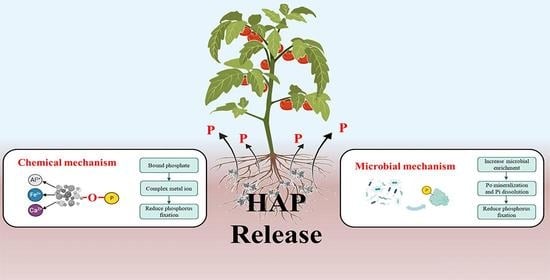
The Effective Combination of Humic Acid Phosphate Fertilizer Regulating the Form Transformation of Phosphorus and the Chemical and Microbial Mechanism of Its Phosphorus Availability
Abstract
1. Introduction
2. Methods
2.1. Preparation for Materials
2.2. Plant Material and Test Methods
2.2.1. Materials and Test Procedure
2.2.2. Sampling and Test Methods
2.3. Characterization Methods
2.4. High-Throughput Sequencing and Bioinformatic Analysis of Microbial Community
2.5. Calculation and Statistical Analysis
3. Results and Discussion
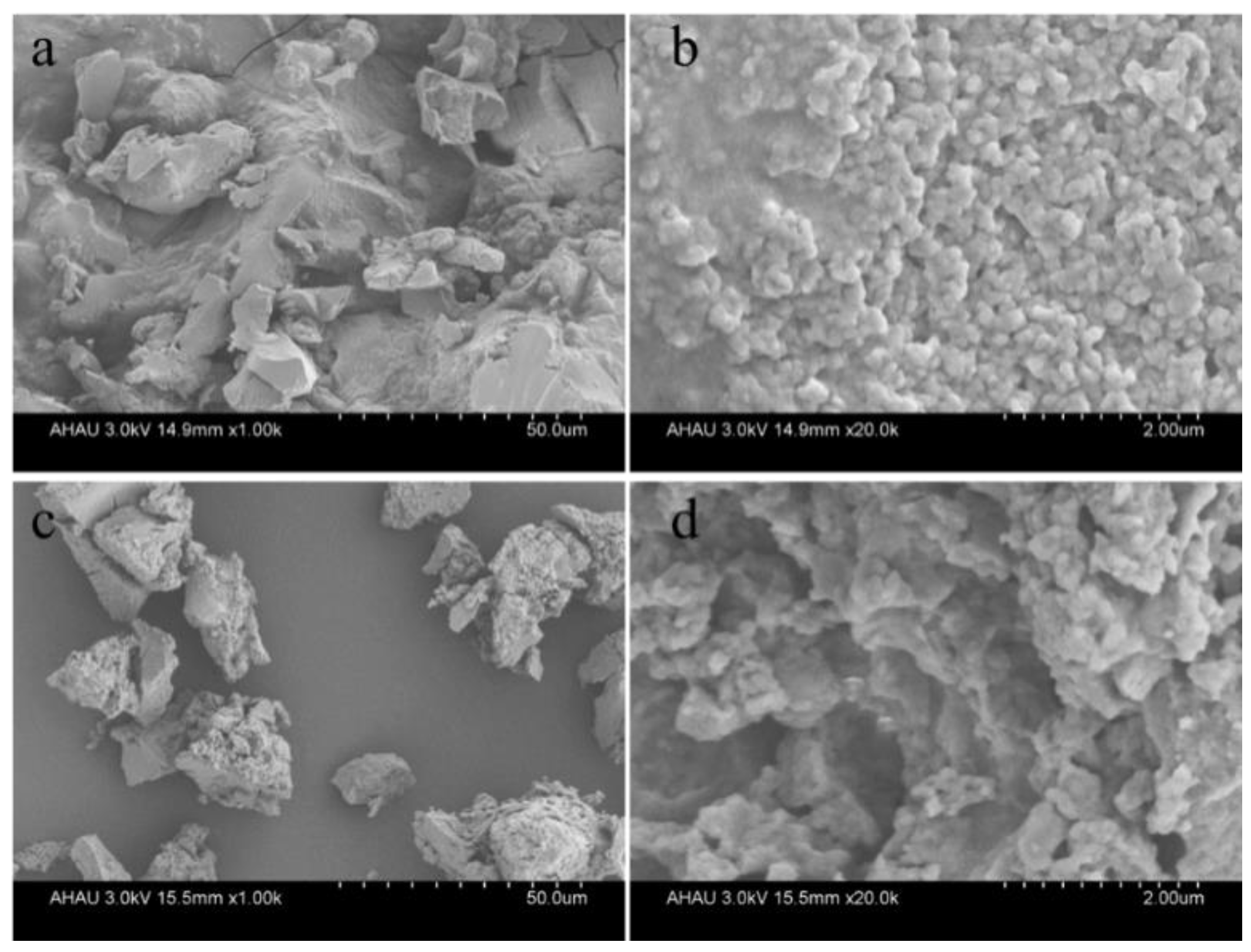
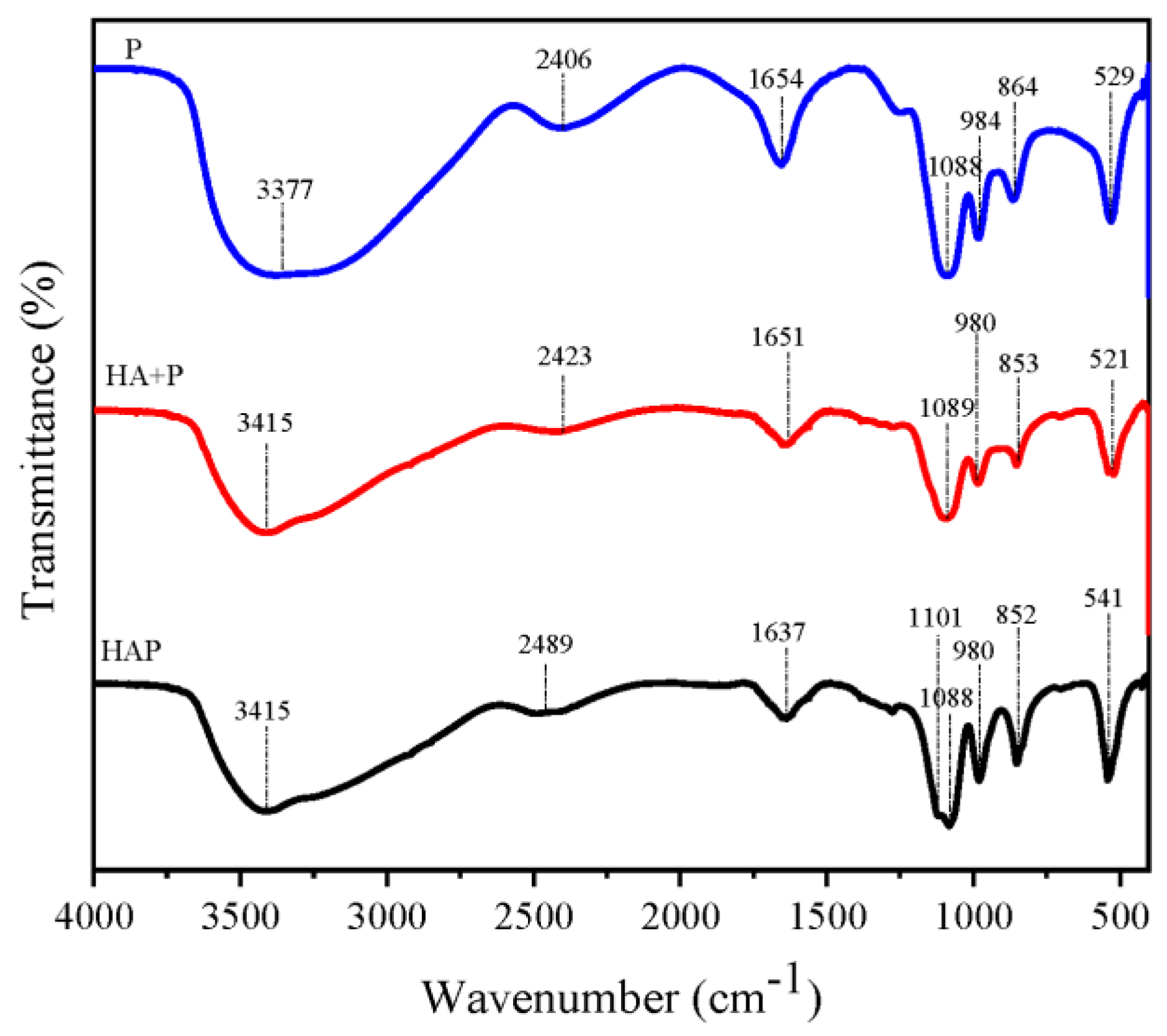
3.1. Structural Characterization of Synthetic Fertilizers
3.2. Mechanism of Chemical Synergistic Effect of Humic acid Phosphate Fertilizer on Phosphorus Availability
3.3. Biological Enhancement Mechanism of Phosphorus Effectiveness by Humic Acid Phosphate Fertilizer
3.4. Growth and Nutrient Absorption Analysis of Tomato Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaya, C.; Şenbayram, M.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Sulfur-enriched leonardite and humic acid soil amendments enhance tolerance to drought and phosphorus deficiency stress in maize (Zea mays L.). Sci. Rep. 2020, 10, 6432. [Google Scholar] [CrossRef] [PubMed]
- Izhar Shafi, M.; Adnan, M.; Fahad, S.; Wahid, F.; Khan, A.; Yue, Z.; Danish, S.; Zafar-ul-Hyu, M.; Brtnicky, M.; Datta, R. Application of Single Superphosphate with Humic Acid Improves the Growth, Yield and Phosphorus Uptake of Wheat (Triticum aestivum L.) in Calcareous Soil. Agronomy 2020, 10, 1224. [Google Scholar] [CrossRef]
- Busato, J.G.; Ferrari, L.H.; Chagas Junior, A.F.; da Silva, D.B.; dos Santos Pereira, T.; de Paula A., M. Trichoderma strains accelerate maturation and increase available phosphorus during vermicomposting enriched with rock phosphate. J. Appl. Microbiol. 2021, 130, 1208–1216. [Google Scholar] [CrossRef]
- Smith, W.F.; Mudge, R.S.; Rae, L.A.; Glassop, D. Phosphate transport in plants. Plant and Soil 2003, 248, 71–83. [Google Scholar] [CrossRef]
- Cheng, L.Y.; Bucciarelli, B.; Shen, J.B.; Allan, D.; Vance, C.P. Update on whitelupin cluster root acclimation to phosphorus deficiency. Plant Physiol. 2011, 156, 1025–1032. [Google Scholar] [CrossRef]
- Rashad, M.; Hafez, M.; Popov, A.I. Humic substances composition and properties as an environmentally sustainable system: A review and way forward to soil conservation. J. Plant Nutr. 2022, 45, 1072–1122. [Google Scholar] [CrossRef]
- Ji, Q.-K.; Wang, D.; Yang, W.-B.; Han, Y.-R.; Ma, W.-Q.; Wei, J. Effects of long-term phosphorus application on crop yield, phosphorus absorption, and soil phosphorus accumulation in maize-wheat rotation system. J. Appl. Ecol. 2021, 32, 2469. [Google Scholar]
- Hafez, M.; Popov, A.I.; Rashad, M. Evaluation of the effects of new environmental additives compared to mineral fertilizers on the leaching characteristics of some anions and cations under greenhouse plant growth of saline-sodic soils. Open Agric. J. 2020, 14, 246–256. [Google Scholar] [CrossRef]
- Li, B.Y.; Zhou, D.M.; Cang, L.; Zhang, H.L.; Fan, X.H.; Qin, S.W. Soil micronutrient availability to crops as affected by long-term inorganic and organic fertilizer applications. Soil Tillage Res. 2007, 96, 166–173. [Google Scholar] [CrossRef]
- Barrow, N.J. Soil phosphate chemistry and the P-sparing effect of previous phosphate applications. Plant Soil 2015, 397, 401–409. [Google Scholar] [CrossRef]
- Balemi, T.; Negisho, K. Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: A review. J. Soil Sci. Plant Nutr. 2012, 12, 547–561. [Google Scholar] [CrossRef]
- Mpanga, I.K.; Ludewig, U.; Dapaah, H.K.; Neumann, G. Acquisition of rock phosphate by combined application of ammonium fertilizers and Bacillus amyloliquefaciens FZB42 in maize as affected by soil pH. J. Appl. Microbiol. 2020, 129, 947–957. [Google Scholar] [CrossRef]
- Tian, D.; Li, Z.; O’Connor, D.; Shen, Z.; Hou, D. The need to prioritize sustainable phosphate-based fertilizers. Soil Use Manag. 2020, 36, 351. [Google Scholar] [CrossRef]
- Heuer, S.; Gaxiola, R.; Schilling, R.; Herrera-Estrella, L.; López-Arredondo, D. Wissuwa, M.; Delhaize, E.; Rouached, H. Improving phosphorus use efficiency: A complex trait with emerging opportunities. Plant J. 2017, 90, 868–885. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci. Total Environ. 2017, 612, 522–537. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, L.; Li, Y.; Zhao, B. Characterization of ultrafiltration-fractionated humic acid derived from chinese weathered coal by spectroscopic techniques. Agronomy 2021, 11, 2030. [Google Scholar] [CrossRef]
- Guo, X.X.; Liu, H.T.; Wu, S.B. Humic substances developed during organic waste composting: Formation mechanisms, structural properties, and agronomic functions. Sci. Total Environ. 2019, 662, 501–510. [Google Scholar] [CrossRef]
- Ghaderimokri, L.; Rezaei-Chiyaneh, E.; Ghiyasi, M.; Gheshlaghi, M.; Battaglia, M.L.; Siddique, K.H.M. Application of humic acid and biofertilizers changes oil and phenolic compounds of fennel and fenugreek in intercropping systems. Sci. Rep. 2022, 12, 5946. [Google Scholar] [CrossRef]
- Zanin, L.; Tomasi, N.; Cesco, S.; Varanini, Z.; Pinton, R. Humic Substances Contribute to Plant Iron Nutrition Acting as Chelators and Biostimulants. Front. Plant Sci. 2019, 10, 675. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Song, J.; Du, Q.; Li, G.; Tarakina, N.V.; Antonietti, M. Synthetic humic acids solubilize otherwise insoluble phosphates to improve soil fertility. Angew. Chem. Int. Ed. 2019, 58, 18813–18816. [Google Scholar] [CrossRef]
- Tang, W.W.; Zeng, G.M.; Gong, J.L.; Liang, J.; Xu, P.; Zhang, C.; Huang, B.B. Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: A review. Sci. Total Environ. 2014, 468–469, 1014–1027. [Google Scholar] [CrossRef]
- Olk, D.C.; Bloom, P.R.; Perdue, E.M.; McKnight, D.M.; Chen, Y.; Farenhorst, A.; Senesi, N.; Chin, Y.-P.; Schmitt-Kopplin, P.; Hertkorn, N.; et al. Environmental and agricultural relevance of humic fractions extracted by alkali from soils and natural waters. J. Environ. Qual. 2019, 48, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liu, J.; Huo, L.; Li, Y.; Xia, L.; Zhou, Z.; Zhang, M.; Li, B. Humic acids derived from Leonardite to improve enzymatic activities and bioavailability of nutrients in a calcareous soil. Int. J. Agric. Biol. Eng. 2020, 13, 200–205. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Yang, X. Effect of organic matter on phosphorus adsorption and desorption in a black soil from Northeast China. Soil Tillage Res. 2019, 187, 85–91. [Google Scholar] [CrossRef]
- Jing, J.; Zhang, S.; Yuan, L.; Li, Y.; Lin, Z.; Xiong, Q.; Zhao, B. Combining humic acid with phosphate fertilizer affects humic acid structure and its stimulating efficacy on the growth and nutrient uptake of maize seedlings. Sci. Rep. 2020, 10, 17502. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, S.; Chen, G. Impact of humic acids on phosphorus retention and transport. J. Soil Sci. Plant Nutr. 2020, 20, 2431–2439. [Google Scholar] [CrossRef]
- Lin, Y.; Muoroe, P.; Joseph, S.; Henderson, R.; Ziolkowski, A. Water extractable organic carbon in untreated and chemical treated biochars. Chemosphere 2012, 87, 151–157. [Google Scholar] [CrossRef]
- Yan, L.J.; Bai, Y.H.; Zhao, R.F.; Li, F.; Xie, K. Correlation between coal structure and release of the two organic compounds during pyrolysis. Fuel 2015, 145, 12–17. [Google Scholar] [CrossRef]
- Sun, R.; Niu, J.; Luo, B.; Wang, X.; Li, W.; Zhang, W.; Wang, F.; Zhang, C.; Ye, X. Substitution of manure for mineral P fertilizers increases P availability by enhancing microbial potential for organic P mineralization in greenhouse soil. Front. Bioeng. Biotechnol. 2022, 10, 1078226. [Google Scholar] [CrossRef]
- Sun, R.; Wang, X.; Tian, Y.; Guo, Y.; Feng, X.; Sun, H.; Liu, X.; Liu, B. Long-term amelioration practices reshape the soil microbiome in a coastal saline soil and alter the richness and vertical distribution differently among bacterial, archaeal, and fungal communities. Front. Microbiol. 2022, 12, 4143. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Ding, J.; Li, H.; Wang, X.; Li, W.; Li, K.; Ye, X.; Sun, S. Mitigating nitrate leaching in cropland by enhancing microbial nitrate transformation through the addition of liquid biogas slurry. Agric. Ecosyst. Environ. 2023, 345, 108324. [Google Scholar] [CrossRef]
- Sintax, E.R.C. A simple non-bayesian taxonomy classifier for 16S and ITS sequences/RC edgar. bioRxiv, 2016; bioRxiv:074161. [Google Scholar]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, W.; Liu, Y.; Yun, W.; Luo, B.; Chai, R.; Zhang, C.; Xiang, X.; Su, X. Changes in phosphorus mobilization and community assembly of bacterial and fungal communities in rice rhizosphere under phosphate deficiency. Front. Microbiol. 2022, 13, 953340. [Google Scholar] [CrossRef]
- Sun, R.; Wang, F.; Hu, C.; Liu, B. Metagenomics reveals taxon-specific responses of the nitrogen-cycling microbial community to long-term nitrogen fertilization. Soil Biol. Biochem. 2021, 156, 108214. [Google Scholar] [CrossRef]
- Sun, R.; Chen, Y.; Han, W.; Dong, W.; Zhang, Y.; Hu, C.; Liu, B.; Wang, F. Different contribution of species sorting and exogenous species immigration from manure to soil fungal diversity and community assemblage under long-term fertilization. Soil Biol. Biochem. 2020, 151, 108049. [Google Scholar] [CrossRef]
- Joo, J.C.; Shackelford, C.D.; Reardon, K.F. Association of humic acid with metal (hydr)oxide-coated sands at solid–water interfaces. J. Colloid Interface Sci. 2008, 317, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Negre, M.; Leone, P.; Trichet, J.; Défarge, C.; Boero, V.; Gennari, M. Characterization of model soil colloids by cryo-scanning electron microscopy. Geoderma 2004, 121, 1–16. [Google Scholar] [CrossRef]
- Sun, G.F.; Jin, J.Y.; Shi, Y.L. Advances in the effect of humic acidand modified lignin on availability to crops. Chin. J. Soil Sci. 2011, 42, 1003–1009. [Google Scholar]
- Wang, X.L.; Liang, C.H.; Ma, Z.H.; Han, Y. Effects of phosphate and zeolite on the transformation of Cd speciation in Soil. Environ. Sci. 2015, 36, 1437–1444. [Google Scholar]
- Nasir, S.; Sarfaraz, T.B.; Verheyen, T.V.; Chaffee, A.L. Structural elucidation of humic acids extracted from Pakistani lignite usingspectroscopic and thermal degradative techniques. Fuel Process Technol. 2011, 92, 983–991. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Gao, Q.; Xie, Z.L.; Yang, J.; Liu, J. Adsorption of nitrification inhibitor nitrapyrin by humic acid and fulvic acid in black soil:characteristics and mechanism. RSC Adv. 2020, 11, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Schild, B.D.; Marquardt, C.M. Analysis of Th(IV)-humate by XPS. Radiochim. Acta 2000, 88, 587–591. [Google Scholar] [CrossRef]
- Huang, R.X.; Tang, Y.Z. Speciation dynamics of phosphorus during (Hydro)thermal treatments of sewage sludge. Environ. Sci. Technol. 2015, 49, 14466–14474. [Google Scholar] [CrossRef]
- Qian, T.T.; Yang, Q.; Chu, F.J.D.; Dong, F.; Zhou, Y. Transformation of phosphorus in sewage sludge biochar mediated by a phosphate-solubilizing microorganism. Chem. Eng. J. 2018, 359, 1573–1580. [Google Scholar] [CrossRef]
- Cao, N.; Chen, X.P.; Cui, Z.L.; Zhang, F. Change in soil available phosphorus in relation to the phosphorus budget in China. Nutr. Cycl. Agroecosystems 2012, 94, 161–170. [Google Scholar] [CrossRef]
- Rosling, A.; Midgley, M.G.; Cheeke, T.; Urbina, H.; Fransson, P.; Phillips, R.P. Phosphorus cycling in deciduous forest soil differs between stands dominated by ecto-and arbuscular mycorrhizal trees. New Phytol. 2016, 209, 1184–1195. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Zhou, L.P.; Yuan, L.; Zhao, B.Q.; Li, Y.; Lin, Z. Advances in research on the mechanism of humic acid promoting root growth of crops. Humic. Acid 2019, 23, 13–18. [Google Scholar]
- Baghaie, A.H.; Aghili, F. Contribution of Piriformospora indica on improving the nutritional quality of greenhouse tomato and its resistance against cu toxicity after humic acid addition to soil. Environ. Sci. Pollut. Res. Int. 2021, 28, 64572–64585. [Google Scholar] [CrossRef] [PubMed]
- Canellas, L.P.; Dobbss, L.B.; Oliveira, A.L.; Chagas, J.G.; AAguiar, N.O.; Rumjanek, V.M.; Novotny, E.H.; Olivares, F.L.; Spaccini, R. Piccolo, A. Chemical properties of humic matter as related to induction of plant lateral roots. Eur. J. Soil Sci. 2012, 63, 315–324. [Google Scholar] [CrossRef]
- Wojciula, A.; Wiater, J. Trace Elements in Consumer Plants. J. Ecol. Eng. 2019, 20, 252–256. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Yuan, L.; Li, Y.; Lin, Z.; Wang, L.; Zhao, B. Structure analysis of citric acid modified phosphorus fertilizer and its effect on fixation rate of water-soluble phosphorus. Plant Nutr. Fertil. Sci. 2021, 27, 878–885. [Google Scholar]
- Gerke, J. Review Article: The effect of humic substances on phosphate and iron acquisition by higher plants: Qualitative and quantitative aspects. J. Plant Nutr. Soil Sci. 2021, 184, 329–338. [Google Scholar] [CrossRef]
- Erro, J.; Urrutia, O.; Baigorri, R.; Aparicio-Tejo, P.; Irigoyen, I.; Storino, F.; Mandado, M.; Yvin, J.C.; Garcia-Mina, J.M. Organic complexed superphosphates (CSP): Physicochemical characterization and agronomical properties. J. Agric. Food Chem. 2012, 60, 2008–2017. [Google Scholar] [CrossRef]

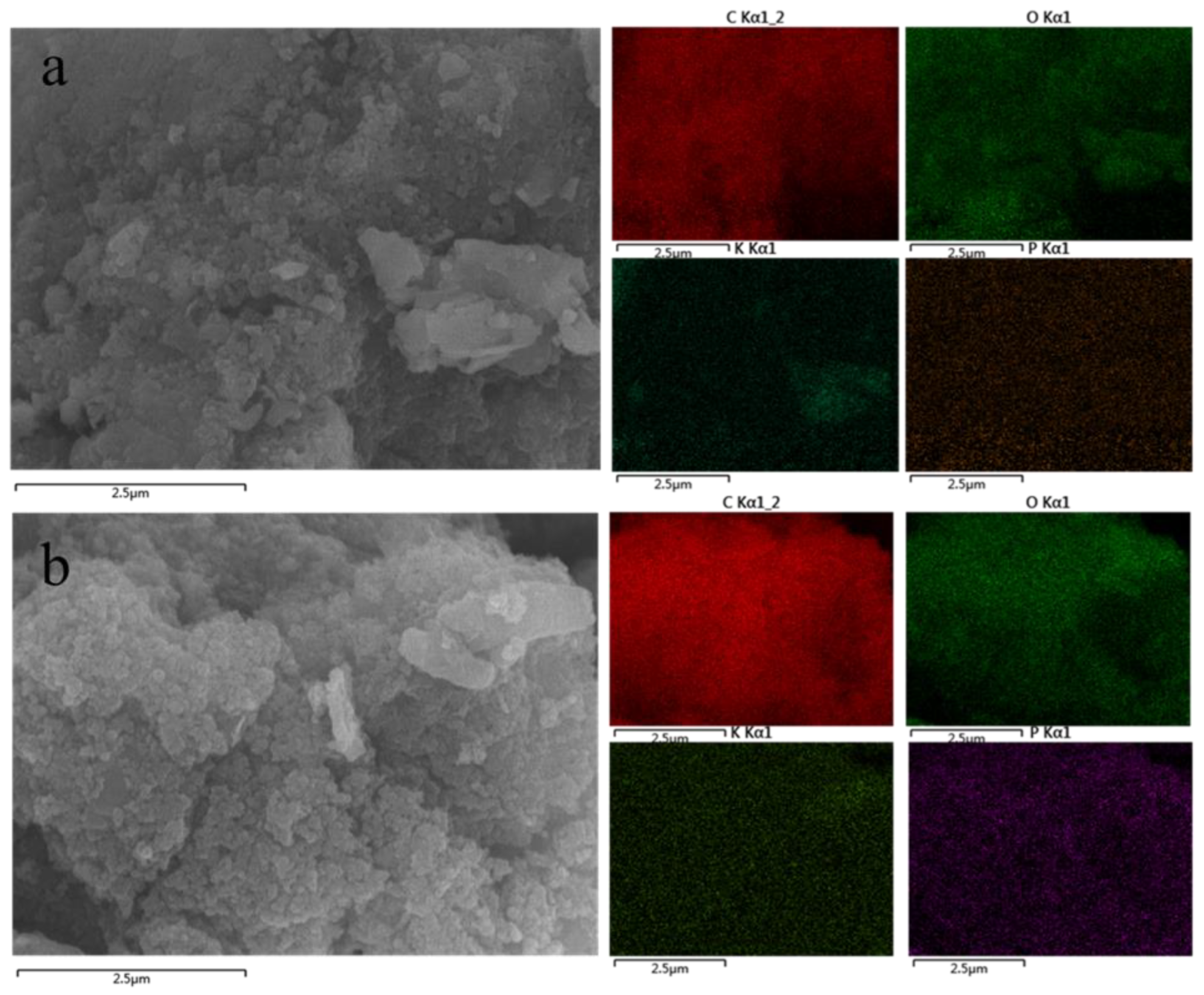
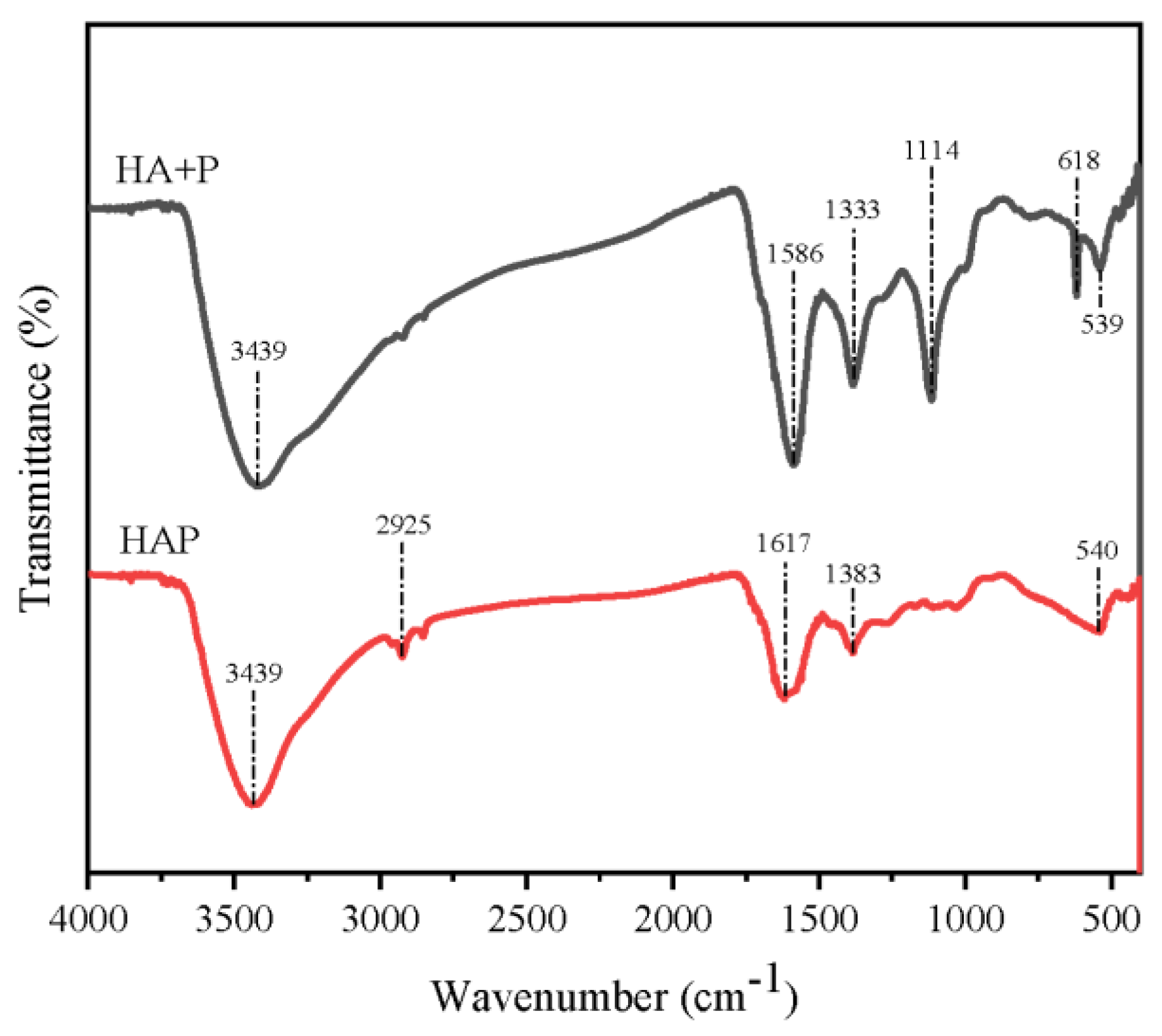



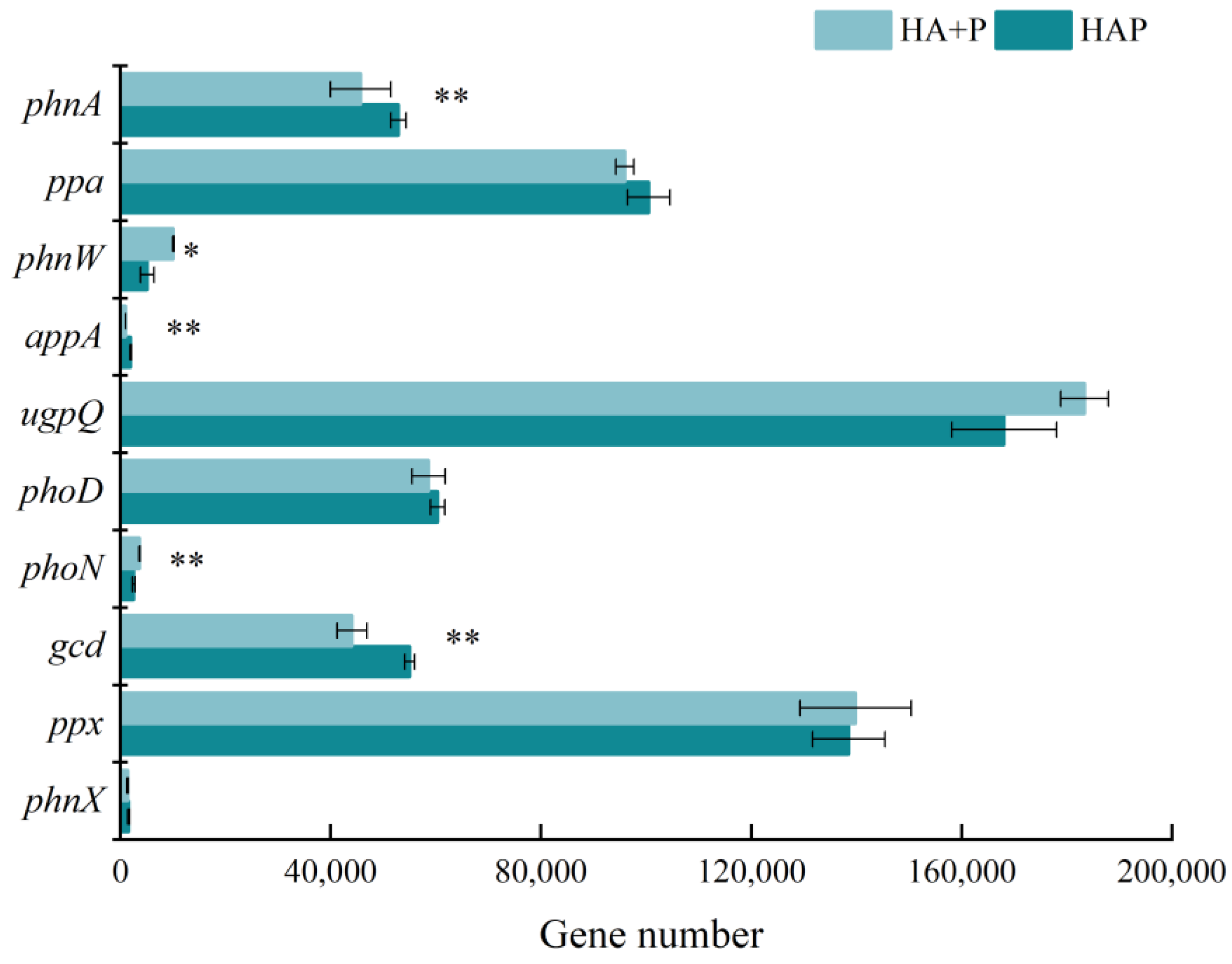

| Treatments | Mass Fraction of HA+P and HAP Residue Elements (%) | |||
|---|---|---|---|---|
| C | O | K | P | |
| HA+P | 69.8 | 25.6 | 3.8 | 0.8 |
| HAP | 78.0 | 18.7 | 2.9 | 0.3 |
| Treatments | Relative Content of Functional Groups Containing Carbon (%) | |||||
|---|---|---|---|---|---|---|
| CAlk 0–65 ppm | CAlk-O 65–90 ppm | CAr-H,R 90–140 ppm | CAr-O 140–156 ppm | CCOO-H,R 156–190 ppm | CC=O 190–250 ppm | |
| HA+P | 8.04 | 1.64 | 63.86 | 6.99 | 12.87 | 6.60 |
| HAP | 7.51 | 1.44 | 63.22 | 7.49 | 13.66 | 6.68 |
| P Type | HA+P | HAP | ||
|---|---|---|---|---|
| Binding Energy (eV) | Relative Content (%) | Binding Energy (eV) | Relative Content (%) | |
| H2PO4− | 133.66 | 30.12 | 133.78 | 31.83 |
| HPO42− | 132.82 | 45.94 | 133.10 | 38.95 |
| PO43− | 132.18 | 23.94 | 132.26 | 29.22 |
| Treatments | Plant Height (cm) | Stem Diameter (cm) | Biomass (g·Plant−1) | |
|---|---|---|---|---|
| Shoot | Root | |||
| CK | 36.95 ± 3.35 b | 6.27 ± 0.70 a | 3.31 ± 0.31 b | 0.18 ± 0.05 b |
| P | 41.10 ± 0.70 ab | 7.33 ± 0.68 a | 5.20 ± 0.69 a | 0.44 ± 0.14 a |
| HAP | 44.83 ± 1.46 a | 7.23 ± 0.51 a | 5.33 ± 1.33 a | 0.50 ± 0.05 a |
| HA+P | 40.77 ± 2.06 ab | 6.37 ± 2.00 a | 4.70 ± 0.31 ab | 0.34 ± 0.10 ab |
| SHAP | 42.10 ± 3.32 a | 6.50 ± 0.53 a | 4.87 ± 0.73 a | 0.36 ± 0.12 ab |
| Treatments | Plant Underground Part | Plant Above-Ground Part | ||||
|---|---|---|---|---|---|---|
| N Accumulation (g·Plant−1) | P Accumulation (g·Plant−1) | K Accumulation (g·Plant−1) | N Accumulation (g·Plant−1) | P Accumulation (g·Plant−1) | K Accumulation (g·Plant−1) | |
| CK | 0.82 ± 0.10 ab | 0.77 ± 0.23 c | 5.53 ± 2.86 b | 0.67 ± 0.21 a | 1.80 ± 0.17 b | 26.14 ± 1.20 a |
| P | 0.82 ± 0.04 ab | 1.22 ± 0.13 ab | 15.12 ± 5.92 a | 0.78 ± 0.02 a | 1.85 ± 0.18 b | 28.69 ± 2.06 a |
| HAP | 0.82 ± 0.03 ab | 1.49 ± 0.24 a | 16.05 ± 1.44 a | 0.77 ± 0.01 a | 1.88 ± 0.21 b | 28.73 ± 1.49 a |
| HA+P | 0.90 ± 0.02 a | 1.17 ± 0.17 abc | 12.76 ± 2.89 ab | 0.78 ± 0.01 a | 1.92 ± 0.21 b | 27.89 ± 2.01 a |
| SHAP | 0.79 ± 0.03 b | 0.93 ± 0.32 bc | 12.59 ± 5.27 ab | 0.70 ± 0.20 a | 2.50 ± 0.35 a | 29.9 ± 2.82 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Q.; Wang, S.; Lu, X.; Xu, Y.; Zhang, L.; Chen, X.; Xu, G.; Tian, D.; Zhang, L.; Jing, J.; et al. The Effective Combination of Humic Acid Phosphate Fertilizer Regulating the Form Transformation of Phosphorus and the Chemical and Microbial Mechanism of Its Phosphorus Availability. Agronomy 2023, 13, 1581. https://doi.org/10.3390/agronomy13061581
Xiong Q, Wang S, Lu X, Xu Y, Zhang L, Chen X, Xu G, Tian D, Zhang L, Jing J, et al. The Effective Combination of Humic Acid Phosphate Fertilizer Regulating the Form Transformation of Phosphorus and the Chemical and Microbial Mechanism of Its Phosphorus Availability. Agronomy. 2023; 13(6):1581. https://doi.org/10.3390/agronomy13061581
Chicago/Turabian StyleXiong, Qizhong, Shaojie Wang, Xuewei Lu, Yating Xu, Lei Zhang, Xiaohui Chen, Gang Xu, Da Tian, Ligan Zhang, Jianyuan Jing, and et al. 2023. "The Effective Combination of Humic Acid Phosphate Fertilizer Regulating the Form Transformation of Phosphorus and the Chemical and Microbial Mechanism of Its Phosphorus Availability" Agronomy 13, no. 6: 1581. https://doi.org/10.3390/agronomy13061581
APA StyleXiong, Q., Wang, S., Lu, X., Xu, Y., Zhang, L., Chen, X., Xu, G., Tian, D., Zhang, L., Jing, J., & Ye, X. (2023). The Effective Combination of Humic Acid Phosphate Fertilizer Regulating the Form Transformation of Phosphorus and the Chemical and Microbial Mechanism of Its Phosphorus Availability. Agronomy, 13(6), 1581. https://doi.org/10.3390/agronomy13061581