Effects of Long-Term Grazing on Feed Intake and Digestibility of Cattle in Meadow Steppe
Abstract
1. Introduction
2. Materials and Methods
2.1. Location of the Study Site
2.2. Experiment Design
2.2.1. Experiment Platform
2.2.2. Animals–Plant Sampling
- Animals
- Plants
2.2.3. Recovery Experiment
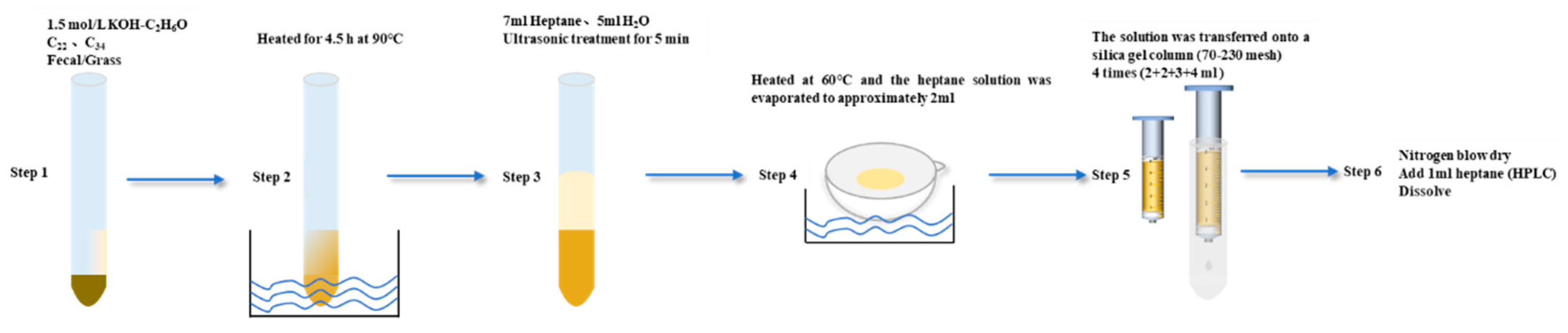
2.3. Alkane and Nutrient Analysis
2.3.1. Alkane Analysis
2.3.2. Nutrient Analysis
2.4. Estimation of Feed Intake and Digestibility
2.5. Statistical Analysis
3. Results
3.1. Chemical Composition and n-Alkanes Pattern of Plant and Fecal Matter
3.2. C31 Recovery Rate
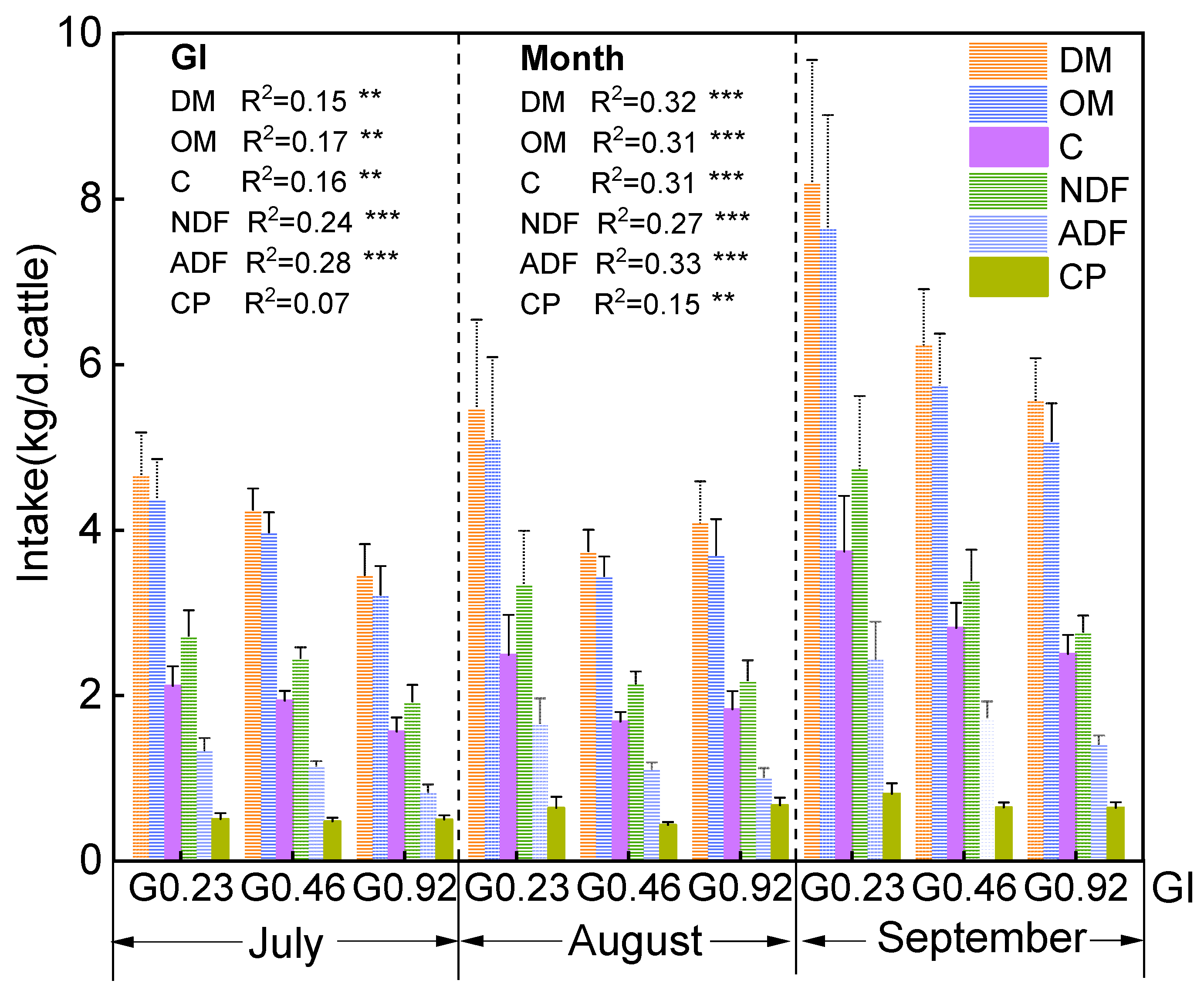
3.3. Intake and Digestibility of Cattle under Different Grazing Intensities and Months
3.4. Plant Species Composition Influencing Cattle Intake
4. Discussion
4.1. Chemical Composition and Alkanes Recovery Rate
4.2. Animal Intake and Digestibility
4.3. Factors Influencing Feed Intake
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Month | GI | DM | NDF | ADF | AIA | OM | C | CP | Gross Energy |
|---|---|---|---|---|---|---|---|---|---|
| 7 | G0.23 | 429.5 ± 20.83 | 592.05 ± 31.61 | 287.38 ± 26.98 | 18.22 ± 1.22 | 938.36 ± 2.73 | 454.57 ± 2.47 | 109.14 ± 20.26 | 18.21 ± 0.11 |
| 7 | G0.46 | 429.94 ± 35.01 | 575.77 ± 21.58 | 273.5 ± 22.12 | 20.77 ± 4.97 | 936.7 ± 3.13 | 457.43 ± 0.71 | 112.11 ± 13.09 | 18.09 ± 0.06 |
| 7 | G0.92 | 388.92 ± 28.64 | 571.93 ± 10.24 | 238.85 ± 5.86 | 22.65 ± 1.59 | 930.53 ± 0.39 | 453.77 ± 0.89 | 149.03 ± 14.41 | 18.01 ± 0.1 |
| 8 | G0.23 | 430.36 ± 12.23 | 607.53 ± 5.22 | 306.42 ± 11.22 | 24.92 ± 7.17 | 932.11 ± 1.98 | 456.42 ± 2.15 | 117.53 ± 11.03 | 18 ± 0.08 |
| 8 | G0.46 | 411.56 ± 49.47 | 565.76 ± 41.03 | 296.95 ± 20.15 | 26.75 ± 2.92 | 919.54 ± 3.89 | 451.1 ± 2.28 | 116.62 ± 9.81 | 17.92 ± 0.13 |
| 8 | G0.92 | 338.82 ± 31.18 | 540.17 ± 33.39 | 247.84 ± 18.21 | 29.45 ± 8.08 | 905.7 ± 13.96 | 450.2 ± 4.32 | 162.71 ± 14.08 | 17.52 ± 0.17 |
| 9 | G0.23 | 494.93 ± 15.48 | 581 ± 12.49 | 296.85 ± 7.76 | 27.19 ± 3.85 | 932.89 ± 3.34 | 456.45 ± 1.62 | 101.05 ± 10.74 | 18.29 ± 0.18 |
| 9 | G0.46 | 481.61 ± 49.01 | 550.17 ± 57.07 | 277.21 ± 36.13 | 39.69 ± 3.94 | 921.8 ± 1.79 | 451.43 ± 1.73 | 105.18 ± 9.14 | 17.53 ± 0.07 |
| 9 | G0.92 | 424.52 ± 55.78 | 507.83 ± 68.8 | 256.23 ± 33.26 | 31.83 ± 10.02 | 911.68 ± 5.05 | 449.7 ± 1.4 | 115.5 ± 5.16 | 17.33 ± 0.25 |
| Main effect | |||||||||
| Month | 7 | 416.122b | 579.915a | 266.577a | 20.544c | 935.197a | 455.258a | 123.425b | 18.099a |
| 8 | 393.582b | 571.152a | 283.736a | 27.042b | 919.114b | 452.572b | 132.287a | 17.817b | |
| 9 | 467.016a | 546.331a | 276.763a | 32.901a | 922.122b | 452.526b | 107.242a | 17.72b | |
| GI | G0.23 | 451.596A | 593.527A | 296.883A | 23.443A | 934.453A | 455.811A | 109.24B | 18.168A |
| G0.46 | 441.037A | 563.897AB | 282.55A | 29.07A | 926.013B | 453.317B | 111.302B | 17.848B | |
| G0.92 | 384.087B | 539.975B | 247.643B | 27.975A | 915.967C | 451.227B | 142.412A | 17.621C | |
| p-value | Month | 0.001 | 0.17 | 0.293 | 0.001 | <0.001 | 0.025 | 0.002 | <0.001 |
| GI | 0.002 | 0.024 | 0.001 | 0.107 | <0.001 | 0.001 | <0.001 | <0.001 | |
| Month ∗ GI | 0.818 | 0.739 | 0.875 | 0.382 | 0.093 | 0.026 | 0.145 | 0.001 |
Appendix B
| Month | GI | DM | NDF | ADF | AIA | OM | C | CP | Gross Energy |
|---|---|---|---|---|---|---|---|---|---|
| 7 | G0.23 | 163.91 ± 17.5 | 615.78 ± 10.87 | 366.56 ± 8.11 | 82.55 ± 8.44 | 856.23 ± 10.35 | 439.9 ± 7.1 | 131.19 ± 5.5 | 18.09 ± 0.18 |
| 7 | G0.46 | 177.73 ± 8.87 | 608.92 ± 26.16 | 361.05 ± 11.64 | 94.78 ± 6.75 | 844.25 ± 8.12 | 436.05 ± 6.03 | 130.45 ± 8.94 | 17.87 ± 0.37 |
| 7 | G0.92 | 168.95 ± 12.89 | 572.88 ± 19.15 | 350.37 ± 16.5 | 137.39 ± 17.85 | 783.33 ± 24.66 | 403.39 ± 14.27 | 143.05 ± 6.72 | 17.04 ± 0.33 |
| 8 | G0.23 | 165.11 ± 16.62 | 616.15 ± 14.06 | 389.84 ± 13.99 | 107.74 ± 10.28 | 827.17 ± 11.63 | 424.76 ± 19.57 | 123.05 ± 5.79 | 17.84 ± 0.58 |
| 8 | G0.46 | 179.94 ± 10.41 | 606.76 ± 32.26 | 374.72 ± 20.55 | 118.77 ± 17.26 | 813.48 ± 18.16 | 425.56 ± 5.07 | 124.97 ± 9.17 | 17.51 ± 0.37 |
| 8 | G0.92 | 169.3 ± 10.51 | 571.93 ± 41.66 | 372.13 ± 28.56 | 167.86 ± 20.71 | 755.25 ± 25.98 | 422.85 ± 7.35 | 133.05 ± 8.01 | 16.24 ± 0.57 |
| 9 | G0.23 | 182.14 ± 14.74 | 587.13 ± 36.56 | 367.15 ± 14.06 | 83.17 ± 5.69 | 852.46 ± 13.9 | 438.6 ± 5.83 | 109.55 ± 8.95 | 17.91 ± 0.2 |
| 9 | G0.46 | 195.02 ± 7.54 | 578.12 ± 38.36 | 370.12 ± 16.93 | 90.6 ± 7.33 | 837.1 ± 10.97 | 435.68 ± 3.9 | 113.66 ± 4.93 | 18.03 ± 0.23 |
| 9 | G0.92 | 191.27 ± 10.27 | 560.02 ± 31.38 | 360.06 ± 21.5 | 144.42 ± 15.31 | 775.4 ± 18.87 | 401.43 ± 10.25 | 126.3 ± 4.39 | 16.55 ± 0.65 |
| Main effect | |||||||||
| Month | 7 | 170.198b | 599.192a | 359.328b | 104.906b | 827.937a | 426.444a | 134.897a | 17.665a |
| 8 | 171.45b | 598.277a | 378.893a | 131.458a | 798.635b | 411.84b | 127.022b | 17.197b | |
| 9 | 189.476a | 575.09b | 365.777b | 106.063b | 821.654a | 425.234a | 116.503c | 17.497a | |
| GI | G0.23 | 170.387B | 606.351A | 374.517A | 91.152C | 845.287A | 434.686A | 121.262B | 17.946A |
| G0.46 | 184.232A | 597.932A | 368.63AB | 101.382B | 831.612B | 431.525A | 123.025B | 17.802A | |
| G0.92 | 176.505B | 568.275B | 360.852B | 149.893A | 771.327C | 397.307B | 134.134A | 16.611B | |
| p-value | Month | <0.001 | 0.013 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 |
| GI | 0.001 | <0.001 | 0.052 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Month ∗ GI | 0.931 | 0.88 | 0.75 | 0.819 | 0.994 | 0.993 | 0.651 | 0.138 |
References
- Brun, P.; Zimmermann, N.E.; Graham, C.H.; Lavergne, S.; Pellissier, L.; Munkemuller, T.; Thuiller, W. The productivity-biodiversity relationship varies across diversity dimensions. Nat. Commun. 2019, 10, 5691. [Google Scholar] [CrossRef] [PubMed]
- Scurlock, J.M.O.; Johnson, K.; Olson, R.J. Estimating net primary productivity from grassland biomass dynamics measurements. Glob. Change Biol. 2002, 8, 736–753. [Google Scholar] [CrossRef]
- Keli, A.; Andueza, D.; de Vega, A.; Guada, J.A. Validation of the n-alkane and NIRS techniques to estimate intake, digestibility and diet composition in sheep fed mixed lucerne: Ryegrass diets. Livest. Sci. 2008, 119, 42–54. [Google Scholar] [CrossRef]
- Wegi, T.; Hassen, A.; Bezabih, M.; Nurfeta, A.; Yigrem, S.; Tolera, A. Estimation of feed intake and digestibility in Zebu type Arsi steers fed natural pasture using the n-alkane technique. Anim. Feed Sci. Technol. 2021, 271, 114765. [Google Scholar] [CrossRef]
- Mcnaughton, S.J.; Milchunas, D.G.; Frank, D.A. How can net Primary Productivity be Measured in Grazing Ecosystems? Ecology 1996, 77, 974–977. [Google Scholar] [CrossRef]
- Hellwing, A.L.F.; Lund, P.; Weisbjerg, M.R.; Oudshoorn, F.W.; Munksgaard, L.; Kristensen, T. Comparison of methods for estimating herbage intake in grazing dairy cows. Livest. Sci. 2015, 176, 61–74. [Google Scholar] [CrossRef]
- Mayes, R.W.; Lamb, C.S.; Colgrove, P.M. The use of dosed and herbage n-alkanes as markers for the determination of herbage intake. J. Agric. Sci. 1986, 107, 161–170. [Google Scholar] [CrossRef]
- Dove, H.; Mayes, R.W. Plant wax components: A new approach to estimating intake and diet composition in herbivores. J. Nutr. 1996, 126, 13–26. [Google Scholar] [CrossRef]
- Unal, Y.; Garnsworthy, P.C. Estimation of intake and digestibility of forage-based diets in group-fed dairy cows using alkanes as markers. J. Agric. Sci. 1999, 133, 419–425. [Google Scholar] [CrossRef]
- Mayes, H.D. The use of plant wax alkanes as marker substances in studies of the nutrition of herbivores: A review. Crop. Pasture Sci. 1991, 42, 913–952. [Google Scholar]
- Ding, L.M.; Long, R.J. The Use of Herbage N-alkanes as Markers to Estimate the Diet Composition of Yaks on the Qinghai-Tibetan Plateau. Asian-Australas. J. Anim. Sci. 2010, 23, 61–67. [Google Scholar] [CrossRef]
- Heublein, C.; Suedekum, K.H.; Gill, F.L.; Dohme-Meier, F.; Schori, F. Using plant wax markers to estimate the diet composition of grazing Holstein dairy cows. J. Dairy Sci. 2017, 100, 1019–1036. [Google Scholar] [CrossRef]
- Barcia, P.; Bugalho, M.N.; Campagnolo, M.L.; Cerdeira, J.O. Using n-alkanes to estimate diet composition of herbivores: A novel mathematical approach. Animal 2007, 1, 141–149. [Google Scholar] [CrossRef]
- Ferreira, L.M.M.; Garcia, U.; Rodrigue, M.A.M.; Celaya, R.; Dias-da-Silva, A.; Osoro, K. The application of the n-alkane technique for estimating the composition of diets consumed by equines and cattle feeding on upland vegetation communities. Anim. Feed Sci. Technol. 2007, 138, 47–60. [Google Scholar] [CrossRef]
- Poppi, D.; Hughes, T.; L’Hullier, P. Intake of pasture by grazing ruminants. In Livestock Feeding on Pasture; NZSAP–New Zealand Society of Animal Production: Hamilton, New Zealand, 1987; pp. 55–63. [Google Scholar]
- Fonseca, L.; Mezzalira, J.C.; Bremm, C.; Filho, R.S.A.; Gonda, H.L.; Carvalho, P.C.d.F. Management targets for maximising the short-term herbage intake rate of cattle grazing in Sorghum bicolor. Livest. Sci. 2012, 145, 205–211. [Google Scholar] [CrossRef]
- Long, R.; Dong, S.; Wang, Y.; Guo, Y. Concepts and investigative methods of ruminant forage intake. Acta Prataculturae Sin. 2003, 12, 8–17. (In Chinese) [Google Scholar]
- Nyachoti, C.M.; Zijlstra, R.T.; de Lange, C.F.M.; Patience, J.F. Voluntary feed intake in growing-finishing pigs: A review of the main determining factors and potential approaches for accurate predictions. Can. J. Anim. Sci. 2004, 84, 549–566. [Google Scholar] [CrossRef]
- Wilcox, K.R.; Shi, Z.; Gherardi, L.A.; Lemoine, N.P.; Koerner, S.E.; Hoover, D.L.; Bork, E.; Byrne, K.M.; Cahill, J., Jr.; Collins, S.L.; et al. Asymmetric responses of primary productivity to precipitation extremes: A synthesis of grassland precipitation manipulation experiments. Glob. Change Biol. 2017, 23, 4376–4385. [Google Scholar] [CrossRef]
- Yan, Y.; Yan, R.; Chen, J.; Xin, X.; Eldridge, D.J.; Shao, C.; Wang, X.; Lv, S.; Jin, D.; Chen, J.; et al. Grazing modulates soil temperature and moisture in a Eurasian steppe. Agric. For. Meteorol. 2018, 262, 157–165. [Google Scholar] [CrossRef]
- Oba, G.; Vetaas, O.R.; Stenseth, N.C. Relationships between biomass and plant species richness in arid-zone grazing lands. J. Appl. Ecol. 2001, 38, 836–845. [Google Scholar] [CrossRef]
- Zainelabdeen, Y.M.; Yan, R.; Xin, X.; Yan, Y.; Ahmed, A.I.; Hou, L.; Zhang, Y. The Impact of Grazing on the Grass Composition in Temperate Grassland. Agronomy 2020, 10, 1230. [Google Scholar] [CrossRef]
- Ferreira, L.M.M.; Celaya, R.; Garcia, U.; Rodrigues, M.A.M.; Osoro, K. Differences between domestic herbivores species in alkane faecal recoveries and the accuracy of subsequent estimates of diet composition. Anim. Feed Sci. Technol. 2009, 151, 128–142. [Google Scholar] [CrossRef]
- Sun, H.X.; Zhou, D.W. Seasonal Changes in Voluntary Intake and Digestibility by Sheep Grazing Introduced Leymus chinensis Pasture. Asian Australas. J. Anim. Sci. 2007, 20, 872–879. [Google Scholar] [CrossRef]
- Dove, H.; Mayes, R.W. Protocol for the analysis of n-alkanes and other plant-wax compounds and for their use as markers for quantifying the nutrient supply of large mammalian herbivores. Nat. Protoc. 2006, 1, 1680–1697. [Google Scholar] [CrossRef]
- Soest, P.V.; Robertson, J.B. Analysis of Forrage and Fibrous Foods: A Laboratory Manual for Animal Science; Cornell University: Ithaca, NY, USA, 1985. [Google Scholar]
- Zhang, Y.; Zhao, J.; Xin, X.; Wang, M.; Pan, F.; Yan, R.; Li, L. Effects of stocking rate on the interannual patterns of ecosystem biomass and soil nitrogen mineralization in a meadow steppe of northeast China. Plant Soil 2022, 473, 9–31. [Google Scholar] [CrossRef]
- de Souza Filho, W.; Nunes, P.A.d.A.; Barro, R.S.; Kunrath, T.R.; de Almeida, G.M.; Genro, T.C.M.; Bayer, C.; de Faccio Carvalho, P.C. Mitigation of enteric methane emissions through pasture management in integrated crop-livestock systems: Trade-offs between animal performance and environmental impacts. J. Clean. Prod. 2019, 213, 968–975. [Google Scholar] [CrossRef]
- Jarillo-Rodriguez, J.; Castillo-Gallegos, E.; Ramirez y Aviles, L.; Valles de la Mora, B.; Ocana-Zavaleta, E. Milk production, grazing behaviour and biomass quality in native tropical pastures grazed to different stocking rate during two years. Trop. Subtrop. Agroecosystems 2018, 21, 373–386. [Google Scholar]
- Hardy, M.B.; Meissner, H.H.; O’Reagain, P.J. Forage intake and free-ranging ruminants: A tropical perspective. In Proceedings of the 18th International Grassland Congrees, Winnepeg and Saskatoon, AB, Canada, 8–17 June 1997; pp. 45–52. [Google Scholar]
- Hou, L.; Xin, X.; Sun, H.; Tao, Y.; Chen, J.; Yan, R.; Zhang, X.; Shen, B.; Altome, A.I.A.; Hamed, Y.M.Z.; et al. Grazing-induced cattle behaviour modulates the secondary production in a Eurasian steppe ecosystem. Sci. Total Environ. 2023, 889, 164191. [Google Scholar] [CrossRef]
- He, M.; Pan, Y.; Zhou, G.; Barry, K.E.; Fu, Y.; Zhou, X. Grazing and global change factors differentially affect biodiversity-ecosystem functioning relationships in grassland ecosystems. Glob. Change Biol. 2022, 28, 5492–5504. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, L.; Yan, R.; Xin, X. Effects of Grazing Intensity on Plant Community Characteristics and Nutrient Quality of Herbage in a Meadow Steppe. Sci. Agric. Sin. 2020, 53, 2550–2561. (In Chinese) [Google Scholar]
- Hou, L.; Yan, R.; Zhang, Y.; Xin, X. Effects of Grazing Intensity on Functional Traits of Leymus chinensis in Meadow Steppe. Sci. Agric. Sin. 2020, 53, 2562–2572. (In Chinese) [Google Scholar]
- Price, J.N.; Sitters, J.; Ohlert, T.; Tognetti, P.M.; Brown, C.S.; Seabloom, E.W.; Borer, E.T.; Prober, S.M.; Bakker, E.S.; MacDougall, A.S.; et al. Evolutionary history of grazing and resources determine herbivore exclusion effects on plant diversity. Nat. Ecol. Evol. 2022, 6, 1290–1298. [Google Scholar] [CrossRef]
- Hsu, J.S.; Powell, J.; Adler, P.B. Sensitivity of mean annual primary production to precipitation. Glob. Change Biol. 2012, 18, 2246–2255. [Google Scholar] [CrossRef]
- Adler, P.B.; Seabloom, E.W.; Borer, E.T.; Hillebrand, H.; Hautier, Y.; Hector, A.; Harpole, W.S.; O’Halloran, L.R.; Grace, J.B.; Anderson, T.M.; et al. Productivity Is a Poor Predictor of Plant Species Richness. Science 2011, 333, 1750–1753. [Google Scholar] [CrossRef]
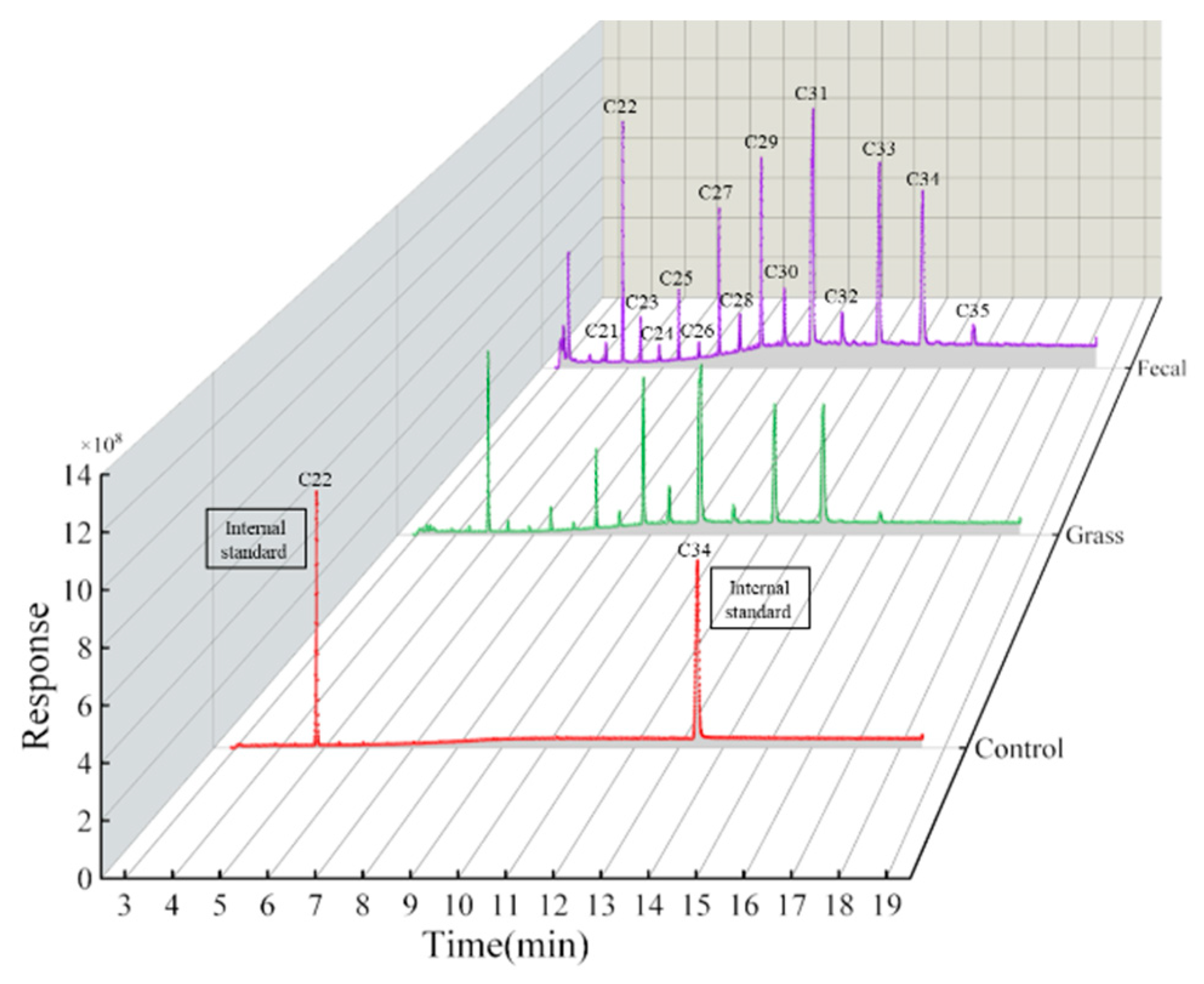

| Fecal Alkane Concentrations | Grass Alkane Concentrations | |||||
|---|---|---|---|---|---|---|
| G0.23 | G0.46 | G0.92 | G0.23 | G0.46 | G0.92 | |
| C21 | 7.22 | 7.91 | 7.49 | 1.68 | 1.85 | 2.16 |
| C22 | 200.97 | 200.92 | 200.87 | 100.38 | 100.43 | 100.39 |
| C23 | 15.32 | 15.08 | 13.15 | 4.73 | 3.93 | 3.84 |
| C24 | 6.08 | 7.40 | 6.32 | 1.66 | 1.80 | 1.81 |
| C25 | 30.95 | 34.56 | 30.90 | 9.80 | 11.24 | 10.88 |
| C26 | 8.01 | 8.82 | 6.99 | 3.32 | 3.59 | 2.60 |
| C27 | 75.13 | 77.54 | 74.87 | 30.14 | 32.07 | 28.83 |
| C28 | 15.51 | 17.35 | 15.85 | 5.30 | 6.58 | 5.96 |
| C29 | 131.64 | 134.55 | 140.73 | 62.94 | 74.85 | 75.37 |
| C30 | 26.29 | 30.55 | 32.30 | 8.59 | 13.40 | 13.94 |
| C31 | 224.84 | 242.02 | 244.55 | 118.71 | 134.68 | 131.71 |
| C32 | 18.50 | 18.53 | 20.15 | 4.62 | 7.16 | 6.95 |
| C33 | 160.29 | 148.72 | 145.86 | 65.00 | 66.90 | 58.80 |
| C34 | 200.67 | 200.70 | 200.69 | 100.03 | 100.08 | 100.04 |
| C35 | 12.03 | 14.59 | 9.96 | 5.15 | 4.24 | 2.58 |
| Total | 731.80 | 757.62 | 749.09 | 321.64 | 362.28 | 345.42 |
| Total even chain | 657.42 | 674.97 | 667.49 | 298.15 | 329.75 | 314.17 |
| GI | Feed Intake (kg/d) | Fecal (kg/d) | Digestibility (%) | C31 (Grass) Concentration (mg/kg DM) | C31 (Fecal) Concentration (mg/kg DM) | C31 Recovery (%) |
|---|---|---|---|---|---|---|
| G0.23 | 4.61 ± 0.15 | 1.55 ± 0.27 | 66.15 ± 5.82 | 106.79 ± 0.72 | 246.35 ± 1.83 | 77.63 ± 12.30 a |
| G0.46 | 4.67 ± 0.24 | 1.68 ± 0.20 | 64.23 ± 2.49 | 145.34 ± 2.89 | 295.69 ± 3.16 | 75.25 ± 7.69 a |
| G0.92 | 3.49 ± 0.29 | 1.06 ± 0.22 | 70.33 ± 4.34 | 120.06 ± 0.78 | 334.36 ± 2.15 | 81.09 ± 5.80 a |
| Month | GI | DM | OM | NDF | ADF | C | CP | GE | ME |
|---|---|---|---|---|---|---|---|---|---|
| 7 | G0.23 | 65.3 ± 3.66 | 68.34 ± 3.35 | 63.26 ± 3.62 | 56.18 ± 3.43 | 66.35 ± 3.67 | 56.07 ± 7.41 | 65.54 ± 3.63 | 9.79 ± 0.55 |
| 7 | G0.46 | 65.06 ± 1.3 | 68.53 ± 1.11 | 64.11 ± 1.04 | 53.97 ± 1.05 | 66.72 ± 1.17 | 58.79 ± 2.57 | 65.51 ± 1.23 | 9.72 ± 0.19 |
| 7 | G0.92 | 60.99 ± 1.18 | 67.23 ± 0.74 | 60.19 ± 1.23 | 42.73 ± 2.11 | 65.39 ± 0.87 | 62.4 ± 1.44 | 63.11 ± 1.06 | 9.32 ± 0.17 |
| 8 | G0.23 | 57 ± 4.86 | 61.84 ± 4.36 | 57.11 ± 4.93 | 45.68 ± 5.56 | 59.89 ± 4.59 | 54.55 ± 5.76 | 57.38 ± 4.87 | 8.48 ± 0.73 |
| 8 | G0.46 | 57.5 ± 2.89 | 62.42 ± 2.56 | 55.38 ± 2.65 | 46.31 ± 3.6 | 60.18 ± 2.71 | 54.17 ± 3.58 | 58.56 ± 2.73 | 8.6 ± 0.39 |
| 8 | G0.92 | 56.23 ± 1.4 | 63.53 ± 1.15 | 53.07 ± 2.51 | 33.76 ± 3.82 | 62.38 ± 1.28 | 64.11 ± 1.44 | 59.44 ± 1.35 | 8.54 ± 0.21 |
| 9 | G0.23 | 51.91 ± 6.23 | 55.97 ± 5.87 | 50.82 ± 6.53 | 40.73 ± 7.21 | 53.69 ± 6.16 | 48 ± 6.47 | 52.9 ± 6.13 | 7.92 ± 0.9 |
| 9 | G0.46 | 50.37 ± 2.37 | 54.87 ± 2.32 | 46.15 ± 3.28 | 32.69 ± 4.7 | 52.05 ± 2.41 | 46.43 ± 2.22 | 49.03 ± 2.29 | 7.05 ± 0.32 |
| 9 | G0.92 | 49.68 ± 4.69 | 57.36 ± 3.78 | 45.03 ± 3.3 | 30.31 ± 4.23 | 55.23 ± 3.98 | 44.85 ± 5.28 | 52.35 ± 3.86 | 7.42 ± 0.53 |
| Main effect | |||||||||
| Month | 7 | 63.60a | 68.00a | 62.42a | 50.31a | 66.13a | 59.47a | 64.62a | 9.59a |
| 8 | 56.90b | 62.69b | 54.95b | 41.45b | 60.93b | 58.00a | 58.60b | 8.55b | |
| 9 | 50.50c | 56.08c | 46.90c | 33.81c | 53.65c | 46.23b | 51.24c | 7.41c | |
| GI | G0.23 | 58.07A | 62.05A | 57.06A | 47.53A | 59.98A | 52.88A | 58.61A | 8.73A |
| G0.46 | 57.65A | 61.94A | 55.21A | 44.32A | 59.65A | 53.13A | 57.70A | 8.45A | |
| G0.92 | 55.64A | 62.71A | 52.76A | 35.60B | 61.00A | 57.12A | 58.30A | 8.43A | |
| p-value | Month | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| GI | 0.61 | 0.93 | 0.28 | <0.001 | 0.84 | 0.33 | 0.93 | 0.69 | |
| Month ∗ GI | 0.97 | 0.96 | 0.95 | 0.67 | 0.94 | 0.48 | 0.82 | 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, L.; Xin, X.; Shen, B.; Qin, Q.; Altome, A.I.A.; Hamed, Y.M.Z.; Yan, R.; Nurlan, S.; Adilbek, N.; Balzhan, A.; et al. Effects of Long-Term Grazing on Feed Intake and Digestibility of Cattle in Meadow Steppe. Agronomy 2023, 13, 1760. https://doi.org/10.3390/agronomy13071760
Hou L, Xin X, Shen B, Qin Q, Altome AIA, Hamed YMZ, Yan R, Nurlan S, Adilbek N, Balzhan A, et al. Effects of Long-Term Grazing on Feed Intake and Digestibility of Cattle in Meadow Steppe. Agronomy. 2023; 13(7):1760. https://doi.org/10.3390/agronomy13071760
Chicago/Turabian StyleHou, Lulu, Xiaoping Xin, Beibei Shen, Qi Qin, Ahmed Ibrahim Ahmed Altome, Yousif Mohamed Zainelabdeen Hamed, Ruirui Yan, Serekpaev Nurlan, Nogayev Adilbek, Akhylbekova Balzhan, and et al. 2023. "Effects of Long-Term Grazing on Feed Intake and Digestibility of Cattle in Meadow Steppe" Agronomy 13, no. 7: 1760. https://doi.org/10.3390/agronomy13071760
APA StyleHou, L., Xin, X., Shen, B., Qin, Q., Altome, A. I. A., Hamed, Y. M. Z., Yan, R., Nurlan, S., Adilbek, N., Balzhan, A., Kussainova, M., Amarjargal, A., Fang, W., Pulatov, A., Zhou, W., & Sun, H. (2023). Effects of Long-Term Grazing on Feed Intake and Digestibility of Cattle in Meadow Steppe. Agronomy, 13(7), 1760. https://doi.org/10.3390/agronomy13071760









