Abstract
Seed germination is a complex process involving several stages, starting with the imbibition of water and ending with the emergence of the radicle. In the current study, we address the observation of an unexpected pH shift during the imbibition of maize grains. We used direct pH measurements of soak water, the pH indicator methyl red, and anatomical analysis to shed light on the acidification associated with maize (Zea mays L.) germination, a largely overlooked phenomenon. Our work shows that acidification during imbibition of maize grains is a two-step process: (i) early, rapid acidification (pH values up to 4.4), in which protons stored in the (dead) pericarp/testa are mobilised and rapidly diffuse into the surrounding medium, and (ii) late, delayed acidification (pH values just below 6), starting hours after contact of grains with water, representing an active transport process caused by living cells of the seed. We discuss the physiological mechanisms and ecological relevance of environmental acidification during maize grain germination.
1. Introduction
Maize (Zea mays L.) is one of the most important crops worldwide and has been cultivated for around 6000–10,000 years for human nutrition and feed production. This cereal is a diploid monocot tropical grass belonging to the Poaceae family and is grown all over the world. The most valuable part of the plant is the fruit, the caryopsis, or grain, which is densely filled with starch. Each fruit contains a single seed whose outer seed coat (testa) is tightly fused to the surrounding fruit wall (pericarp) and is called a caryopsis [1,2]. It is the reproductive organ of the plant, which cannot reproduce asexually and is difficult to propagate vegetatively. In our work with maize grains, we performed several procedures, e.g., the electrical conductivity test (EC test) [3], to assess germination and vigour. In EC tests, grains are incubated in deionised water, and conductivity of the leachate is measured. In addition, we also analysed the pH value of the soak water. Surprisingly, maize grains appeared to have acidified the solution to pH values around 4−5. To the best of our knowledge, this phenomenon has not been reported yet (but see [4]) and the causes and mechanisms of this pH drop during maize grain imbibition are not known. The imbibition of the grain upon contact with water is the first step in seed germination. The seed rapidly takes up water until it is fully hydrated. During imbibition, several processes take place inside the seed, including the reconstitution of membranes and reactivation of enzymes [5,6,7]. Such distinct acidification of the soaking water during seed imbibition, as described above, may play an important role in germination. In fact, there is evidence that slightly acidic pH values of the germination medium may promote germination and play a role in ecological interactions with the environment and/or other organisms [7,8,9,10,11]. Profound knowledge and understanding of the biology, biochemistry as well as biophysics of seeds and germination is essential to optimally grow crop plants to sustainably feed humanity, especially in times of climate change.
The current study was designed to shed light on the pH effect in maize grains during imbibition. Our work shows that acidification of the soak water by maize grains can be divided into (i) an immediate and strong (passive) diffusion of protons from the pericarp/testa into the surrounding solution, causing the pH to drop significantly within minutes, and (ii) a late and weaker but continuous acidification hours after immersion in the water, representing an active transport process caused by living cells of the seed. Finally, we discuss that the acidification of Zea mays grains can be seen in an ecological context.
2. Materials and Methods
Six different maize seed lots (Zea mays var. dent corn; SeedForward GmbH, Osnabrück, Germany, indicated by Zm1–Zm6) were used for the trials. The study experiments were carried out in the laboratories of the University of Osnabrück as part of a master’s thesis in 2022.
For the progress graphs, individual maize grains (Zm2) were incubated in test tubes with 4 mL deionised water each or 10 g of seeds in beakers with 40 mL deionised water and measured with a COMBI5000 pH meter (STEP Systems). Graphs were then generated using Excel to visualise and compare the results.
Light microscopic examinations were performed to identify the different tissues and structures of Zea mays grains. Whole caryopses were soaked for several hours to soften them and make them less brittle. Thin cross sections (~50–70 µm) were then prepared using a microtome (REICHERT, Munich, Germany). The sample slices were stained in safranin/astra blue (1:10 ratio) for a few seconds or in Sudan III for a few minutes, and the solution was carefully heated to enhance the reaction. The cross sections were then put under a BX43 microscope (OLYMPUS, Tokyo, Japan), and images were captured using an SC50 camera (OLYMPUS). The scale bar was added via cellSens software (version 1.18).
Methyl red was used as an indicator to visualise pH changes in the soak water. The marker indicates pH values between 4.4 and 6.2 by shifting colours from yellow (neutral) to red and subsequently to pink with decreasing pH values. A stock solution of methyl red was prepared according to the manufacturer’s instructions (0.1% w/v; ROTH) and diluted (1:10) before use. To evaluate the colours of the pH indicator, we created a colour scale by gradually decreasing the pH of the methyl red solution with hydrochloric acid (HCl) titration and taking samples at each step of 0.1 pH units. These samples were then photographed, and the colours were selected in an image editing program (GIMP) to create the scale digitally.
Experiments with the methyl red pH indicator were performed in 48-well plates to incubate individual grains (Zm1) with 500 µL of the solution each at 21 °C. Also, three replicates were used on each plate for the control (pH indicator alone) and the blank (water). Grains were dehulled by first placing them between damp paper for half an hour and then removing the outermost layer (pericarp/testa) with tweezers. For measurements and photographs, 100 µL of each well was transferred to a 96-well plate after incubation. Measurements were performed using the SPECTROstar Nano plate reader (BMG LABTECH, Ortenberg, Germany) using the SPECTROstar Nano software (version 5.50) and MARS (version 3.33) for data management. To monitor the pH changes at different time points, the samples (whole grains, stripped grains, or isolated pericarp/testae) were rinsed three times under deionised water after incubation in methyl red and immersed in 1 mL of water for 24 h each. The next day, the water was removed, the samples were rinsed again, and another incubation in 500 µL of 0.01% methyl red was performed. This procedure was repeated for 72 h.
3. Results
3.1. Acidification of the Milieu by Zea mays Grains upon Imbibition
Maize grains rapidly acidify the medium after contact with water (Figure 1). Figure 1A illustrates the pH curves of ten individual maize grains in 4 mL deionised water, each over 4 h at 21 °C. The graph demonstrates that the initial acidification of the water occurs quite rapidly within the first two minutes of immersion, and the pH decreased by up to two units. There are differences in the magnitude of the effect between grains, but all samples initially lower the pH very rapidly and then stabilise over 4 h at pH values between 5.6 and 5.2. In Figure 1B, the pH effect of six different maize seed lots is shown in comparison with seeds of other crop plants of different families, other cereals included. In each case, 10 g of seed was incubated in 40 mL of deionised water, and the pH was monitored constantly over a period of 4 h and measured again after 24 h. In the first measurement, the pH of the water surrounding the maize samples dropped rapidly from an initial neutral pH of 7 to values between 5.5 and 5 after just 5 min, which is considerably lower than pH values of all other species tested within the same period. The soaking water of the other species (Secale cereale, Avena sativa, Raphanus sativus, Sinapis alba, Helianthus annuus, Glycine max, and Phaseolus vulgaris) showed a neutral pH between 6.5 and 7, while Avena sativa (pH 8.2) and Phaseolus vulgaris (pH 7.5) even raised the pH to slightly alkaline values. During the following 4 h, the pH of the Zea mays grains remained relatively stable, except for one seed lot (Zm1) that dropped to a pH of 4.5. The pH values of the other tested species decreased slightly during this time but did not fall significantly below pH 6. After 24 h of incubation, the pH of all samples dropped uniformly by approximately 0.4 pH units, except for Avena sativa, whose previous alkaline pH (after 4 h) dropped by 1.2 units. The Zea mays samples finally ended up with a pH value between 4.4 and 5, while the soak water of all other species showed a pH of about 5.7 after a day of immersion.
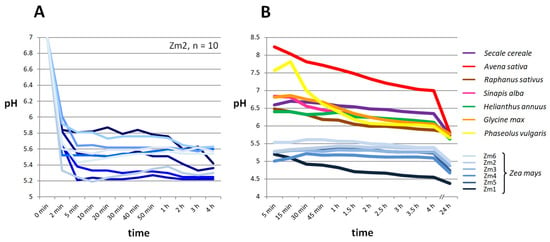
Figure 1.
Zea mays grains acidify the medium within the first minutes after contact with water. (A) Individual grains of Zea mays (Zm2) were each incubated in 4 mL of deionised water, and the pH was monitored. The pH values decreased rapidly from the initial pH of 7 within the first 5 min and stabilised between 5.8 and 5.2. (B) For each species, 10 g of seeds were incubated in 40 mL of deionised water, and the pH was measured over 4 h and after 24 h. Six seed lots of Zea mays (blue) already showed a considerably lower pH value after five minutes than all seven other species. For all species tested, pH values dropped slightly within the first 4 h and continued to drop after 24 h.
3.2. Anatomy of Maize Grains
To understand the causes and mechanisms of this pH effect in maize, the anatomy of maize caryopses was studied using light microscopy and specific staining techniques of cross-sections of maize grains with safranin/astra blue and Sudan III. The grain tissues described in the literature were found and identified (Figure 2). The outermost coat structure of the caryopsis is the pericarp and testa. These two tissues are tightly fused and compressed during development so that it seems impossible to distinguish them from each other and thus are treated here as pericarp/testa. This layer is stained red with safranin, indicating that cell walls are lignified and the cells are metabolically dead. The grain can be dehulled by manually removing the pericarp/testa after soaking in water (Figure 2C,D). Directly beneath the pericarp/testa is a thin suberised layer-stained orange with Sudan III due to its hydrophobic properties. Semi-permeable layers (endosperm-associated cuticles) stained with Sudan III (lipid/suberin/cutin-containing) are also known from Arabidopsis thaliana, Allium spp. as well as from Solanaceae and Cucurbitaceae seeds [12,13,14]. This very thin suberised layer, about 1 µm thick [15], adheres to the seed when the grain is peeled. The embryo is localised below the suberised layer on one side of the grain, and the aleurone layer covers the starchy endosperm (but not the embryo, Figure 2a). The non-lignified cell walls of the living aleurone cells turn blue upon safranine/astra blue staining. The starchy endosperm fills the entire remaining interior of the grain and consists of dead cells containing mainly accumulated starch that is mobilised and nourishes the embryo during germination [2]. This tissue does not stain with the dyes used.
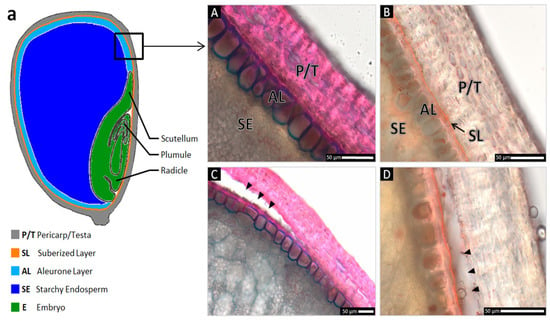
Figure 2.
Light microscopic anatomical details of intact Zea mays grain peripheral layers (A,B) and manual pericarp removal (C,D), stained with safranine/astra blue (A,C), and Sudan III (B,D). Specific staining of cross sections illustrates a lignified pericarp/testa (P/T) separated from the aleurone layer (AL) below by a suberised layer (SL). The multilayered pericarp is anatomically indistinguishable from the thin testa that is fused to it on the inner side of this tissue. (a) Schematic overview of the tissues of maize grain in a longitudinal section. (A) Astra blue stains the non-lignified cell walls of the living aleurone layer blue; lignified cell walls of the dead pericarp/testa cells are stained red by safranine. (B) A thin suberised layer overlying the aleurone cells is stained orange due to its hydrophobic properties, potentially functioning as a selectively permeable layer. (C,D) When the grains are dehulled, the pericarp/testa is completely removed, while the living cells of the aleurone layer and the suberised layer remain intact on the seed. Arrowheads indicate the artificial removal of the pericarp/testa.
3.3. Acidification Process of Maize Grains Is Mediated by a Two-Step Process and Caused by Different Tissues
To better understand this acidification process, we searched for the source of the hydrogen ions that lower the pH value. For this purpose, Zea mays caryopses were dissected into different tissues, namely the embryo, the starchy endosperm, the aleurone layer, and the pericarp/testa. These tissues were incubated in 48-well plates in 500 µL each of methyl red pH indicator for 30 min (Figure 3B). This pH indicator was chosen because it covers the pH range of interest (pH 4.4–6.2) previously determined (Figure 1A). Measurements and photographs were taken after transferring 100 µL of the solution of each well into a 96-well plate. An immediate colour change was visible in the wells containing the pericarp/testa, while none of the other tissues showed any effect within the incubation period.
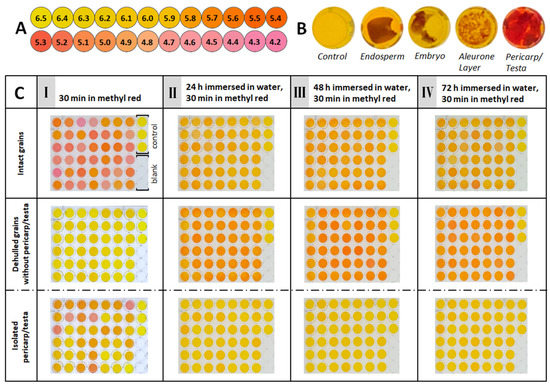
Figure 3.
Early, rapid acidification of the medium after 30 min is caused by the maize grain’s pericarp/testa, and late acidification after 24 h is caused by the seed. (A) Colour scale of methyl red at pH values between 6.5 and 4.2. (B) Different tissues of individual maize grains incubated for 30 min in 500 µL of 0.01% (w/v) methyl red. (C) A total of 42 grains (Zm1) intact (top), grains without pericarp/testa (middle), and their isolated pericarp/testa (bottom) were each incubated in 500 µL 0.01% (w/v) methyl red for 30 min. For measurement, 100 µL of each well was transferred to a new 96-well plate. The rightmost column on each plate represents the control (methyl red solution, top three wells) and blank (water, bottom three wells). After measurement, samples were washed in water and immersed in 1 mL of water each. This protocol was repeated at 24-h intervals for 3 d, indicated by I–IV.
In a subsequent experiment, individual intact fruit (n = 126) was incubated in methyl red, and the pH effect was monitored over 72 h (3 d) (Figure 3C). This period covers all three stages of seed germination, from imbibition to radicle emergence [5]. In addition to whole grains being analysed, grains were separated from their pericarp/testa, and the dehulled seeds and pericarp/testa were incubated separately (Figure 3C) to assess the contribution of the different tissues to the acidification over time. To evaluate the colours of the pH indicator, we created a colour scale (Figure 3A), as described in Materials and Methods. On the first day, just after 30 min of incubation, the whole, intact grains change the colour of the indicator significantly, reaching pH values ranging from about 5.8 to about 4.4. Each grain had rapidly acidified the solution, but there are clear differences in acidification between the individual grains, which is consistent with the results shown in Figure 1A. In contrast, the dehulled grains without pericarp/testa only very slightly changed the pH indicator’s colour, indicating that no notable acidification had occurred. However, the isolated pericarp/testa of the same grains caused a significant change in colour of the indicator, although not as much as the intact grains. The individual pericarp/testa also differed significantly in terms of acidification. After 24 h submerged in water, the intact grains resulted in slight colour changes of the indicator (Figure 3CII), but compared to the first day’s result with dry grains (Figure 3CI), the colours appeared more uniform, and the acidification was less strong. The corresponding pH values were just below 6. The dehulled grains without pericarp/testa, which showed no effect in the first round of the experiment (Figure 3CI), now resulted in obvious colour changes of the pH indicator (Figure 3CII). Comparison with the colour scale indicated relatively uniform pH values of about 5.6. On the other hand, the corresponding manually removed pericarp/testae that resulted in a clear pH change after the first 30 min (Figure 3CI) now stopped changing colours, leaving the indicator as yellow as the control (Figure 3CII). When this protocol is repeated after 48 h under water, the results (Figure 3CIII) do not differ from those obtained after 24 h (Figure 3CII): Dehulled grains uniformly acidify the solution slightly, dehulled grains change the colour of the indicator slightly more, while wells with removed pericarp/testa alone remain yellow. This pattern remains unchanged after 72 h underwater (Figure 3CIV). At this time, the grains complete their germination by emerging visible radicles.
4. Discussion
Acidification of the medium by Zea mays caryopses incubated in water can be divided into two separate steps.
4.1. Early Acidification Is a Passive Proton Diffusion of Protons Stored in the Pericarp/Testa
The first effect is a rapid decrease in pH that occurs immediately after contact with water and is shown in Figure 1A and Figure 3C. The speed of this effect indicates that it is purely physical and passive, caused by concentration-dependent diffusion of hydrogen ions from the grain into the water. Furthermore, Figure 3B,C show that the hydrogen ions causing the immediate drop in the pH of the soak water originate solely from the pericarp/testa. Therefore, the pericarp/testa alone is the source of the rapid acidification that occurs immediately after imbibition. The difference in early acidification between dry, intact grains (very strong acidification) and isolated pericarp/testa (less strong acidification) is most likely due to the fact that removal of the pericarp/testa required prior soaking of the grains in moist paper to soften the tissue, which probably allowed some protons to diffuse prior to the experiment. This also argues for a diffusion effect, since the pericarp/testa consists of dead cells that cannot contribute to any active metabolic process, e.g., active ion transport (Figure 2). Therefore, we envision the pericarp/testa as a proton reservoir filled with hydrogen ions that are mobilized during imbibition. These protons could either be reversibly fixed to the negative charges of the cell walls [16] or dissociate from an acid that has accumulated in the pericarp/testa during fruit development, e.g., ferulic acid, which has been found in large amounts in the pericarp of maize grain [17]. In the presence of water, these protons are then no longer bound and diffuse into the medium, following the osmotic gradient, and the pH value of the surrounding aqueous solution decreases. Therefore, the removal of the pericarp/testa from the seed stops this immediate acidification.
4.2. The Late, Delayed Acidification Is Caused by Active Enzymatic Transport of Protons from the Living Cells of the Seed
The second effect is late acidification, which occurs hours after the first contact of the maize grain with water. Figure 3CIV shows that immersed grains lower the pH even after 72 h with repeated washing, which does not occur with the isolated (manually removed) pericarp/testa. We interpret this phenomenon to mean that the “proton reservoir” (pericarp/testa) is emptied during the initial 24 h of the experiment, and no more hydrogen ions can diffuse out of the pericarp/testa and change the pH of the soak water. Meanwhile, the remaining dehulled seed (grains without pericarp/testa), which had no effect on the pH value change during the first 30 min of incubation (first acidification step), start to release protons after 24 h (second acidification step). This could be indicative of an active biological process of living seed cells that is not detected within the first 24 h of incubation in water (Figure 3CI). During this period, the grain has fully imbibed and reconstituted, including protein/enzyme synthesis, membrane reconstitution, etc. [7]. The late (second) acidification effect is even amplified when the pericarp/testa, as a natural physical barrier for ion diffusion processes, is removed. There is reason to believe that the late acidification is caused by the plasma membrane H+-ATPase [17,18,19]. This enzyme is an electrogenic pump that exports cytosolic protons to the apoplast, which is characterised by a much lower pH value than the cytosol (apoplast pH: 4.8–5, cytosol pH: 7.7) [8,20]. Its activity is energetically covered by ATP hydrolysis. The plasma membrane H+-ATPase thereby generates an electrochemical gradient across the plasma membrane: an electrical gradient due to molecular charges (negative on the inside) and a chemical gradient of H+ (acidic on the outside). This gradient is understood to drive various cellular processes such as secondary transport, pH regulation, and cell enlargement [21,22,23]. Enríquez-Arredondo et al. [24] described this proton pump as a key element in plant cell physiology and demonstrated its presence (with various functions) in Zea mays fruits in living seed tissue, namely the scutellum, plumule, and radicle and in the aleurone layer. Upon first contact with water, the enzyme is not yet in its full physiologically active state and must be reactivated/resynthesized before it can start to work [21]. This explains the delay in acidification, as shown in Figure 3CII. Manually removed pericarp/testae, which do not contain living cells, did not show this late acidification (Figure 3CII). In this context, one might add that this important universal enzyme is present in seeds of all higher plants and explains why all tested seed species showed some acidification after 24 h incubation in water (Figure 1B).
4.3. Physiological Mechanisms and Ecological Relevance of Acidification of the Environment during Maize Grain Germination
As just outlined, we have detected early rapid and subsequently delayed acidification. There are several ideas on how these pH phenomena might be linked to important physiological and ecological functions. (i) Shifts to low pH values can be seen in the context of “acid growth” [8,18] during cell elongation. The mechanisms of acid-induced cell wall extension/growth are not fully understood, but it has been repeatedly shown that a milieu of low pH (4.5 and below) promotes cell elongation [6,7,8,25,26,27,28]. Initial radicle and plumule growth occur by cell elongation and is a turgor-controlled process [5]. This cell elongation is promoted by acid-induced loosening of cell walls at which expansions are active, and penetration of embryo surrounding coat layers (endosperm, pericarp, testa) is likely facilitated by cell wall weakening ([27,29], and references therein). Therefore, acidification of the cellular environment surrounding the embryo’s growth zones and pericarp/testa during germination likely promotes germination in Zea mays. (ii) The second suggestion explaining the pH shift is the establishment of an electrochemical H+ gradient between the seed and its soil environment, promoting secondary transport and thus the uptake of ions and nutrients into the germinating seed. This mechanism is known for the roots of Zea mays plants [30] and could also apply to the germinating seed. (iii) During initial contact with water, membrane reconstitution of the seed tissues occurs, restoring membrane integrity, membrane transport mechanisms, and selective permeability [31,32]. In this context, the third explanation we offer argues that hydrogen ions in close proximity to the grain reduce potential osmotic stress acting on the imbibing grain (e.g., uncontrolled water influx resulting in improper rehydration and reconstitution) [33,34]. We tentatively conclude that reducing the difference in osmotic potential between the cells of the seed and the soil environment results in a decrease in the rate of water uptake, and thus the potential of uncontrolled water influx and corresponding damage is less probable. (iv) Acidification of the surrounding soil changes its biochemistry and makes it more favourable for the germinating grain. Soil pH is referred to as the “master soil variable” and affects many biological, chemical, and physical properties, such as the leaching of basic ions from weathered minerals or the growth conditions for microorganisms [10]. Therefore, it is conceivable that acidification supports the proper development of seeds into vigorous seedlings either by creating favourable conditions for these seeds and/or even making life unfavourable for competing organisms. (v) The final hypothesis we propose is that the acidification effects serve as a defence mechanism for the grain. The grain, filled with high-energy starch plus proteins and fats [35,36], is susceptible to attack and digestion by bacteria and fungi. Thus, seeds need protection. It is well-known that the pericarp/testa also represents a protective tissue that functions as a physical barrier and chemical defence against pathogens and herbivores [37,38]. This was specifically shown for maize grains in which polyphenolic components in the pericarp mediated resistance against Aspergillus flavus (a saprotrophic and pathogenic fungus) and Spodoptera frugiperda (fall armyworm, a pest) [39,40]. We suggest that the above-outlined acidification of the maize grain environment might represent a mechanism that reduces microbial growth around maize grains and protects them from pest attacks during germination. A similar mechanism has already been described by Wade and Beuchard [9] in tomato, where acidic pericarp and fruit juice inhibit the growth of foodborne pathogens. On the other hand, there are also mutualistic interactions between plants and soil organisms that might be affected by acidification. In this context, Msimbira et al. [11] demonstrated that plant-growth-promoting microbes such as Lactobacillus helveticus and Bacillus subtilis increased the germination rate and plant growth of maize and tomato. This effect was enhanced when these beneficial microorganisms were incubated at an acidic pH value of 5. Hence, reducing the growth of pathogens and/or promoting the growth of mutualists could explain the environmental acidification by maize grains.
5. Conclusions
The early stages of maize grain germination are accompanied by a two-step acidification process: early, rapid but passive acidification caused by the diffusion of protons stored in the (dead) pericarp/testa and a late, delayed, and active transport process caused by living cells of the seed. Though we have provided evidence for the causes and potential mechanisms of pH shifts, further studies are needed to shed light on the beneficial role of this acidification phenomenon on seed germination and to exploit potential applications of this knowledge.
Author Contributions
Conceptualization, K.W., J.V. and K.M.; Funding acquisition, J.V. and K.M.; Investigation, K.W.; Methodology, K.W., J.V. and K.M.; Project administration, K.M.; Resources, J.V.; Supervision, J.V. and K.M.; Validation, K.W., J.V. and K.M.; Visualization, K.W., J.V. and K.M.; Writing—original draft, K.W. and K.M.; Writing—review & editing, G.L.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Aloys and Brigitte Coppenrath Stiftung Osnabrück (funding no. 2022-02-01). Furthermore, open access publishing was supported by Deutsche Forschungsgemeinschaft (DFG) and the Open Access Publishing Fund of the University of Osnabrück (BO 5110/2-1, 491052604).
Data Availability Statement
All data supporting our findings are presented in the article.
Acknowledgments
We thank Ulrike Coja (University of Osnabrück) and Richard Wagner (Jacobs University, Bremen) for fruitful discussions, SeedForward GmbH for the donation of maize seed samples, and the Aloys and Brigitte Coppenrath Stiftung for generous support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rodríguez, M.V.; Barrero, J.M.; Corbineau, F.; Gubler, F.; Benech-Arnold, R.L. Dormancy in cereals (not too much, not so little): About the mechanisms behind this trait. Seed Sci. Res. 2015, 25, 99–119. [Google Scholar] [CrossRef]
- Awata, L.O.A.; Tongoona, P.; Danquah, E.; Ilfie, B.E.; Suresh, L.M.; Jumbo, M.B.; Marchelo-D’ragga, P.W.; Sitonik, C. Understanding tropical maize (Zea mays L.): The major monocot in modernization and sustainability of agriculture in sub-Saharan Africa. IJAAR 2019, 7, 32–77. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; International Seed Testing Association: Zurich, Switzerland, 2021. [Google Scholar]
- Lado, P.; Rasi-Caldogno, F.; Colombo, R. Acidification of the Medium Associated with Normal and Fusicoccin-Induced Seed Germination. Physiol. Plant 1975, 34, 359–364. [Google Scholar] [CrossRef]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Weitbrecht, K.; Müller, K.; Leubner-Metzger, G. First off the mark: Early seed germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef]
- Mulkey, T.J.; Kuzmanoff, K.M.; Evans, M.L. Promotion of growth and hydrogen ion efflux by auxin in roots of maize pretreated with ethylene biosynthesis inhibitors. Plant Physiol. 1982, 70, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Winch, S.; Pritchard, J. Acid-induced wall loosening is confined to the accelerating region of the root growing zone. J. Exp. Biol. 1999, 338, 1481–1487. [Google Scholar] [CrossRef]
- Wade, W.N.; Beuchat, L.R. Proteolytic fungi isolated from decayed and damaged raw tomatoes and implications associated with changes in pericarp pH favorable for survival and growth of foodborne pathogens. J. Food Prot. 2003, 66, 911–917. [Google Scholar] [CrossRef]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 3, 5794869. [Google Scholar] [CrossRef]
- Msimbira, L.A.; Naamala, J.; Antar, M.; Subramanian, S.; Smith, D.L. Effect of Microbial Cell-Free Supernatants Extracted from a Range of pH Levels on Corn (Zea mays L.) and Tomato (Solanum lycopersicum L.) Seed Germination and Seedling Growth. Front. Sustain. Food Syst. 2022, 6, 789335. [Google Scholar] [CrossRef]
- De Giorgi, J.; Piskurewicz, U.; Loubery, S.; Utz-Pugin, A.; Bailly, C.; Mene-Saffrane, L.; Lopez-Molina, L. An endosperm-associated cuticle is required for Arabidopsis seed viability, dormancy and early control of germination. PLoS Genet. 2015, 11, e1005708. [Google Scholar] [CrossRef] [PubMed]
- Beresniewicz, M.M.; Taylor, A.G.; Goffinet, M.C.; Koeller, W.D. Chemical Nature of a Semipermeable Layer in Seed Coats of Leek, Onion (Liliaceae), Tomato and Pepper (Solanaceae). Seed Sci. Technol. 1995, 23, 135–145. [Google Scholar]
- Yim, K.O.; Bradford, K.J. Callose deposition is responsible for apoplastic semipermeability of the endosperm envelope of muskmelon seeds. Plant Physiol. 1998, 118, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kiesselbach, T.A.; Walker, E.R. Structure of certain specialized tissue in the kernel of corn. Am. J. Bot. 1952, 39, 561–569. [Google Scholar] [CrossRef]
- Delsart, C. Plant cell wall: Description, role in transport, and effect of electroporation. In Handbook of Electroporation; Miklavčič, D., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–22. [Google Scholar] [CrossRef]
- Chateigner-Boutin, A.L.; Ordaz-Ortiz, J.J.; Alvarado, C.; Bouchet, B.; Durand, S.; Verhertbruggen, Y.; Barrière, Y.; Saulnier, L. Developing pericarp of maize: A model to study arabinoxylan synthesis and feruloylation. Front. Plant Sci. 2016, 7, 1476. [Google Scholar] [CrossRef]
- Sánchez-Linares, L.; Gavilanes-Ruíz, M.; Díaz-Pontones, D.; Guzmán-Chávez, F.; Calzada-Alejo, V.; Zurita-Villegas, V.; Luna-Loaiza, V.; Moreno-Sánchez, R.; Bernal-Lugo, I.; Sánchez-Nieto, S. Early carbon mobilization and radicle protrusion in maize germination. J. Exp. Bot. 2012, 63, 4513–4526. [Google Scholar] [CrossRef]
- Pielot, R.; Kohl, S.; Manz, B.; Rutten, T.; Weier, D.; Tarkowská, D.; Rolčík, J.; Strnad, M.; Volke, F.; Weber, H.; et al. Hormone-mediated growth dynamics of the barley pericarp as revealed by magnetic resonance imaging and transcript profiling. J. Exp. Bot. 2015, 66, 6927–6943. [Google Scholar] [CrossRef]
- Planes, M.D.; Niñoles, R.; Rubio, L.; Bissoli, G.; Bueso, E.; García-Sánchez, M.J.; Alejandro, S.; Gonzalez-Guzmán, M.; Hedrich, R.; Rodriguez, P.L.; et al. A mechanism of growth inhibition by abscisic acid in germinating seeds of Arabidopsis thaliana based on inhibition of plasma membrane H+-ATPase and decreased cytosolic pH, K+, and anions. J. Exp. Bot. 2015, 66, 813–825. [Google Scholar] [CrossRef]
- Sánchez-Nieto, S.; Enríquez-Arredondo, C.; Guzmán-Chávez, F.; Hernández-Muñoz, R.; Ramírez, J.; Gavilanes-Ruíz, M. Kinetics of the H+-ATPase from dry and 5-hours-imbibed maize embryos in its native, solubilized, and reconstituted forms. Mol. Plant 2011, 4, 505–515. [Google Scholar] [CrossRef]
- Falhof, J.; Pedersen, J.T.; Fuglsang, A.T.; Palmgren, M. Plasma membrane H+-ATPase regulation in the center of plant physiology. Mol. Plant 2016, 9, 323–337. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, J.; Li, D.; Kong, X.; Rengel, Z.; Chen, L.; Yang, Y.; Cui, X.; Chen, Q. The role of the plasma membrane H+-ATPase in plant responses to aluminum toxicity. Front. Plant Sci. 2017, 8, 1757. [Google Scholar] [CrossRef]
- Enríquez-Arredondo, C.; Sánchez-Nieto, S.; Rendón-Huerta, E.; González-Halphen, D.; Gavilanes-Ruíz, M.; Díaz-Pontones, D. The plasma membrane H+-ATPase of maize embryos localizes in regions that are critical during the onset of germination. Plant Sci. 2005, 169, 11–19. [Google Scholar] [CrossRef]
- Williams, S.E.; Bennett, A.B. Leaf closure in the venus flytrap: An Acid growth response. Science 1982, 218, 1120–1122. [Google Scholar] [CrossRef]
- Rayle, D.L.; Cleland, R.E. The Acid Growth Theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992, 99, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Majda, M.; Robert, S. The Role of Auxin in Cell Wall Expansion. Int. J. Mol. Sci. 2018, 19, 951. [Google Scholar] [CrossRef] [PubMed]
- Gámez-Arjona, F.; Sánchez-Rodríguez, C.; Montesinos Lopez, J.C. The root apoplastic pH as an integrator of plant signaling. Front. Plant Sci. 2022, 13, 931979. [Google Scholar] [CrossRef] [PubMed]
- Steinbrecher, T.; Leubner-Metzger, G. Tissue and cellular mechanics of seeds. Curr. Opin. Genet. Dev. 2018, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Schäffer, S.; Fortmeier, H.; Schubert, S. Adaptation of active proton pumping and plasmalemma ATPase activity of corn roots to low root medium pH. Plant Physiol. 1998, 117, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.H.; Crowe, L.M. Membrane integrity in anhydrobiotic organisms: Towards a mechanism for stabilizing dry cells. In Water and Life; Somero, G.N., Osmond, C.B., Bolis, C.L., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 87–103. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Jarma-Orozco, A.; Rodriguez-Páez, L.A. Imbibition and Germination of Seeds with Economic and Ecological Interest: Physical and Biochemical Factors Involved. Sustainability 2023, 15, 5394. [Google Scholar] [CrossRef]
- Powell, A.A. Cell membranes and seed leachate conductivity in relation to the quality of seed for sowing. J. Seed Technol. 1986, 10, 81–100. [Google Scholar]
- Ocvirk, D.; Špoljarević, M.; Marković, S.; Lisjak, M.; Hanzer, R.; Teklić, T. Seed germinability after imbibition in electrical conductivity test and relations among maize seed vigour parameters. J. Food Agric. Environ. 2014, 12, 140–145. [Google Scholar]
- Hallauer, A.R. Specialty Corns; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Mayland-Quellhorst, S.; Müller, C.; Mummenhoff, K. Two-tier morpho-chemical defence tactic in Aethionema via fruit morph plasticity and glucosinolates allocation in diaspores. Plant Cell Environ. 2018, 42, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Bhattacharya, S.; Gesing, M.A.; Klupsch, K.; Theißen, G.; Mummenhoff, K.; Müller, C. Morphologically and physiologically diverse fruits of two Lepidium species differ in allocation of glucosinolates into immature and mature seed and pericarp. PLoS ONE 2020, 15, e0227528. [Google Scholar] [CrossRef] [PubMed]
- Gembeh, S.V.; Brown, R.L.; Grimm, C.; Cleveland, T.E. Identification of chemical components of corn kernel pericarp wax associated with resistance to Aspergillus flavus infection and aflatoxin production. J. Agric. Food Chem. 2001, 49, 4635–4641. [Google Scholar] [CrossRef]
- Singh, S.; Kariyat, R.R. Exposure to polyphenol-rich purple corn pericarp extract restricts fall armyworm (Spodoptera frugiperda) growth. Plant Signal. Behav. 2020, 15, 1784545. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).