Abstract
Gray mold disease, caused by Botrytis cinerea, has reduced grape’s output and market quality globally. In this study, the antifungal activity of a mixed microbial combination of Brevibacillus brevis FJAT-0809-GLX and Bacillus sp. strains was investigated. The results showed that the inhibition rate against B. cinerea was 85.10% when B. brevis FJAT-0809-GLX was mixed with a mixture of B. brevis FJAT-10623 and Bacillus velezensis FJAT-55034 at a proportion of 80%:20%, which was significantly higher than those of other combination proportions. The inhibitory rates of the mixed microbial combination diluted 0 times, 10 times, and 100 times were 89.14%, 88.10%, and 86.33%, respectively, with no significant differences between each other. The mixed microbial combination appeared to be temperature-insensitive and significantly stable from pH 3 to pH 7. Furthermore, it was discovered that its antifungal activity was significantly stable with UV radiation for 30 min, 60 min, and 90 min, with values of 84.82%, 83.89%, and 82.22%, respectively. An amount of 0.025 mol mL−1 of KCl, CuSO4, and MgCl2 had no effects on the antifungal activity of the mixed microbial combination, while 0.025 mol mL−1 of NaCl, ZnSO4, FeSO4, CaCl2, and MnSO4 reduced the inhibition rate. The mixed microbial combination demonstrated antifungal activities against a variety of fungi, with inhibition rates ranging from 68.78% to 85.10%. The grape fruits and grape leaves treated with the mixed microbial combination decayed at 27.27% and 48.34%, respectively. Additionally, the mixed microbial combination improved grape fruit resistance by increasing the activity of defense enzymes polyphenol oxidase (PPO) and catalase (CAT). Therefore, the results indicated that the mixed microbial combination had great biocontrol potential against gray mold in grape fruits.
1. Introduction
Grape is a popular annual fruit all over the world due to its high nutritious content and wonderful flavor [1]. China’s total grape production in 2017 was 1.37 million tons, ranking first in total world production. However, its shelf life has been shortened due to fungal pathogen infection during cultivation, postharvest storage, and transit [2]. Gray mold, caused by Botrytis cinerea Pers., is one of the most destructive postharvest diseases in grape production [3,4]. B. cinerea is able to grow and spread at very low temperatures and develop under different conditions such as in the field, during storage, and even after customer purchase [5,6]. Various fungicides were applied to control gray mold on grapes, resulting in environmental pollution [7,8,9]. In addition, chemical residues of chemical fungicides in grape fruits are also potential threats to human health [10]. Therefore, it is urgent to develop a safe and desirable alternative control agent to manage gray mold disease on grapes caused by B. cinerea [11,12].
Microbial antagonists against gray mold on fruits have been reported [13,14]. Traditional biological control focuses on a single plant biocontrol agent [15]. Different strains, such as Pichia pastoris [16], Candida sake [17], Candida membranifaciens [15], Paenibacillus polymyxa [18], Serratia proteamaculans [19], Bacillus subtilis, and Bacillus amyloliquefaciens [20], have been reported as promising agents to control the gray mold on grapes [1]. They have different action modes including competition for space and nutrients, direct parasitism, the production of secondary metabolites, and the induction of host resistance [21]. Nutritional competition and parasites phenomena occurred between P. pastoris G5 and B. cinerea [16]. P. polymyxa SCHC33 could produce antifungal metabolites fusaricidin [18]. S. proteamaculans could increase antidisease metabolites and activities of SOD, POD, and PPO in grapevine [19]. However, compared with synthetic fungicides, most of the biocontrol agents are less effective because of the weak colonization ability, low field control effect, and instability [22]. Therefore, there is a need to find effective approaches to improve the efficacy of biological agents.
The mixed microbial combination has become a new research hotspot in recent research [23,24,25]. Various biocontrol strains have different activity spectra and mixtures of the compatible biocontrol strains could enhance the suppressive effects against plant pathogens [26,27]. The mixed cultures of Metschnikowia pulcherrima and Cryptococcus laurentii showed greater antifungal activity on blue mold (P. expansum) than either yeast applied alone [28]. Combination treatment with Stenotrophomonas rhizophila and Debaryomyces hansenii was effective for the biocontrol of fruit rot on postharvest muskmelon [29]. Four microbial preparations, whose active microorganisms were composed of Bacillus sp., Trichoderma sp., Purureocillium lilacinum, and Clonostachys rosea, had a high effect against pepper anthracnose [30]. Brevibacillus brevis strains have been used to inhibit different plant pathogens, such as Fusarium oxysporum [31], B. cinerea [32], Ralstonia solanacearum [33], and Lasiodiplodia theobromae [34]. No studies have been conducted to investigate the antifungal potential of mixed B. brevis with other Bacillus strains in order to improve its biological control activity against B. cinerea on grapes.
The main objectives of this study were to analyze characteristics of a mixture of B. brevis with Bacillus velezensis, to assess its biological control activities against different fungi in vitro, and to evaluate its antifungal activity against gray mold on grape fruits and grape leaves in vivo, as well as its effect on defense-related enzymes in grape fruits. The present results could provide a potential mixture biocontrol agent against gray mold disease on grapes.
2. Materials and Methods
2.1. Microbial Strains and Cultural Conditions
B. brevis strain FJAT-0809-GLX was isolated from soil in Yontai, Fujian Province, PR China. B. brevis strain FJAT-10623 was isolated from leaves of mandarin orange in Shunchang, Fujian Province, PR China. Bacillus velezensis strain FJAT-55034 was obtained from leaves of grapes in Fuan, Fujian Province, PR China. These strains were all isolated in our laboratory and cultured in Luria–Bertani (LB) medium [35,36].
All fungal strains used in this study were described as follows: Colletotrichum orbiculare FJAT-30256, Colletotrichum acutatum FJAT-31072, Lasiodiplodia theobromae FJAT-9860, Botrytis cinerea FJAT-32835, and Lasiodiplodia pseudotheobromae FJAT-3586 were obtained from loquat fruits, pear fruits, wax apple fruits, sweet potato, and longan fruits, respectively, in our lab [37]. Botrytis cinerea FJAT-32835 was provided by Guizhou University. The fungi were cultured on potato dextrose agar (PDA) medium at 25–28 °C for 5 d before they were used in this study.
2.2. Compatibility between Bacterial Isolates
Different bacterial isolates were cultured as described above in Section 2.1. Every two isolates were cultured in perpendicular lines on LB plates at 30 °C for 48 h. The inhibition zones between every two isolates were observed. Bacterial isolates that grow over each other were compatible; otherwise, they were incompatible. The experiment was performed three times.
2.3. Inhibitory Activities of Different Microbial Combinations
A single colony of the B. brevis strains FJAT-0809-GLX, FJAT-10623, and B. velezensis FJAT-55034 was, respectively, cultured in 20 mL of LB medium for 48 h at 180 r min−1 and 30 °C. First, culture supernatants of B. brevis FJAT-10623 (2 × 108 CFU mL−1) and B. velezensis FJAT-55034 (2 × 108 CFU mL−1) were collected and combined in equal parts. Then, the culture supernatant of B. brevis FJAT-0809-GLX (2 × 108 CFU mL−1) was mixed in various quantities with this mixture (90%:10%, 80%:20%, 70%:30%, 60%:40%, and 50%:50%). The activity of microbial combinations was determined by measuring mycelial growth rate. The mixed microbial combination was added to PDA at a final concentration of 10%. An equal volume of LB liquid medium was used as a control. The B. cinerea FJAT-32835 was cultured on PDA medium at 25 °C for 5 d, and a mycelial plug, whose diameter was 0.6 cm, was placed on the center of the PDA plates and cultured at 25 °C for 5 d. The diameter of mycelial growth was determined and the inhibition rate was calculated as follows: inhibition rate (%) = [(diameter of mycelium in control group − diameter of mycelium in mixed microbial combination group)/(diameter of mycelium in control group − 0.6)] × 100% [19]. There were three replicates for each treatment.
To further test the inhibitory activity of different dilutions of the mixed microbial combination, the mixed microbial combination was diluted 10, 100, 200, and 500 times with LB medium and added to PDA. An equal volume of LB liquid medium was used as a control. A mycelial plug of B. cinerea FJAT-32835, whose diameter was 0.6 cm, was placed on the center of the PDA plates and cultured at 25 °C for 5 d. The diameters of mycelial growth were determined to evaluate the inhibition rates. There were three replicates for each treatment.
2.4. Sensitivity of Mixed Microbial Combination to Different Conditions
The mixed microbial combination was incubated at different temperatures (50 °C, 70 °C, 90 °C, 100 °C, 121 °C) for 30 min and then cooled to room temperature. The mixed microbial combination was added to PDA at a final concentration of 10%. The antifungal activity of the mixed microbial combination was tested as described above in Section 2.3. The plates were incubated at 25 °C for 5 days and the diameters of mycelial growth were determined to assess the inhibition rates. The experiment was conducted three times.
The mixed microbial combination was adjusted to pH 3.0, 5.0, 7.0, 9.0, and 11.0 and stood for 24 h. It was adjusted to pH 7.0 again and added to PDA at a final concentration of 10%. The antifungal activity of the mixed microbial combination was determined as described above in Section 2.3. The plates were incubated at 25 °C for 5 days and the diameters of mycelial growth were determined to assess the inhibition rates. The experiment was conducted three times.
A 5 mL mixed microbial combination was placed in Petri plates and irradiated with UV radiation using an X-30 G UV lamp (Spectronics Corporation, Westbury, NY, USA) with 254 nm light (30 W) for 30 min, 60 min, 90 min, 120 min, 150 min, and 180 min. The antifungal activity of the mixed microbial combination was determined as described above in Section 2.3. The plates were incubated at 25 °C for 5 days and the diameters of mycelial growth were determined to assess the inhibition rates. Three replicates were used for each treatment.
Amounts of 100 µL of 1 mol L−1 KCl, MgCl2, CuSO4, NaCl, ZnSO4, FeSO4, CaCl2, and MnSO4 (final concentration was 0.025 mol mL−1) were added to the 4 mL mixed microbial combinations, respectively [38]. The antifungal activity of the mixed microbial combination was tested as described above in Section 2.3. The plates were incubated at 25 °C for 5 days and the diameters of mycelial growth were determined to assess the inhibition rates. Three replicates were carried out for each treatment.
2.5. Spectrum of Inhibitory Activity of Mixed Microbial Combination In Vitro
The mixed microbial combination was added to PDA at a final concentration of 10%. The fungal strains were cultured on potato dextrose agar (PDA) medium at 25–28 °C for 5 d. Antifungal activity of the mixed microbial combination was tested as described above in Section 2.3. The plates were incubated at 25–28 °C for 5 days and the diameters of mycelial growth were determined to assess the inhibition rates. Three replicates were carried out for each treatment.
2.6. Effect of Mixed Microbial Combination on Decay of Grape Fruits and Leaves In Vivo
Grape fruits (Vitis vinifera × Vitis labrusca, Kyoho grape) and grape leaves (Vitis vinifera × Vitis labrusca, Kyoho grape) were soaked in 75% alcohol for 30 s, washed 3 times in sterile water, and air-dried. They were wounded (approximately 3 mm in diameter and 3 mm deep) using sterile toothpicks, soaked in the spore suspension of B. cinerea FJAT-32835 (1 × 105 spore mL−1) for 10 min, and then naturally air-dried. The treated grape fruits and grape leaves were then immersed in the mixed microbial combination for 10 min. A total of 50 grape fruits and 9 leaves were used for the treatment and control, respectively. They were cultured in a growth chamber (TRP-1000D, Zhejiang Tuopu) with a high humidity (85%) and 25 °C for 10 d and 5 d, respectively. An equal amount of LB was used as a negative control. Three replicates were carried out for each treatment. The numbers of decayed grape fruits and grape leaves were determined. The percentage of decay was calculated as follows: decay percentage (%) = (number of decay/the total number of grape fruits and grape leaves) × 100%.
2.7. Effects of Mixed Microbial Combination on Defense Enzymes
The effect of microbial combination on the induction of defense-related enzyme production, such as polyphenol oxidase (PPO) and catalase (CAT), was determined in mixed-microbial-combination-treated grape fruits (Vitis vinifera × Vitis labrusca, Kyoho grape) as described above in Section 2.6. The grape samples were collected at different times (0, 2, 4, and 5 days after treatment) and stored at −80 °C. The defense-related enzymes’ activities were assayed using the method described by AboElyousr et al. [39]. A reaction mixture lacking the sample was used as the bank. The change in light absorption value at 410 nm by 0.005 per minute per g tissue per mL reaction system was defined as a unit of PPO enzyme activity (U·g−1). At 25 °C, the degradation of H2O2 catalyzed by 1 µmoL per minute per g tissue was defined as a unit of CAT enzyme activity (U·g−1).
2.8. Data Analysis
The data from the three experiments were analyzed using the DPS V12.01 software for the analysis of variance in this study. The means were separated using Duncan’s multiple range test (p = 0.05) when the treatments were significant.
3. Results
3.1. In Vitro Compatibility between Bacterial Isolates
The in vitro compatibility between every two bacterial isolates was investigated. The results showed that different combinations among the three bacterial isolates were compatible (Figure 1). Hence, all the isolates were tested in further experiments.
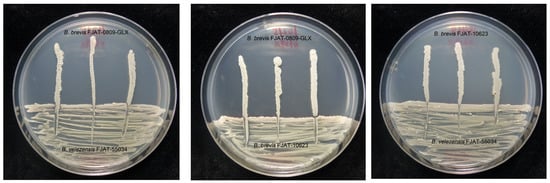
Figure 1.
In vitro compatibility between every two bacterial isolates.
3.2. Inhibitory Activities of Different Microbial Combinations
The inhibition rates of different microbial combination proportions (90%:10%, 80%:20%, 70%:30%) were significantly higher than those of B. brevis FJAT-0809-GLX, FJAT-10623, and FJAT-55034 (Figure 2). The inhibition rate of the microbial combination proportion 80%:20% was significantly higher than those of other combination proportions. The combination proportion of 50%:50% resulted in the lowest inhibition rate. Therefore, the microbial combination proportion of 80%:20% was chosen for further study.
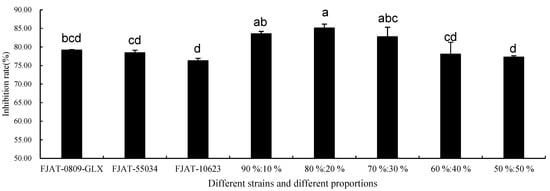
Figure 2.
Inhibition rate of different microbial combinations against B. cinerea FJAT-32835 at 25 °C for 5 d. Vertical bars represent standard error. Values followed by different letters are significantly different according to Duncan’s multiple range test (p < 0.05).
The inhibitory effect of different dilutions of the mixed microbial combination against B. cinerea FJAT-32835 was different (Figure 3). There was no significant difference among the inhibitory rates of 0, 10, and 100 dilutions. Additionally, gradually decreasing inhibition rates were observed with 200 and 500 dilutions.
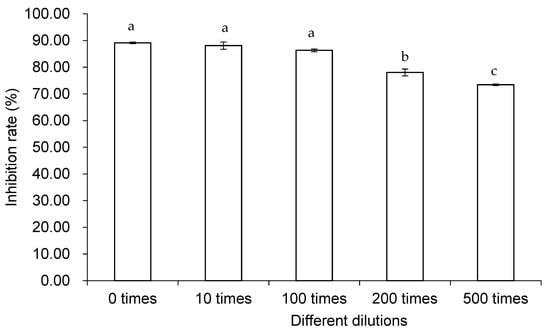
Figure 3.
Inhibition rates of different dilutions of the mixed microbial combination against B. cinerea FJAT-32835 at 25 °C for 5 d. Vertical bars represent standard error. Values followed by different letters are significantly different according to Duncan’s multiple range test (p < 0.05).
3.3. Sensitivity of Mixed Microbial Combination to Different Conditions
Different heat treatments at various temperatures (30 °C, 50 °C, 70 °C, 100 °C, and 121 °C) were found to have an impact on the antifungal activity of the mixed microbial combination. The mixed microbial combination appeared to be insensitive to 30 °C but sensitive to higher temperatures. Its inhibition rates significantly decreased with increasing temperature (Figure 4). The mixed microbial combination displayed no antifungal activity at 100 °C or above.
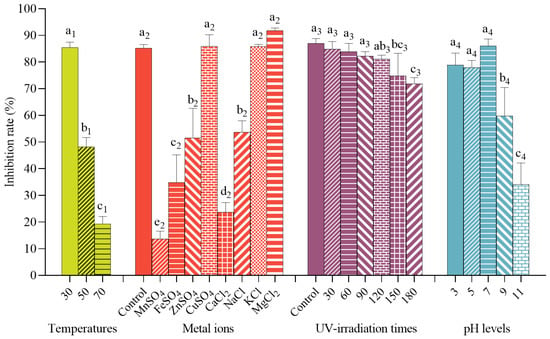
Figure 4.
Antifungal activities of the mixed microbial combination against B. cinerea FJAT-32835 under different conditions at 25 °C for 5 d. Vertical bars represent standard error. Values followed by different letters are significantly different according to Duncan’s multiple range test (p < 0.05).
Antifungal activities were found to be significantly stable from pH 3 to pH 7 (Figure 4). The mixed microbial combination had the strongest inhibition rate at pH 7.0. It showed the lowest inhibition rate at pH 11.
The stability of the mixed microbial combination treated with UV radiation at different times was assessed. Antifungal activities exhibited significant stability with UV radiation for 30 min, 60 min, and 90 min (Figure 4). However, the antifungal activity was stable up to 120 min of UV radiation.
Volumes of 100 µL of 1 mol/L KCl, MgCl2, CuSO4, NaCl, ZnSO4, FeSO4, CaCl2, and MnSO4 were added to the 4 mL of mixed microbial combinations, respectively, and the antifungal activities are presented in Figure 4. Amounts of 0.025 mol mL−1 KCl, CuSO4, and MgCl2 had no effects on the antifungal activity of the mixed microbial combination. In contrast, 0.025 mol mL−1 NaCl, ZnSO4, FeSO4, CaCl2, and MnSO4 could decrease the inhibition rates of the mixed microbial combination. Among them, the mixed microbial combination treated with 0.025 mol mL−1 MnSO4 showed the lowest inhibition rate.
3.4. Antifungal Activity of Mixed Microbial Combination In Vitro
The in vitro antifungal activity of the mixed microbial combination against different pathogens was significantly different. The mixed microbial combination had the strongest antifungal activities against B. cinerea FJAT-32045 and FJAT-32835. Additionally, it also exhibited antifungal activities against fungal pathogens such as L. theobromae and L. pseudotheobromae. However, its inhibition rates against C. orbiculare and C.acutatum were comparatively lower.
3.5. Effect of Mixed Microbial Combination on Decay of Grape Fruits and Leaves In Vivo
As shown in Figure 5 and Figure 6, there were significant differences in the decay percentages between the treatment group and the control group. The decay percentages of grape fruits and leaves in the treatment group were significantly lower than that of the control group.
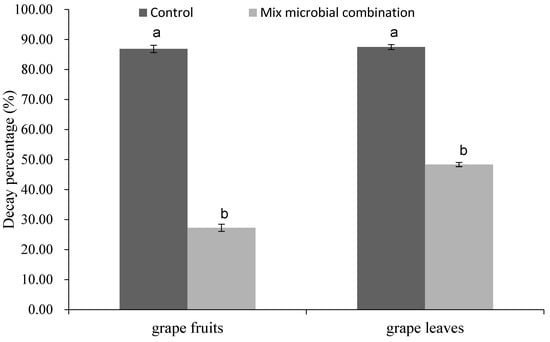
Figure 5.
The decay percentages of the grape fruits and grape leaves treated with mixed microbial combination for 10 d and 5 d at 25 °C, respectively. Vertical bars represent standard error. Values followed by different letters are significantly different according to Duncan’s multiple range test (p < 0.05).

Figure 6.
Effects of mixed microbial combination treatment on the rot of grape fruits and grape leaves ((A): grape fruits, (B): grape leaves). Photographs were taken on day 10 (A) and 5 (B) of incubation at 25 °C.
3.6. PPO and CAT Activities of Grape Fruits Treated with Mixed Microbial Combination
To further investigate the mechanism of the mixed microbial combination against B. cinerea, we analyzed the PPO and CAT activities. Our results showed that the PPO activity of grape fruits initially increased and subsequently decreased upon treatment with the mixed microbial combination, as well as in the control group. However, the PPO activity was consistently higher in the treatment group compared to the control group during the culture time (Figure 7A). Furthermore, while the CAT activity increased during the whole culture period in the treatment group, it first increased and then decreased in the control group. Notably, the CAT activity of treatment at 5 days was higher than that of the control group (Figure 7B).

Figure 7.
Changes in PPO and CAT activities of grape fruits treated with mixed microbial combination for 0, 2, 4, and 5 days at 25 °C. ((A). Activities of PPO, (B). Activities of CAT). Vertical bars represent standard error. Values followed by different letters are significantly different according to Duncan’s multiple range test (p < 0.05).
4. Discussion
The utilization of the mixed microbial combination could broaden the scope of adaptation and enhance the biocontrol effect [40,41]. The disease incidence caused by F. proliferatum on muskmelon was significantly reduced when fruit was inoculated with the mixture of D. hansenii + S. rhizophila [29]. Trichoderma spp. and Pseudomonas fluorescence alone were able to prevent 92% and 96% of Ralstonia solanacearum infection, and the combination of both was more effective, preventing 97% [42]. Our investigation also indicated that B. brevis FJAT-0809-GLX mixed with other Bacillus sp. had a significantly higher suppression rate than either alone. Additionally, we found that the inhibition rates were different when the microbial combination proportion was different. This result is consistent with Li et al. [43], who reported that the biocontrol ability of different ratios of mixed bacteria A1 and C-9 was significantly different, with the best ratio being 1:9.
The inhibitory effect of the biocontrol agent was influenced by various factors, including temperature, pH, and UV radiation [33]. The inhibitory effects of the mixture of Bacillus subtilis GLB191 and Bacillus pumilus GLB197 decreased gradually with different dilutions [15], which we also observed in our study while comparing the biocontrol ability of different dilutions. The mixed microbial combination seemed to be insensitive to 30 °C and to be stable to pH and UV radiation. These results are consistent with Li et al. [44], who reported that filtrates of Bacillus amyloliquefaciens BA17 were sensitive to heat, had an optimum efficacy at pH 8.0, and were relatively resistant to ultraviolet irradiation. Additionally, these results are also in line with the partial properties of B. brevis FJAT-0809-GLX, which was stable against pH changes [34]. In our study, FeSO4 could decrease the inhibition rate of the mixed microbial combination. However, this is not the case for Bacillus subtilis, where the combination of ferrous sulfate and B. subtilis had a synergistic effect on the prevention and control of pepper blight [38].
Microbial biological control agents could reduce the use of chemical pesticides due to their wide-ranging antimicrobial activity [45]. The mixed microbial combination exhibited a broad spectrum of antifungal activity, leading to the biocontrol of two or more postharvest diseases [26]. Our study also showed that the mixed microbial combination had antifungal activity against various fungi. In addition, the mixed microbial combination could significantly decrease the decay percentage of grape fruits and grape leaves. The data obtained are also lower than those reported in previous studies where the decay percentages of Bacillus sp. Ka3, A10, and kh26 on grape bunches ranged from 50% to 70% in their ability to reduce B. cinerea growth [14]. To our knowledge, this is the first report demonstrating that a combination of B. brevis FJAT-0809-GLX with other bacterial strains has the capability to reduce the decay rate of grape fruits and leaves.
One of the most important mechanisms of biological control agents is the induction of systemic resistance [46]. Different types of defense enzymes, such as PPO and CAT, have been found to play important roles in inducing disease resistance in plants [39]. GABA-treated Sporidiobolus pararoseus Y16 was found to effectively increase the activities of PPO, POD, PAL, and the content of total phenols and flavonoids in grapes [47]. According to our findings, PPO and CAT activities were significantly increased in grape fruits treated with the mixed microbial combination. Similar results were observed by Kabdwal et al. [48], who found that the combination of Trichoderma harzianum and Pseudomonas could increase the PPO activities of tomato plants. Wu et al. [49] also reported that yeast T-2 could decrease the rot rate of grape fruits caused by B. cinerea and increase the activities of catalase (CAT) during the longest storage time.
5. Conclusions
In summary, we introduced a novel biological control agent comprising Brevibacillus brevis and Bacillus velezensis for the control of B. cinerea. The research findings demonstrated that the mixed microbial combination retained its antifungal activity under various conditions. The mixed microbial combination effectively suppressed the growth of B. cinerea in vitro, and reduced the decay percentage of grape fruits and grape leaves in vivo. In addition, the mixed microbial combination increased the activities of defense enzymes PPO and CAT. We believe that this mixed microbial combination can be used as a potential biocontrol agent for the green control of grape gray mold disease.
Author Contributions
J.C., C.L. and B.L. designed the experiments, Q.C. and G.L. (Guohong Liu) prepared the materials. J.C., C.L. and G.L. (Gongti Lai) performed the experiments. J.C. and G.L. (Gongti Lai) analyzed the data and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by the Fujian Province Public Welfare Project (2021R1034001, 2021R10320013) and High Quality Development ‘5511’ Collaborative Innovation Project between Fujian and Chinese Academy of Agricultural Sciences Agricultural (XTCXGC2021019).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Solairaj, D.; Legrand, N.N.G.; Yang, Q.Y.; Zhang, H.Y. Isolation of pathogenic fungi causing postharvest decay in table grapes and in vivo biocontrol activity of selected yeasts against them. Physiol. Molecul. Plant Pathol. 2020, 110, 101478. [Google Scholar] [CrossRef]
- Renzo, F.; Lucia, L.; Gianfranco, R. Analyses of sylem vessel size on grapevine cultivars and relationship with incidence of esca disease a threat to grape quality. Appl. Sci. 2022, 12, 1177. [Google Scholar]
- Xu, W.T.; Huang, K.L.; Guo, F.; Qu, W.; Yang, J.J.; Liang, Z.H.; Luo, Y.B. Postharvest grapefruit seed extract and chitosan treatments of table grapes to control Botrytis cinerea. Postharvest Biol. Technol. 2007, 46, 86–94. [Google Scholar] [CrossRef]
- Evelyn, S.M.; Jocelyn, B.E.; Miguel, L.; Juan, R.; Iván, B.; Reinaldo, C.V.; Rubén, P. Effect of cuticular waxes compounds from table grapes on growth, germination and gene expression in Botrytis cinerea. World J. Microbiol. Biotechnol. 2016, 32, 74. [Google Scholar]
- Marsico, A.D.; Velenosi, M.; Perniola, R.; Bergamini, C.; Sinonin, S.; David, V.V.; Maggiolini, F.A.M.; Hervè, A.; Cardone, M.F.; Ventura, M. Native vineyard non-saccharomyces yeasts used for biological control of Botrytis cinerea in stored table grape. Microorganisms 2021, 9, 457. [Google Scholar] [CrossRef]
- Junior, O.J.C.; Youssef, K.; Koyama, R.; Ahmed, S.; Dominguez, A.R.; Mühlbeier, D.T.; Roberto, S.R. Control of gray mold on clamshell-packaged ‘benitaka’ table grapes using sulphur dioxide pads and perforated liners. Pathogens 2019, 8, 271. [Google Scholar] [CrossRef]
- Karabulut, O.A.; Gabler, F.M.; Mansour, M.; Smilanick, J.L. Postharvest ethanol and hot water treatments of table grapes to control gray mold. Postharvest Biol. Technol. 2004, 34, 169–177. [Google Scholar] [CrossRef]
- Caterina, R.; Rita, M.D.M.A.; Crescenza, D.; Stefania, P.; Giulio, F.; Michele, D.C.; Donato, P.; Patrizia, N.; Francesco, F. Use of biocontrol agents and botanicals in integrated management of Botrytis cinerea in table grape vineyards. Pest Manag. Sci. 2018, 74, 715–725. [Google Scholar]
- Zhang, H.Y.; Godana, E.A.; Sui, Y.; Yang, Q.Y.; Zhang, X.Y.; Zhao, L.N. Biological control as an alternative to synthetic fungicides for the management of grey and blue mould diseases of table grapes: A review. Crit. Rev. Microbiol. 2020, 46, 450–462. [Google Scholar] [CrossRef]
- Latorre, B.A.; Spadaro, I.; Rioja, M.E. Occurrence of resistant strains of Botrytis cinerea to anilinopyrimidine fungicides in table grapes in Chile. Crop Protect. 2002, 21, 957–961. [Google Scholar] [CrossRef]
- Calvo, H.; Marco, P.; Blanco, D.; Oria, R.; Venturini, M.E. Potential of a new strain of Bacillus amyloliquefaciens BUZ-14 as a biocontrol agent of postharvest fruit diseases. Food Microbiol. 2017, 63, 101–110. [Google Scholar] [CrossRef]
- Jing, X.; Zheng, Z.; Li, X.P.; Wei, J.; Wu, B. Effect of nitrous oxide against Botrytis cinerea and phenylpropanoid pathway metabolism in table grapes. Sci. Horticult. 2019, 254, 99–105. [Google Scholar]
- Wafaa, M.H. Isolation of bioactive antibiotic peptides from Bacillus brevis and Bacillus polymyxa against Botrytis grey mould in strawberry. Arch. Phytopathol. Plant Protect. 2008, 41, 477–491. [Google Scholar]
- Kasfi, K.; Taheri, P.; Jafarpour, B.; Saeed, T. Identification of epiphytic yeasts and bacteria with potential for biocontrol of grey mold disease on table grapes caused by Botrytis cinerea. Span. J. Agric. Res. 2018, 16, 23. [Google Scholar] [CrossRef]
- Wang, H.L.; Shan, W.H.; Hu, H.Y.; Li, Y.; Wang, Q.; Wang, K.; Bian, F.G. Control effect of mixed inoculation of different biocontrol strains on Botrytis cinerea. Chin. J. Biol. Control 2020, 36, 265–271. [Google Scholar]
- Luo, L.; Zhou, L.X.; Liu, Y. Preliminary probe on antagonistic mechanisms of the Pichia pastoris G5 against Botrytis cinerea. Biotech. Bullet. 2017, 33, 210–215. [Google Scholar]
- Garrido, C.C.; Usall, J.; Torres, R.; Teixidό, N. Effective control of Botrytis bunch rot in commercial vineyards by large-scale application of Candida sake CPA-1. BioControl 2017, 62, 161–173. [Google Scholar] [CrossRef]
- Santiago, R.; Huiliñir, C.; Cottet, L.; Castillo, A. Microbiological characterization for a new wild strain of Paenibacillus polymyxa with antifungal activity against Botrytis cinerea. BioControl 2016, 103, 251–260. [Google Scholar] [CrossRef]
- Zheng, T.; Zhang, P.A.; Zhang, K.K.; Jiu, S.T.; Zhu, X.D.; Song, C.N.; Jia, H.F.; Fang, J.G. Isolation and identification of endophytes from grape bleeding sap and their disease resistance function analysis. Acta Hortic. Sin. 2018, 45, 2106–2120. [Google Scholar]
- Boubakri, H.; Hadj-Brahim, A.; Schmitt, C.; Soustre-Gacougnolle, I.; Mliki, A. Biocontrol potential of chenodeoxycholic acid (CDCA) and endophytic Bacillus subtilis strains against the most destructive grapevine pathogens. N. Z. J. Crop Hort. 2015, 43, 261–274. [Google Scholar] [CrossRef]
- Steinke, K.; Mohite, O.S.; Weber, T.; Kovács, Á.T. Phylogenetic distribution of secondary metabolites in the Bacillus subtilis species complex. mSystems 2021, 6, e00057-21. [Google Scholar] [CrossRef]
- Nunes, C.A. Biological control of postharvest diseases of fruit. Eur. J. Plant Pathol. 2012, 133, 181–196. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, J.; Zhou, Y.H.; Deng, L.L.; Yao, S.X.; Zeng, K.F. Inhibitory effect of Pichia membranaefaciens and Kloeckera apiculata against Monilinia fructicola and their biocontrol ability of brown rot in postharvest plum. Biol. Control 2017, 114, 51–58. [Google Scholar] [CrossRef]
- Barbosa, L.O.; Lima, J.S.; Magalhães, V.C.; Gava, C.A.T.; Soares, A.C.F.; Marbach, P.A.S.; Souza, J.T.D. Compatibility and combination of selected bacterial antagonists in the biocontrol of sisal bole rot disease. BioControl 2018, 63, 595–605. [Google Scholar] [CrossRef]
- Nada, O.; Vallance, J.; Gerbore, J.; Yacoub, A.; Rey, P. Combining potential oomycete and bacterial biocontrol agents as a tool to fight tomato rhizoctonia root rot. BioControl 2021, 155, 104521–104532. [Google Scholar]
- Sharma, R.R.; Singh, D.; Singh, R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonistis: A review. BioControl 2009, 50, 205–221. [Google Scholar]
- Gao, Y.H.; Zheng, Z.H.; Zhang, Y.; Hu, Y.G.; Wang, X.F. Mechanism of rhizosphere micro-ecology in controlling soil-borne fungal diseases: A review. J. China Agric. Univ. 2021, 26, 100–113. [Google Scholar]
- Janisiewicz, W.J.; Korsten, L. Biological control of postharvest diseases of fruit. Annu. Rev. Phytopathol. 2002, 40, 411–441. [Google Scholar] [CrossRef]
- Tomas, R.G.; Bernardo, M.A.; Alejandra, N.G.; Gabriel, R.E.; Roberto, G.C.C.; Luis, G. Hernandez-Montiel. Enhanced biocontrol of fruit rot on muskmelon by combination treatment with marine Debaryomyces hansenii and Stenotrophomonas rhizophila and their potential modes of action. Postharvest Biol. Technol. 2019, 151, 61–67. [Google Scholar]
- Mao, T.T.; Tao, G.; Zhao, X.L.; Wang, Q.; Li, S.D. Biological control of four kinds of microbial preparations against main diseases of pepper. Chin. J. Biol. Control 2020, 36, 258–264. [Google Scholar]
- Bouqellah, N.; Naureen, Z.; Woodwards, S. Multitrophic interactions between Fusarium oxysporum f. sp. lycopersici and Brevibacillus brevis and their role in induction of defense enzymes in tomato plants. Curr. Opin. Biotech. 2011, 22, 47. [Google Scholar] [CrossRef]
- Edwards, S.G.; Seddon, B. Mode of antagonism of Brevibacillus brevis against Botrytis cinerea in vitro. J. Appl. Microbiol. 2001, 91, 652–659. [Google Scholar] [CrossRef]
- Che, J.M.; Liu, B.; Chen, Z.; Shi, H.; Liu, G.H.; Ge, C.B. Identification of ethylparaben as the antimicrobial substance produced by Brevibacillus brevis FJAT-0809-GLX. Microbiol. Res. 2015, 172, 48–56. [Google Scholar]
- Che, J.M.; Liu, B.; Liu, G.H.; Chen, Q.Q.; Huang, D.D. Induced mutation breeding of Brevibacillus brevis FJAT-0809-GLX for improving ethylparaben production and its application in the biocontrol of Lasiodiplodia theobromae. Postharvest Biol. Technol. 2018, 146, 60–67. [Google Scholar] [CrossRef]
- Che, J.M.; Liu, B.; Lin, Y.Z.; Tang, W.Q.; Tang, J.Y. Draft genome sequence of biocontrol bacterium Brevibacillus brevis strain FJAT-0809-GLX. Genome Announc. 2013, 1, e00160-13. [Google Scholar] [CrossRef]
- Che, J.M.; Liu, B.; Ruan, C.Q.; Tang, J.Y.; Huang, D.D. Biocontrol of Lasiodiplodia theobromae, which causes black spot disease of harvested wax apple fruit, using a strain of Brevibacillus brevis FJAT-0809-GLX. Crop Prot. 2015, 67, 178–183. [Google Scholar] [CrossRef]
- Che, J.M.; Liu, G.H.; Liu, B.; Chen, Q.Q.; Chen, M.C. Control of banana postharvest antracnose by Brevibacillu sp. strains. Microbiol. China 2020, 47, 1753–1762. [Google Scholar]
- Du, G.F.; Li, X.L.; Qi, Z.Q.; Shen, L.B.; Han, X.; Qin, Y.L.; Cao, Z.M.; Yang, Y. Inhibitory mechanism of ferrous sulfate combined with Bacillus subtilis on Phytophthora capsici. Plant Protect. 2020, 46, 142–149. [Google Scholar]
- AboElyousr, K.A.M.; Ibrahim, O.H.M.; AlQurashi, A.D.; Mousa, M.A.A.; Saad, M.M. Biocontrol potential of endophytic fungi for the eco-friendly management of root rot of Cuminum cyminum caused by Fusarium solani. Agronomy 2022, 12, 2612. [Google Scholar] [CrossRef]
- Mao, W.; Lumsden, R.D.; Lewis, J.A.; Hebbar, P.K. Seed treatment using pre-infiltration and biocontrol agents to reduce damping-off of corn caused by species of Pythium and Fusarium. Plant Dis. 1998, 3, 294–299. [Google Scholar] [CrossRef]
- Zhimo, V.Y.; Biasi, A.; Kumar, A.; Feygenberg, O.; Salim, S.; Vero, S.; Wisniewski, M.; Droby, S. Yeasts and bacterial consortia from kefir grains are effective biocontrol agents of postharvest diseases of fruits. Microorgainisms 2020, 8, 428. [Google Scholar] [CrossRef]
- Shiva, Y.; Ramesh, G.C.; Binayak, R.P. Evaluation of Trichoderma spp., Pseudomonas fluorescence and Bacillus subtilis for biological control of Ralstonia wilt of tomato. F1000Research 2017, 6, 2028. [Google Scholar]
- Li, G.; Zhao, H.; Liu, Y.Y.; Zhang, M.T.; Pang, X.B.; Guo, J.Q.; Li, X.Y.; Zhu, J.B.; Wang, A.Y. Antagonistic effect of the mixed bacteria culture on Verticillium dahlia. Chin. J. Biol. Control 2018, 34, 431–439. [Google Scholar]
- Li, Y.; Cai, Y.; Liang, Y.; Ji, P.; Xu, L. Assessment of antifungal activities of a biocontrol bacterium BA17 for managing postharvest gray mold of green bean caused by Botrytis cinerea. Postharvest Biol. Technol. 2020, 161, 111086. [Google Scholar] [CrossRef]
- Sui, X.N.; Han, X.B.; Cao, J.M.; Li, Y.Q.; Yuan, Y.; Gou, J.Y.; Zheng, Y.F.; Meng, C.; Zhang, C.S. Biocontrol potential of Bacillus velezensis EM-1 associated with suppressive rhizosphere soil microbes against tobacco bacterial wilt. Front. Microbiol. 2022, 13, 940156. [Google Scholar] [CrossRef]
- Kashyap, A.S.; Manzar, N.; Nebapure, S.M.; Rajawat, M.V.S.; Deo, M.M.; Singh, J.P.; Kesharwani, A.K.; Singh, R.P.; Dubey, S.C.; Singh, D. Unraveling microbial volatile elicitors using a transparent methodology for induction of systemic resistance and regulation of antioxidant genes at expression levels in Chili against bacterial wilt disease. Antioxidants 2022, 11, 404. [Google Scholar] [CrossRef]
- Xiao, J.W.; Zhao, L.N.; Bai, Y.H.; Lin, R.L.; Legrand, N.N.G.; Dhanasekaran, S.; Li, B.; Gu, X.Y.; Zhang, X.Y.; Zhang, H.Y. The biocontrol efficacy of Sporidiobolus pararoseus Y16 cultured with gamma-aminobutyric acid and its effects on the resistant substances of postharvest grapes. BioControl 2022, 169, 104900. [Google Scholar] [CrossRef]
- Kabdwal, B.C.; Sharma, R.; Kumar, A.; Kumar, S.; Singh, K.P.; Srivastava, R.M. Efficacy of different combinations of microbial biocontrol agents against sheath blight of rice caused by Rhizoctonia solani. Egypt. J. Biol. Pest Control 2023, 33, 29. [Google Scholar] [CrossRef]
- Wu, C.Y.; Wang, Y.C.; Ai, D.; Li, Z.R.; Wang, Y.H. Biocontrol yeast T-2 improves the postharvest disease resistance of grape by stimulation of the antioxidant system. Food Sci. Nutr. 2022, 10, 3219–3229. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).