Abstract
The use of zinc oxide nanoparticles (ZnO NPs), applied as a possible micronutrient source, in conjunction with organic pesticides in agricultural soils has the potential to alter the environmental behavior and toxicity of these chemicals to soil biota. This research examines the joint effects of ZnO NPs and the herbicide metribuzin (MTZ) on phytotoxicity to plants, toxicity to soil microorganisms, and the accumulation of Zn and MTZ in plants. After 23 days, effects on growth, photosynthetic pigment content, and oxidative stress biomarkers in bean plants (Phaseolus vulgaris) and soil enzymatic activities were evaluated. Additionally, the amounts of Zn and MTZ (and the latter’s main metabolites) in soil and plant tissues were quantified. ZnO NPs reduced ammonium oxidase activity and growth among MTZ-stressed plants while reducing photosynthetic pigment levels and enhancing antioxidant enzymatic activities. MTZ had a marginal impact on the availability and accumulation of Zn in plant tissues, although significant effects were observed in some specific cases. In turn, ZnO NPs drastically affected MTZ degradation in soil and influenced MTZ accumulation/metabolization in the bean plants. Our findings indicate that the indirect effects of ZnO NPs, through their interaction with commonly used organic pesticides, may be relevant and should be taken into account in agricultural soils.
1. Introduction
The increasing importance of nanotechnology in the agricultural sector is evidenced by the development and promising results of nanoparticles (NPs) in various applications. These include the supply of pesticides, fertilizers, and genetic material for plant transformation. Among the widely used NPs, ZnO NPs are extensively used in pharmaceuticals, cosmetics, textiles, electronics, and other industries, including the agricultural and food sectors [1].
ZnO NPs are being explored for their potential use as pesticides or fertilizers in agricultural formulations [2,3]. Although the agricultural use of ZnO NPs is promising, a better understanding of their potential environmental hazards is needed [4,5], especially regarding their interaction with other agricultural products in the soil. The introduction of ZnO NPs into the soil through the application of contaminated amendments or the use of pesticides or fertilizers containing ZnO NPs together with uncontrolled releases may lead to the coexistence of ZnO NPs with other agricultural products, such as pesticides. The use of pesticides has been a primary area of development in the agricultural industry in recent decades. Metribuzin (MTZ, 4-amino-6-tert-butyl-3-methylthio-1,2,4-triazin-5(4H)-one) is a systemic selective herbicide of the triazine class that inhibits DNA synthesis in target weeds and acts on photosystem II, ultimately inhibiting photosynthesis. As a result, MTZ provides good control of important annual grasses and broadleaf weeds. It is a widely used herbicide in agricultural production worldwide and is usually applied during pre- and post-emergence periods. MTZ’s extensive and sometimes unjustified use has led to various agri-environmental problems related to soil and water contamination, which have been reported by several authors [6,7,8]. MTZ residues can be toxic to freshwater macrophytes and algae and also cause physiological disorders in higher animals due to endocrine disruption. Soil microbial populations and their activities may also be affected by the residues of this herbicide. In addition, MTZ residues in soil can significantly affect the germination of successive crops. The coexistence of such different molecules (ZnO NPs and MTZ) in agricultural soils may modify the environmental fate and toxic effects of the individual chemicals on the soil biota. Joint toxicity assessments have so far focused on hydroponic cultures [9,10,11], although the interactions and effects of such mixtures on soil are much more complex. Soil components can interact with contaminants to affect their availability and toxicity, as well as root uptake and transport in plants [1,12]. Moreover, soil properties can play an important role in the degradation of organic contaminants. If the availability or the persistence of the herbicide increases, it can affect the current and subsequent crops. Therefore, studies conducted in natural soils with low relevant concentrations of NPs may show different effects than those reported in the literature. However, studies in soil media are scarce, and most have been conducted at high NPs concentrations [13,14].
The mechanisms of interaction between two compounds in a mixture are complex and not clearly understood. One chemical may affect the availability of another, hence affecting the accumulation. Josko et al. [15] studied the behavior of NP mixtures of CuO and ZnO in soil and found that extractable concentrations of Cu and Zn decreased compared to single exposure in soil. Interactions can also alter the uptake and internalization of co-contaminants in plant cells [16]. In addition, NPs can interfere with the metabolic processes associated with the mechanisms of action or tolerance or inhibit the detoxification systems of another chemical, leading to higher than expected toxicity [17]. Another possible mechanism of joint action between two compounds could be their ability to alter the degradation of parent compounds or facilitate degradation to more reactive metabolites. Several authors have investigated the contribution of NPs to the accumulation of metals and organic compounds in plants as well as their toxicity, generally in soilless media. Metallic NPs have been shown in a number of studies to significantly reduce the uptake and accumulation of organic compounds in plants. For example, Ag NPs decreased dichlorodiphenyldichloroethylene (DDE) in soybean and zucchini [18], and ZVI (zero-valent iron) NPs reduced quinclorac in rice shoots and roots [19]. In turn, coexistence with other compounds may affect the uptake and accumulation of NPs in plants. For example, kinetin reduced Cu in roots and increased Cu in the leaves of bean plants exposed to Cu NPs [20]. However, the potential effect of NPs on the metabolism of organic compounds and the accumulation of degradation products in plants has hardly been studied [21].
Biomarkers can provide information about the mechanism of the toxic action of chemicals. Increased levels of reactive oxygen species (ROS) can cause oxidative stress in organisms, leading to cellular damage, e.g., in the form of lipid peroxidation. To protect cells from the harmful effects of ROS stress, plants activate their antioxidant defense system. The use of biomarkers related to oxidative stress has allowed some authors to observe joint effects on plants due to co-exposure to NPs and metals [10,14] or organic compounds [11,22].
On the other hand, most NP toxicity studies on soil microorganisms have generally only addressed the impacts of single NPs. Some studies have shown that the presence of NPs can enhance or mitigate the toxicity of other contaminants compared to single exposure [23,24]. Soil enzymes are involved in the cycling of nutrients and organic matter breakdown; therefore, they can be used as indicators of changes in soil quality caused by co-exposure to NPs and other contaminants. In addition, plants can modify soil properties and strongly influence the toxicity of contaminants to soil microorganisms. Soil–plant systems help to simulate complex natural scenarios and thus provide more relevant information. However, studies on the effects of interactions between NPs and other contaminants on soil microorganisms using these more complex systems are scarce [23,24].
The working hypothesis of this study was that significant interactions may occur between ZnO NPs and MTZ when they coexist in agricultural soil. These interactions could modify the toxicity of these compounds as well as their fate or behavior in soil and plants. Accordingly, the objectives of this work were as follows: (i) to explore the impact of the co-presence of ZnO NPs at low concentrations and MTZ in soil on the toxicity to plants and soil microorganisms, (ii) to evaluate the influence of co-exposure on the accumulation and translocation of Zn and MTZ in plants, and (iii) to determine the effects of ZnO NPs on the metabolism of MTZ in soil and plants.
To complete these objectives, the biological effects of ZnO NPs and MTZ on bean (Phaseolus vulgaris var. contender) and soil microorganisms were initially evaluated individually and subsequently in mixtures. Representative parameters such as changes in growth, photosynthetic pigment content, and those related to plant oxidative stress and soil enzymatic activities were measured.
In order to assess the influence of ZnO NPs on the levels of the main MTZ degradation products in soils and plants, the analysis of these products was of particular interest. According to the literature, the most relevant metabolites of MTZ in soil and plants are desamino-metribuzin (DA-M), formed by the elimination of the amino-group; diketo-metribuzin (DK-M), derived from the elimination of the S-methyl-group and oxidation; and desamino-diketo-metribuzin (DADK-M), derived from DA-M and DK-M [25].
2. Materials and Methods
2.1. Soil Properties, Chemicals and Reagents
Topsoil (0–20 cm) from an agricultural field located in the “Comunidad de Madrid” (Spain) at GPS coordinates 40°27′20.2″ N 3°44′56.4″ W was air-dried and sieved (2 mm mesh). The soil’s characteristics were as follows: 7.8% clay, 18.8% silt, 73.4% sand, pHw (1:2.5) 7.4, and 1.9% organic matter. The concentrations of MTZ and Zn in the soil were <limit of detection (LOD) and 37 ± 5 mg/kg soil dry weight (DW), respectively. The concentration of Zn extracted from the soil (12 ± 1 mg/kg, DW) with the reagent diethylenetriaminepentaacetic acid-triethanolamine (DTPA-TEA) was sufficient for the correct development of any crop [26].
Standards of metribuzin and its metabolites (purity ≥ 99%) were provided by the supplier, Dr. Ehrenstorfer (Augsburg, Germany). SENCOR, a commercial preparation (600 g of MTZ per liter), was purchased from J.V.MONTALVO SL (Madrid, Spain) and used to add metribuzin to the soil.
The ZnO NPs (particle size < 100 nm and specific surface area of 15–25 m2/g) were supplied by Sigma-Aldrich (Stenheim, Germany). The morphology and particle size of the ZnO NPs were determined via transmission electron microscopy (TEM) at the ICTS Centro Nacional de Microscopía Electrónica as previously described [27]. The TEM images revealed that the ZnO NPs had an elongated shape with a mean length for the longer dimension of 55 ± 27 nm (median = 35 nm).
2.2. Experimental Design
The following treatments were applied to the soil: ZnO NPs, MTZ, or a mixture of both. ZnO NPs were added as a dry powder at 0, 20, and 50 mg/kg soil (DW, Zn basis) and were thoroughly stirred by hand. SENCOR was added to a soil subsample to give a MTZ concentration of 80 mg/kg soil. The MTZ soil was then diluted with control soil to give MTZ concentrations of 0, 2, and 3 mg/kg soil DW. After the addition of the test compounds, the soil was homogenized by sieving (2 mm mesh) and thoroughly mixed by hand shaking. These MTZ concentrations were chosen on the basis of the effects observed in a preliminary assay performed with MTZ at concentrations ranging from 0.5 to 4 mg/kg. In this range-finding test, concentrations below 2 mg/kg had no effect on germination, growth, and the biochemical parameters of the bean plants, and the highest concentration (4 mg/kg) caused plant mortality throughout the test period. Four binary mixtures were designed: 2 mg/kg of MTZ + 20 mg/kg of ZnO NPs; 2 mg/kg of MTZ + 50 mg Zn/kg of ZnO NPs; 3 mg/kg of MTZ + 20 mg/kg of ZnO NPs; 3 mg/kg of MTZ + 50 mg/kg of ZnO NPs. Before sowing, the treated soils were allowed to stabilize for 24 h in the dark at 20 °C.
The control (no added Zn or MTZ) and treated soils (600 g, DW) were placed in pots; four common bean seeds (Phaseolus vulgaris L. cv. Contender) were sown in each pot. A total of 27 plastic pots (12 cm wide and 10 cm high) were used with three replicates of each treatment in a randomized design. The pots were irrigated with dechlorinated water until 75% of the water holding capacity (WHC) of the soil was reached, corresponding to a total moisture content of 45%. Moisture was checked by weight every three days. The soil Zn and MTZ concentrations were measured one day after treatment to determine the initial soil concentrations. The pots were grown in a climate-controlled room (20 ± 2 °C) under a 16:8 h (light/dark) photoperiod. The shoots and roots were harvested separately, and the fresh weight of the aerial parts was recorded after 23 days. This is the period when the foliage of the plant is at its maximum. The bean plants developed their third triflorate leaves at node 5 (V3), and the trial finished just before pre-flowering (R1). They were then washed twice with deionized water. To remove the Zn adsorbed on the root surface, the roots were ultrasonicated (35 kHz, 15 min) with a solution of ethylenediaminetetraacetic acid (EDTA) (0.010 M, pH 6).
The different parts of the plants (root, stem, and leaf) were separated and distributed in groups. In the first group, the plant components were lyophilized, ground, and kept at −20 °C until MTZ analysis. In the second group, the components were dried at 60 °C for 24 h for Zn analysis. A subsample of the plant leaves was cut and stored in aliquots at −80 °C until biochemical analysis.
2.3. Zinc Concentration in Soil and Plant Tissues
For the determination of total Zn content in soil and plant tissues, the samples were digested in a microwave oven using an acid mixture of HNO3:HF:ultrapure water (1:1:1, v/v/v) and HNO3:HCl:ultrapure water (1:1:1, v/v/v), respectively. Details of the methods can be found in García-Gómez et al. [28].
The availability of Zn in soil to the common bean was assessed using a low-molecular-weight organic acids solution (LMWOAs): 2 g of soil in 20 mL of a mixture of acetic, lactic, citric, malic, and formic acids in a molar ratio of 4:2:1:1:1, respectively [29].
The DTPA-TEA extraction method was applied to determine the potentially available Zn in the control soil [26]. Extraction was performed with a combined solution of 5 mM DTPA, 0.01 M CaCl2, and 0.1 M TEA at pH 7.3, with the soil/reagent ratio in the solution being 10:20 (w:v).
All procedures were performed in triplicate. The Zn concentration in the different extracts from plants and soils was determined by flame atomic absorption spectrometry (FAAS) using a spectrometer AAnalyst 800 (Perkin Elmer, Waltham, MA, USA), which was equipped with a deuterium lamp, Zn-hollow cathode lamp, and air-acetylene burner. The working range using this technique typically spans from 0.02 to 1.0 mg Zn/L. For the determination of Zn in such extracts, graphite furnace atomic absorption spectrometry (GF-AAS) is not usually suitable as the detection limits in the main absorption line for this element are in the sub-μg/L range.
2.4. MTZ and Metabolites Concentration in Soil and Plant Tissues
Soil concentrations of MTZ and its metabolites were measured on days 1 and 23, whereas their concentrations in bean plants were determined in plants collected after 23 days. The analytical methods were developed by our group [30]. Briefly, the extraction of MTZ and its metabolites was carried out by ultrasound-assisted extraction followed by GC-MS/MS determination. For both matrices, the solvent was ethyl acetate, although, for plants, a cleanup step by dispersive solid-phase extraction was necessary.
2.5. Biochemical Analysis of Plant Leaves
Levels of chlorophyll a (CHLa) and chlorophyll b (CHLb), carotenoids, ROS, malondialdehyde (MDA), proteins, and enzymatic activities (catalase (CAT), ascorbate peroxidase (APX), and guaiacol peroxidase (GPOD)) in the fresh leaves were determined as previously described [31]. Analyses were performed in duplicate for the replicates of each treatment or control (n = 6), and the details are provided in the Supplementary Materials (Text S1).
2.6. Enzymatic Activity in Soil
Dehydrogenase (DH) and phosphatase activities were measured as previously described [32]. Details are provided in the Supplementary Materials (Text S2).
Ammonium oxidation in the soil was determined by nitrite production using ammonium ions as substrate [33]. Fresh soil (2 g, DW) was amended with 8 mL of 1 mM (NH4)2SO4 and 40 µL of 1.5 M sodium chlorate, which was added to inhibit nitrite oxidation during incubation. After an incubation period of 5 h at 25 °C, the released nitrite was extracted with 2 mL of 2 M KCl. The nitrite concentration in the supernatant was determined colorimetrically at 520 nm after the addition of the reactive solution (0.25 g of sulfanilamide, 12.5 mg of N-(1-naphthyl)-ethylenediamine hydrochloride, and 2.5 mL of H3PO4 in 25 mL of water). Ammonium oxidase activities were calculated as µg of nitrite-N released per g dry soil for 5 h.
The determination of urease activity was based on the determination of ammonium released from urea-treated soil [34]. Fresh soil (1 g, DW) was treated with urea (20 mg) and incubated for 2 h at 37 °C. Ammonium was extracted with 5 mL of 1 M KCl and analyzed via a reaction with phenol nitroprusside solution (2.67 g of NaOH, 7 g of phenol and 0.034 g of sodium nitroprusside in 100 mL of water), which forms a green-colored complex in the presence of an oxidant reactive (1.5 g of NaOH, 6.10 g of K2HPO4, and 4.25 mL of NaClO 10% (w/v) in 100 mL of water). The developed color was measured at 636 nm. The urease activity was expressed as µg ammonium-N released per g dry soil for 2 h.
2.7. Statistical Analysis
STATGRAPHICS software (version XVII) was used for the statistical analysis of the data. Chemical and toxicological data were statistically analyzed using one-way analysis of variance (ANOVA) with Fisher’s least significant difference procedure (LSD, p < 0.05) for each concentration of MTZ and for each concentration of ZnO NPs in the analysis of Zn in soil and plants. Two-way analysis of variance (ANOVA) was used to determine the significance of the variable ZnO NP and MTZ concentrations and their interactions in the measured parameters. The LSD test at a 5% confidence level was used to analyze the pair-wise differences and multiple comparisons between the study groups.
The bioconcentration factor (BCF) was calculated to evaluate the distribution of Zn and MTZ between the soil and biota. BCF values were calculated as the ratio of the measured concentrations of the chemical in the plants (root, stem, and leaf) to that in the soil; all these measurements were related to dry weight. The translocation factor (TF), calculated as the ratio of Zn and MTZ concentrations (on a DW basis) in the different tissues, was used to determine the capacity to translocate Zn and MTZ between the different plant parts (root to stem and stem to leaf).
The relationship between the Zn and MTZ concentrations in the soil and plant parts and the toxicological data was determined using Pearson correlation analysis. Only significant analyses with a regression fit greater than R2 of 0.75 are presented.
3. Results
3.1. Initial Soil Zn and MTZ Concentrations and Soil pH
Our chemical analysis showed that the Zn concentrations in the treated soils were 55 ± 2 and 84 ± 3 mg/kg for the 20 and 50 mg/kg soil treatments, respectively. The initial MTZ concentration in the soil samples, calculated as the mean from the single and combined assays after 24 h, was 2.2 ± 0.1 and 3.0 ± 0.3 mg/kg for the nominal treatments of 2 and 3 mg/kg soil, respectively.
One day after treatment, the pHw (1:2.5) of the control and treated soils was 7.36 ± 0.03, with no differences between treatments. During the experiment, the pH of the control soil and soils treated with ZnO NPs alone decreased by about 0.3 pH units (Supplementary Materials Table S1). Single or combined treatments with MTZ showed pH values similar to the initial values, except for the treatment with 2 mg/kg of MTZ and 50 mg/kg of ZnO NPs, where a slight increase in pH was observed. After 23 days, significant differences were found between the control soil and all soils containing MTZ (p < 0.05), but the maximum difference was 0.5 pH units. The addition of MTZ significantly increased soil pH in the ZnO NPs treatments.
3.2. Zn Concentration in Soil and Plants
The potentially available Zn concentrations for bean plants as a result of the soil treatment applied are summarized in Figure 1A. The application of ZnO NPs significantly increased the concentration of phyto-available Zn in the soil, and this increase was also dependent on the dose of Zn applied (p = 0.0001). The addition of MZT to soils treated with ZnO NPs had no effect on Zn availability, except for the mixture containing 50 mg/kg of ZnO NPs and 3 mg/kg of MTZ (p = 0.0483).
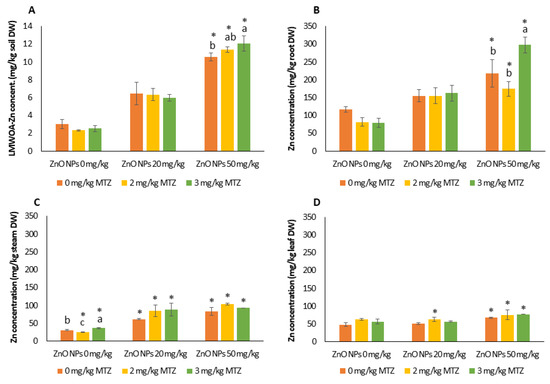
Figure 1.
Data on the available Zn soil concentration determined via the LMOWA extraction method (A) and Zn concentration in bean plants (root (B), stem (C), and leaf (D)) grown in control soil (no Zn or metribuzin (MTZ) addition) and in soils spiked with ZnO NPs, MTZ, and a ZnO NPs/MTZ combination at the time of harvest (23 days). Error bars represent the mean ± SD of the three replicates of each treatment or control performed in triplicate (n = 3 × 3). * indicates a significant difference from the control (LSD test, p < 0.05). Different letters indicate significant differences (LSD test, p < 0.05) in each ZnO NP treatment (0, 20, and 50 mg/kg of Zn). Within each treatment group, columns without letters indicate no significant difference.
Zinc mainly accumulated in the roots, and the translocation factors were low (TF < 1), regardless of the treatment. Two-way ANOVA analysis showed that the Zn concentration in the different plant parts was significantly influenced by the addition of ZnO NPs (p < 0.001). The Zn levels in roots and leaves were also affected by MTZ (p < 0.05) and, in the case of roots, by the interaction between them (p = 0.0034).
Our analysis of Zn in the roots (Figure 1B) and leaves (Figure 1D) generally showed only significant differences between the control soil and the soil treated with the highest Zn concentration. In stems, increased Zn levels were observed in plants exposed to single and combined treatments at both exposure levels of ZnO NPs (Figure 1C). The application of MTZ at 3 mg/kg significantly increased root Zn accumulation (p = 0.039), ranging from 217 ± 39 in the individual treatment to 298 ± 23 mg/kg in the combined treatment.
3.3. Concentration of MTZ and Its Metabolites in Soil
In the single exposure to MTZ, the concentrations of parent and metabolites in soil were independent of the doses of MTZ applied, except for DADK-M, which showed a higher concentration at 2 mg/kg MTZ dose (p = 0.0031). Based on the two-way ANOVA results, the levels of MTZ and its metabolites in soil varied with the concentration of ZnO NPs added but were independent of the MTZ concentration added. The levels of MTZ and DADK-M were significantly influenced by the interactions between the two variables (Table 1).

Table 1.
Significance levels (p-value) of the effects of ZnO NPs, metribuzin (MTZ,) and their interactions (ZnO NPs × MTZ) on bean plant growth and biochemical parameters on soil enzymatic activities and on the concentration of MTZ and its metabolites (desamino-metribuzin (DA-M), diketo-metribuzin (DK-M), and desamino-diketo-metribuzin (DADK-M)) in soil and bean plants (root, stem, and leaf) based on two-way ANOVA analysis. Data were obtained from a soil–plant test performed on soil spiked with ZnO NPs (0, 10 and 50 mg/kg), MTZ (0, 2, 20 mg/kg), and their mixtures for 23 days; NS: not significant.
The concentrations of MTZ and DADK-M were the most affected by ZnO NPs (Figure 2). At the end of the assay, MTZ residue levels in the soil were higher in the ZnO NP-co-exposed soil than in the group solely treated with MTZ. This was particularly noticeable in the 2 mg/kg MTZ treatment, where MTZ residues increased by two- and three-fold with increasing levels of ZnO NPs (Figure 2A). In contrast, the soil concentration of DADK-M decreased in the treatments containing ZnO NPs (Figure 2D). The DADK-M concentration for the mixture of 2 mg/kg MTZ with ZnO NPs 20 and 50 mg/kg, was about 1% and 14% of the value measured in the individual treatment with MTZ at 2 mg/kg, respectively. Likewise, the DADK-M concentration decreased at values of 47% and 31% of the concentration measured in the individual treatment with 3 mg/kg of MTZ in the presence of 20 and 50 mg/kg of ZnO NPs.
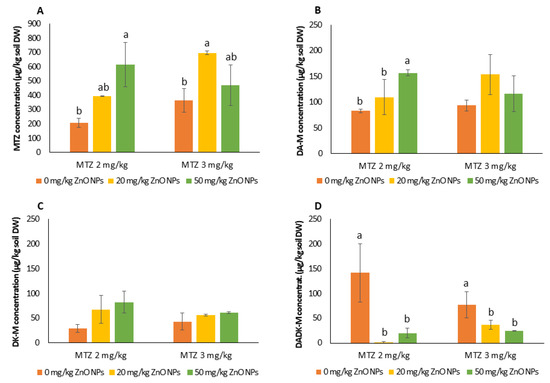
Figure 2.
Levels of MTZ (A) and its metabolites (desamino-metribuzin (DA-M) (B), diketo-metribuzin (DK-M) (C), and desamino-diketo-metribuzin (DADK-M) (D)) in soils spiked with MTZ and ZnO NPs/MTZ mixtures at the end of the assay (23 days). Error bars represent the mean ± SD of the three replicates of each treatment performed in triplicate (n = 3 × 3). Different letters indicate significant differences (LSD test, p < 0.05) in each MTZ treatment (2 and 3 mg/kg of MTZ). Within each treatment group, columns without letters indicate no significant difference.
3.4. Concentration of MTZ and Its Metabolites in Plants
MTZ was predominantly accumulated in plant leaves, whether exposed individually or in combination, in the following order: MTZleaf > MTZstem > MTZroot (Figure 3). MTZ was the main product, and at lower concentrations, its metabolites were mainly found in the stem. The influence of ZnO NPs, MTZ, and a mixture of both on the concentration of MTZ and its metabolites in plants based on two-way ANOVA analysis is shown in Table 1. In general, significant differences were observed in the concentration of MTZ and its metabolites in the roots and leaves depending on the MTZ dose applied but not on the ZnO NP concentration, whereas in the stems, ZnO NPs affected the measured concentrations for all with the exception of DADK-M. The interaction between MTZ and ZnO NPs significantly affected the concentration of MTZ and DA-M in the roots and of DADK-M in the stems and leaves.
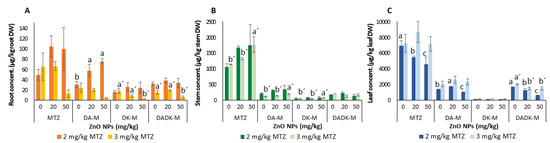
Figure 3.
Levels of MTZ and its metabolites (DA-M, DK-M, and DADK-M) in bean plants (root (A), stem (B), and leaf (C)) grown in soils spiked with MTZ and ZnO NPs/MTZ mixtures at the end of the assay (23 days). Error bars represent the mean ± SD of three replicates of each treatment performed in triplicate (n = 3 × 3). Different letters indicate significant differences (LSD test, p < 0.05) in each MTZ treatment (2 and 3 mg/kg of MTZ). Within each treatment group, columns without letters indicate no significant difference.
The concentration of these chemical compounds in the bean plant roots was always higher in the plants grown in soils treated with 2 mg/kg of MTZ than in those treated with 3 mg/kg of MTZ (Figure 3). The presence of ZnO NPs significantly increased root DA-M levels in plants exposed to 2 mg/kg of MTZ, whereas DK-M and DADK-M concentrations decreased in plants treated with 3 mg/kg of MTZ at the highest Zn level (Figure 3A) compared to treatment with MTZ alone. Combined and individual MTZ treatment showed significant differences for MTZ, DA-M, and DK-M contents in the stems of the plants grown in soils treated with 3 mg/kg of MTZ, which were significantly higher for mixtures containing 50 mg/kg of ZnO NPs (Figure 3B). For treatments with 2 mg/kg of MTZ, the content of MTZ in the bean leaves decreased with the increase in NPs. The effect of ZnO NPs on the DA-M concentration in plants exposed to 2 mg/kg of MTZ varied depending on concentration, increasing and decreasing for mixtures containing 20 mg/kg and 50 mg/kg of ZnO NPs, respectively. The DADK-M concentration in bean leaves exposed to 2 and 3 mg/kg of MTZ decreased in the presence of ZnO NPs at both levels (p = 0.0000) (Figure 3C). At all doses, the DK-M concentration in leaves was not affected by the co-exposure of MTZ and ZnO NPs.
BCF and TF were calculated to further investigate the effects of ZnO NPs on MTZ translocation (Supplementary Materials Table S2). The addition of ZnO NPs did not affect TF from root to stem at any concentration of MTZ but reduced TF from stem to leaf in bean plants exposed to 2 mg/kg of MTZ. BCFleaf/soil for MTZ decreased markedly with the addition of the NPs (p < 0.05). At 2 mg/kg of MTZ, the BCFleaf/soil values decreased from 34 ± 1 in the individual MTZ treatment to 14 ± 1 and 9 ± 3 for mixtures containing 20 and 50 mg/kg of ZnO NPs, respectively. For the 3 mg/kg MTZ treatments, the differences were smaller, and BCFleaf/soil for MTZ decreased from 24 ± 1 in the individual MTZ treatment to 13 ± 1 and 16 ±4 when 20 and 50 mg/kg of ZnO NPs were added, respectively.
3.5. Plant Toxicity
3.5.1. Germination and Plant Growth
Exposure of plants to MTZ and ZnO NPs had no effect on plant germination compared to the control in the individual and combined assays. In the individual experiments, ZnO NPs did not affect plant growth at any concentration tested (Figure 4A). In contrast, MTZ inhibited plant growth by 24 ± 2% and 36 ± 3% compared to the control at concentrations of 2 and 3 mg/kg, respectively. Two-way analysis of variance revealed a significant effect of ZnO NPs on plant weight, which decreased after treatment with NPs compared to treatment without NPs (p = 0.0236) (Table 1).
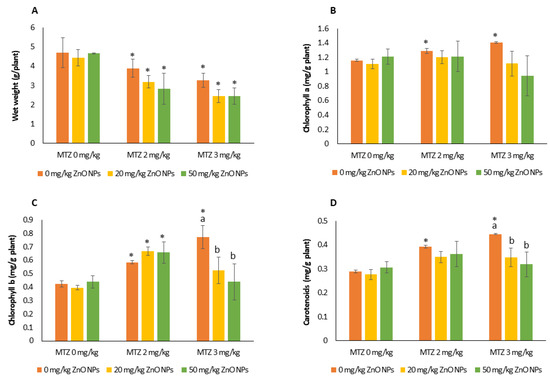
Figure 4.
Fresh weight of seedlings (A) and concentrations of photosynthetic pigments (chlorophyll a (B), chlorophyll b (C), and carotenoids (D) in the leaves of bean plants grown for 23 days in control soil (no Zn or MTZ addition) and soils spiked with ZnO NPs, MTZ, and ZnO NPs/MTZ combined at different concentrations. Error bars represent the mean ± SD of three replicates of each treatment or control performed in duplicate (n = 3 and n = 3 × 2, for growth and photosynthetic pigments, respectively). * Indicates significant difference from the control (LSD test, p > 0.05). Different letters indicate significant differences (LSD test, p < 0.05) within each MTZ treatment (0, 2, and 3 mg/kg of MTZ). Within each treatment, columns without letters indicate no significant difference.
3.5.2. Photosynthetic Pigments
In the single assay, the level of photosynthetic pigments (Figure 4B–D) was unaffected by ZnO NPs, whereas MTZ dose-dependently increased the chlorophyll and carotenoid contents (p < 0.05) and decreased the CHLa/b ratio compared to the control (p = 0.0027). The increase in CHLb was greatest at the highest MTZ concentration tested, being 64 ± 5% over the control soil.
In the combined treatments with MTZ at 3 mg/kg, the addition of ZnO NPs significantly decreased the CHLb (p = 0.0221) and carotenoid content (p = 0.0477) in relation to the individual exposure to MTZ. Multifactorial analysis showed that the CHLb and the CHLa/b ratio were influenced by MTZ and the interactions between ZnO NPs and MTZ at p < 0.05. The carotenoid content was influenced by the three aforementioned variables: the concentration of MTZ and ZnO NPs and their interaction (Table 1).
3.5.3. Oxidative Stress-Related Parameters
Compared to the control, the treatments tested did not alter protein levels in the plants, but some significant changes were observed in ROS generation, antioxidant enzyme activity, and MDA levels (Figure 5). Two-way ANOVA showed the significant effects of MTZ and ZnO NPs on the biomarkers of oxidative stress (except for ROS) and for MDA and CAT, respectively. The interaction of ZnO NPs and MTZ significantly affected GPOD and APX activities and MDA levels at p < 0.01 (Table 1).
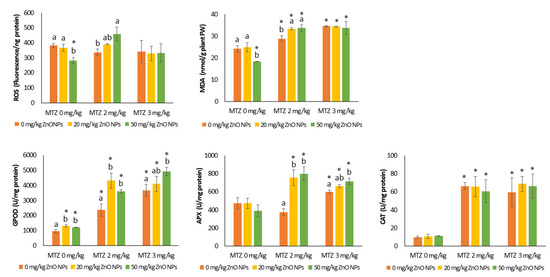
Figure 5.
Levels of reactive oxygen species (ROS) and malondialdehyde (MDA) and enzymatic activities (guaiacol peroxidase (GPOD), ascorbate peroxidase (APX), and catalase (CAT)) in the leaves of bean plants grown in control soil (no Zn or MTZ addition) and soil spiked with ZnO NPs, MTZ, and ZnO NPs/MTZ mixtures at the time of harvest (23 days). Error bars represent the mean ± SD of the three replicates of each treatment or control performed in duplicate (n = 3 × 2). * Indicates significant difference from the control (LSD test, p < 0.05). Different letters indicate significant differences (LSD test, p < 0.05) within each MTZ treatment (0, 2, and 3 mg/kg of MTZ). Columns without letters indicate no significant difference within each treatment group. Note: For GPOD and APX, one unit of enzymatic activity was defined as the amount of enzyme that caused a change of 0.1 absorbance units per minute per mg of protein. One unit of CAT activity corresponded to 1 μmol of H2O2 consumed per minute per mg of protein using an extinction coefficient of 40 M−1 cm−1.
ZnO NPs reduced ROS and MDA levels in bean leaves at the highest Zn concentration tested and induced GPOD activity at both Zn concentrations. In plants growing in soils treated with MTZ alone, the levels of enzymatic activity and MDA increased markedly at both exposure levels, except for APX activity in the treatments with 2 mg/kg of MTZ. These effects were dose-dependent, except for the inhibition of CAT activity (p < 0.001) (Figure 5).
In the combined assay, the presence of ZnO NPs did not alter CAT activity, whereas an increase in APX and GPOD activities was observed compared to single exposure to MTZ. This effect was especially notable in the treatments with 2 mg/kg of MTZ, where the addition of ZnO NPs at 20 and 50 mg/kg increased APX activity by 61 ± 8% and 70 ± 9%, respectively, compared to the plants grown in soils treated with MTZ alone (Figure 5). Similarly, the accumulation of peroxidation products in leaves increased in the presence of ZnO NPs in the treatments with 2 mg/kg of MTZ, whereas this did not cause any changes in the MDA levels of the plants grown in soils treated with the highest concentration of MTZ.
3.6. Enzymatic Activity in Soil
Urease activity was not significantly affected by the addition of ZnO NPs or MTZ in either the individual or combined assays. DH activity decreased in all treatments, with inhibition ranging from 23 to 39%. However, the differences were not significant, probably due to the high variability in the control samples (p = 0.0515) (Figure 6A). Based on the two-way ANOVA results, the activity of ammonium oxidase was significantly influenced by the three variables, whereas phosphatase activity was significantly influenced by MTZ and the interaction between MTZ and ZnO NPs but not by ZnO NPs (Table 1). Single exposure to MTZ caused significant changes (p = 0.0020) in ammonium oxidase activity compared to the control soil (Figure 6B), but these were always less than 10%. In the co-exposure experiments, the addition of ZnO NPs at both exposure levels significantly reduced ammonium oxidase activity at each MTZ level (p < 0.05).
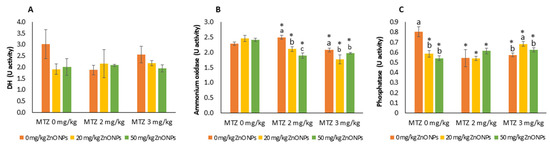
Figure 6.
Dehydrogenase (A), ammonium oxidase (B), and phosphatase (C) activities in control soil (without the addition of Zn or MTZ) and in soils spiked with ZnO NPs, MTZ, and ZnO NPs/MTZ combined after 23 days of exposure. Error bars represent the mean ± SD of three replicates of each treatment, where each treatment or control was performed in duplicate (n = 3 × 2). * indicates significant difference from the control (LSD test, p < 0.05). Different letters indicate significant differences (LSD test, p < 0.05) within each MTZ treatment (0, 2 and 3 mg/kg of MTZ). Within each treatment group, the columns without letters indicate no significant difference. Note: DH activity was calculated as µg of TPF formed per g of dry soil during 24 h; phosphatase activity was calculated as µg of MU formed per g dry soil; ammonium oxidase activity was calculated as µg of nitrite-N released per g dry soil for 5 h.
Single exposure to ZnO NPs and MTZ decreased soil phosphatase activity compared to the control (Figure 6C). The mixtures of MTZ and ZnO NPs inhibited phosphatase activity at the same or lower levels than the individual compounds.
3.7. Correlation Analysis
Pearson correlation analysis of the data showed that soil MTZ concentration at the end of the experiment was negatively correlated with ammonium oxidase activity (R2 = 0.90, p = 0.004) and with plant growth (R2 = 0.75, p = 0.0258). A strong relationship was also found between plant growth and some oxidative stress parameters. The loss of plant weight correlated with the increase in enzymatic activities—especially GPOD activity (R2 = 0.92, p = 0.0000)—and MDA levels (R2 = 0.83, p = 0.0007).
4. Discussion
4.1. Zn Concentration in Soil and Plants
Zinc accumulated mainly in the roots, with TF values always lower than 1, indicating the low capacity of bean plants to transfer Zn applied as ZnO NPs from the root to the above-ground plant parts [31]. It is known that organic compounds can influence the accumulation of metal-based NPs in soil [20]. In our study, MTZ had a marginal effect on Zn availability, accumulation, and translocation in plant tissues, although significant effects were observed for combined exposure to 3 mg/kg of MTZ and 50 mg/kg of ZnO NPs. This treatment increased the LMWOA-Zn extractable concentration in soil, which was associated with an increase in Zn content in the roots of the bean plants. Although ZnO NPs and metal ions can coexist in soil, the uptake, translocation, and accumulation of Zn in plants occur mainly as Zn ions [35].
The increase in Zn availability in the soil treated with MTZ was probably due to the dissolution of the ZnO NPs. In this study, soil pH was higher in the combined treatment group than in the group solely treated with ZnO NPs. In line with this, the availability of ZnO NPs [31] and the Zn solubility [36] should have been reduced. However, the presence of MTZ did not affect Zn availability in the treatments with low ZnO NP concentration and increased it in the treatments with high ZnO NP concentration. Zinc ions released from ZnO NP dissolution are subject to sorption processes in the soil that reduce the Zn concentration in the soluble fraction [37]. Previous studies have shown that organic compounds such as perfluoroalkyl substances contribute to an increase in free metal ions in soil solution [38]. Therefore, the increased available concentration of Zn in treatments with 50 mg/kg of ZnO NPs with the increase in MTZ could be attributed to MTZ helping to stabilize the free metal ions in solution, thus counteracting Zn adsorption to the soil components.
4.2. Concentration of MTZ and Its Metabolites in Soil
One of the most prominent observations from this study was the effect of ZnO NPs on the degradation rate of MTZ in soil. The remaining concentration of MTZ increased drastically with the addition of ZnO NPs, especially at low MTZ doses, indicating that MTZ was less degradable in the presence of ZnO NPs. The concentration of DADK-M in soil was also greatly reduced by the addition ZnO NPs, suggesting that ZnO NPs primarily inhibited the degradation of the DK-M or DA-M intermediates. MTZ decomposes in soil primarily through microbial degradation [25].
Several factors could explain the slower degradation rate. MTZ could become biologically unavailable to the microorganisms responsible for MTZ degradation due to adsorption on the NP surface. On the other hand, the combined addition of MTZ and ZnO NPs could induce changes responsible for the biodegradation of MTZ in the soil microbial communities, which could contribute, at least in part, to the increase in the residual concentration of MTZ in the soil [39,40]. Previous studies in the literature have shown that the addition of Cu NPs (500 and 1500 mg/kg) [23] and Cu(OH)2 NPs (5 and 50 mg/kg) [41] can mitigate the degradation of triazine and thiacloprid, respectively. The authors of these studies attributed the delayed degradation of the pesticides to changes in the microbial community rather than to changes in the availability of the organic compounds. In this study, a reduction in the biological availability of MTZ due to its adsorption on the NP surface cannot be excluded, although the observed changes in soil enzyme activities seem to indicate changes in the microbial community.
4.3. Concentration of MTZ and Its Metabolites in Plants
Another important unexpected result was that the presence of ZnO NPs strongly determined the concentration of MTZ and its metabolites in plants. In addition to the type of exposure (single or combined), the application rate and the plant tissue were also important factors for their accumulation in plants.
The MTZ applied to the soil was absorbed through the pore-water by the roots of the plants and translocated to the aerial parts through the xylem with the flow of water through transpiration. Metabolites in plants could result from the degradation of the parent compound in soil and the subsequent uptake of metabolites by plants or from the uptake of MTZ from soil and further degradation in plants [25]. Although, the dominant MTZ-based species in plant tissues was always the parent compound, the metabolic profile in roots was different from that in stems and leaves. In addition, ZnO NPs significantly increased the levels of the pool of MTZ and its metabolites in the roots of the plants. Previous studies in hydroponic media have generally reported a reduced accumulation of organic compounds due to the addition of NPs [18,19]. In most cases, the effects were due to the reduced availability of organic compounds because of sorption to NPs and removal from the media. Uwizeyimana et al. [42] showed that some herbicides, such as atrazine, could potentially form complexes with metal ions and affect the absorption of both compounds.
The opposite trend was observed in bean leaves, where a decrease in MTZ was observed in the presence of ZnO NPs as well as a notable decrease in BCF from soil to leaves. This could be due to a lower translocation of metabolites from the root to the aerial parts of the plants compared to MTZ or to a faster MTZ degradation rate in roots. The metabolization rate of MTZ did not seem to be affected in this tissue since the percentage of degradation did not vary with the addition of ZnO NPs at 2 mg/kg and even decreased at 3 mg/kg. Furthermore, the concentration of DADK-M decreased with the addition of ZnO NPs at both concentrations. Therefore, the differences observed in the leaves of plants exposed to the mixtures seemed to be due to ZnO NPs decreasing the translocation of MTZ. Wu et al. [21] found differences in the accumulation and translocation of decabromodiphenyl ether and its debrominated metabolites in Chinese cabbage plants due to the addition of bimetallic NPs of Ni/Fe. However, the reasons for these results were different. The authors suggested that the debromination occurred mainly in the soil affected by NPs before being taken up by plants and that plant metabolism was less important. Nevertheless, the effects were observed at very high concentrations of Ni/Fe NPs (30,000 mg/kg). The influence of ZnO NPs on the final metabolic profile of MTZ in plant tissues is complex and difficult to explain due to the balance between MTZ accumulation and metabolism. However, the results of this study reflect the ability of ZnO NPs to influence the accumulation of MTZ and its metabolites in plant tissues.
4.4. Effects of ZnO NPs and MTZ on Plants
In general, ZnO NPs did not affect most of the measured parameters beyond their influence on MTZ toxicity. The lack of adverse effects of ZnO NPs on plant biomass was expected since exposure to similar concentrations did not result in observable phytotoxicity in previous studies [31]. Zn is a trace element that is essential for plant growth and development. The lack of improvement in plant growth or photosynthetic pigments when Zn was added was probably because the control soil was not Zn deficient in terms of DTPA-TEA-Zn levels [26]. However, this study showed that the application of ZnO NPs at low concentrations can modify the toxicity of MTZ in bean plants. Although ZnO NPs alone had no negative effect on plant growth, two-way ANOVA analysis showed that ZnO NPs aggravated the toxic effects of MTZ on bean growth. The increased toxicity of MTZ in the presence of ZnO NPs could have resulted from the fact that the lowest degradation of MTZ was in soils exposed to the mixture, according to the strong negative correlation found between plant growth and remaining soil MTZ concentration. ZnO NPs also modified the effects of MTZ on photosynthetic pigments. MTZ acts mainly on chloroplasts, where it disrupts the flow of electrons in photosystem II (Hill reaction) during photosynthesis, increasing the production of ROS [43,44,45]. The abundance of ROS in chloroplasts disrupts the CHL structure and decreases the CHLa/b ratio, resulting in a reduced net photosynthetic rate. In this work, MTZ increased CHL and carotenoid content, in contrast to most previous studies [43,46]. The increase in chlorophyll may be a defense mechanism of MTZ-stressed plants to improve the leaf photosystem and thus maintain photosynthetic processes [47]. Similarly, the increase in carotenoids could be stimulated by the production of ROS in leaves, as they are very potent scavengers of singlet oxygen (1O2) and other free radicals usually generated in chloroplasts [48]. The application of ZnO NPs restored the levels of CHLb and carotenoids in plants exposed to 3 mg/kg of MTZ back to those of the controls. This may indicate that the addition of Zn suppressed chlorophyll stress in the MTZ-treated plants. However, Zn had no effect on the ratio between CHLa and CHLb, which was lower than the control, indicating that ZnO NPs were not able to restore the thylakoid stacking and electron transport ability of the photosynthetic system in plants damaged by MTZ [49].
In this work, the lower growth observed in plants exposed to the mixtures could be explained by studying the effects on biochemical parameters. One of the most important mechanisms of the toxicity of metallic NPs is their capacity to trigger the generation of ROS due to their redox activity and thus cause oxidative stress [31,50]. Zinc, in turn, is critical in the control of ROS generation and detoxification [51]. In the present study, ZnO NPs at 50 mg/kg reduced ROS generation and MDA content in plant leaves compared to the control group. MDA is the end product of lipid peroxidation, and its decrease after the addition of Zn was consistent with the protective role Zn plays against the damaging attacks of ROS on cells. However, despite the increased enzymatic activity of the mixtures, ZnO NPs increased ROS and MDA levels in the combined assay at low MTZ concentrations.
Previous studies have evaluated the influence of the co-exposure of NPs and metals or organic compounds on enzymatic activity and have found activation or inhibition depending on the enzyme type, contaminant concentration, and plant tissue [11,52,53,54]. In the present study, the activity of APX and GPOD, as influenced by MTZ, changed significantly in the presence of ZnO NPs, whereas no changes were observed for CAT. The different response of CAT compared to APX and GPOD could be due to differences in H2O2 detoxification pathways and substrate affinities, as well as APX and GPOD’s localization in different cell organelles [22,52]. Interestingly, APX activity increased in plants exposed to the mixture of 2 mg/kg of MTZ and ZnO NPs, although neither of these chemicals alone had an effect on this enzyme at the concentrations in the mixture. This suggests that ZnO NPs and MTZ worked together to generate ROS and induce antioxidant activity. The increase in oxidative stress seemed to lead to a decrease in plant weight, as evidenced by the strong negative correlation found between plant weight and the increase in enzymatic activities, especially GPOD activity, and the increase in MDA (determined by Pearson analysis). In addition, previous studies have indicated that GPOD could also cause root damage, which could contribute to reduced plant weight [14].
4.5. Enzymatic Activity in Soil
Combined exposure to ZnO NPs and metals can mitigate [24] or enhance [55] the inhibition of microbial enzymatic activity exerted by individual treatments. Regarding organic compounds, Parada et al. [23] observed the enhanced activation of soil nitrification in a soil–plant system exposed to a mixture of atrazine (3 mg/kg) and Cu NPs at very high concentrations (500 and 1500 mg/kg). Our research showed that phosphatase and ammonium oxidase enzymes were the most sensitive to soil contamination caused by the application of ZnO NPs and MTZ and their mixtures. Phosphatase enzymes participate in the phosphorous cycle and are responsible for the mineralization of organic phosphorus compounds. Ammonia oxidation is a crucial stage in the terrestrial nitrogen cycle as it is the rate-limiting step in the nitrification process. The co-exposure of ZnO NPs and MTZ showed an interesting dual behavior. Phosphatase activity was inhibited to a similar or even smaller extent in the mixtures than in the single exposure, although both compounds contributed to the toxicity, indicating an antagonistic effect. In contrast, the toxicity to ammonium oxidase was higher in the mixture than in the individual tests.
In soil ammonium oxidase, toxicity was caused by MTZ, as ZnO NPs did not show any toxicity in the individual assays. In this case, the increase in enzymatic inhibition in the combined treatments could be due to the decrease in MTZ degradation in the NP-amended soils and the consequent increase in MTZ levels in the soil. In agreement with this, our Pearson analysis showed that ammonium oxidase activity was negatively correlated with the MTZ concentration measured in the soil at the end of the experiment rather than with the applied concentration of MTZ. In contrast, the ZnO NPs and MTZ applied individually were toxic to phosphatase at the same level, and antagonistic behavior could result from interactions between biota and contaminants, indicating the importance of biological responses in the joint effects of both contaminants on this enzyme. A possible explanation for the differences observed between phosphatase and ammonium oxidation could be that both MTZ [39] and ZnO NPs [32] could alter the microbial composition of the soil and thus affect the nitrogen and phosphorus cycles in the soil differently [40]. Another factor that can strongly alter the microbial communities is soil pH [56]. However, in this experiment, the differences in soil pH between treatments ranged from 7.0 to 7.6. These small changes in soil pH cannot justify the changes observed in enzyme activities [40]. Furthermore, the effects on phosphatase or ammonium oxidase activities did not follow a trend similar to the changes in soil pH.
In this study, several processes appear to be involved in the changes in toxicity and accumulation due to ZnO NPs/MTZ mixtures compared to single exposures. In some cases (plant growth and ammonium oxidase activity), ZnO NPs could increase the toxicity of MTZ by inhibiting its degradation in soil [17]. NPs could also alter the toxicity of a contaminant to plants by affecting its bioaccumulation [9,22]. However, changes in the accumulation of MTZ and its metabolites with the addition of NPs did not always correlate with changes in toxicity to plants. For example, the addition of ZnO NPs increased APX and GPOD activities and MDA levels in bean leaves even though MTZ concentrations decreased in plants exposed to a soil concentration of 2 mg/kg of MTZ. Finally, the effects of combined exposure could be due to changes in the metabolic processes related to toxicity/detoxification mechanisms or the tolerance to contaminant stress [52,53,57]. ZnO NPs increased the activity of the antioxidant enzymes involved in coping with MTZ-induced oxidative stress. However, this was not reflected in an increase in plant growth or a decrease in lipid peroxidation.
5. Conclusions
The findings of this study indicate that, although low concentrations of NPs may not be of direct concern, their indirect and interactive effects may be relevant and should be taken into account in agricultural soils. Interestingly, combined exposure to ZnO NPs and MTZ resulted in dual synergistic or antagonistic effects depending on the endpoint measured. The mechanisms underlying the joint toxicity observed in this study are unclear but did not appear to be related to increased MTZ accumulation in plants. MTZ had the ability to increase Zn availability in agricultural soils treated with ZnO NPs and thus potentially enhance Zn uptake by plant roots. However, this effect was only observed in limited cases, suggesting that the interaction between MTZ and ZnO NPs is influenced by particular conditions and may not always result in increased Zn accumulation in plants. On the other hand, these experiments clearly demonstrated the ability of ZnO NPs to influence the rate of MTZ degradation in soil and its accumulation and metabolization in plants under more realistic conditions (i.e., low ZnO NP concentrations of and soil–plant systems). The influence of ZnO NPs on MTZ transformation was most pronounced in soil, where ZnO NPs drastically reduced the degradation rate of MTZ, which could increase the risk of MTZ transfer to other environmental compartments and its accumulation in food. Although the results obtained reflect the experimental conditions of the study, they indicate the need to assess the potential consequences of such coexistence to ensure sustainable agricultural practices and minimize any undesirable adverse effects. To our knowledge, this is the first published study on the influence of ZnO NPs on the toxicity and accumulation/metabolism of a herbicide to crops grown in soil.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13082004/s1, Text S1. Methods for the analysis of biochemical parameters in bean plants; Text S2. Methods for the analysis of dehydrogenase and phosphatase activities; Table S1. Soil pHw (1:2.5) data measured at the end of the experiment; Table S2. Translocation factor and bioconcentration factor values for MTZ in bean plants. References [58,59] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, C.G.-G. and M.D.F.; Formal analysis, C.G.-G. and M.D.F.; Investigation, C.G.-G., R.A.P., B.A., A.O. and M.D.F.; Validation, C.G.-G., R.A.P., P.A. and M.D.F.; Visualization, P.A.; Writing—original draft, M.D.F.; Writing—review and editing, C.G.-G., R.A.P., B.A., A.O. and M.D.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Community of Madrid (project S2018/BAA-4330).
Data Availability Statement
Data will be made available from the corresponding author upon request.
Acknowledgments
The authors are grateful to Pilar Ortiz and Javier Sánchez for the technical assistance they provided.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sheteiwy, M.S.; Shaghaleh, H.; Hamoud, Y.A.; Holford, P.; Shao, H.; Qi, W.; Hashmi, M.Z.; Wu, T. Zinc oxide nanoparticles: Potential effects on soil properties, crop production, food processing, and food quality. Environ. Sci. Pollut. Res. 2021, 28, 36942–36966. [Google Scholar] [CrossRef]
- Šebesta, M.; Kolenčík, M.; Sunil, B.R.; Illa, R.; Mosnáček, J.; Ingle, A.P.; Urík, M. Field Application of ZnO and TiO2 Nanoparticles on Agricultural Plants. Agronomy 2021, 11, 2281. [Google Scholar] [CrossRef]
- Thounaojam, T.C.; Meetei, T.T.; Devi, Y.B.; Panda, S.K.; Upadhyaya, H. Zinc oxide nanoparticles (ZnO-NPs): A promising nanoparticle in renovating plant science. Acta Physiol. Plant. 2021, 43, 136. [Google Scholar] [CrossRef]
- Gauba, A.; Hari, S.K.; Ramamoorthy, V.; Vellasamy, S.; Govindan, G.; Arasu, M.V. The versatility of green synthesized zinc oxide nanoparticles in sustainable agriculture: A review on metal-microbe interaction that rewards agriculture. Physiol. Mol. Plant Pathol. 2023, 125, 102023. [Google Scholar] [CrossRef]
- Liu, L.; Nian, H.; Lian, T. Plants and rhizospheric environment: Affected by zinc oxide nanoparticles (ZnO NPs). A review. Plant Physiol. Biochem. 2022, 185, 91–100. [Google Scholar] [CrossRef]
- Wahla, A.Q.; Iqbal, S.; Anwar, S.; Firdous, S.; Mueller, J.A. Optimizing the metribuzin degrading potential of a novel bacterial consortium based on Taguchi design of experiment. J. Hazard. Mater. 2019, 366, 1–9. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, L.; Wang, S.; Dong, M.; Gao, A.; Han, Z.; Liang, S.; Zhang, H. Bioremediation of metribuzin-contaminated soil by corn straw biochar-immobilized Bacillus cereus N1. Process Biochem. 2023, 130, 520–533. [Google Scholar] [CrossRef]
- Mohamed, I.A.; Abdalla, R.M. Weed control, growth, and yield of tomato after application of metribuzin and different pendimethalin products in Upper Egypt. J. Soil Sci. Plant Nutr. 2023, 23, 924–937. [Google Scholar] [CrossRef]
- Ji, Y.; Zhou, Y.; Ma, C.; Feng, Y.; Hao, Y.; Rui, Y.; Wu, W.; Gui, X.; Le, V.N.; Han, Y.; et al. Jointed toxicity of TiO2 NPs and Cd to rice seedlings: NPs alleviated Cd toxicity and Cd promoted NPs uptake. Plant Physiol. Biochem. 2017, 110, 82–93. [Google Scholar] [CrossRef]
- Venkatachalam, P.; Jayaraj, M.; Manikandan, R.; Geetha, N.; Rene, E.R.; Sharma, N.; Sahi, S. Zinc oxide nanoparticles (ZnONPs) alleviate heavy metal-induced toxicity in Leucaena leucocephala seedlings: A physiochemical analysis. Plant Physiol. Biochem. 2017, 110, 59–69. [Google Scholar] [CrossRef]
- Zhu, J.; Zou, Z.; Shen, Y.; Li, J.; Shi, S.; Han, S.; Zhan, X. Increased ZnO nanoparticle toxicity to wheat upon co-exposure to phenanthrene. Environ. Pollut. 2019, 247, 108–117. [Google Scholar] [CrossRef]
- Usman, M.; Zia-Ur-Rehman, M.; Rizwan, M.; Abbas, T.; Ayub, M.A.; Naeem, A.; Alharby, H.F.; Alabdallah, N.M.; Alharbi, B.M.; Qamar, M.J.; et al. Effect of soil texture and zinc oxide nanoparticles on growth and accumulation of cadmium by wheat: A life cycle study. Environ. Res. 2023, 216, 114397. [Google Scholar] [CrossRef]
- Zhang, W.; Long, J.; Li, J.; Zhang, M.; Xiao, G.; Ye, X.; Chang, W.; Zeng, H. Impact of ZnO nanoparticles on Cd toxicity and bioaccumulation in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. 2019, 26, 23119–23128. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, Y.; Shi, Y.; Li, Z.; Zhang, X.; Liu, T.; Farooq, T.H.; Pan, Y.; Chen, X.; Yan, W. Combined toxicity of zinc oxide nanoparticles and cadmium inducing root damage in Phytolacca americana L. Sci. Total. Environ. 2022, 806, 151211. [Google Scholar] [CrossRef]
- Josko, I.; Kusiak, M.; Xing, B.; Oleszczuk, P. Combined effect of nano-CuO and nano-ZnO in plant-related system: From bioavailability in soil to transcriptional regulation of metal homeostasis in barley. J. Hazard. Mater. 2021, 416, 126230. [Google Scholar] [CrossRef]
- Naasz, S.; Altenburger, R.; Kühnel, D. Environmental mixtures of nanomaterials and chemicals: The Trojan-horse phenomenon and its relevance for ecotoxicity. Sci. Total. Environ. 2018, 635, 1170–1181. [Google Scholar] [CrossRef]
- Deng, R.; Lin, D.; Zhu, L.; Majumdar, S.; White, J.C.; Gardea-Torresdey, J.L.; Xing, B. Nanoparticle interactions with co-existing contaminants: Joint toxicity, bioaccumulation and risk. Nanotoxicology 2017, 11, 591–612. [Google Scholar] [CrossRef]
- De La Torre-Roche, R.; Hawthorne, J.; Musante, C.; Xing, B.; Newman, L.A.; Ma, X.; White, J.C. Impact of Ag nanoparticle exposure on p,p′-DDE bioaccumulation by Cucurbita pepo (Zucchini) and Glycine max (Soybean). Environ. Sci. Technol. 2013, 47, 718–725. [Google Scholar] [CrossRef]
- Zhang, R.; Bai, X.; Shao, J.; Chen, A.; Wu, H.; Luo, S. Effects of zero-valent iron nanoparticles and quinclorac coexposure on the growth and antioxidant system of rice (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2020, 203, 111054. [Google Scholar] [CrossRef]
- Apodaca, S.A.; Tan, W.; Dominguez, O.E.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Physiological and biochemical effects of nanoparticulate copper, bulk copper, copper chloride, and kinetin in kidney bean (Phaseolus vulgaris) plants. Sci. Total. Environ. 2017, 599–600, 2085–2094. [Google Scholar] [CrossRef]
- Wu, J.; Xie, Y.; Fang, Z.; Cheng, W.; Tsang, P.E. Effects of Ni/Fe bimetallic nanoparticles on phytotoxicity and translocation of polybrominated diphenyl ethers in contaminated soil. Chemosphere 2016, 162, 235–242. [Google Scholar] [CrossRef]
- Ma, C.; Liu, H.; Chen, G.; Zhao, Q.; Eitzer, B.; Wang, Z.; Cai, W.; Newman, L.A.; White, J.C.; Dhankher, O.P.; et al. Effects of titanium oxide nanoparticles on tetracycline accumulation and toxicity in Oryza sativa (L.). Environ. Sci. Nano 2017, 4, 1827–1839. [Google Scholar] [CrossRef]
- Parada, J.; Rubilar, O.; Sousa, D.; Martínez, M.; Fernández-Baldo, M.; Tortella, G. Short term changes in the abundance of nitrifying microorganisms in a soil-plant system simultaneously exposed to copper nanoparticles and atrazine. Sci. Total Environ. 2019, 670, 1068–1074. [Google Scholar] [CrossRef]
- Wang, F.; Adams, C.A.; Shi, Z.; Sun, Y. Combined effects of ZnO NPs and Cd on sweet sorghum as influenced by an arbuscular mycorrhizal fungus. Chemosphere 2018, 209, 421–429. [Google Scholar] [CrossRef]
- European Commision (EC). Renewal Assessment Report under Regulation 1107/2009. Metribuzin. Sencor SC 600. 2018. Available online: https://www.efsa.europa.eu/en/consultations/call/181115# (accessed on 26 July 2023).
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- García-Gómez, C.; García, S.; Obrador, A.F.; González, D.; Babín, M.; Fernández, M.D. Effects of aged ZnO NPs and soil type on Zn availability, accumulation and toxicity to pea and beet in a greenhouse experiment. Ecotoxicol. Environ. Saf. 2018, 160, 222–230. [Google Scholar] [CrossRef]
- García-Gómez, C.; Babin, M.; Obrador, A.; Álvarez, J.M.; Fernández, M.D. Integrating ecotoxicity and chemical approaches to compare the effects of ZnO nanoparticles, ZnO bulk, and ZnCl2 on plants and microorganisms in a natural soil. Environ. Sci. Pollut. Res. 2015, 22, 16803–16813. [Google Scholar] [CrossRef]
- Feng, M.-H.; Shan, X.-Q.; Zhang, S.; Wen, B. A comparison of the rhizosphere-based method with DTPA, EDTA, CaCl2, and NaNO3 extraction methods for prediction of bioavailability of metals in soil to barley. Environ. Pollut. 2005, 137, 231–240. [Google Scholar] [CrossRef]
- Albero, B.; Fernández, M.D.; García-Gómez, C.; Pérez, R.A. Rapid determination of metribuzin and three major transformation products in soil and plant by Gas Chromatography–Tandem Mass Spectrometry. Separations 2022, 9, 386. [Google Scholar] [CrossRef]
- García-Gómez, C.; Obrador, A.; González, D.; Babín, M.; Fernández, M.D. Comparative study of the phytotoxicity of ZnO nanoparticles and Zn accumulation in nine crops grown in a calcareous soil and an acidic soil. Sci. Total Environ. 2018, 644, 770–780. [Google Scholar] [CrossRef]
- García-Gómez, C.; Fernández, M.D.; García, S.; Obrador, A.F.; Letón, M.; Babín, M. Soil pH effects on the toxicity of zinc oxide nanoparticles to soil microbial community. Environ. Sci. Pollut. Res. 2018, 25, 28140–28152. [Google Scholar] [CrossRef]
- Kandeler, E. Potential nitrification. Methods Soil Biol. 1996, 4, 146–149. [Google Scholar]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Lv, J.; Christie, P.; Zhang, S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: Recent advances and methodological challenges. Environ. Sci. Nano 2019, 6, 41–59. [Google Scholar] [CrossRef]
- Adriano, D.C. Trace elements in terrestrial environments. In Biogeochemistry, Bioavailability and Risk of Metals, 2nd ed.; Springer: New York, NY, USA, 2001. [Google Scholar]
- Waalewijn-Kool, P.L.; Ortiz, M.D.; van Straalen, N.M.; van Gestel, C.A.M. Sorption, dissolution and pH determine the long-term equilibration and toxicity of coated and uncoated ZnO nanoparticles in soil. Environ. Pollut. 2013, 178, 59–64. [Google Scholar] [CrossRef]
- Zhao, S.; Fan, Z.; Sun, L.; Zhou, T.; Xing, Y.; Liu, L. Interaction effects on uptake and toxicity of perfluoroalkyl substances and cadmium in wheat (Triticum aestivum L.) and rapeseed (Brassica campestris L.) from co-contaminated soil. Ecotoxicol. Environ. Saf. 2017, 137, 194–201. [Google Scholar] [CrossRef]
- Safarpoor, M.; Faraji, M.; Kamali, M.; Sharififar, A.; Sahami, B.; Sadeghianfar, P.; Nazari, M. Effect of Biofertilizers and Metribuzin on Weeds and Yield of Tomato. Agric. Res. 2018, 7, 89–92. [Google Scholar] [CrossRef]
- He, G.; Yang, Y.; Liu, G.; Zhang, Q.; Liu, W. Global analysis of the perturbation effects of metal-based nanoparticles on soil nitrogen cycling. Glob. Chang. Biol. 2023, 29, 4001–4017. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Z.; Wu, M.; Qian, X.; Lin, D.; Zhang, H.; Tang, J.; Zeng, T.; Yao, W.; Filser, J.; et al. Potential environmental risks of nanopesticides: Application of Cu(OH)2 nanopesticides to soil mitigates the degradation of neonicotinoid thiacloprid. Environ. Int. 2019, 129, 42–50. [Google Scholar] [CrossRef]
- Uwizeyimana, H.; Wang, M.; Chen, W.; Khan, K. The eco-toxic effects of pesticide and heavy metal mixtures towards earthworms in soil. Environ. Toxicol. Pharmacol. 2017, 55, 20–29. [Google Scholar] [CrossRef]
- Bartucca, M.L.; Mimmo, T.; Cesco, S.; Panfili, I.; Del Buono, D. Effect of metribuzin on nitrogen metabolism and iron acquisition in Zea mays. Chem. Ecol. 2019, 35, 720–731. [Google Scholar] [CrossRef]
- Pilcher, W.; Zandkamiri, H.; Arceneaux, K.; Harrison, S.; Baisakh, N. Genome-wide microarray analysis leads to identification of genes in response to herbicide, metribuzin in wheat leaves. PLoS ONE 2017, 12, e0189639. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yu, Q.; Han, H.; Owen, M.J.; Powles, S.B. Metribuzin resistance in a wild radish (Raphanus raphanistrum) population via both psbA gene mutation and enhanced metabolism. J. Agric. Food Chem. 2019, 67, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Bhoite, R.; Onyemaobi, I.; Si, P.; Siddique, K.H.M.; Yan, G. Identification and validation of QTL and their associated genes for pre-emergent metribuzin tolerance in hexaploid wheat (Triticum aestivum L.). BMC Genet. 2018, 19, 102. [Google Scholar] [CrossRef]
- González-Moscoso, M.; Juárez-Maldonado, A.; Cadenas-Pliego, G.; Meza-Figueroa, D.; SenGupta, B.; Martínez-Villegas, N. Silicon nanoparticles decrease arsenic translocation and mitigate phytotoxicity in tomato plants. Environ. Sci. Pollut. Res. 2022, 29, 34147–34163. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Cao, W.C.; Gong, J.L.; Zeng, G.M.; Song, B.; Zhang, P.; Li, J.; Fang, S.Y.; Qin, L.; Ye, J.; Cai, Z. Mutual effects of silver nanoparticles and antimony(III)/(V) co-exposed to Glycine max (L.) Merr. in hydroponic systems: Uptake, translocation, physiochemical responses, and potential mechanisms. Environ. Sci. Nano 2020, 7, 2691–2707. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Rizwan, M.; Zia ur Rehman, M.; Javed, M.R.; Imran, M.; Chatha, S.A.S.; Nazir, R. Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ. Pollut. 2018, 242, 1518–1526. [Google Scholar] [CrossRef]
- Cakmak, I. Tansley review No. 111—Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef]
- Jośko, I.; Kusiak, M.; Oleszczuk, P.; Świeca, M.; Kończak, M.; Sikora, M. Transcriptional and biochemical response of barley to co-exposure of metal-based nanoparticles. Sci. Total. Environ. 2021, 782, 146883. [Google Scholar] [CrossRef]
- Kamali-Andani, N.; Fallah, S.; Peralta-Videa, J.R.; Golkar, P. A comprehensive study of selenium and cerium oxide nanoparticles on mung bean: Individual and synergistic effect on photosynthesis pigments, antioxidants, and dry matter accumulation. Sci. Total Environ. 2022, 830, 154837. [Google Scholar] [CrossRef]
- González-Moscoso, M.; Martínez-Villegas, N.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Effect of Silicon Nanoparticles on Tomato Plants Exposed to Two Forms of Inorganic Arsenic. Agronomy 2022, 12, 2366. [Google Scholar] [CrossRef]
- Yu, R.; Wu, J.; Liu, M.; Zhu, G.; Chen, L.; Chang, Y.; Lu, H. Toxicity of binary mixtures of metal oxide nanoparticles to Nitrosomonas europaea. Chemosphere 2016, 153, 187–197. [Google Scholar] [CrossRef]
- Pietri, J.A.; Brookes, P. Substrate inputs and pH as factors controlling microbial biomass, activity and community structure in an arable soil. Soil Biol. Biochem. 2009, 41, 1396–1405. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; ur Rehman, M.Z.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of 846 protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).