Integrating Soil pH, Clay, and Neutralizing Value of Lime into a New Lime Requirement Model for Acidic Soils in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Characterization of Soil and Lime Samples
2.2. Neutralizing Value of Lime
2.3. Soil-Lime Incubation
2.4. Modelling
2.5. Model Validation
2.6. Statistical Analysis
3. Results
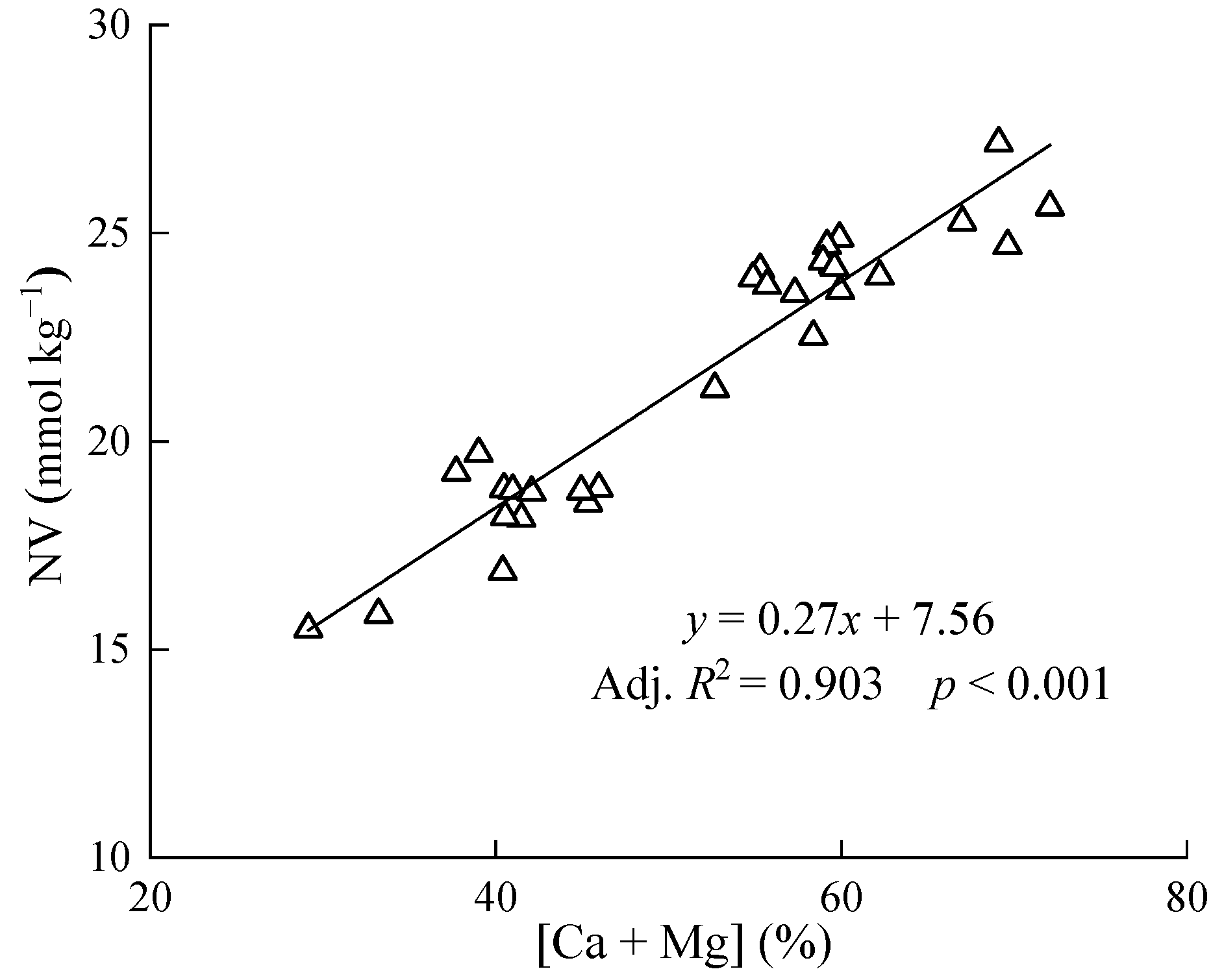
3.1. Relationship between Neutralizing Value and [Ca+Mg] in Lime
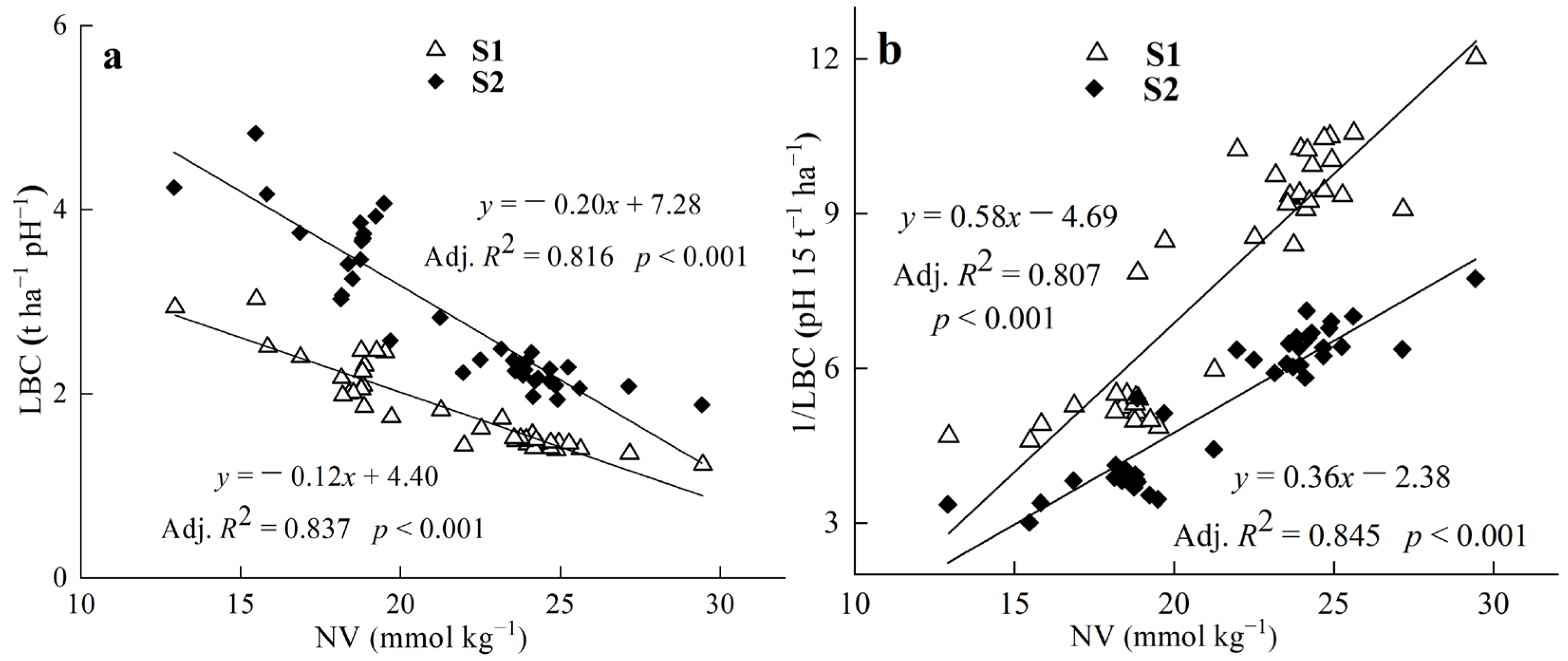
3.2. Relationships between LBC or 1/LBC and Soil pH, Clay Content, the NV of Lime
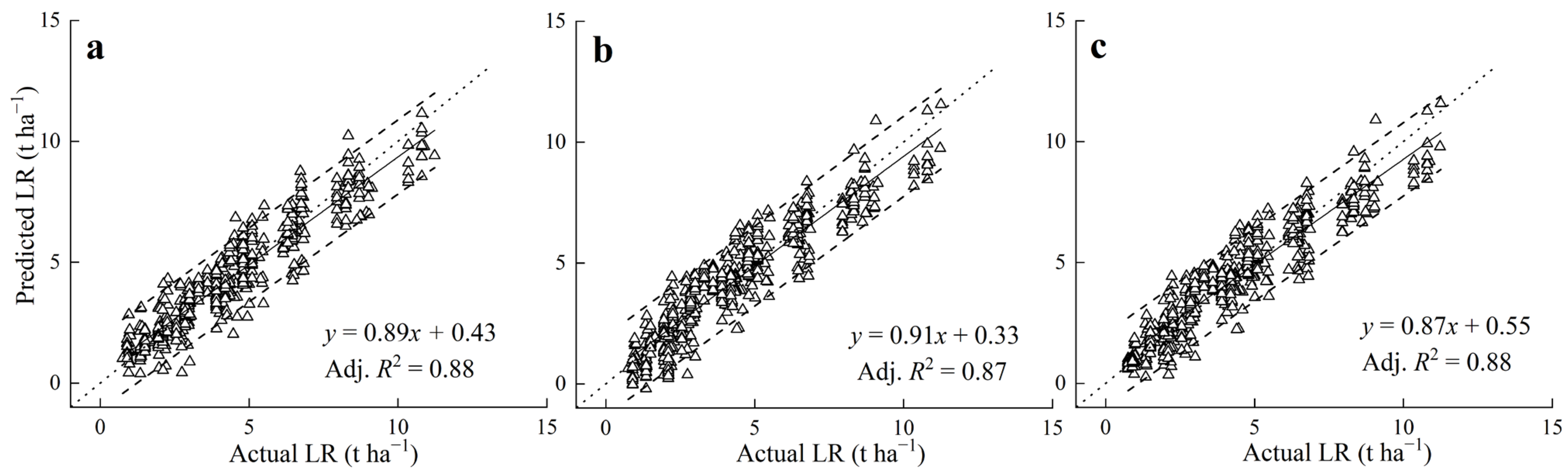
3.3. Modelling Based on Soil-Lime Incubation
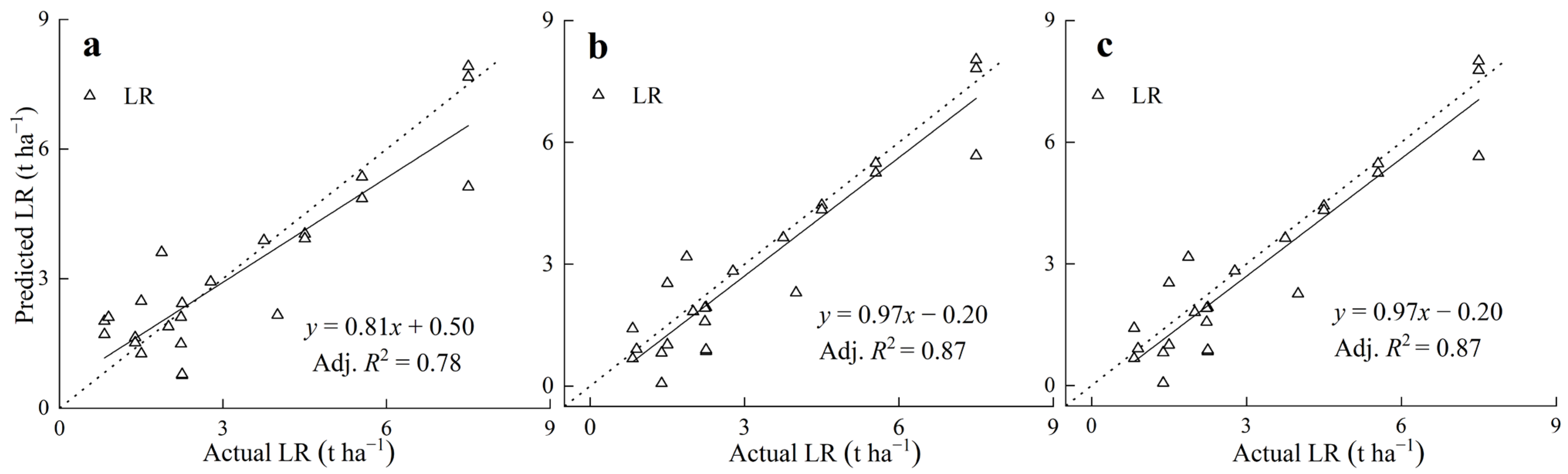
3.4. Model Validation with Field Studies
4. Discussion
4.1. Relationship between NV and [Ca+Mg] in Lime
4.2. Relationship between the LBC or LR and NV of Lime
4.3. The Relationship between the LBC or LR and Soil pH, Clay Content
4.4. The Predictive Equation for the LBC or LR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- De Vries, W.; Breeuwsma, A. The relation between soil acidification and element cycling. Water Air Soil Pollut. 1987, 35, 293–310. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.B.; Tian, Y.; Zhang, H.C.; Cheng, Y.; Zhang, J.B. A soil management strategy for ameliorating soil acidification and reducing nitrification in tea plantations. Eur. J. Soil. Biol. 2018, 88, 36–40. [Google Scholar] [CrossRef]
- Athanase, N.; Vicky, R.; Jayne, M.N.; Sylvestre, H. Soil acidification and lime quality: Sources of soil acidity, its effects on plant nutrients, efficiency of lime and liming requirements. Agric. Adv. 2013, 9, 259–269. [Google Scholar]
- Goulding, K.W.T. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef]
- Meng, C.F.; Lv, X.N.; Cao, Z.H.; Hu, Z.Y.; Ma, W.Z. Long-term effects of lime application on soil acidity and crop yields on a red soil in Central Zhejiang. Plant Soil 2004, 265, 101–109. [Google Scholar] [CrossRef]
- Daba, N.A.; Li, D.C.; Huang, J.; Han, T.F.; Zhang, L.; Ali, S.; Khan, M.N.; Du, J.X.; Liu, S.J.; Legesse, T.G.; et al. Long-term fertilization and lime-induced soil pH changes affect nitrogen use efficiency and grain yields in acidic soil under wheat-maize rotation. Agronomy 2021, 11, 2069. [Google Scholar] [CrossRef]
- Caires, E.F.; Garbuio, F.J.; Churka, S.; Barth, G.; Corrêa, J.C.L. Effects of soil acidity amelioration by surface liming on no-till corn, soybean, and wheat root growth and yield. Eur. J. Agron. 2008, 28, 57–64. [Google Scholar] [CrossRef]
- Follett, R.H.; Follett, R.F. Soil and lime requirement tests for the 50 states and Puerto Rico. J. Agron. Educ. 1983, 12, 9–17. [Google Scholar] [CrossRef]
- Vogel, S.; Bönecke, E.; Kling, C.; Kramer, E.; Lück, K.; Philipp, G.; Rühlmann, J.; Schröter, I.; Gebbers, R. Direct prediction of site-specific lime requirement of arable fields using the base neutralizing capacity and a multi-sensor platform for on-the-go soil mapping. Precis. Agric. 2021, 23, 127–149. [Google Scholar] [CrossRef]
- Bailey, J.S.; Stevens, R.J.; Kilpatrick, D.J. A rapid method for predicting the lime requirement of acidic temperate soils with widely varying organic matter contents. Dev. Plant. Soil Sci. 1991, 45, 253–262. [Google Scholar] [CrossRef]
- Teixeira, W.G.; Alvarez, V.; Neves, J.; Paulucio, R.B. Evaluation of traditional methods for estimating lime requirement in Brazilian soils. Rev. Bras. Cien. Solo 2020, 44, e0200078. [Google Scholar] [CrossRef]
- McBratney, A.B.; Minasny, B.; Cattle, S.R.; Vervoort, R.W. From pedotransfer functions to soil inference systems. Geoderma 2002, 109, 41–73. [Google Scholar] [CrossRef]
- Combatt Caballero, E.; Jarma Orozco, A.; Palencia Luna, M. Modeling the requirements of agricultural limestone in acid sulfate soils of Brazil and Colombia. Commun. Soil Sci. Plant Anal. 2019, 50, 935–947. [Google Scholar] [CrossRef]
- Nelson, P.N.; Su, N. Soil pH buffering capacity: A descriptive function and its application to some acidic tropical soils. Soil Res. 2010, 48, 201–207. [Google Scholar] [CrossRef]
- Thompson, J.S.; Kissel, D.E.; Cabrera, M.L.; Sonon, L.S. Equilibration Reaction from Single Addition of Base to Determine Soil Lime Requirement. Soil Sci. Soc. Am. J. 2010, 74, 663–669. [Google Scholar] [CrossRef]
- Vogel, S.; Bönecke, E.; Kling, C.; Kramer, E.; Lück, K.; Nagel, A.; Philipp, G.; Rühlmann, J.; Schröter, I.; Gebbers, R. Base neutralizing capacity of agricultural soils in a quaternary landscape of north-east Germany and its relationship to best management practices in lime requirement determination. Agronomy 2020, 10, 877. [Google Scholar] [CrossRef]
- Kissel, D.E.; Isaac, R.A.; Hitchcock, R.; Sonon, L.S.; Vendrell, P.F. Implementation of soil lime requirement by a single-addition titration method. Commun. Soil Sci. Plant Anal. 2007, 38, 1341–1352. [Google Scholar] [CrossRef]
- Kissel, D.E.; Sonon, L.S.; Cabrera, M.L. Rapid measurement of soil pH buffering capacity. Soil Sci. Soc. Am. J. 2012, 76, 694–699. [Google Scholar] [CrossRef]
- Wong, M.T.F.; Webb, M.J.; Wittwer, K. Development of buffer methods and evaluation of pedotransfer functions to estimate pH buffer capacity of highly weathered soils. Soil Use Manag. 2013, 29, 30–38. [Google Scholar] [CrossRef]
- Jalali, M.; Moradi, A. Measuring and simulating pH buffer capacity of calcareous soils using empirical and mechanistic models. Arch. Agron. Soil Sci. 2020, 66, 559–571. [Google Scholar] [CrossRef]
- Hui, W.; Yang, J.Y.; Liu, Z.Q.; Zhang, J.E. Data integration analysis indicates that soil texture and pH greatly influence the acid buffering capacity of global surface soils. Sustainability 2022, 14, 3017. [Google Scholar] [CrossRef]
- Aitken, R.; Moody, P.; McKinley, P. Lime requirement of acidic Queensland soils. I. Relationships between soil properties and pH buffer capacity. Aust. J. Soil Res. 1990, 28, 695–701. [Google Scholar] [CrossRef]
- Keeney, D.R.; Corey, R.B. Factors affecting the lime requirements of Wisconsin soils. Soil Sci. Soc. Am. J. 1963, 3, 277–280. [Google Scholar] [CrossRef]
- Li, Y.; Cui, S.; Chang, S.X.; Zhang, Q.P. Liming effects on soil pH and crop yield depend on lime material type, application method and rate, and crop species: A global meta-analysis. J. Soils Sediments 2018, 19, 1393–1406. [Google Scholar] [CrossRef]
- Logan, K.; Floate, M. Acidity in upland and hill soils: Cation exchange capacity, pH and lime requirement. J. Sci. Food Agric. 2010, 36, 1084–1092. [Google Scholar] [CrossRef]
- Nguyen, T.; Tran, T.T.H. The contribution of various components to pH buffering capacity of acrisols in southeastern Vietnam. Commun. Soil Sci. Plant Anal. 2019, 50, 1170–1177. [Google Scholar] [CrossRef]
- Chairiyah, R.R.; Ramija, K.E.; Batubara, S.F. Liming of acid soil and the interaction with soil pH and corn productivity. Earth Environ. Sci. 2021, 807, 042071. [Google Scholar] [CrossRef]
- Raboin, L.; Razafimahafaly, A.H.D.; Rabenjarisoa, M.B.; Rabary, B.; Dusserre, J.; Becquer, T. Improving the fertility of tropical acid soils: Liming versus biochar application? A long-term comparison in the highlands of Madagascar. Field Crops Res. 2016, 199, 99–108. [Google Scholar] [CrossRef]
- Vieira Fontoura, S.M.; de Castro Pias, O.H.; Tiecher, T.; Cherubin, M.R.; de Moraes, R.P.; Bayer, C. Effect of gypsum rates and lime with different reactivity on soil acidity and crop grain yields in a subtropical oxisol under no-tillage. Soil Till. Res. 2019, 193, 27–41. [Google Scholar] [CrossRef]
- Yang, R.; Mitchell, C.C.; Howe, J.A. Relative neutralizing value as an indicator of actual liming ability of limestone and byproduct materials. Commun. Soil Sci. Plant Anal. 2018, 49, 1144–1156. [Google Scholar] [CrossRef]
- Jones, J.D.; Mallarino, A.P. Influence of source and particle size on agricultural limestone efficiency at increasing soil pH. Soil Sci. Soc. Am. J. 2018, 82, 271–282. [Google Scholar] [CrossRef]
- Aramburu Merlos, F.; Silva, J.V.; Baudron, F.; Hijmans, R.J. Estimating lime requirements for tropical soils: Model comparison and development. Geoderma 2023, 432, 116421. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, W.G.; Alvarez, V.V.H.; Neves, J.C.L. New methods for estimating lime requirement to attain desirable pH values in Brazilian soils. Rev. Bras. De Ciência Do Solo 2020, 44, e0200008. [Google Scholar] [CrossRef]
- Goulding, K.W.T.; McGrath, S.P.; Johnston, A.E. Predicting the lime requirement of soils under permanent grassland and arable crops. Soil Use Manag. 1989, 5, 54–58. [Google Scholar] [CrossRef]
- McFarland, C.; Shiwakoti, S.; Boggs, L.C.; Schroeder, K.; Brown, T.; Huggins, D.R. Evaluating buffer methods for determining lime requirement on acidified agricultural soils of the Palouse. Soil Sci. Soc. Am. J. 2020, 84, 1769–1781. [Google Scholar] [CrossRef]
- Xu, D.H.; Zhu, Q.C.; Ros, G.; Cai, Z.J.; Wen, S.L.; Xu, M.G.; Zhang, F.S.; de Vries, W. Calculation of spatially explicit amounts and intervals of agricultural lime applications at county-level in China. Sci. Total Environ. 2022, 806, 150955. [Google Scholar] [CrossRef]
- Yin, F.; Li, Y.J.; Zhao, Q.; Li, C.; Li, J.; Tian, S.L. Experimental and model predictive investigation into the relationship of pH alternation with addition of lime in acid Tin Tailing treatments. Bull. Environ. Contam. Toxicol. 2021, 107, 1202–1207. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Yan, P.; Wu, L.Q.; Wang, D.H.; Fu, J.Y.; Shen, C.; Li, X.; Zhang, L.P.; Zhang, L.; Fan, L.C.; Han, W.Y. Soil acidification in Chinese tea plantations. Sci. Total Environ. 2020, 715, 136963. [Google Scholar] [CrossRef]
- Yang, Y.H.; Ji, C.J.; Ma, W.H.; Wang, S.F.; Wang, S.P.; Han, W.X.; Mohammat, A.; Robinson, D.; Smith, P. Significant soil acidification across northern China’s grasslands during 1980s–2000s. Glob. Chang. Biol. 2012, 18, 2292–2300. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Kilmer, V.J.; Alexander, L.T. Methods of making mechanical analyses of soils. Soil Sci. 1949, 68, 15–24. [Google Scholar] [CrossRef]
- Leng, Y.P.; Chu, D.R.; Shang, Z.C. Study of determination of neutralizing value of calcareous materials by chemical titration and formulation of international standard. Fert. Health 2019, 46, 19–26. [Google Scholar] [CrossRef]
- Libohova, Z.; Wills, S.; Odgers, N.P.; Ferguson, R.; Nesser, R.; Thompson, J.A.; West, L.T.; Hempel, J.W. Converting pH 1:1 H2O and 1:2 CaCl2 to 1:5 H2O to contribute to a harmonized global soil database. Geoderma 2014, 213, 544–550. [Google Scholar] [CrossRef]
- Jones, C.A. Effect of soil texture on critical bulk densities for root growth. Soil Sci. Soc. Am. J. 1983, 47, 1208–1211. [Google Scholar] [CrossRef]
- Holland, J.E.; Bennett, A.E.; Newton, A.C.; White, P.J.; McKenzie, B.M.; George, T.S.; Pakeman, R.J.; Bailey, J.S.; Fornara, D.A.; Hayes, R.C. Liming impacts on soils, crops and biodiversity in the UK: A review. Sci. Total Environ. 2018, 610–611, 316–332. [Google Scholar] [CrossRef]
- du Toit, D.J.; Swanepoel, P.A.; Hardie, A.G. Effect of lime source, fineness and granulation on neutralization of soil pH. S. Afr. J. Plant Soil 2022, 39, 163–174. [Google Scholar] [CrossRef]
- Ohno, T.; Erich, M.S. Incubation-derived calcium carbonate equivalence of papermill boiler ashes derived from sludge and wood sources. Environ. Pollut. 1993, 79, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, M.; Russelle, M.P.; Rosen, C.J.; Nater, E.A. Nutrient supply and neutralizing value of alfalfa stem gasification ash. Soil Sci. Soc. Am. J. 2002, 66, 171–178. [Google Scholar] [CrossRef]
- Chen, H.P.; Zhang, W.W.; Yang, X.P.; Wang, P.; McGrath, S.P.; Zhao, F.J. Effective methods to reduce cadmium accumulation in rice grain. Chemosphere 2018, 207, 699–707. [Google Scholar] [CrossRef]
- Huang, G.X.; Ding, C.F.; Hu, Z.Y.; Cui, C.H.; Zhang, T.L.; Wang, X.X. Topdressing iron fertilizer coupled with pre-immobilization in acidic paddy fields reduced cadmium uptake by rice (Oryza sativa L.). Sci. Total Environ. 2018, 636, 1040–1047. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, X.; Zeng, M.; Liao, B.H.; Liu, L.; Yang, W.T.; Wu, Y.M.; Qiu, Q.Y.; Wang, Y.J. Effects of combined amendments on heavy metal accumulation in rice (Oryza sativa L.) planted on contaminated paddy soil. Ecotoxicol. Environ. Saf. 2014, 101, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Niyozov, S.; Amonova, H.I.; Rizvonova, M.; Murodova, M.A. Mineralogical, chemical composition of uchtut dolomite mineral and physico-chemical basis of production of magnesium chloride. J. Integr. Educ. Res. 2022, 6, 32–38. [Google Scholar]
- Gregg, J.M.; Bish, D.L.; Kaczmarek, S.E.; Machel, H.G. Mineralogy, nucleation and growth of dolomite in the laboratory and sedimentary environment: A review. Sedimentology 2015, 62, 1749–1769. [Google Scholar] [CrossRef]
- Liu, Z.; Yuan, D.; Dreybrodt, W. Comparative study of dissolution rate-determining mechanisms of limestone and dolomite. Environ. Geol. 2005, 49, 274–279. [Google Scholar] [CrossRef]
- Liu, M.; Kissel, D.E.; Cabrera, M.L.; Vendrell, P.F. Soil lime requirement by direct titration with a single addition of calcium hydroxide. Soil Sci. Soc. Am. J. 2005, 69, 522–530. [Google Scholar] [CrossRef]
- Kapembwa, C.; Shitumbanuma, V.; Chabu, K.; Van Ranst, E. Evaluation of the effectiveness of a new method of measuring the chemical reactivity of liming materials on two acid soils from Zambia. Commun. Soil. Sci. Plant Anal. 2020, 51, 327–340. [Google Scholar] [CrossRef]
- Nguyen, T. The pH buffering capacity of acrisols under cassava production in Southeastern Vietnam, Chau Thanh district. Soil Use Manag. 2018, 34, 554–562. [Google Scholar] [CrossRef]
- Nye, P.H.; Ameloko, A.Y. Predicting the rate of dissolution of lime in soil. J. Soil Sci. 1987, 38, 641–649. [Google Scholar] [CrossRef]
- Jansen Van Rensburg, H.G.; Claassens, A.S.; Beukes, D.J. Relationships between soil buffer capacity and selected soil properties in a resource-poor farming area in the Mpumalanga Province of South Africa. S. Afr. J. Plant Soil 2009, 26, 237–243. [Google Scholar] [CrossRef]
- Lierop, W.V. Conversion of organic soil pH values measured in water, 0.01 M CaCl2 or 1 N KCl. Can. J. Soil Sci. 1981, 61, 577–579. [Google Scholar] [CrossRef]
- Kabala, C.; Musztyfaga, E.; Gałka, B.; Łabuńska, D.; Mańczyńska, P. Conversion of soil pH 1:2.5 KCl and 1:2.5 H2O to 1:5 H2O: Conclusions for soil management, environmental monitoring, and international soil databases. Pol. J. Environ. Stud. 2016, 25, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Machacha, S. Comparison of laboratory pH buffer methods for predicting lime requirement for acidic soils of eastern Botswana. Commun. Soil Sci. Plant Anal. 2004, 35, 2675–2687. [Google Scholar] [CrossRef]
- Meng, C.F. Effects of limestone powder on barley growth and soil chemical properties in red soil paddy field. J. Zhejiang Agri. Sci. 1985, 5, 222–224+237. (In Chinese) [Google Scholar]
- Chen, Y.H. Effects of different soil conditioners on lead control in lead contaminated vegetable-rice rotation farmland. Bull. Agric. Sci. Technol. 2021, 123–130. (In Chinese) [Google Scholar] [CrossRef]
- Duan, S.H.; Chen, S.B.; Li, X.Y.; Xiao, Y.S.; Shan, X.H.; Deng, X.; Liao, Y.H.; Liu, Y.J.; Zhou, Z.C. Effects of different agronomic practices on Cd uptake by tobacco plant and on tobacco quality under field conditions. Earth Sci. Front. 2019, 26, 112–117. [Google Scholar] [CrossRef]
- Wu, Y.J.; Yan, J.L.; Zhang, M.K.; Wang, D.Z.; Lv, X.H. Effects of fertilizer and conditioner on properties of newly cultivated red soil and soybean yield. Acta. Agric. Jiangxi 2020, 32, 68–72. (In Chinese) [Google Scholar] [CrossRef]
- Xing, S.H.; Xiong, D.Z.; Zhou, B.Q.; Xu, Q.; Liu, C.Y. Effects of various modifiers on soil fertility properties and tobacco yields and qualities. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2004, 33, 384–389. (In Chinese) [Google Scholar] [CrossRef]
- Duan, M.M.; Wang, S.; Huang, D.Y.; Zhu, Q.H.; Liu, S.L.; Zhang, Q.; Zhu, H.H.; Xu, C. Effectiveness of simultaneous applications of lime and zinc/iron foliar sprays to minimize cadmium accumulation in rice. Ecotoxicol. Environ. Saf. 2018, 165, 510–515. [Google Scholar] [CrossRef]
- Guo, F.Y.; Ding, C.F.; Zhou, Z.G.; Huang, G.X.; Wang, X.X. Effects of combined amendments on crop yield and cadmium uptake in two cadmium contaminated soils under rice-wheat rotation. Ecotoxicol. Environ. Saf. 2017, 148, 303–310. [Google Scholar] [CrossRef]
| Method | Equation |
|---|---|
| Exponential method | |
| LBC-exponential method | |
| 1/LBC-exponential method |
| Statistical Indicator | Quicklime | Hydrated Lime | Limestone | Dolomite | ||||
|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | |
| Mean | 2.19 | 1.47 | 2.36 | 1.68 | 3.50 | 2.58 | 3.72 | 2.58 |
| Standard deviation | 0.04 | 0.02 | 0.10 | 0.08 | 0.15 | 0.11 | 0.23 | 0.13 |
| Median | 2.21 | 1.45 | 2.31 | 1.61 | 3.53 | 2.63 | 3.86 | 2.72 |
| Minimum | 1.98 | 1.39 | 1.96 | 1.46 | 2.44 | 1.86 | 2.59 | 1.75 |
| Maximum | 2.36 | 1.60 | 3.19 | 2.35 | 4.25 | 3.15 | 4.84 | 3.02 |
| Relative value | 0.59 | 0.57 | 0.63 | 0.65 | 0.94 | 1.00 | 1.00 | 1.00 |
| Equation | Predictive Equation | R2 | n |
|---|---|---|---|
| (6) | 0.75 | 122 | |
| (7) | 0.82 | 122 |
| Equation | Generic Predictive Equation | R2 | n |
|---|---|---|---|
| (8) | 0.88 | 542 | |
| (9) | 0.87 | 542 | |
| (10) | 0.88 | 542 |
| Equation | Simplified Predictive Equation | Lime Source | NV (mmol kg−1) | Clay (%) | Bulk Density (g cm−3) | Soil Texture |
|---|---|---|---|---|---|---|
| (11) | Quicklime | 25 | 5 | 1.49 | Sandy soil | |
| (12) | Quicklime | 25 | 20 | 1.39 | Loamy soil | |
| (13) | Quicklime | 25 | 40 | 1.26 | Clayed soil | |
| (14) | Limestone | 18 | 5 | 1.49 | Sandy soil | |
| (15) | Limestone | 18 | 20 | 1.39 | Loamy soil | |
| (16) | Limestone | 18 | 40 | 1.26 | Clayed soil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Zeng, S.; Zhang, X.; Li, J.; Ma, Y. Integrating Soil pH, Clay, and Neutralizing Value of Lime into a New Lime Requirement Model for Acidic Soils in China. Agronomy 2023, 13, 1860. https://doi.org/10.3390/agronomy13071860
Han D, Zeng S, Zhang X, Li J, Ma Y. Integrating Soil pH, Clay, and Neutralizing Value of Lime into a New Lime Requirement Model for Acidic Soils in China. Agronomy. 2023; 13(7):1860. https://doi.org/10.3390/agronomy13071860
Chicago/Turabian StyleHan, Dandan, Saiqi Zeng, Xi Zhang, Jumei Li, and Yibing Ma. 2023. "Integrating Soil pH, Clay, and Neutralizing Value of Lime into a New Lime Requirement Model for Acidic Soils in China" Agronomy 13, no. 7: 1860. https://doi.org/10.3390/agronomy13071860
APA StyleHan, D., Zeng, S., Zhang, X., Li, J., & Ma, Y. (2023). Integrating Soil pH, Clay, and Neutralizing Value of Lime into a New Lime Requirement Model for Acidic Soils in China. Agronomy, 13(7), 1860. https://doi.org/10.3390/agronomy13071860




