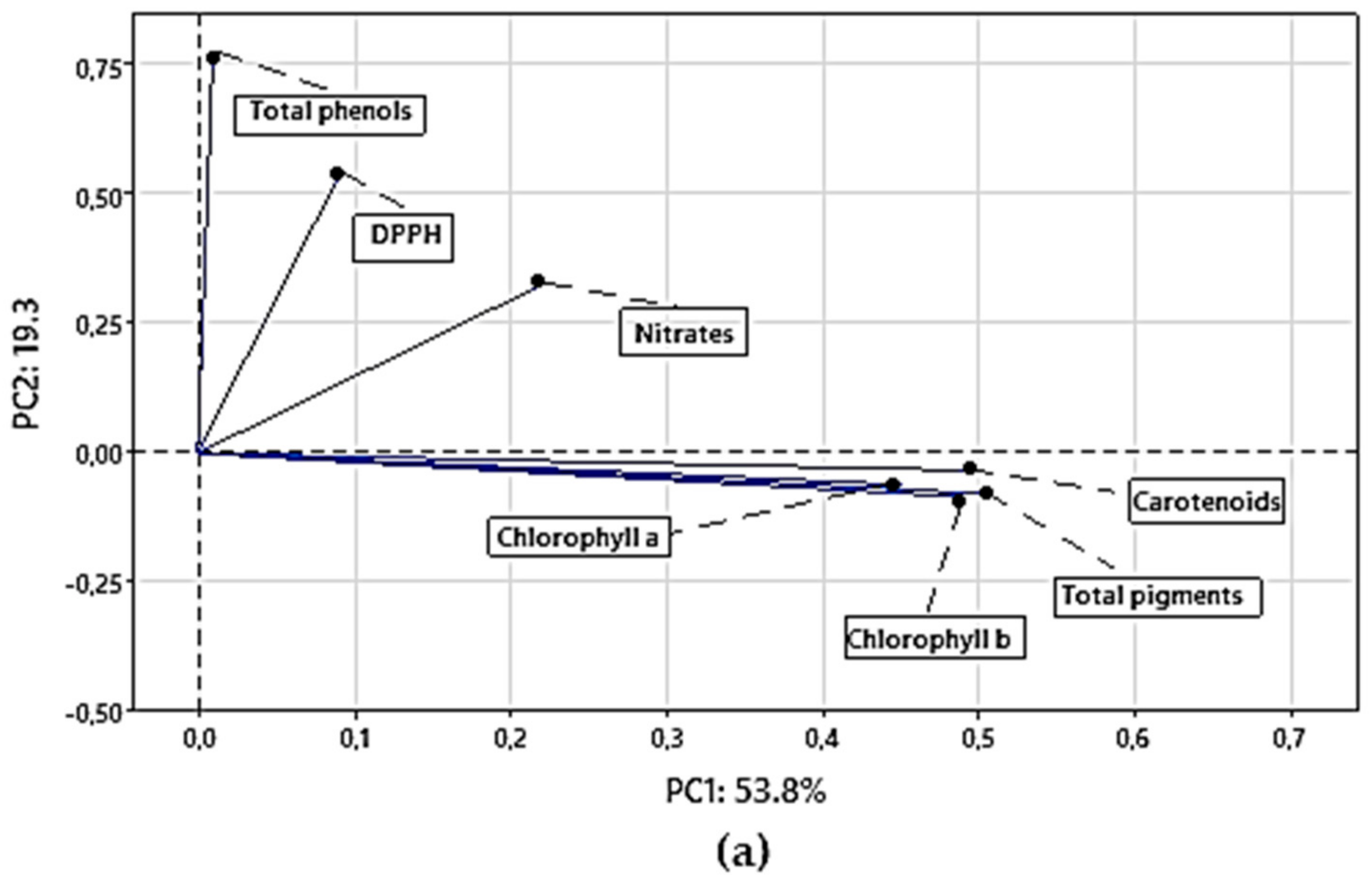
3.4.1. Principal Component Analysis (PCA) for Chemical Parameters
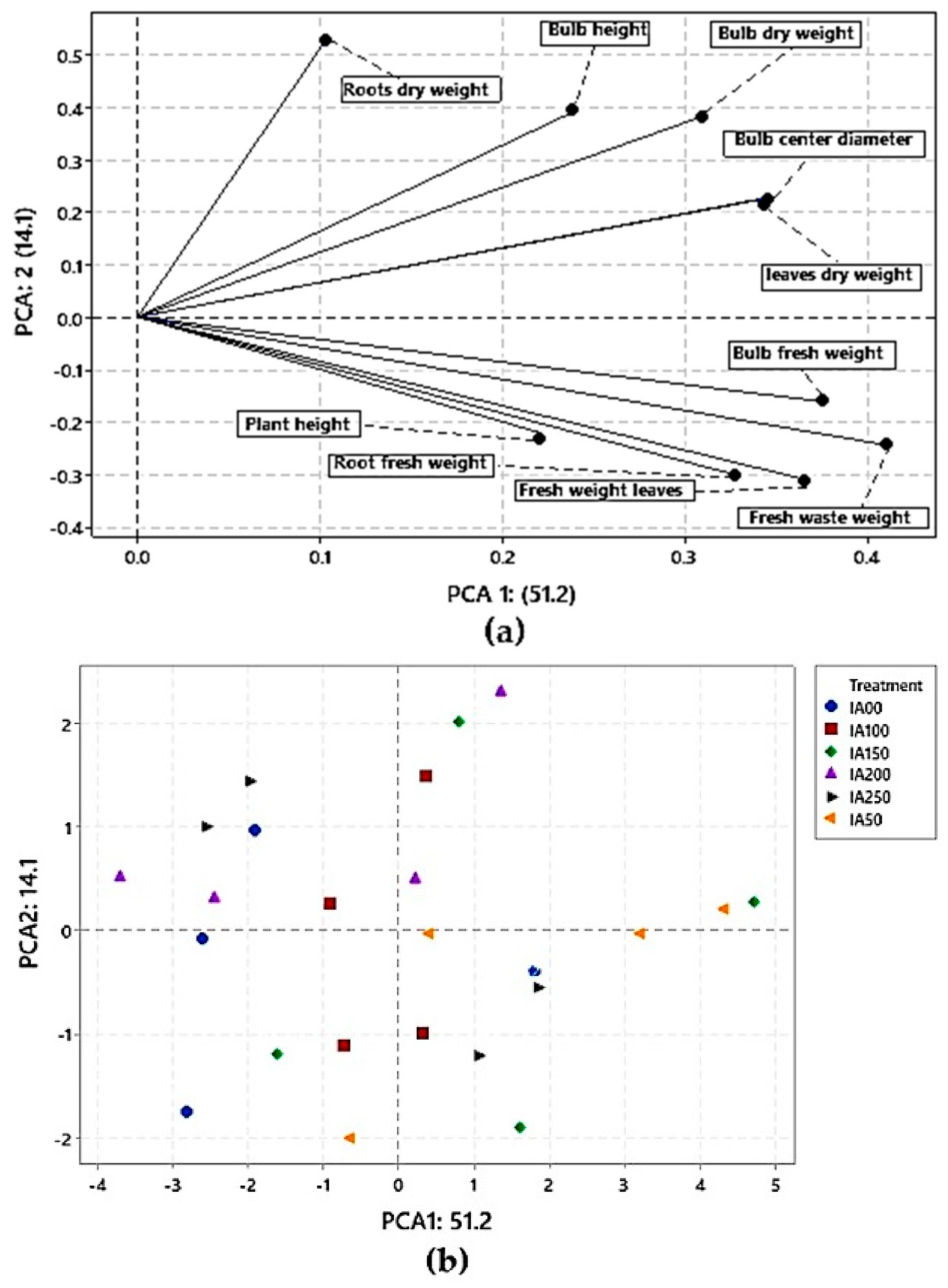
3.4.2. Principal Component Analysis (PCA) for Agronomic Properties
3.4.3. Combined Agronomic Properties and Chemical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, K.; Mahato, N.; Nile, S.H.; Lee, E.T.; Lee, Y.R. Economical and environmentally-friendly approaches for usage of onion (Allium cepa L.) Waste. Food Funct. 2016, 7, 3354–3369. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska, M.A.; Jeleń, H.H. Role of sulfur compounds in vegetable and mushroom aroma. Molecules 2022, 27, 6116. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.; Caruso, G. Chapter 5—Onion. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Jaiswal, A.K., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 73–87. ISBN 978-0-12-812780-3. [Google Scholar]
- Murtado, A.; Mubarik, N.R.; Tjahjoleksono, A. Isolation and characterization endophytic bacteria as biological control of fungus Colletotrichum sp. on Onion Plants (Allium cepa L.). IOP Conf. Ser. Earth Environ. Sci. 2020, 457, 012043. [Google Scholar] [CrossRef]
- Nepomuceno, R.A.; Brown, C.M.B.; Mojica, P.N.; Brown, M.B. Biological control potential of vesicular arbuscular mycorrhizal root inoculant (VAMRI) and associated phosphate solubilizing bacteria, Pseudochrobactrum asaccharolyticum against soilborne phytopathogens of onion (Allium cepa L. var. Red Creole). Arch. Phytopathol. Plant Prot. 2019, 52, 714–732. [Google Scholar] [CrossRef]
- Dutta, R.; K, J.; Nadig, S.M.; Manjunathagowda, D.C.; Gurav, V.S.; Singh, M. Anthracnose of onion (Allium cepa L.): A twister disease. Pathogens 2022, 11, 884. [Google Scholar] [CrossRef] [PubMed]
- Kalman, B.; Abraham, D.; Graph, S.; Perl-Treves, R.; Meller Harel, Y.; Degani, O. Isolation and identification of Fusarium Spp., the causal agents of onion (Allium cepa) Basal rot in northeastern Israel. Biology 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Ratnarajah, V.R.; Gnanachelvam, N.G. Effect of abiotic stress on onion yield. Adv. Technol. 2021, 1, 147–160. [Google Scholar] [CrossRef]
- Khar, A.; Singh, H.; Verma, P. Mitigating Abiotic Stresses in Allium under Changing Climatic Scenario. In Genomic Designing for Abiotic Stress Resistant Vegetable Crops; Kole, C., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 253–278. ISBN 978-3-031-03964-5. [Google Scholar]
- Carvalho, F.P. Agriculture, Pesticides, food security and food safety. Environ. Sci. Policy 2006, 9, 685–692. [Google Scholar] [CrossRef]
- Yeshiwas, Y.; Alemayehu, M.; Adgo, E. The rise and fall of onion production; Its multiple constraints on pre-harvest and post-harvest management issues along the supply chain in northwest Ethiopia. Heliyon 2023, 9, e15905. [Google Scholar] [CrossRef]
- Li, N.; Euring, D.; Cha, J.Y.; Lin, Z.; Lu, M.; Huang, L.-J.; Kim, W.Y. Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front. Plant Sci. 2021, 11, 627969. [Google Scholar] [CrossRef]
- Campos, E.V.R.; do Espirito Santo Pereira, A.; Aleksieienko, I.; do Carmo, G.C.; Gohari, G.; Santaella, C.; Fraceto, L.F.; Oliveira, H.C. Encapsulated plant growth regulators and associative microorganisms: Nature-based solutions to mitigate the effects of climate change on plants. Plant Sci. 2023, 331, 111688. [Google Scholar] [CrossRef]
- Fu, S.-F.; Wei, J.-Y.; Chen, H.-W.; Liu, Y.-Y.; Lu, H.-Y.; Chou, J.-Y. Indole-3-Acetic Acid: A Widespread physiological code in interactions of fungi with other organisms. Plant Signal. Behav. 2015, 10, e1048052. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, C.; Xu, L.; Niu, H.; Liu, Q.; Huang, Y.; Lv, G.; Yang, H.; Li, M. Melatonin and Indole-3-Acetic Acid Synergistically regulate plant growth and stress resistance. Cells 2022, 11, 3250. [Google Scholar] [CrossRef]
- Hagaggi, N.S.A.; Mohamed, A.A.A. Enhancement of Zea mays (L.) Growth performance using Indole Acetic Acid producing endophyte mixta theicola isolated from Solenostemma argel (Hayne). S. Afr. J. Bot. 2020, 134, 64–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Paschold, A.; Marcon, C.; Liu, S.; Tai, H.; Nestler, J.; Yeh, C.-T.; Opitz, N.; Lanz, C.; Schnable, P.S.; et al. The Aux/IAA Gene Rum1 involved in seminal and lateral root formation controls vascular patterning in maize (Zea mays L.) primary roots. J. Exp. Bot. 2014, 65, 4919–4930. [Google Scholar] [CrossRef] [PubMed]
- Ogwu, M.C. Effects of Indole-3-Acetic Acid on the Growth Parameters of Citrullus lanatus (Thunberg) matsum and nakai. Momona Ethiop. J. Sci. 2018, 10, 109–125. [Google Scholar] [CrossRef]
- Bermejo, A.; Granero, B.; Mesejo, C.; Reig, C.; Tejedo, V.; Agustí, M.; Primo-Millo, E.; Iglesias, D.J. Auxin and gibberellin interact in citrus fruit Set. J. Plant Growth Regul. 2018, 37, 491–501. [Google Scholar] [CrossRef]
- Masmoudi, F.; Tounsi, S.; Dunlap, C.A.; Trigui, M. Halotolerant Bacillus spizizenii FMH45 Promoting growth, physiological, and antioxidant parameters of tomato plants exposed to salt stress. Plant Cell Rep. 2021, 40, 1199–1213. [Google Scholar] [CrossRef]
- Mir, A.R.; Siddiqui, H.; Alam, P.; Hayat, S. Foliar Spray of Auxin/IAA Modulates photosynthesis, elemental composition, ros localization and antioxidant machinery to promote growth of Brassica juncea. Physiol. Mol. Biol. Plants 2020, 26, 2503–2520. [Google Scholar] [CrossRef]
- Yadav, A.N. Plant Microbiomes for Sustainable Agriculture: Current Research and Future Challenges. In Plant Microbiomes for Sustainable Agriculture; Desarrollo Sostenible y, Biodiversidad; Yadav, A.N., Singh, J., Rastegari, A.A., Yadav, N., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 475–482. ISBN 978-3-030-38453-1. [Google Scholar]
- Li, Z.; Zhang, X.; Zhao, Y.; Li, Y.; Zhang, G.; Peng, Z.; Zhang, J. Enhancing auxin accumulation in maize root tips improves root growth and dwarfs plant height. Plant Biotechnol. J. 2018, 16, 86–99. [Google Scholar] [CrossRef]
- Bista, D.; Sapkota, D.; Paudel, H.; Adhikari, G. Effect of foliar application of growth regulators on growth and yield of onion (Allium cepa). Int. J. Hortic. Sci. Technol. 2022, 9, 247–254. [Google Scholar] [CrossRef]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant Growth Enhancement using Rhizospheric Halotolerant Phosphate Solubilizing Bacterium Bacillus licheniformis QA1 and Enterobacter asburiae QF11 Isolated from Chenopodium quinoa Willd. Microorganisms 2020, 8, 948. [Google Scholar] [CrossRef] [PubMed]
- Susilowati, D.N.; Riyanti, E.I.; Setyowati, M.; Mulya, K. Indole-3-Acetic Acid Producing bacteria and its application on the growth of rice. AIP Conf. Proc. 2018, 2002, 020016. [Google Scholar] [CrossRef]
- Porras, R.C.S.; Artola, A.; Barrena, R.; Ghoreishi, G.; Matos, C.B.; Sánchez, A. Breaking new ground: Exploring the promising role of Solid-State Fermentation in harnessing natural biostimulants for sustainable agriculture. Processes 2023, 11, 2300. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Abdel-Motaal, F.; El-Sayed, M.; Jogaiah, S.; Shigyo, M.; Ito, S.-i.; Tran, L.S.P. Dissection of Trichoderma longibrachiatum-Induced defense in onion (Allium cepa L.) against Fusarium oxysporum f. sp. cepa by target metabolite profiling. Plant Sci. 2016, 246, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, D.; Rupinder, S.; Ishita, W. Effect of Foliar Application of GA3 and NAA on Onion-a Review. Plant Arch. 2018, 18, 1209–1214. [Google Scholar]
- Publicaciones Fertilizantes. Available online: https://www.mapa.gob.es/es/agricultura/publicaciones/Publicaciones-fertilizantes.aspx (accessed on 9 June 2023).
- BOE-A-2022-860; Real Decreto 47/2022, de 18 de Enero, Sobre Protección de las Aguas Contra la Contaminación Difusa Producida por los Nitratos Procedentes de Fuentes Agrarias. Ministerio de la Presidencia, Relaciones con las Cortes y Memoria Democrática: Madrid, Spain, 2022; pp. 5664–5684.
- Biehler, E.; Mayer, F.; Hoffmann, L.; Krause, E.; Bohn, T. Comparison of 3 Spectrophotometric methods for carotenoid determination in frequently consumed fruits and vegetables. J. Food Sci. 2010, 75, C55–C61. [Google Scholar] [CrossRef]
- Nitrate Assay for Plant Tissues. Available online: https://en.bio-protocol.org/en/bpdetail?id=2029&type=0 (accessed on 9 August 2023).
- Cruzado, M.; Pastor, A.; Castro, N.; Cedrón, J.C. Determinación de compuestos fenólicos y actividad antioxidante de extractos de alcachofa (Cynara scolymus L.). Rev. Soc. Química Perú 2013, 79, 57–63. [Google Scholar]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH Assays to measure antioxidant capacity in popular antioxidant-rich us foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- He, Y.; Zhang, T.; Sun, Y.; Wang, X.; Cao, Q.; Fang, Z.; Chang, M.; Cai, Q.; Lou, L. Exogenous IAA alleviates arsenic toxicity to rice and reduces arsenic accumulation in rice grains. J. Plant Growth Regul. 2022, 41, 734–741. [Google Scholar] [CrossRef]
- Hu, Q.-Q.; Shu, J.-Q.; Li, W.-M.; Wang, G.-Z. Role of auxin and nitrate signaling in the development of root system architecture. Front. Plant Sci. 2021, 12, 690363. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Li, Y.; Li, G.; Liu, D.; Zhao, M.; Cai, N. Growth promotion of Yunnan pine early seedlings in response to foliar application of IAA and IBA. Int. J. Mol. Sci. 2012, 13, 6507–6520. [Google Scholar] [CrossRef] [PubMed]
- PflaPflanzen, G.; Alam, M.; Khan, M.; Khan, A.; Imtiaz, M.; Khan, A.; Naeem, M.; Asim Shah Bacha, S.; Ahmad Shah, S.; Khan, L.; et al. Indole-3-Acetic Acid rescues plant growth and yield of salinity stressed tomato (Lycopersicon esculentum L.). Gesunde Pflanz. 2019, 72, 87–95. [Google Scholar] [CrossRef]
- Ivanchenko, M.G.; den Os, D.; Monshausen, G.B.; Dubrovsky, J.G.; Bednářová, A.; Krishnan, N. Auxin increases the hydrogen peroxide (H2O2) concentration in tomato (Solanum lycopersicum) root tips while inhibiting root growth. Ann. Bot. 2013, 112, 1107–1116. [Google Scholar] [CrossRef]
- Hye, M.; Haque, M.; Karim, M. Influence of growth regulators and their time of application on yield of onion. Pak. J. Biol. Sci. 2002, 5, 1021–1023. [Google Scholar] [CrossRef][Green Version]
- Talukdar, M.; Swain, D.K.; Bhadoria, P.B.S. Effect of IAA and bap application in varying concentration on seed yield and oil quality of Guizotia abyssinica (L.f.) Cass. Ann. Agric. Sci. 2022, 67, 15–23. [Google Scholar] [CrossRef]
- Gupta, S.; Stirk, W.A.; Plačková, L.; Kulkarni, M.G.; Doležal, K.; Van Staden, J. Interactive effects of plant growth-promoting rhizobacteria and a seaweed extract on the growth and physiology of Allium cepa L. (Onion). J. Plant Physiol. 2021, 262, 153437. [Google Scholar] [CrossRef]
- Kondhare, K.R.; Patil, A.B.; Giri, A.P. Auxin: An emerging regulator of tuber and storage root development. Plant Sci. 2021, 306, 110854. [Google Scholar] [CrossRef]
- Lobo, L.L.B.; de Andrade da Silva, M.S.R.; Castellane, T.C.L.; Carvalho, R.F.; Rigobelo, E.C. Effect of Indole-3-Acetic Acid on tomato plant growth. Microorganisms 2022, 10, 2212. [Google Scholar] [CrossRef]
- Masciarelli, O.; Urbani, L.; Reinoso, H.; Luna, V. Alternative mechanism for the evaluation of Indole-3-Acetic Acid (IAA) Production by Azospirillum brasilense strains and its effects on the germination and growth of maize seedlings. J. Microbiol. 2013, 51, 590–597. [Google Scholar] [CrossRef]
- Figueredo, E.F.; da Cruz, T.A.; de Almeida, J.R.; Batista, B.D.; Marcon, J.; de Andrade, P.A.M.; de A. Hayashibara, C.A.; Rosa, M.S.; Azevedo, J.L.; Quecine, M.C. The Key Role of Indole-3-Acetic Acid biosynthesis by Bacillus thuringiensis RZ2MS9 in Promoting maize growth revealed by the IPDC Gene knockout mediated by the CRISPR-Cas9 System. Microbiol. Res. 2023, 266, 127218. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cheng, K.; Huang, G.; Chen, G.; Zhou, S.; Huang, Y.; Zhang, J.; Duan, H.; Fan, H. Effects of Exogenous 3-Indoleacetic Acid and cadmium stress on the physiological and biochemical characteristics of Cinnamomum camphora. Ecotoxicol. Environ. Saf. 2020, 191, 109998. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, J.; Chen, K.; Wang, Y.; Ai, Y.; Zhang, C.; Zhou, S. Effect of Indole-3-Acetic Acid supplementation on the physiology of Lolium perenne L. and Microbial activity in cadmium-contaminated soil. Environ. Sci Pollut. Res. 2022, 29, 52483–52492. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Zhang, X.; Mao, Z.; Qin, S.; Lv, D. Integration of root architecture, root nitrogen metabolism, and photosynthesis of ‘Hanfu’apple trees under the cross-talk between glucose and IAA. Hortic. Plant J. 2023, 9, 631–644. [Google Scholar] [CrossRef]
- Garnica, M.; Houdusse, F.; Zamarreño, A.M.; Garcia-Mina, J.M. The signal effect of nitrate supply enhances active forms of cytokinins and Indole Acetic content and reduces abscisic acid in wheat plants grown with ammonium. J. Plant Physiol. 2010, 167, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Medici, A.; Krouk, G. The primary nitrate response: A multifaceted signalling pathway. J. Exp. Bot. 2014, 65, 5567–5576. [Google Scholar] [CrossRef] [PubMed]
- Beeckman, F.; Motte, H.; Beeckman, T. Nitrification in agricultural soils: Impact, actors and mitigation. Curr. Opin. Biotechnol. 2018, 50, 166–173. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, Y.; Liu, Z.; Jin, W.; Sun, Y. Effects of melatonin on seedling growth, mineral nutrition, and nitrogen metabolism in cucumber under nitrate stress. J. Pineal Res. 2017, 62, e12403. [Google Scholar] [CrossRef]
- Fu, Y.-F.; Zhang, Z.-W.; Yang, X.-Y.; Wang, C.-Q.; Lan, T.; Tang, X.-Y.; Chen, G.-D.; Zeng, J.; Yuan, S. Nitrate reductase is a key enzyme responsible for nitrogen-regulated auxin accumulation in Arabidopsis roots. Biochem. Biophys. Res. 2020, 532, 633–639. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, X.; Guo, X.; Qi, X.; Feng, F.; Zhang, Y.; Zhao, Q.; Han, D.; Sun, H. Nitrate modulates lateral root formation by regulating the auxin response and transport in rice. Genes 2021, 12, 850. [Google Scholar] [CrossRef] [PubMed]
- Atif, M.; Perveen, S.; Parveen, A.; Mahmood, S.; Saeed, M.; Zafar, S. Thiamine and Indole-3-Acetic Acid Induced modulations in physiological and biochemical characteristics of maize (Zea mays L.) under arsenic stress. Sustainability 2022, 14, 13288. [Google Scholar] [CrossRef]
- Kecis, H.; Bagues, M.; Abdelouhab, Y.; Mekircha, F.; Gali, L.; Kadi, K.; Addad, D.; Nagaz, K.; Brahmi, F.; Kouba, Y. Different Indole-3-Acetic Acid and 6 Benzyl Amino Purine concentrations affect biomass, phenolic profile, and bioactivity in Mentha rotundifolia L. J. Food Meas. Charact. 2023, 1–14. [Google Scholar] [CrossRef]
- Mona, S.A.; Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Soliman, D.W.K.; Wirth, S.; Egamberdieva, D. Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J. Integr. Agric. 2017, 16, 1751–1757. [Google Scholar] [CrossRef]
- Arias, J.P.; Zapata, K.; Rojano, B.; Arias, M. Effect of light wavelength on cell growth, content of phenolic compounds and antioxidant activity in cell suspension cultures of Thevetia peruviana. J. Photochem. Photobiol. B Biol. 2016, 163, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Oueslati, S.; Ksouri, R.; Falleh, H.; Pichette, A.; Abdelly, C.; Legault, J. Phenolic content, antioxidant, anti-inflammatory and anticancer activities of the edible halophyte suaeda Fruticosa forssk. Food Chem. 2012, 132, 943–947. [Google Scholar] [CrossRef]
- Mirheidari, F.; Hatami, M.; Ghorbanpour, M. Effect of different concentrations of IAA, GA3 and Chitosan Nano-fiber on physio-morphological characteristics and metabolite contents in Roselle (Hibiscus sabdariffa L.). S. Afr. J. Bot. 2022, 145, 323–333. [Google Scholar] [CrossRef]
- Schauffler, G.P.; dos Anjos Verzutti Fonseca, J.; Di Piero, R.M. Defense mechanisms involved in the resistance of maize cultivars to Bipolaris maydis. Eur. J. Plant Pathol. 2022, 163, 269–277. [Google Scholar] [CrossRef]
- Basu, S.; Roychoudhury, A.; Saha, P.P.; Sengupta, D.N. Differential antioxidative responses of indica rice cultivars to drought stress. Plant Growth Regul. 2010, 60, 51–59. [Google Scholar] [CrossRef]
- Esan, A.M.; Masisi, K.; Dada, F.A.; Olaiya, C.O. Comparative Effects of Indole Acetic Acid and Salicylic Acid on oxidative stress marker and antioxidant potential of okra (Abelmoschus esculentus) Fruit under Salinity Stress. Sci. Hortic. 2017, 216, 278–283. [Google Scholar] [CrossRef]
- Mareček, V.; Mikyška, A.; Hampel, D.; Čejka, P.; Neuwirthová, J.; Malachová, A.; Cerkal, R. ABTS and DPPH Methods as a tool for studying antioxidant capacity of spring barley and malt. J. Cereal Sci. 2017, 73, 40–45. [Google Scholar] [CrossRef]
- Subhasree, B.; Baskar, R.; Laxmi Keerthana, R.; Lijina Susan, R.; Rajasekaran, P. Evaluation of antioxidant potential in selected green leafy vegetables. Food Chem. 2009, 115, 1213–1220. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Kocira, A.; Czerwińska, E.; Wójtowicz, A.; Bronowicka-Mielniczuk, U.; Koszel, M.; Findura, P. Modeling biometric traits, yield and nutritional and antioxidant properties of seeds of three soybean cultivars through the application of biostimulant containing seaweed and amino acids. Front. Plant Sci. 2018, 9, 388. [Google Scholar] [CrossRef] [PubMed]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Kisiriko, M.; Anastasiadi, M.; Terry, L.A.; Yasri, A.; Beale, M.H.; Ward, J.L. Phenolics from medicinal and aromatic plants: Characterisation and potential as biostimulants and bioprotectants. Molecules 2021, 26, 6343. [Google Scholar] [CrossRef]
- Liu, R.; Yang, L.; Zou, Y.; Wu, Q. Root-Associated Endophytic fungi modulate endogenous auxin and cytokinin levels to improve plant biomass and root morphology of trifoliate orange. Hortic. Plant J. 2023, 9, 463–472. [Google Scholar] [CrossRef]
- Sevik, H.; Guney, K. Effects of IAA, IBA, NAA, and GA3 on rooting and morphological features of melissa officinalis l. stem cuttings. Sci. World J. 2013, 2013, 909507. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Park, Y.J.; Morgan, A.M.A.; Valan Arasu, M.; Al-Dhabi, N.A.; Park, S.U. Influence of Indole-3-Acetic Acid and Gibberellic Acid on Phenylpropanoid accumulation in common buckwheat (Fagopyrum esculentum Moench) sprouts. Molecules 2017, 22, 374. [Google Scholar] [CrossRef]
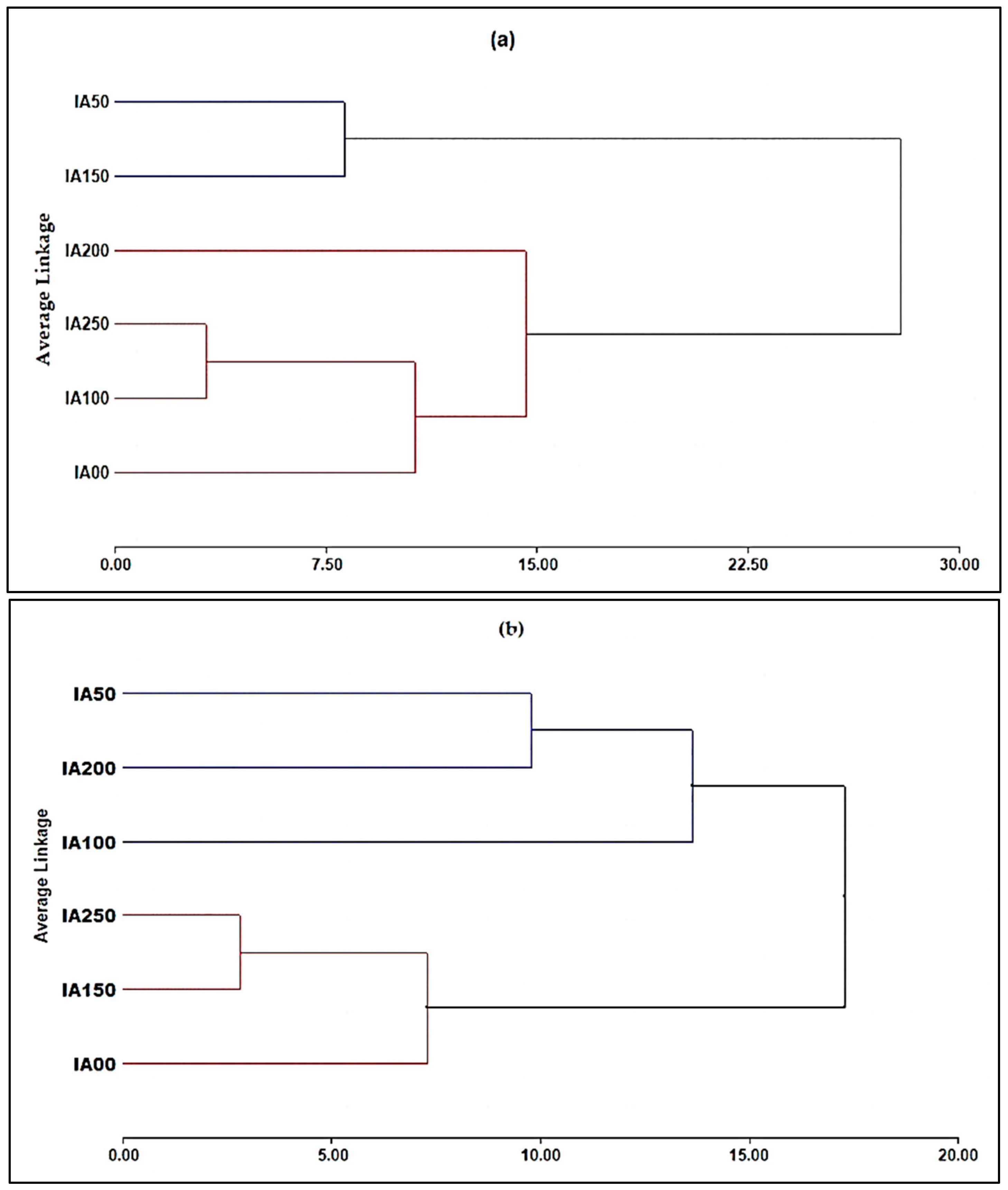
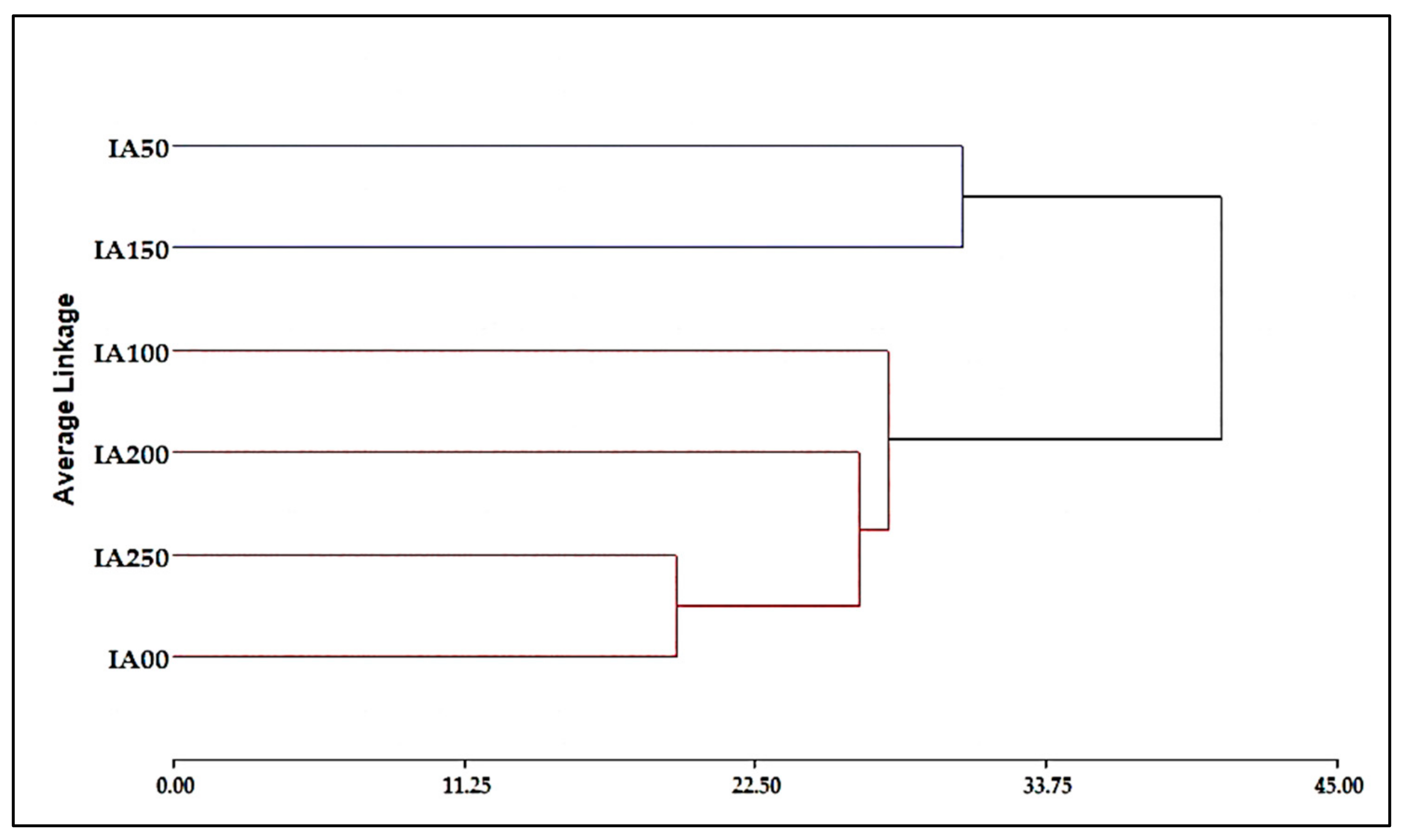

| Treatment | FW (g) | FR (g) | FF (g) | FB (g) |
|---|---|---|---|---|
| IA50 | 92.00 ± 23.30 a | 13.63 ± 2.78 a | 36.47 ± 7.58 a | 3.68 ± 0.35 a |
| IA100 | 63.88 ± 6.05 ab | 9.500 ± 0.70 a | 26.25 ± 6.97 a | 3.32 ± 0.14 ab |
| IA150 | 79.40 ± 32.00 ab | 13.25 ± 5.78 a | 31.87 ± 15.14 a | 3.47 ± 0.37 ab |
| IA200 | 53.00 ± 16.75 b | 9.38 ± 2.02 a | 27.21 ± 13.15 a | 2.79 ± 0.13 c |
| IA250 | 62.50 ± 30.90 ab | 12.00 ± 6.45 a | 25.23 ± 13.50 a | 3.12 ± 0.51 bc |
| Control | ||||
| IA0 | 57.50 ± 26.80 ab | 7.75 ± 3.07 a | 24.13 ± 11.35 a | 3.09 ± 0.41 bc |
| Significance | ||||
| concentration | ns | ns | ns | * |
| Treatment | PH (cm) | BH (cm) | CDB (cm) |
|---|---|---|---|
| IA50 | 56.00 ± 5.23 ab | 5.00 ± 0.89 a | 4.02 ± 0.62 a |
| IA100 | 56.50 ± 4.51 ab | 4.62 ± 0.98 a | 3.22 ± 0.26 a |
| IA150 | 58.25 ± 5.56 a | 4.80 ± 0.78 a | 3.85 ± 0.99 a |
| IA200 | 51.00 ± 4.90 b | 5.25 ± 0.50 a | 3.20 ± 1.23 a |
| IA250 | 54.50 ± 3.87 ab | 4.67 ± 0.57 a | 3.05 ± 0.85 a |
| Control | |||
| IA0 | 52.25 ± 2.06 ab | 4.42 ± 0.83 a | 3.07 ± 0.35 a |
| Significance | |||
| concentration | ns | ns | ns |
| Treatment | RDW (g) | BDW (g) | FDW (g) |
|---|---|---|---|
| IAA | |||
| IA50 | 0.45 ± 0.16 ab | 0.53 ± 0.35 a | 0.793 ± 0.40 a |
| IA100 | 0.45 ± 0.18 ab | 0.50 ± 0.09 a | 0.631 ± 0.21 a |
| IA150 | 0.43 ± 0.15 ab | 0.79 ± 0.60 a | 0.749 ± 0.38 a |
| IA200 | 0.55 ± 0.12 a | 0.44 ± 0.46 a | 0.4419 ± 0.19 a |
| IA250 | 0.53 ± 0.16 a | 0.49 ± 0.29 a | 0.6107 ± 0.14 a |
| Control | |||
| IA0 | 0.31 ± 0.06 b | 0.51 ± 0.35 a | 0.458 ± 0.31 a |
| Significance | |||
| concentration | ns | ns | ns |
| Treatment | Chlorophyll a (mg fw−1) | Chlorophyll b (mg fw−1) | Carotenoids (mg fw−1) | Total Pigments (mg fw−1) | Nitrates (mg kg fw−1) |
|---|---|---|---|---|---|
| IA50 | 0.44 ± 0.23 a | 0.41 ± 0.26 a | 0.20 ± 0.11 a | 0.44 ± 0.35 a | 1424 ± 349 b |
| IA100 | 0.37 ± 0.05 a | 0.45 ± 0.05 a | 0.19 ± 0.04 a | 0.58 ± 0.06 a | 3734 ± 1526 a |
| IA150 | 0.20 ± 0.11 a | 0.29 ± 0.14 a | 0.12 ± 0.10 a | 0.41 ± 0.18 a | 2422 ± 1104 ab |
| IA200 | 0.41 ± 0.11 a | 0.35 ± 0.08 a | 0.15 ± 0.03 a | 0.55 ± 0.08 a | 2278 ± 732 ab |
| IA250 | 0.26 ± 0.11 a | 0.24 ± 0.16 a | 0.12 ± 0.11 a | 0.36 ± 0.18 a | 2104 ± 1239 b |
| Control | |||||
| IA0 | 0.25 ± 0.02 a | 0.35 ± 0.11 a | 0.12 ± 0.04 a | 0.4473 ± 0.07 a | 2072 ± 742 b |
| Significance | |||||
| concentration | ns | ns | ns | ns | ns |
| Treatments | TPs (mg AGE g dm−3) | DPPH (μmol TROLOX g dm−3) |
|---|---|---|
| IA50 | 0.69 ± 0.53 c | 7.57 ± 0.82 ab |
| IA100 | 1.60 ± 1.19 bc | 6.00 ± 0.54 bc |
| IA150 | 2.69 ± 0.72 abc | 5.42 ± 1.85 c |
| IA200 | 3.98 ± 1.53 a | 7.94 ± 2.00 a |
| IA250 | 2.97 ± 0.78 ab | 6.66 ± 1.09 abc |
| Control | ||
| IA0 | 0.63 ± 0.89 c | 7.49 ± 0.72 ab |
| Significance | ||
| concentration | * | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solano, C.; Artola, A.; Barrena, R.; Ballardo, C.; Sánchez, A. Effect of the Exogenous Application of Different Concentrations of Indole-3-Acetic Acid as a Growth Regulator on Onion (Allium cepa L.) Cultivation. Agronomy 2023, 13, 2204. https://doi.org/10.3390/agronomy13092204
Solano C, Artola A, Barrena R, Ballardo C, Sánchez A. Effect of the Exogenous Application of Different Concentrations of Indole-3-Acetic Acid as a Growth Regulator on Onion (Allium cepa L.) Cultivation. Agronomy. 2023; 13(9):2204. https://doi.org/10.3390/agronomy13092204
Chicago/Turabian StyleSolano, Carlos, Adriana Artola, Raquel Barrena, Cindy Ballardo, and Antoni Sánchez. 2023. "Effect of the Exogenous Application of Different Concentrations of Indole-3-Acetic Acid as a Growth Regulator on Onion (Allium cepa L.) Cultivation" Agronomy 13, no. 9: 2204. https://doi.org/10.3390/agronomy13092204
APA StyleSolano, C., Artola, A., Barrena, R., Ballardo, C., & Sánchez, A. (2023). Effect of the Exogenous Application of Different Concentrations of Indole-3-Acetic Acid as a Growth Regulator on Onion (Allium cepa L.) Cultivation. Agronomy, 13(9), 2204. https://doi.org/10.3390/agronomy13092204









