Contribution of Glutathione Transferases in the Selective and Light-Dependent Effect of Flumioxazin on Winter Wheat (Triticum aestivum L.) and Its Typical Weed Common Poppy (Papaver rhoeas L.)
Abstract
1. Introduction
2. Materials and Methods
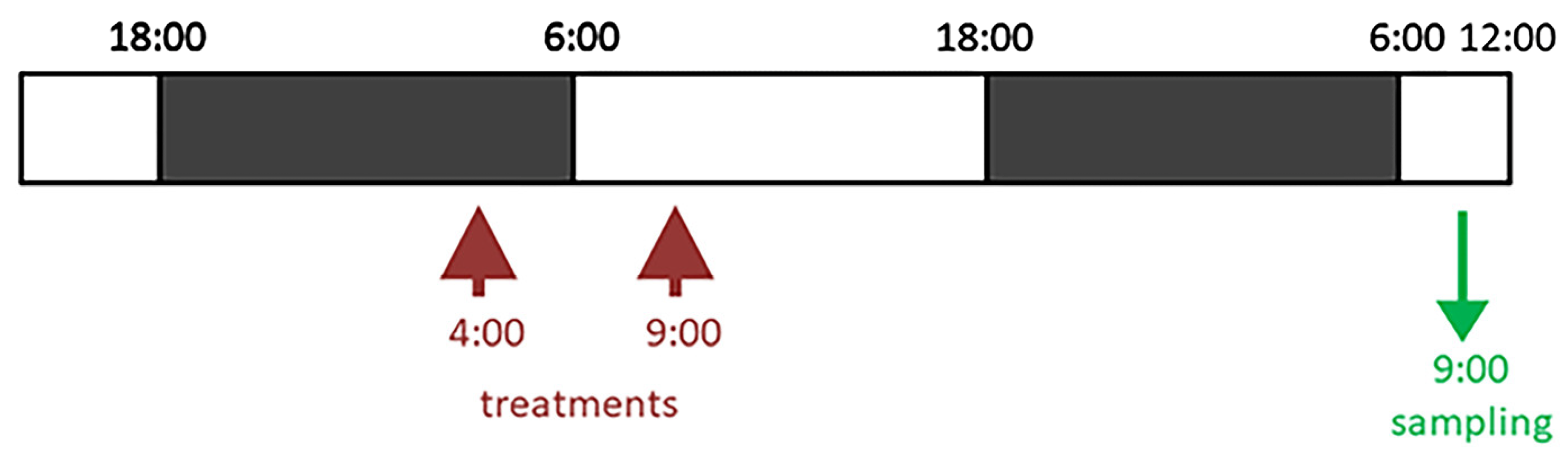
2.1. Plant Materials and Treatments
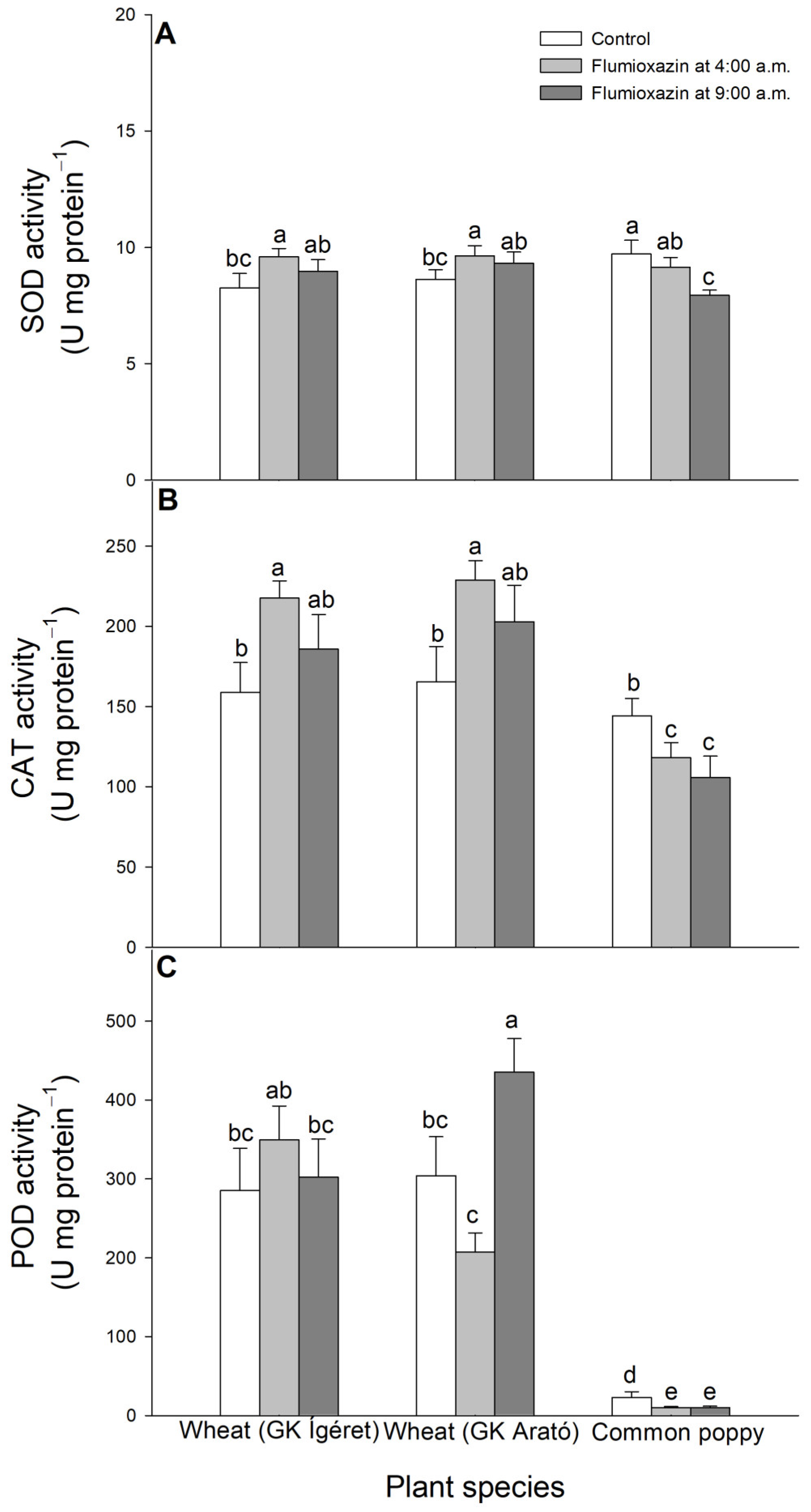
2.2. Determination of Antioxidant Activities
2.3. Measurement of Maximal Quantum Yield of PSII Photochemistry
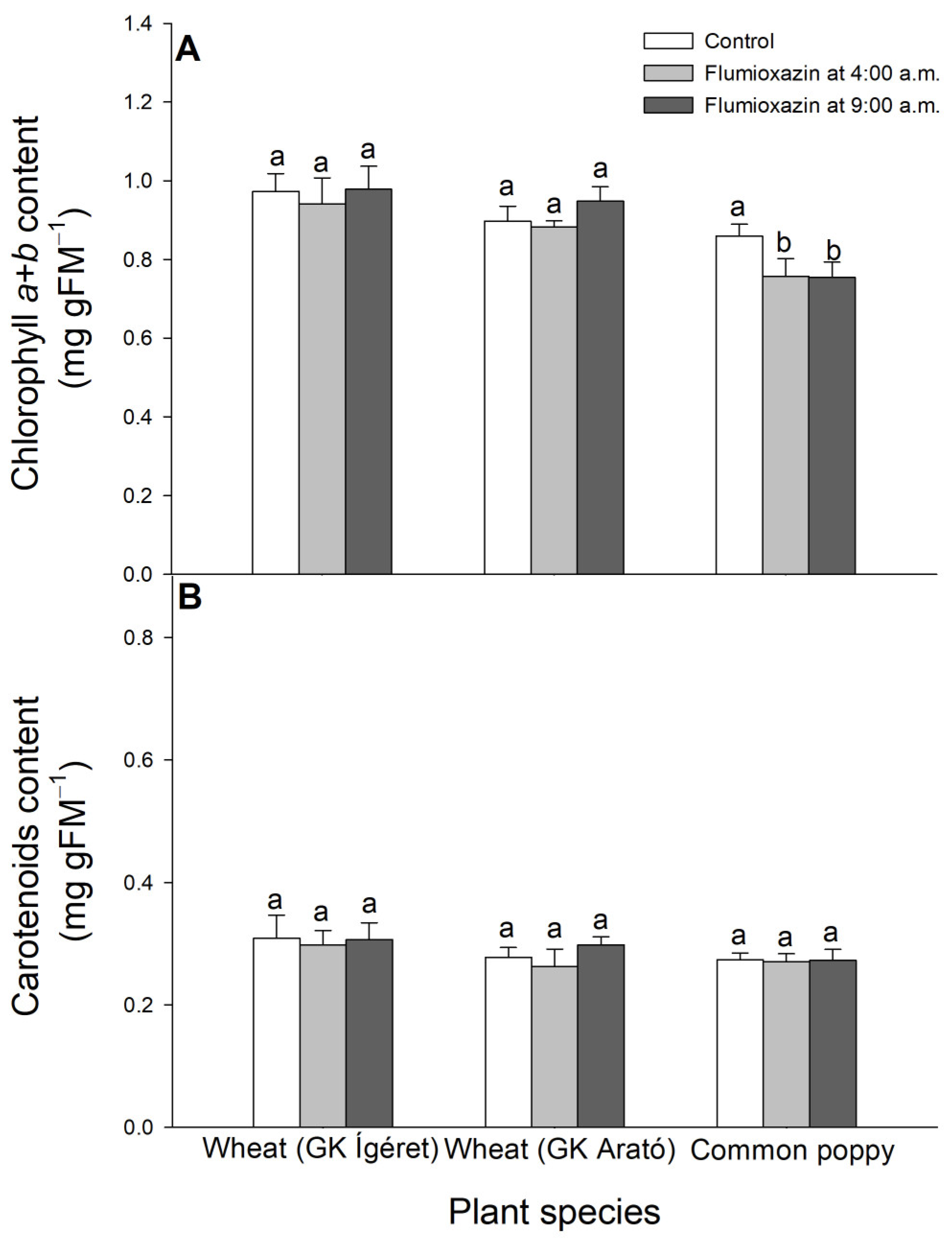
2.4. Determination of Photosynthetic Pigment Concentration
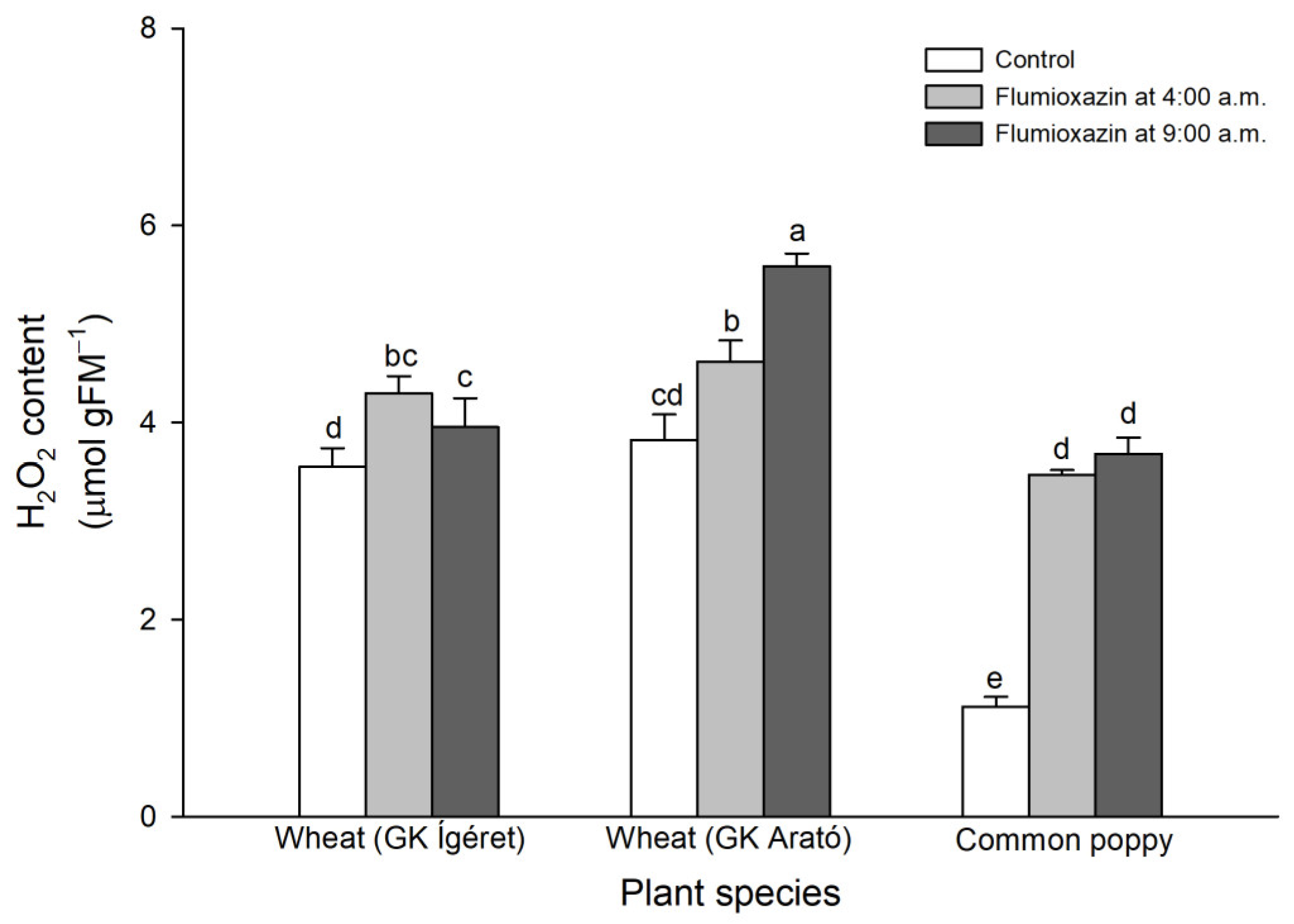
2.5. Determination of H2O2 Concentration
2.6. RNA Extraction, cDNA Synthesis, and Expression Analysis by Quantitative Real-Time PCR
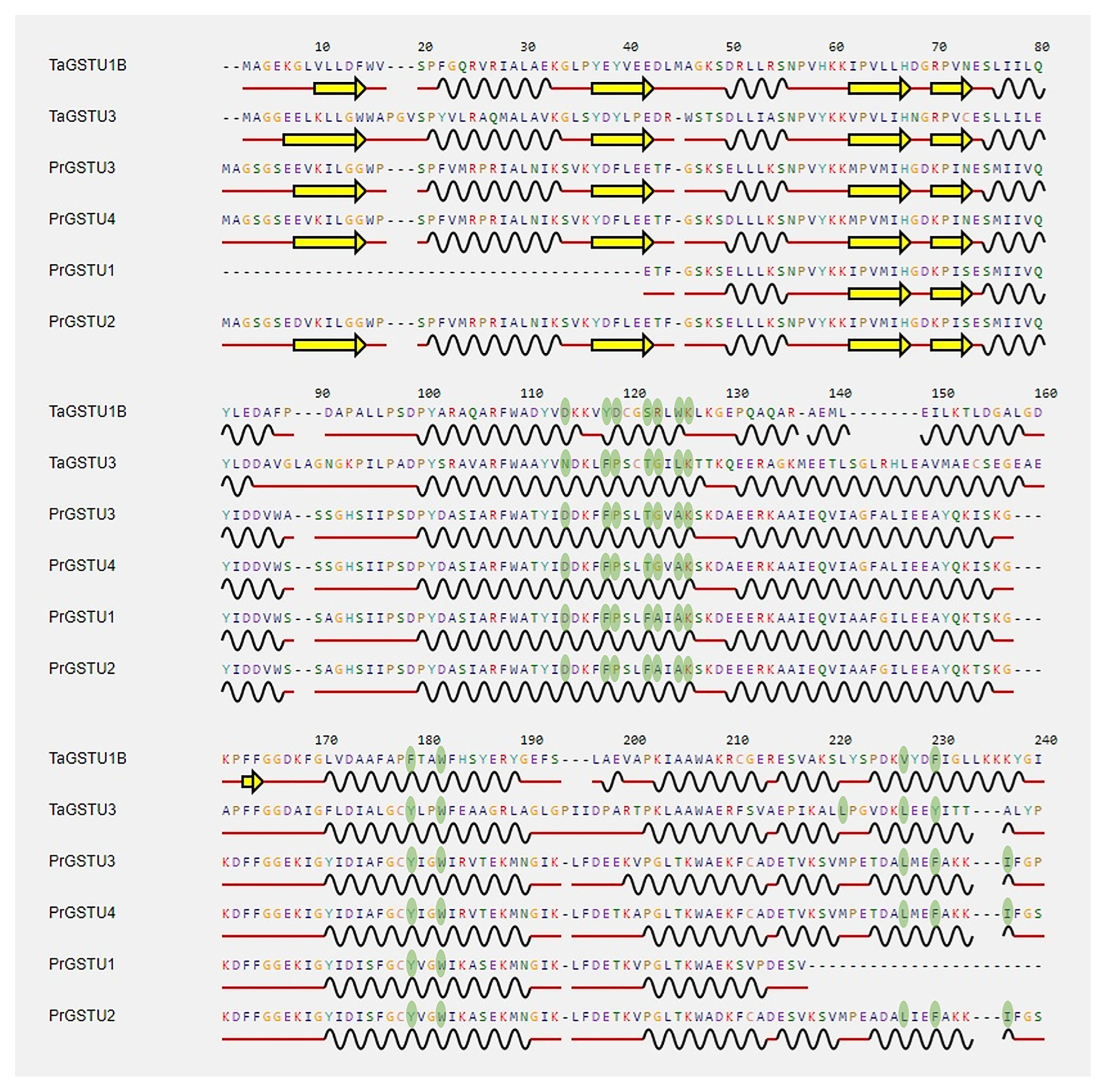
2.7. Alignment of the Secondary Structure
2.8. Statistical Analysis
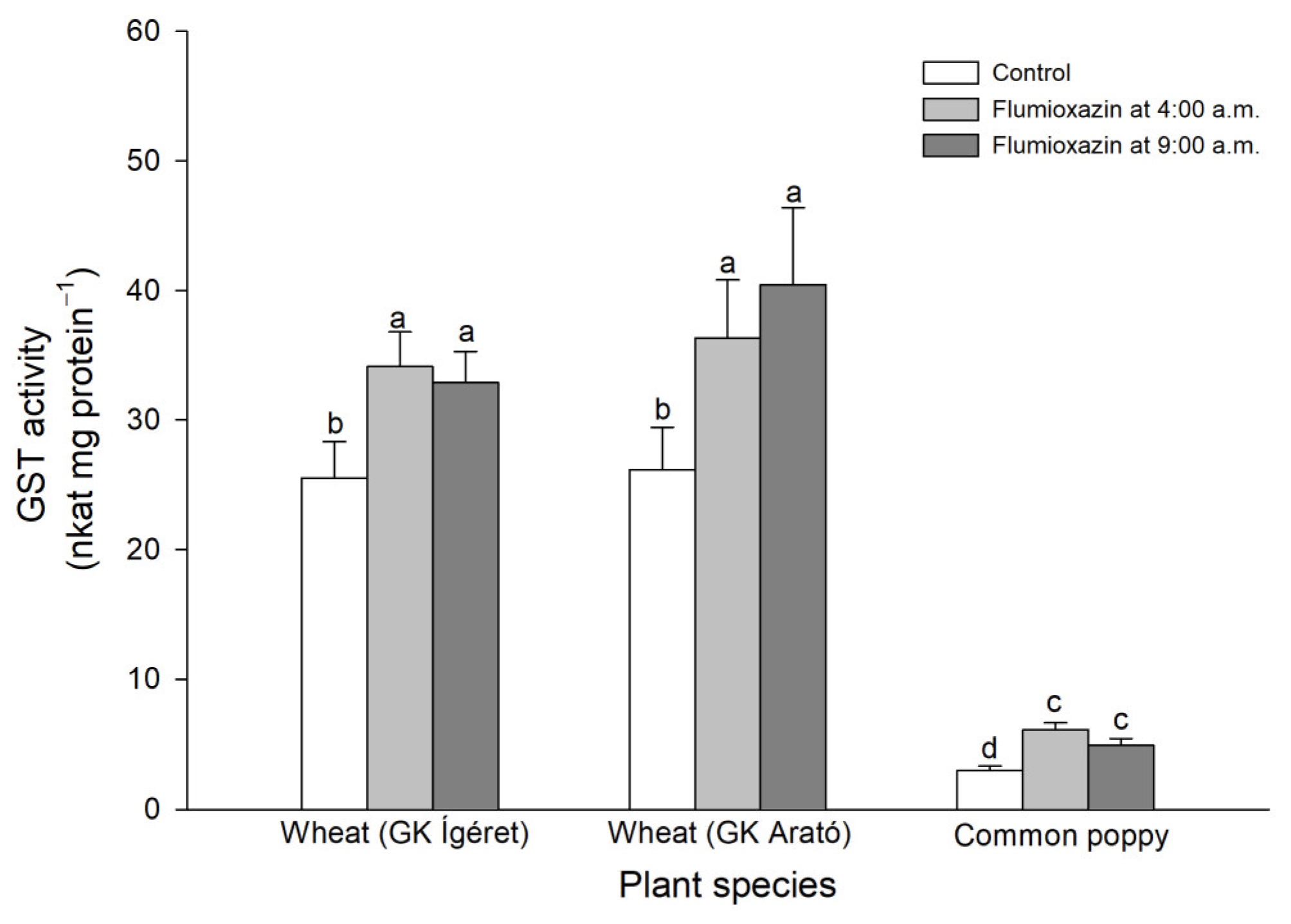
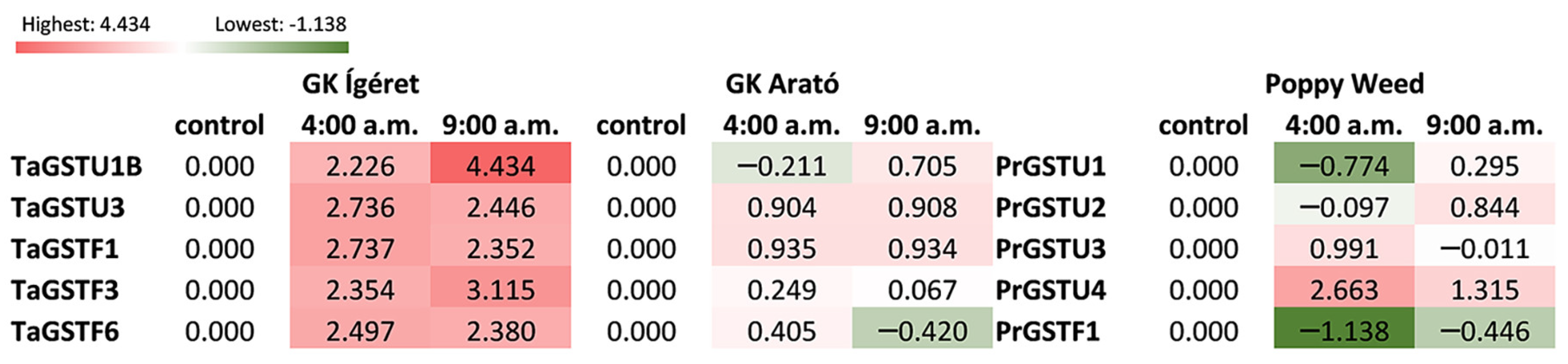
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camargo, E.R.; Senseman, S.A.; McCauley, G.N.; Bowe, S.; Harden, J.; Guice, J.B. Interaction between saflufenacil and imazethapyr in red rice (Oryza ssp.) and hemp sesbania (Sesbania exaltata) as affected by light intensity. Pest. Manag. Sci. 2012, 68, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Lati, R.N.; Mou, B.; Rachuy, J.S.; Fennimore, S.A. Evaluation of cycloate followed by evening two-leaf–stage phenmedipham application in fresh market spinach. Weed Technol. 2016, 30, 464–471. [Google Scholar] [CrossRef]
- Frenkel, E.; Matzrafi, M.; Rubin, B.; Peleg, Z. Effects of Environmental Conditions on the Fitness Penalty in Herbicide Resistant Brachypodium hybridum. Front. Plant Sci. 2017, 8, 94. [Google Scholar] [CrossRef]
- Nagano, E. Herbicidal efficacy of protoporphyrinogen oxidase inhibitors. In Peroxidizing Herbicides; Springer: Berlin/Heidelberg, Germany, 1999; pp. 293–302. [Google Scholar]
- Niekamp, J.W.; Johnson, W.G.; Smeda, R.J. Broadleaf weed control with sulfentrazone and flumioxazin in no-tillage soybean (Glycine max). Weed Technol. 1999, 13, 233–238. [Google Scholar] [CrossRef]
- Geoffroy, L.; Frankart, C.; Eullaffroy, P. Comparison of different physiological parameter responses in Lemna minor and Scenedesmus obliquus exposed to herbicide flumioxazin. Env. Pollut. 2004, 131, 233–241. [Google Scholar] [CrossRef]
- Duke, S.O.; Lydon, J.; Becerril, J.M.; Sherman, T.D.; Lehnen, L.P.; Matsumoto, H. Protoporphyrinogen oxidase-inhibiting herbicides. Weed Sci. 1991, 39, 465–473. [Google Scholar]
- Theodoridis, G.; Bahr, J.T.; Hotzman, F.W.; Sehgel, S.; Suarez, D.P. New generation of protox-inhibiting herbicides. Crop Prot. 2000, 19, 533–535. [Google Scholar]
- Lloyd, K.L.; Johnson, J.M.; Gover, A.E.; Sellmer, J.C. Preemergence and postemergence suppression of kochia on rights-of-way. Weed Technol. 2011, 25, 292–297. [Google Scholar] [CrossRef]
- Sebastian, D.J.; Nissen, S.J.; Westra, P.; Shaner, D.L.; Butters, G. Influence of soil properties and soil moisture on the efficacy of indaziflam and flumioxazin on Kochia scoparia L. Pest. Manag. Sci. 2017, 73, 444–451. [Google Scholar] [CrossRef]
- Glaspie, C.F.; Jones, E.A.; Penner, D.; Pawlak, J.A.; Everman, W.J. Effect of clay, soil organic matter, and soil pH on initial and residual weed control with flumioxazin. Agronomy 2021, 11, 1326. [Google Scholar] [CrossRef]
- Hurdle, N.L.; Grey, T.L.; Pilon, C.; Monfort, W.S.; Prostko, E.P. Peanut seed germination and radicle development response to direct exposure of flumioxazin across multiple temperatures. Peanut Sci. 2020, 47, 89–93. [Google Scholar]
- Czarnocka, W.; Karpiński, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [PubMed]
- Noctor, G.; Reichheld, J.P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. PPB 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Kocsy, G.; Tari, I.; Vankova, R.; Zechmann, B.; Gulyas, Z.; Poor, P.; Galiba, G. Redox control of plant growth and development. Plant Sci. Int. J. Exp. Plant Biol. 2013, 211, 77–91. [Google Scholar] [CrossRef]
- Ślesak, I.; Miszalski, Z.; Karpinska, B.; Niewiadomska, E.; Ratajczak, R.; Karpinski, S. Redox control of oxidative stress responses in the C3–CAM intermediate plant Mesembryanthemum crystallinum. Plant Physiol. Biochem. 2002, 40, 669–677. [Google Scholar]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [PubMed]
- Kerdnaimongkol, K.; Bhatia, A.; Joly, R.J.; Woodson, W.R. Oxidative stress and diurnal variation in chilling sensitivity of tomato seedlings. J. Am. Soc. Hortic. Sci. 1997, 122, 485–490. [Google Scholar]
- Sosa Alderete, L.G.; Guido, M.E.; Agostini, E.; Mas, P. Identification and characterization of key circadian clock genes of tobacco hairy roots: Putative regulatory role in xenobiotic metabolism. Environ. Sci. Pollut. Res. Int. 2018, 25, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Galle, A.; Czekus, Z.; Bela, K.; Horvath, E.; Csiszar, J.; Poor, P. Diurnal changes in tomato glutathione transferase activity and expression. Acta Biol. Hung. 2018, 69, 505–509. [Google Scholar] [CrossRef]
- Pelsőczi, A.; Horváth, E.; Czékus, Z.; Kukri, A.; Poór, P.; Gallé, Á. Nocturnal Red Light Application Modulated the Fumonisin B1-Induced Changes in Glutathione Transferases of Different Wheat Cultivars. J. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Labrou, N.E.; Papageorgiou, A.C.; Pavli, O.; Flemetakis, E. Plant GSTome: Structure and functional role in xenome network and plant stress response. Curr. Opin. Biotechnol. 2015, 32, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Galle, A.; Czekus, Z.; Bela, K.; Horvath, E.; Ordog, A.; Csiszar, J.; Poor, P. Plant Glutathione Transferases and Light. Front. Plant Sci. 2019, 9, 1944. [Google Scholar] [CrossRef]
- Dixon, D.P.; McEwen, A.G.; Lapthorn, A.J.; Edwards, R. Forced evolution of a herbicide detoxifying glutathione transferase. J. Biol. Chem. 2003, 278, 23930–23935. [Google Scholar] [CrossRef]
- Benekos, K.; Kissoudis, C.; Nianiou-Obeidat, I.; Labrou, N.; Madesis, P.; Kalamaki, M.; Makris, A.; Tsaftaris, A. Overexpression of a specific soybean GmGSTU4 isoenzyme improves diphenyl ether and chloroacetanilide herbicide tolerance of transgenic tobacco plants. J. Biotechnol. 2010, 150, 195–201. [Google Scholar] [CrossRef]
- Lo Cicero, L.; Madesis, P.; Tsaftaris, A.; Lo Piero, A.R. Tobacco plants over-expressing the sweet orange tau glutathione transferases (CsGSTUs) acquire tolerance to the diphenyl ether herbicide fluorodifen and to salt and drought stresses. Phytochemistry 2015, 116, 69–77. [Google Scholar] [CrossRef]
- Axarli, I.; Muleta, A.W.; Vlachakis, D.; Kossida, S.; Kotzia, G.; Maltezos, A.; Dhavala, P.; Papageorgiou, A.C.; Labrou, N.E. Directed evolution of Tau class glutathione transferases reveals a site that regulates catalytic efficiency and masks co-operativity. Biochem. J. 2016, 473, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Perperopoulou, F.; Pouliou, F.; Labrou, N.E. Recent advances in protein engineering and biotechnological applications of glutathione transferases. Crit. Rev. Biotechnol. 2018, 38, 511–528. [Google Scholar] [CrossRef]
- Lo Piero, A.R.; Mercurio, V.; Puglisi, I.; Petrone, G. Different roles of functional residues in the hydrophobic binding site of two sweet orange tau glutathione S-transferases. FEBS J. 2010, 277, 255–262. [Google Scholar]
- Yuan, J.S.; Tranel, P.J.; Stewart, C.N., Jr. Non-target-site herbicide resistance: A family business. Trends Plant Sci. 2007, 12, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Cummins, I.; O’Hagan, D.; Jablonkai, I.; Cole, D.J.; Hehn, A.; Werck-Reichhart, D.; Edwards, R. Cloning, characterization and regulation of a family of phi class glutathione transferases from wheat. Plant Mol. Biol. 2003, 52, 591–603. [Google Scholar] [CrossRef]
- Chronopoulou, E.; Georgakis, N.; Nianiou-Obeidat, I.; Madesis, P.; Perperopoulou, F.; Pouliou, F.; Vasilopoulou, E.; Ioannou, E.; Ataya, F.S.; Labrou, N.E. Plant glutathione transferases in abiotic stress response and herbicide resistance. In Glutathione in Plant Growth, Development, and Stress Tolerance; Springer: Cham, Switzerland, 2017; pp. 215–233. [Google Scholar]
- Riechers, D.E.; Irzyk, G.P.; Jones, S.S.; Fuerst, E.P. Partial characterization of glutathione S-transferases from wheat (Triticum spp.) and purification of a safener-induced glutathione S-transferase from Triticum tauschii. Plant Physiol. 1997, 114, 1461–1470. [Google Scholar] [CrossRef]
- Cummins, I.; Cole, D.J.; Edwards, R. Purification of multiple glutathione transferases involved in herbicide detoxification from wheat (Triticum aestivum L.) treated with the safener fenchlorazole-ethyl. Pestic. Biochem. Physiol. 1997, 59, 35–49. [Google Scholar] [CrossRef]
- Koeppe, M.; Barefoot, A.; Cotterman, C.; Zimmerman, W.; Leep, D. Basis of selectivity of the herbicide flupyrsulfuron-methyl in wheat. Pestic. Biochem. Physiol. 1997, 59, 105–117. [Google Scholar] [CrossRef]
- Thom, R.; Cummins, I.; Dixon, D.P.; Edwards, R.; Cole, D.J.; Lapthorn, A.J. Structure of a tau class glutathione S-transferase from wheat active in herbicide detoxification. Biochemistry 2002, 41, 7008–7020. [Google Scholar] [CrossRef] [PubMed]
- Hatton, P.J.; Cummins, I.; Cole, D.J.; Edwards, R. Glutathione transferases involved in herbicide detoxification in the leaves of Setaria faberi (giant foxtail). Physiol. Plant. 1999, 105, 9–16. [Google Scholar] [CrossRef]
- Andrews, C.J.; Cummins, I.; Skipsey, M.; Grundy, N.M.; Jepson, I.; Townson, J.; Edwards, R. Purification and characterisation of a family of glutathione transferases with roles in herbicide detoxification in soybean (Glycine max L.); selective enhancement by herbicides and herbicide safeners. Pestic. Biochem. Physiol. 2005, 82, 205–219. [Google Scholar] [CrossRef]
- Matzenbacher, F.d.O.; Vidal, R.A.; Merotto Jr, A.; Trezzi, M.M. Environmental and physiological factors that affect the efficacy of herbicides that inhibit the enzyme protoporphyrinogen oxidase: A literature review. Planta Daninha 2014, 32, 457–463. [Google Scholar] [CrossRef][Green Version]
- Ando, D. Study of uptake, translocation, and metabolic behavior of pesticides in water milfoil. J. Pestic. Sci. 2020, 45, 151–158. [Google Scholar] [CrossRef]
- Jia, L.; Zhao, L.-X.; Sun, F.; Peng, J.; Wang, J.-Y.; Leng, X.-Y.; Gao, S.; Fu, Y.; Ye, F. Diazabicyclo derivatives as safeners protect cotton from injury caused by flumioxazin. Pestic. Biochem. Physiol. 2022, 187, 105185. [Google Scholar] [CrossRef]
- Loyall, L.; Uchida, K.; Braun, S.; Furuya, M.; Frohnmeyer, H. Glutathione and a UV light–induced glutathione S-transferase are involved in signaling to chalcone synthase in cell cultures. Plant Cell 2000, 12, 1939–1950. [Google Scholar] [PubMed]
- Frohnmeyer, H.; Staiger, D. Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol. 2003, 133, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.W.; Liu, M.J.; Chen, I.C.; Huang, C.H.; Chao, L.Y.; Hsieh, H.L. A glutathione S-transferase regulated by light and hormones participates in the modulation of Arabidopsis seedling development. Plant Physiol. 2010, 154, 1646–1658. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Ho, S.S.; Kuo, T.Y.; Hsieh, H.L.; Cheng, Y.S. Structural basis of jasmonate-amido synthetase FIN219 in complex with glutathione S-transferase FIP1 during the JA signal regulation. Proc. Natl. Acad. Sci. USA 2017, 114, E1815–E1824. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Upadhyaya, A.; Sankhla, D.; Davis, T.D.; Sankhla, N.; Smith, B. Effect of paclobutrazol on the activities of some enzymes of activated oxygen metabolism and lipid peroxidation in senescing soybean leaves. J. Plant Physiol. 1985, 121, 453–461. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Poor, P.; Borbely, P.; Czekus, Z.; Takacs, Z.; Ordog, A.; Popovic, B.; Tari, I. Comparison of changes in water status and photosynthetic parameters in wild type and abscisic acid-deficient sitiens mutant of tomato (Solanum lycopersicum cv. Rheinlands Ruhm) exposed to sublethal and lethal salt stress. J. Plant Physiol. 2019, 232, 130–140. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [PubMed]
- Csiszar, J.; Horvath, E.; Vary, Z.; Galle, A.; Bela, K.; Brunner, S.; Tari, I. Glutathione transferase supergene family in tomato: Salt stress-regulated expression of representative genes from distinct GST classes in plants primed with salicylic acid. Plant Physiol. Biochem. PPB 2014, 78, 15–26. [Google Scholar] [CrossRef]
- Galle, A.; Csiszar, J.; Secenji, M.; Guoth, A.; Cseuz, L.; Tari, I.; Gyorgyey, J.; Erdei, L. Glutathione transferase activity and expression patterns during grain filling in flag leaves of wheat genotypes differing in drought tolerance: Response to water deficit. J. Plant Physiol. 2009, 166, 1878–1891. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar]
- Subramaniyam, S.; Bae, S.; Jung, M.; Shin, Y.; Oh, J.-H. The transcriptome data from the leaves of four Papaver species captured at the plant’s three developmental life cycles. Data Brief. 2020, 28, 104955. [Google Scholar] [PubMed]
- Montgomerie, S.; Cruz, J.A.; Shrivastava, S.; Arndt, D.; Berjanskii, M.; Wishart, D.S. PROTEUS2: A web server for comprehensive protein structure prediction and structure-based annotation. Nucleic Acids Res. 2008, 36, W202–W209. [Google Scholar] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar]
- Goujon, M.; McWilliam, H.; Li, W.; Valentin, F.; Squizzato, S.; Paern, J.; Lopez, R. A new bioinformatics analysis tools framework at EMBL–EBI. Nucleic Acids Res. 2010, 38, W695–W699. [Google Scholar] [CrossRef]
- Lotun, D.P.; Cochard, C.; Vieira, F.R.; Bernardes, J.S. 2dSS: A web server for protein secondary structure visualization. BioRxiv, 2019; preprint. [Google Scholar] [CrossRef]
- Bourguet, D.; Guillemaud, T. The hidden and external costs of pesticide use. Sustain. Agric. Rev. 2016, 19, 35–120. [Google Scholar]
- Czékus, Z.; Farkas, M.; Bakacsy, L.; Ördög, A.; Gallé, Á.; Poór, P. Time-dependent effects of bentazon application on the key antioxidant enzymes of soybean and common ragweed. Sustainability 2020, 12, 3872. [Google Scholar]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [PubMed]
- Grace, S.C.; Logan, B.A. Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2000, 355, 1499–1510. [Google Scholar] [CrossRef]
- Mathe, C.; Barre, A.; Jourda, C.; Dunand, C. Evolution and expression of class III peroxidases. Arch. Biochem. Biophys. 2010, 500, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, W.G.; Ketsa, S. Cross reactivity between ascorbate peroxidase and phenol (guaiacol) peroxidase. Postharvest Biol. Technol. 2014, 95, 64–69. [Google Scholar] [CrossRef]
- Edwards, R.; Dixon, D.P.; Walbot, V. Plant glutathione S-transferases: Enzymes with multiple functions in sickness and in health. Trends Plant Sci. 2000, 5, 193–198. [Google Scholar] [CrossRef]
- Droog, F. Plant glutathione S-transferases, a tale of theta and tau. J. Plant Growth Regul. 1997, 16, 95–107. [Google Scholar] [CrossRef]
- Monticolo, F.; Colantuono, C.; Chiusano, M.L. Shaping the evolutionary tree of green plants: Evidence from the GST family. Sci. Rep. 2017, 7, 14363. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallé, Á.; Farkas, M.; Pelsőczi, A.; Czékus, Z.; Kukri, A.; Dorner, Z.; Ördög, A.; Csiszár, J.; Bela, K.; Poór, P. Contribution of Glutathione Transferases in the Selective and Light-Dependent Effect of Flumioxazin on Winter Wheat (Triticum aestivum L.) and Its Typical Weed Common Poppy (Papaver rhoeas L.). Agronomy 2023, 13, 2053. https://doi.org/10.3390/agronomy13082053
Gallé Á, Farkas M, Pelsőczi A, Czékus Z, Kukri A, Dorner Z, Ördög A, Csiszár J, Bela K, Poór P. Contribution of Glutathione Transferases in the Selective and Light-Dependent Effect of Flumioxazin on Winter Wheat (Triticum aestivum L.) and Its Typical Weed Common Poppy (Papaver rhoeas L.). Agronomy. 2023; 13(8):2053. https://doi.org/10.3390/agronomy13082053
Chicago/Turabian StyleGallé, Ágnes, Máté Farkas, Alina Pelsőczi, Zalán Czékus, András Kukri, Zita Dorner, Attila Ördög, Jolán Csiszár, Krisztina Bela, and Péter Poór. 2023. "Contribution of Glutathione Transferases in the Selective and Light-Dependent Effect of Flumioxazin on Winter Wheat (Triticum aestivum L.) and Its Typical Weed Common Poppy (Papaver rhoeas L.)" Agronomy 13, no. 8: 2053. https://doi.org/10.3390/agronomy13082053
APA StyleGallé, Á., Farkas, M., Pelsőczi, A., Czékus, Z., Kukri, A., Dorner, Z., Ördög, A., Csiszár, J., Bela, K., & Poór, P. (2023). Contribution of Glutathione Transferases in the Selective and Light-Dependent Effect of Flumioxazin on Winter Wheat (Triticum aestivum L.) and Its Typical Weed Common Poppy (Papaver rhoeas L.). Agronomy, 13(8), 2053. https://doi.org/10.3390/agronomy13082053







