Psychrotolerant Strains of Phoma herbarum with Herbicidal Activity
Abstract
1. Introduction
2. Materials and Methods
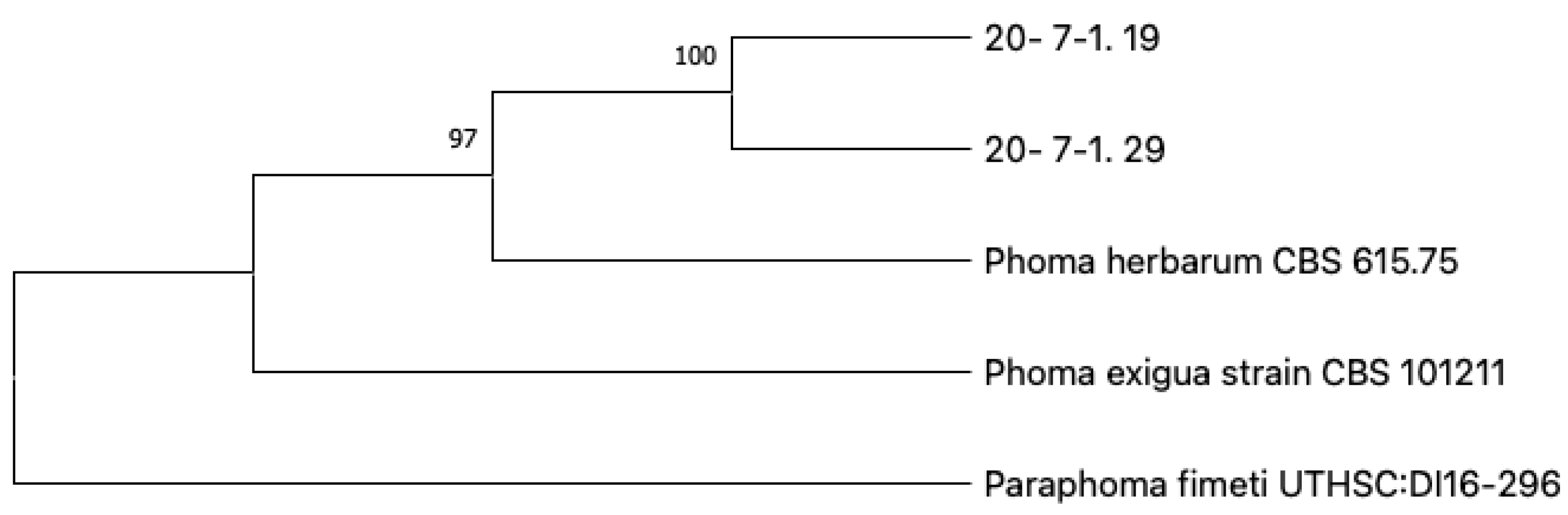
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berestetskiy, A.; Sokornova, S. Production and stabilization of mycoherbicides. In Biological Approaches for Controlling Weeds; Radhakrishnan, R., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Ibrar, M.; Ullah, M.W.; Manan, S.; Farooq, U.; Rafiq, M.; Hasan, F. Fungi from the extremes of life: An untapped treasure for bioactive compounds. Appl. Microbiol. Biotechnol. 2020, 104, 2777–2801. [Google Scholar] [CrossRef]
- Tomova, I.; Stoilova-Disheva, M.; Lazarkevich, I.; Vasileva-Tonkova, E. Antimicrobial activity and resistance to heavy metals and antibiotics of heterotrophic bacteria isolated from sediment and soil samples collected from two Antarctic islands. Front. Life Sci. 2015, 8, 348–357. [Google Scholar] [CrossRef]
- Vaca, I.; Chávez, R. Bioactive compounds produced by Antarctic filamentous fungi. In Fungi of Antarctica; Rosa, L., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A. Synthesis and regulation of fungal secondary metabolites. In Microbial Technology for the Welfare of Society; Arora, P., Ed.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Selbmann, L.; Onofri, S.; Fenice, M.; Federici, F.; Petruccioli, M. Production and structural characterization of the exopolysaccharide of the Antarctic fungus Phoma herbarum CCFEE 5080. Res. Microbiol. 2002, 153, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Gade, A.; Zimowska, B.; Ingle, A.P.; Ingle, P. Marine-derived Phoma—The gold mine of bioactive compounds. Appl. Microbiol. Biotechnol. 2018, 102, 9053–9066. [Google Scholar] [CrossRef] [PubMed]
- Waill, A.E.; Ghoson, M.D. The exceptional endophytic fungi, Emericella (Berk.) and Phoma (Sacc.) genera. Int. J. Res. Pharm. Biosci. 2020, 7, 1–6. [Google Scholar]
- Graupner, P.R.; Carr, A.; Clancy, E.; Gilbert, J.R.; Bailey, K.L.; Derby, J.-A.; Gerwick, B.C. The macrocidins: Novel cyclictetramic acids with herbicidal activity produced by Phoma macrostoma. J. Nat. Prod. 2003, 66, 1558–1561. [Google Scholar] [CrossRef]
- Liu, S.-S.; Jiang, J.-X.; Huang, R.; Wang, Y.-T.; Jiang, B.-G.; Zheng, K.-X.; Wu, S.-H. A new antiviral 14-nordrimane sesquiterpenoid from an endophytic fungus Phoma sp. Phytochem. Lett. 2019, 29, 75–78. [Google Scholar] [CrossRef]
- Ghoran, S.H.; Taktaz, F.; Ayatollahi, S.A.; Kijjoa, A. Anthraquinones and their analogues from marine-derived fungi: Chemistry and biological activities. Mar. Drugs 2022, 20, 474. [Google Scholar] [CrossRef]
- Pitt, W.M.; Bailey, K.L.; Fu, Y.-B.; Peterson, G.W. Biological and genetic characterisation of Phoma macrostoma isolates with bioherbicidal activity. Biocontrol Sci. Technol. 2012, 22, 813–835. [Google Scholar] [CrossRef]
- Tiwari, V.V.; Gade, A.K.; Rai, M.K. A study of phylogenetic variations among Indian Phoma tropica species by RAPD-PCR and ITS-rDNA sequencing. Ind. J. Biotechnol. 2013, 12, 187–194. [Google Scholar]
- Rai, M.K.; Tiwari, V.V.; Irinyi, L.; Kövics, G.J. Advances in taxonomy of genus Phoma: Polyphyletic nature and role of phenotypic traits and molecular systematics. Indian J. Microbiol. 2014, 54, 123–128. [Google Scholar] [CrossRef]
- Chen, Q.; Hou, L.W.; Duan, W.J.; Crous, P.W.; Cai, L. Didymellaceae revisited. Stud. Mycol. 2017, 87, 105–159. [Google Scholar] [CrossRef]
- Boerema, G.H.; de Gruyter, J.; Noordeloos, M.E.; Hamers, M.E.C. Phoma Identification Manual, 1st ed.; CABI Publishing: Cambridge, MA, USA, 2004. [Google Scholar]
- Sambrook, E.A.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989; p. 479. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Aveskamp, M.M.; Verkley, G.J.M.; de Gruyter, J.; Murace, M.A.; Perello, A.; Woudenberg, J.H.C.; Groenewald, J.Z.; Crous, P.V. DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia 2009, 101, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Coimbra, M.C.; Castro, A.H.F. Different methods for surface sterilization of Pyrostegia venusta (Ker Gawl.) Miers (Bignoniaceae) leaf explants. Plant Cell Cult. Micropropag. 2016, 12, 34–38. [Google Scholar]
- Sokornova, S.V.; Berestetskiy, A.O. Liquid fermentation of Stagonospora cirsii C-163, a potential mycoherbicide for Cirsium arvense (L.) Scop. Agricultural biology 2018, 53, 1054–1061. [Google Scholar] [CrossRef]
- Aveskamp, M.; de Gruyter, J.; Crous, P. Biology and recent developments in the systematics of Phoma, a complex genus of major quarantine significance. Fungal Divers. 2008, 31, 1–18. [Google Scholar]
- Poluektova, E.; Tokarev, Y.; Sokornova, S.; Chisty, L.; Evidente, A.; Berestetskiy, A. Curvulin and Phaeosphaeride A from Paraphoma sp. VIZR 1.46 isolated from Cirsium arvense as potential herbicides. Molecules 2018, 23, 2795. [Google Scholar] [CrossRef]
- Caro, Y.; Venkatachalam, M.; Lebeau, J.; Fouillaud, M.; Dufossé, L. Pigments and colorants from filamentous fungi. In Fungal Metabolites. Reference Series in Phytochemistry; Merillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2015; pp. 1–70. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Taylor, J.L.; Morales, V.M. Phomaligin A and other yellow pigments in Phoma lingam and P. wasabiae. Phytochemistry 1995, 38, 1215–1222. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Taylor, J.L.; Morales, V.M. The blackleg fungus of rapeseed: How many species? Acta Hortic. 1996, 407, 441–446. [Google Scholar] [CrossRef]
- Birch, A.J.; Fryer, R.I.; Thomson, P.J.; Smith, H. Pigments of Phoma terrestris Hansen and their Biosynthesis. Nature 1961, 190, 441–442. [Google Scholar] [CrossRef]
- Bick, I.R.; Rhee, C. Anthraquinone pigments from Phoma foveata Foister. Biochem. J. 1966, 98, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Vikrant, P.; Verma, K.K.; Rajak, R.C.; Pandey, A.K. Characterization of a phytotoxin from Phoma herbarum for management of Parthenium hysterophorus L. J. Phytopathol. 2006, 154, 461–468. [Google Scholar] [CrossRef]
- Ji, M.; Liu, X.; Gao, Y.; Li, X. Synthesis and herbicidal activity evaluation of toxins III from Phoma herbarbum derivatives. Chin. J. Pest. Sci. 2015, 17, 520–529. [Google Scholar]
- Neumann, S.; Boland, G.J. Influence of selected adjuvants on disease severity by Phoma herbarum on dandelion (Taraxacum officinale). Weed Technol. 1999, 13, 675–679. [Google Scholar] [CrossRef]
- Gilardi, G.; Matic, S.; Gullino, M.L.; Garibaldi, A. First report of Phoma herbarum causing leaf spot of woodland sage (Salvia nemorosa) in Northern Italy. Plant Dis. 2017, 101, 1824. [Google Scholar] [CrossRef]
- Schnick, P.J.; Boland, G.J. 2, 4-D and Phoma herbarum to control dandelion (Taraxacum officinale). Weed Sci. 2004, 52, 808–814. [Google Scholar] [CrossRef]
- Rai, M.; Zimowska, B.; Shinde, S.; Tres, M.V. Bioherbicidal potential of different species of Phoma: Opportunities and challenges. Appl. Microbiol. Biotechnol. 2021, 105, 3009–3018. [Google Scholar] [CrossRef]
- Hahn, D.; Sallenave, R.; Pornaro, C.; Leinauer, B. Managing cool-season turfgrass without herbicides: Optimizing maintenance practices to control weeds. Crop Sci. 2020, 60, 2204–2220. [Google Scholar] [CrossRef]
- Quereshi, S.; Khan, N.A.; Pandey, A.K. Anthraquinone pigment with herbicidal potential from Phoma herbarum FGCC#54. Chem. Nat. Compd. 2011, 47, 521–523. [Google Scholar] [CrossRef]
- Khan, A.; Ali, S.; Khan, M.; Hamayun, M.; Moon, Y.S. Parthenium hysterophorus’s endophytes: The second layer of defense against biotic and abiotic stresses. Microorganisms 2022, 10, 2217. [Google Scholar] [CrossRef] [PubMed]
- Mahish, P.K.; Singh, S.; Chauhan, R. Bioactive secondary metabolites from endophytic Phoma spp. In Phoma: Diversity, Taxonomy, Bioactivities, and Nanotechnology; Rai, M., Zimowska, B., Kövics, G.J., Eds.; Springer: Cham, Switzerland, 2022; pp. 205–2019. [Google Scholar] [CrossRef]
- Rivero-Cruz, J.F.; García-Aguirre, G.; Cerda-García-Rojas, C.M.; Mata, R. Conformational behavior and absolute stereostructure of two phytotoxic nonenolides from the fungus Phoma herbarum. Tetrahedron 2000, 56, 5337–5344. [Google Scholar] [CrossRef]
- Rivero-Cruz, J.F.; Marcias, M.; Cerda-García-Rojas, C.M.; Mata, J. A new phytotoxic nonenolide from Phoma herbarum. J. Nat. Prod. 2003, 66, 511–514. [Google Scholar] [CrossRef] [PubMed]


| Treatment Samples | Seed Germination, % | |
|---|---|---|
| Taraxacum officinale | Solidago canadensis | |
| Control (H2O) | 70 | 78 |
| Control (0.05% Ethyl acetate) | 68 | 77 |
| CL M19 | 30 | 48 |
| CFCF M19 | 28 | 21 |
| CME M19 | 10 | 31 |
| CL M29 | 40 | 22 |
| CFCF M29 | 30 | 15 |
| CME M29 | 10 | 32 |
| Metabolite Class | Substance | Phoma-like Fungi Species | Pigment Color | UV λmax/nm | Monoisotopic Mass, g/mol | Ref. |
|---|---|---|---|---|---|---|
| Polyketides | Phomaligin | Phoma lingam, P. wasabiae | Bright yellow | 415, 330, 245 | 311.17327290 | [27] |
| Wasabidienone A | P. wasabiae | Yellow | (CHCl3) 406, 275, 245 | 268.13107373 | [28] | |
| Wasabidienone B, C, E | Brownish yellow | 311.17327290 * | ||||
| Hydroxyanthraquinone | Cynodontin (3-methyl-1,4,5,8-tetrahydroxyanthraquinone) | Setophoma terrestris (=Phoma terrestris) | Bronze | No data | 286.04773803 | [29] |
| Pachybasin (1-hydroxy-3-methylanthracene-9,10-dione 9,10 Anthracenedione, 1-hydroxy-3-methyl- 1-Hydroxy-3-methylanthraquinone) | Phoma exigua var foveata (=Phoma foveata) | Yellow | (EtOH) 403, 281, 252, 224 | 238.062994177 | [30] | |
| Emodin (6-methyl-1,3,8-trihydroxyanthraquinone) | Orange | 437, 289, 265, 252, 222 | 270.05282342 | |||
| Chrysophanol (3-Methylchrysazin 1,8-Dihydroxy-3-methylanthraquinone) | Red | 436, 288, 278, 256, 226 | 254.05790880 | |||
| Phomarin (1,6-dihydroxy-3-methyl-9,10-anthraquinone) | Orange | (MeOH) 215,231, 251, 338, 356, 441 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trigubovich, A.; Mandryk-Litvinkovich, M.; Valakhanovich, A.; Gorodilova, E.; Malygin, D.; Kalamiyets, E.; Sokornova, S. Psychrotolerant Strains of Phoma herbarum with Herbicidal Activity. Agronomy 2023, 13, 1619. https://doi.org/10.3390/agronomy13061619
Trigubovich A, Mandryk-Litvinkovich M, Valakhanovich A, Gorodilova E, Malygin D, Kalamiyets E, Sokornova S. Psychrotolerant Strains of Phoma herbarum with Herbicidal Activity. Agronomy. 2023; 13(6):1619. https://doi.org/10.3390/agronomy13061619
Chicago/Turabian StyleTrigubovich, Andrey, Maryna Mandryk-Litvinkovich, Anastasiya Valakhanovich, Elizaveta Gorodilova, Daniil Malygin, Emiliya Kalamiyets, and Sofia Sokornova. 2023. "Psychrotolerant Strains of Phoma herbarum with Herbicidal Activity" Agronomy 13, no. 6: 1619. https://doi.org/10.3390/agronomy13061619
APA StyleTrigubovich, A., Mandryk-Litvinkovich, M., Valakhanovich, A., Gorodilova, E., Malygin, D., Kalamiyets, E., & Sokornova, S. (2023). Psychrotolerant Strains of Phoma herbarum with Herbicidal Activity. Agronomy, 13(6), 1619. https://doi.org/10.3390/agronomy13061619


_Asaduzzaman.jpg)



