Integrated Anatomical and Transcriptomic Analysis Revealed the Molecular Mechanism of the Healing Process in Homografted and Heterografted Seedlings of Acanthopanax senticosus
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Grafting Method
2.3. Microscopic Examination of Anatomical Structures
2.3.1. Scanning Electron Microscopy Examination
2.3.2. Histological Sectioning Examination
2.3.3. Vascular Reconnection Analysis
2.4. RNA Extraction, cDNA Library Construction and Illumina Sequencing
2.5. De Novo Transcriptome Assembly and Functional Annotation
2.6. Screening and Enrichment of DEGs
2.7. Quantitative Real-Time PCR Analysis
2.8. Statistical Analysis
3. Results
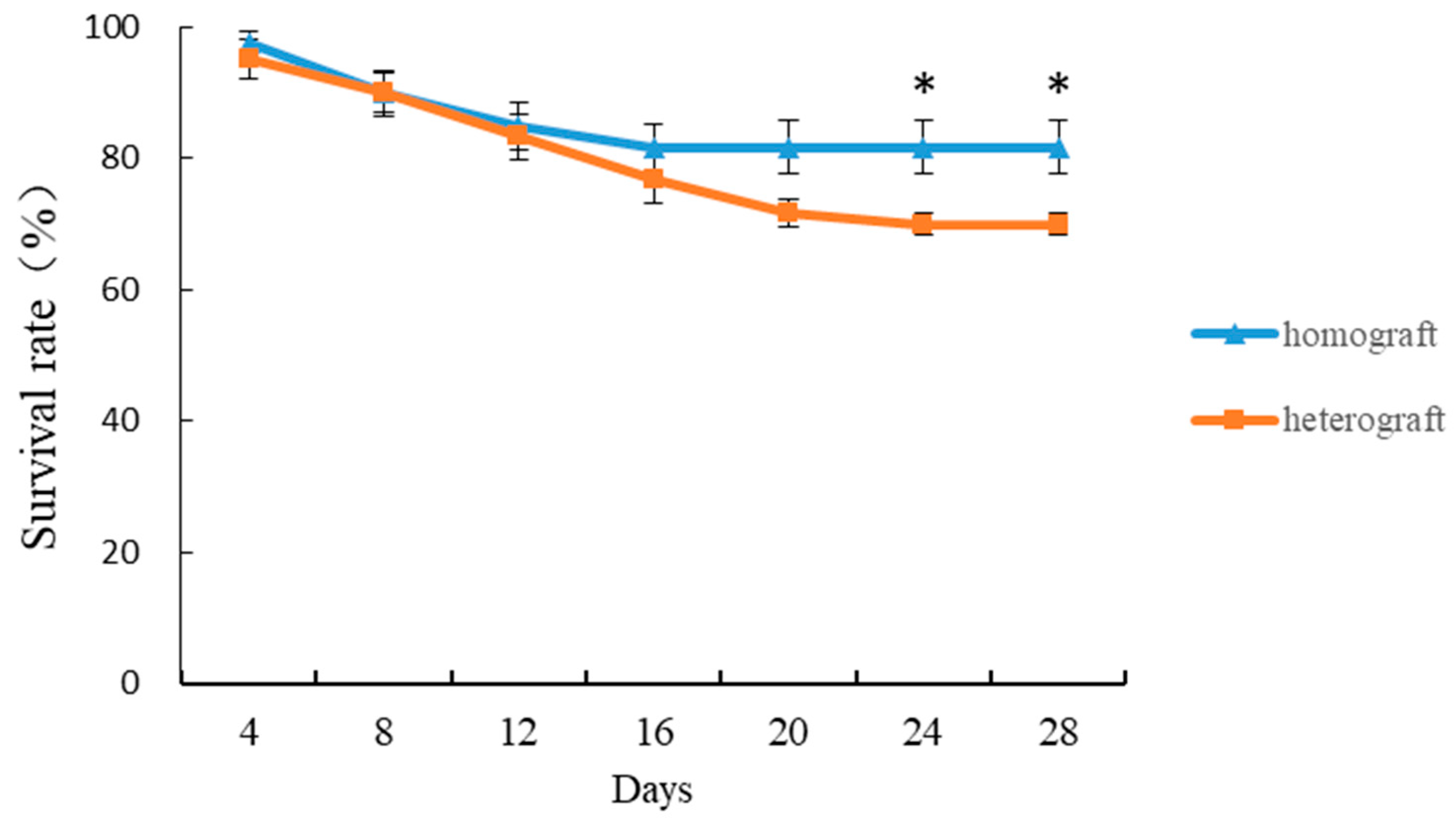
3.1. Survival Rate of Homografted and Heterografted Seedlings
3.2. Morphological Observation of Homografted and Heterografted Seedlings
3.3. Microscopic Observation of Homografted and Heterografted Seedlings
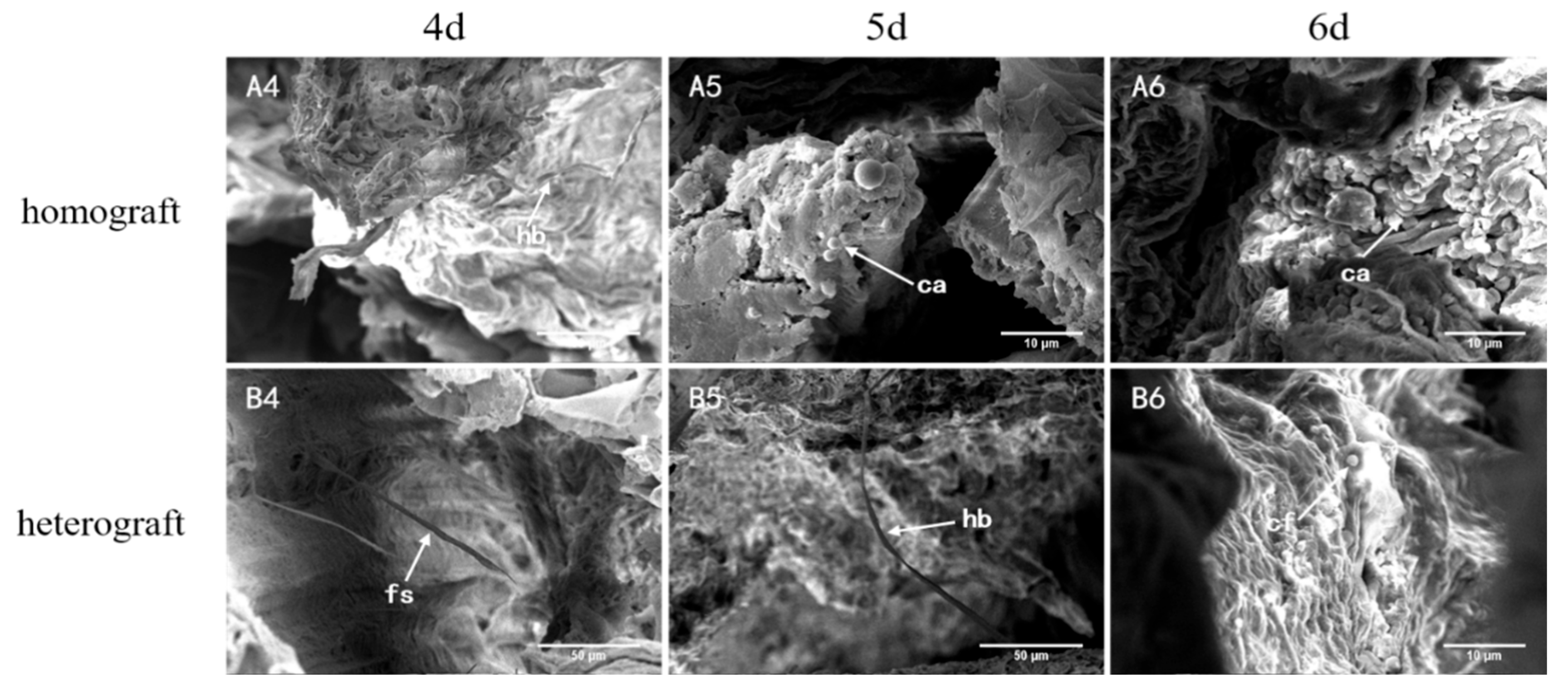
3.3.1. Scanning Electron Microscope Observation
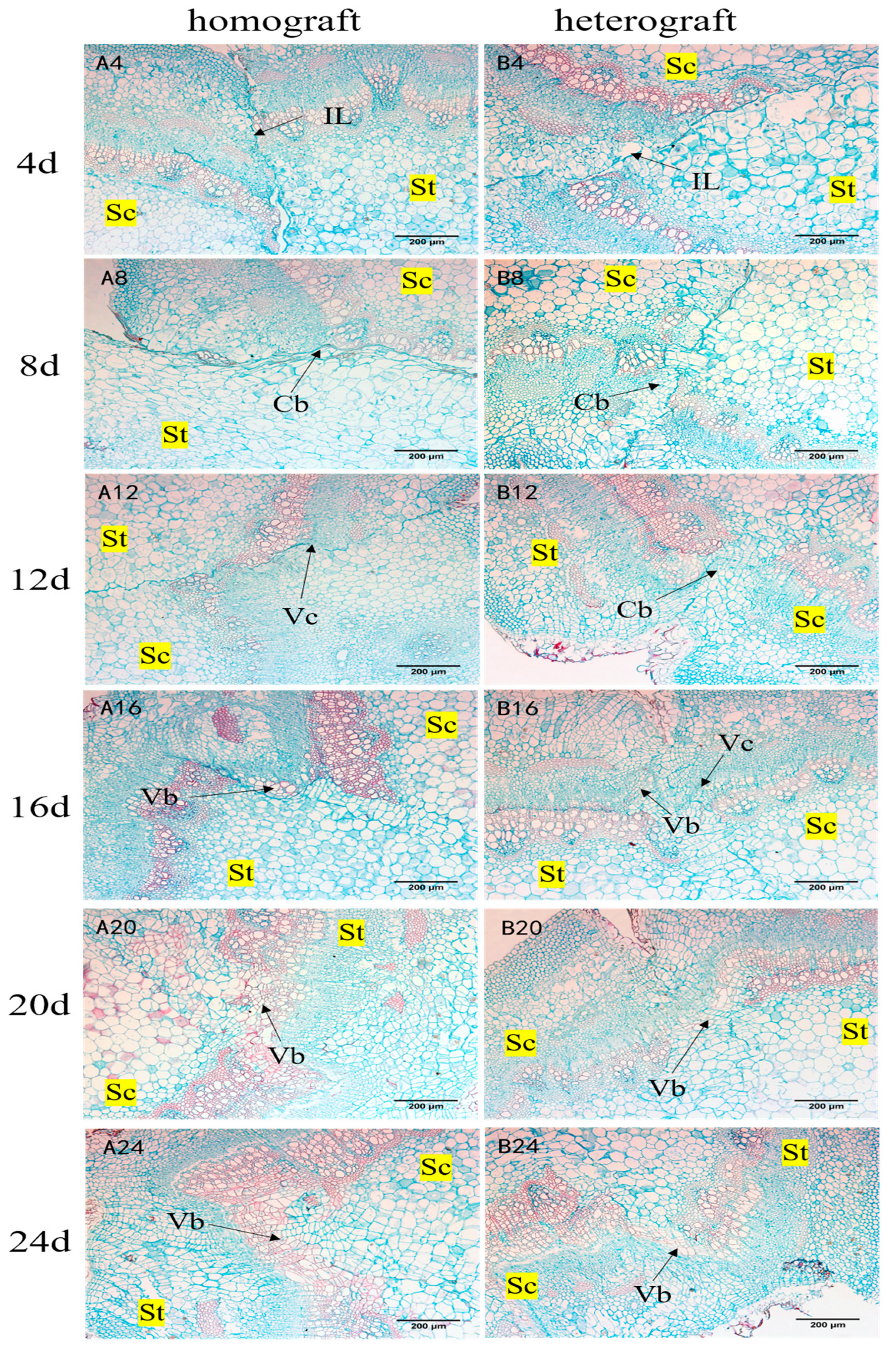
3.3.2. Paraffin Section Observation
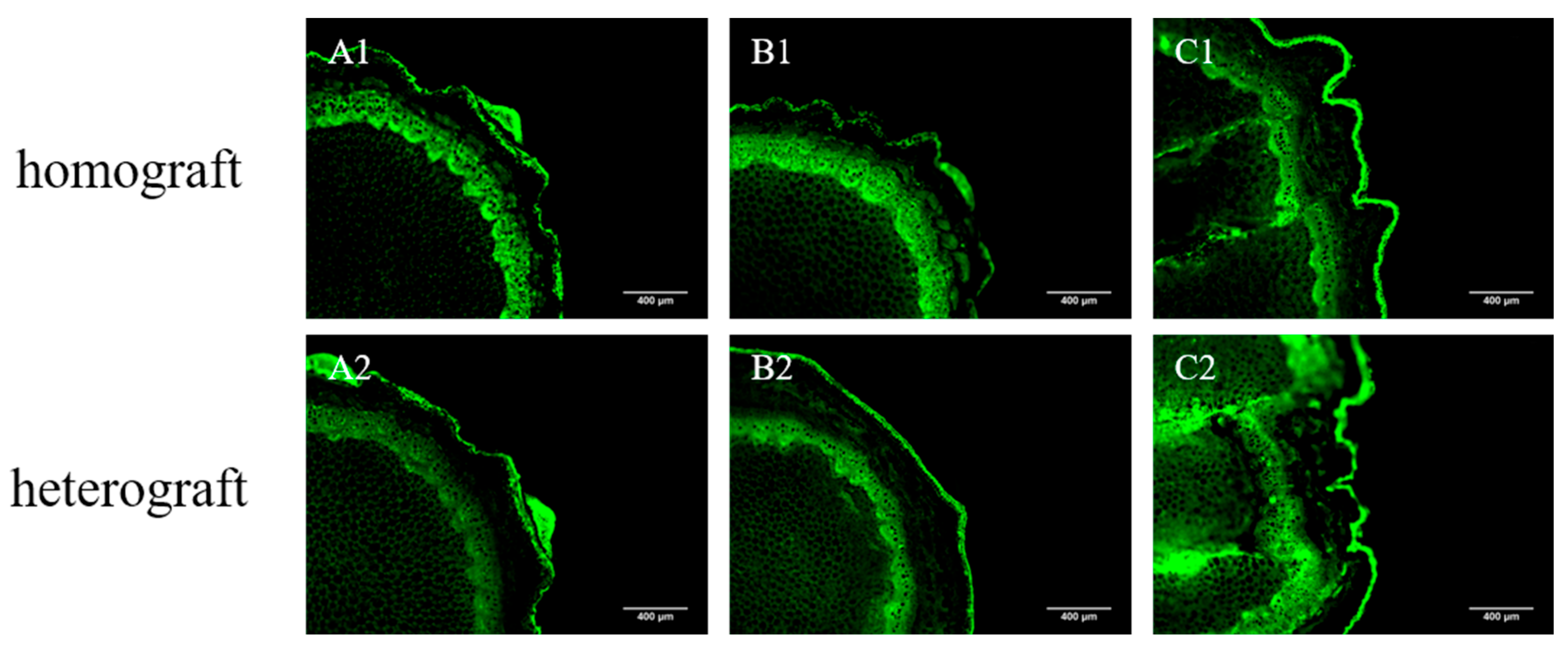
3.4. Vascular Tissue Connectivity of Homografted and Heterografted Seedlings
3.5. RNA Sequence Data Analysis and Functional Annotation
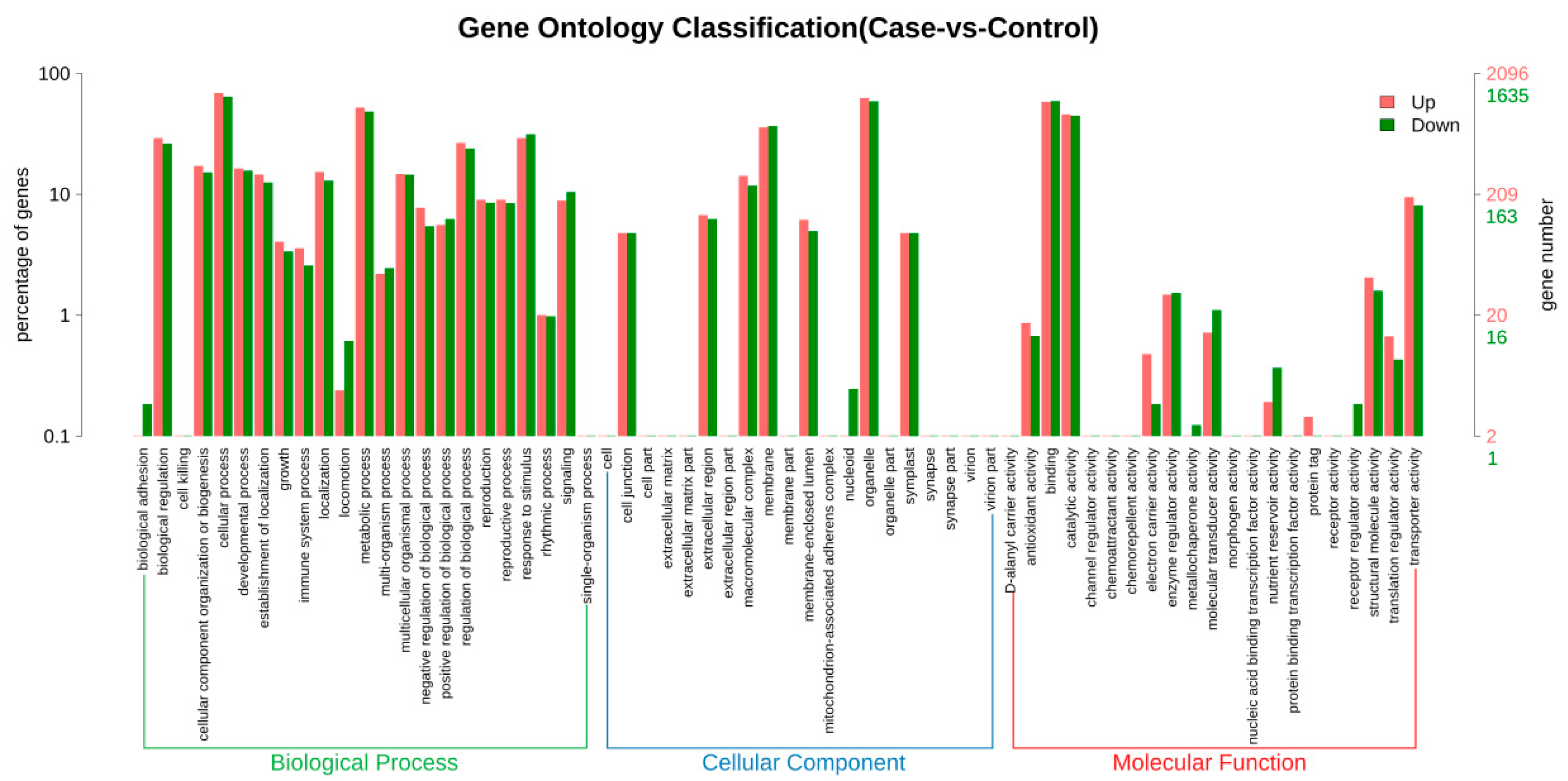
3.6. GO Enrichment Analysis of DEGs
3.7. KEGG Enrichment Analysis of DEGs
3.8. Analysis of DEGs in MAPK Signaling Pathway
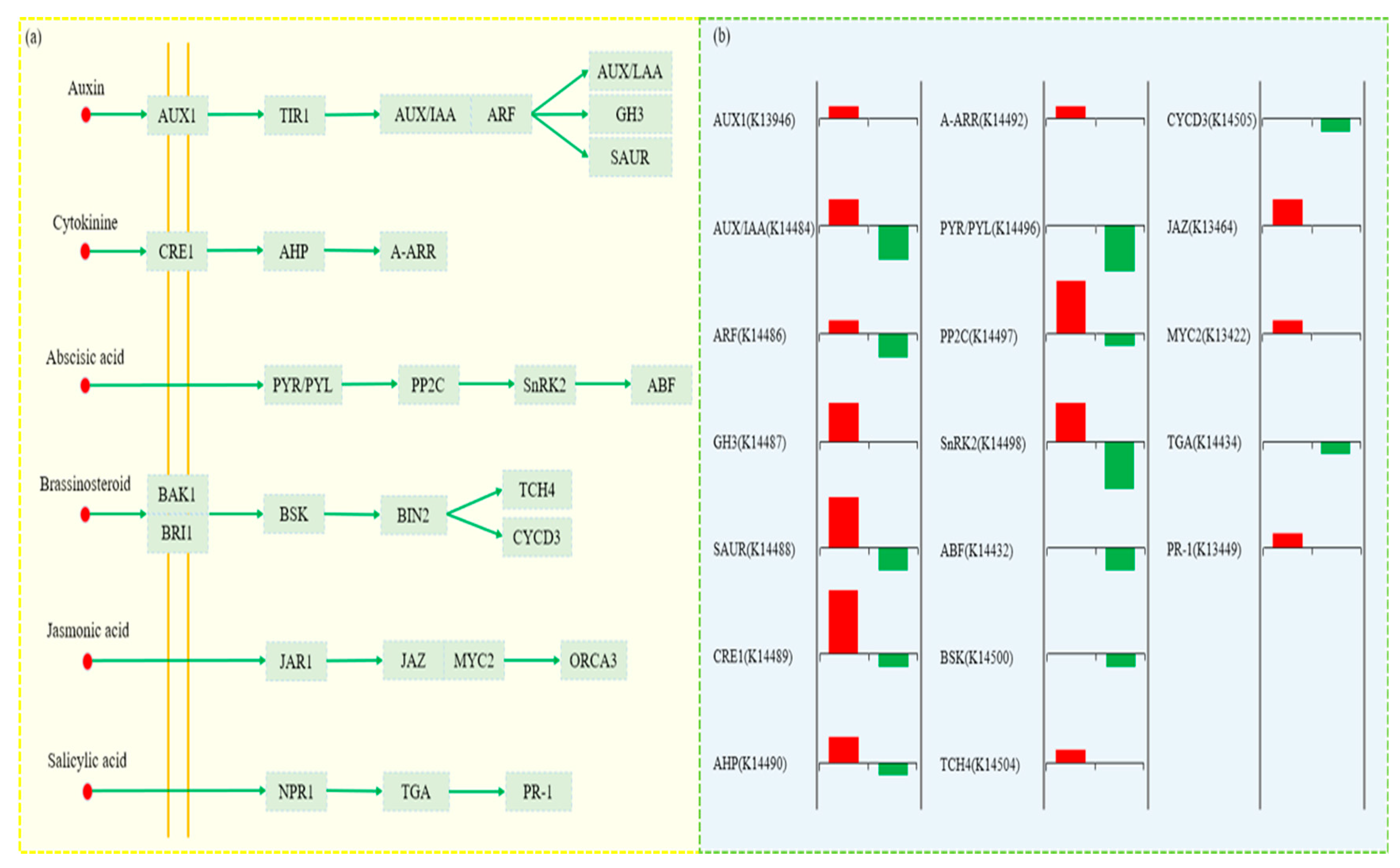
3.9. Analysis of DEGs in Plant Hormone Signaling Pathways
3.10. Transcription Factor Analysis
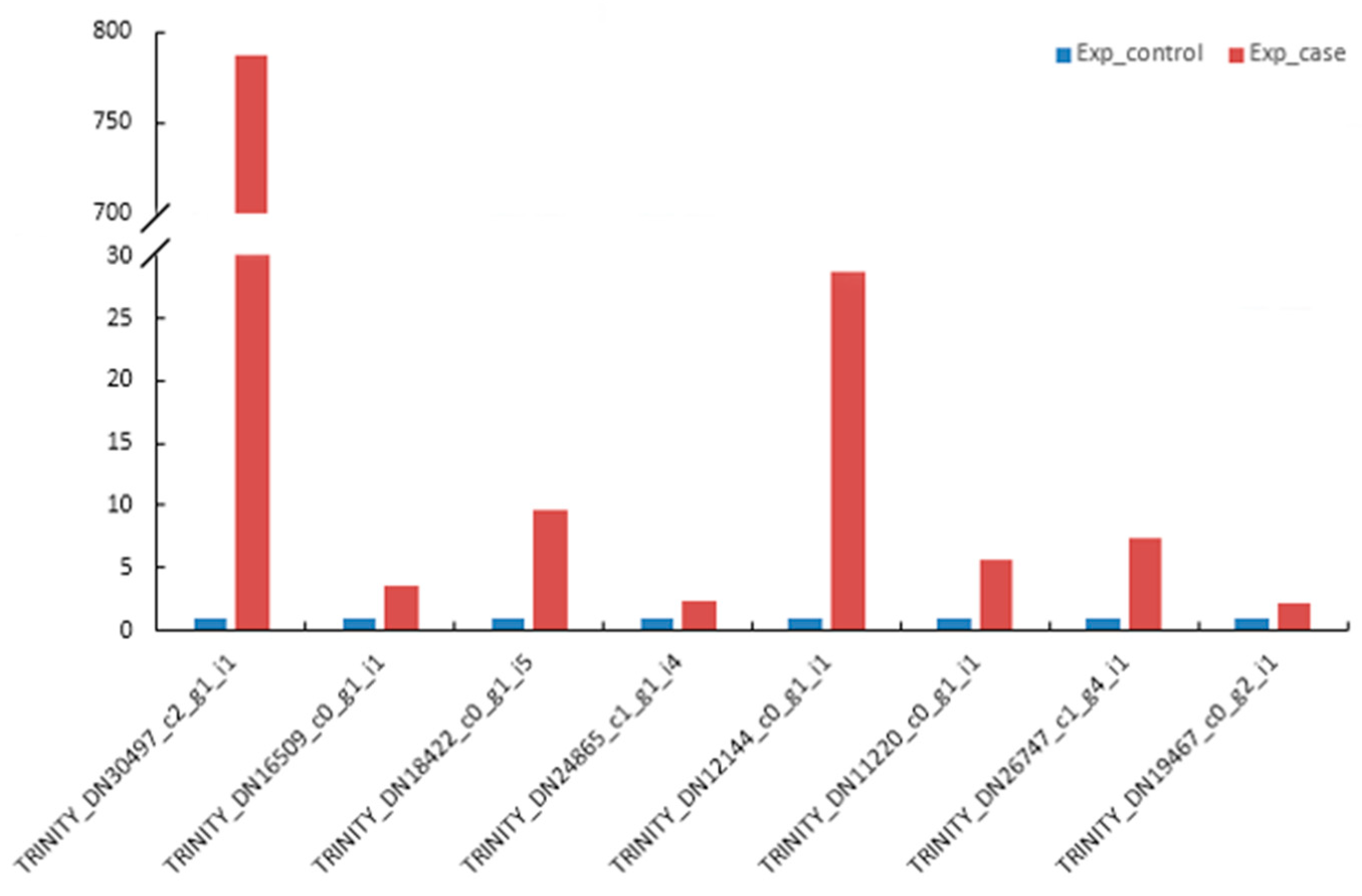
3.11. qRT-PCR Validation of DEGs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, D.; Park, J.; Yoon, J.; Kim, M.-Y.; Choi, H.-Y.; Kim, H. Neuroprotective effects of Eleutherococcus senticosus bark on transient global cerebral ischemia in rats. J. Ethnopharmacol. 2012, 139, 6–11. [Google Scholar] [CrossRef]
- Hibasami, H.; Fujikawa, T.; Takeda, H.; Nishibe, S.; Satoh, T.; Fujisawa, T.; Nakashima, K. Induction of apoptosis by Acanthopanax senticosus HARMS and its component, sesamin in human stomach cancer KATO III cells. Oncol. Rep. 2000, 7, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Sumiyoshi, M. Effects of various Eleutherococcus senticosus cortex on swimming time, natural killer activity and corticosterone level in forced swimming stressed mice. J. Ethnopharmacol. 2004, 95, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, S.G.; Kang, S.K.; Chung, S.H. Acanthopanax senticosus reverses fatty liver disease and hyperglycemia in ob/ob mice. Arch. Pharmacal Res. 2006, 29, 768–776. [Google Scholar] [CrossRef]
- Maslov, L.N.; Guzarova, N.V. Cardio protective and antiarrhythmic properties of preparations from Leuzea carthamoides, Aralia mandshurica, and Eleutherococcus senticosus. Eksperimentalnaia I Klin. Farmakol. 2007, 70, 48–54. [Google Scholar]
- Zhang, Y.Z.; Zhang, A.H.; Zhang, Y.; Sun, H.; Meng, X.C.; Yan, G.L.; Wang, X.J. Application of Ultra-performance Liquid Chromatography with Time-of-Flight Mass Spectrometry for the Rapid Analysis of Constituents and Metabolites from the Extracts of Acanthopanax senticosus Harms Leaf. Pharmacogn. Mag. 2016, 12, 145–152. [Google Scholar] [CrossRef]
- Jin, Z.X.; Xu, S.Y.; Sun, S.Q.; Zhou, Q. Analysis of Acanthopanax senticosus harms for different parts using fourier transform infrared spectroscopy. Spectrosc. Spectr. Anal. 2008, 28, 2859–2863. [Google Scholar] [CrossRef]
- Zhang, X.N.; Sun, Y.; Cao, F. Extraction and GC/MS analysis of volatile constituents from Acanthopanax senticosus (Rupr.et Maxim.) Harms leaf. Chin. J. New Drugs 2011, 20, 936–939. [Google Scholar]
- Dubey, A.K.; Sharma, R.M. Effect of rootstocks on tree growth, yield, quality and leaf mineral composition of lemon (Citrus limon (L.) Burm.). Sci. Hortic. 2016, 200, 131–136. [Google Scholar] [CrossRef]
- Gao, Q.H.; Wu, Y.; Jia, S.S.; Huang, S.H.; Liu, X.M. Effect of rootstock on the growth, photosynthetic capacity and osmotic adjustment of eggplant seedlings under chilling stress and recovery. Pak. J. Bot. 2016, 48, 461–468. [Google Scholar]
- Deng, W.W.; Han, J.Y.; Fan, Y.B.; Tai, Y.L.; Zhu, B.Y.; Lu, M.Q.; Wang, R.J.; Wang, X.C.; Zhang, Z.Z. Uncovering tea-specific secondary metabolism using transcriptomic and metabolomic analyses in grafts of Camellia sinensis and C. oleifera. Tree Genet. Genomes 2018, 14, 23. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Colla, G.; Zrenner, R.; Schwarz, D. Vegetable grafting: The implications of a growing agronomic imperative for vegetable fruit quality and nutritive value. Front. Plant Sci. 2017, 8, 741. [Google Scholar] [CrossRef]
- Duan, X.; Bi, H.G.; Li, T.; Wu, G.X.; Li, Q.; Ai, X.Z. Root characteristics of grafted peppers and their resistance to Fusarium solani. Biol. Plant. 2016, 61, 579–586. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, P.; Zhu, W.; Wang, F. De-novo Comparative Transcriptome Analysis of Genes Differentially Expressed in the Scion of Homografted and Heterografted Tomato Seedlings. Sci. Rep. 2019, 9, 20240. [Google Scholar] [CrossRef] [PubMed]
- Bahar, E.; Korkutal, I.; Carbonneau, A.; Akcay, G. Using magnetic resonance imaging technique (MRI) to investigate graft connection and its relation to reddeningdiscoloration in grape leaves. J. Food Agric. Environ. 2010, 8, 293–297. [Google Scholar] [CrossRef]
- Yin, H.; Yan, B.; Sun, J.; Jia, P.F.; Zhang, Z.J.; Yan, X.S.; Chai, J.; Ren, Z.Z.; Zheng, G.C.; Liu, H. Graft-union development: A delicate process that involves cell-cell communication between scion and stock for local auxin accumulation. J. Exp. Bot. 2012, 63, 4219–4232. [Google Scholar] [CrossRef] [PubMed]
- Aloni, B.; Cohen, R.; Karni, L.; Aktas, H.; Edelstein, M. Hormonal signalling in rootstock-scion interactions. Sci. Hortic. 2010, 127, 119–126. [Google Scholar] [CrossRef]
- Taylor, S.A. Flowering in Pisum: Identifcation of a new ppd allele and its physiological action as revealed by grafing. Physiol. Plant. 2010, 97, 719–723. [Google Scholar] [CrossRef]
- Flaishman, M.A.; Loginovsky, K.; Golobowich, S.; Lev-Yadun, S. Arabidopsis thalianaas a model system for graf union development in homografs and heterografs. J. Plant Growth Regul. 2008, 27, 231–239. [Google Scholar] [CrossRef]
- Lee, E.J.; Malik, A.; Pokharel, S.; Ahmad, S.; Mir, B.A.; Cho, K.H.; Kim, J.; Kong, J.C.; Lee, D.M.; Chung, K.Y.; et al. Identifcation of Genes Diferentially Expressed in Myogenin Knock-Down Bovine Muscle Satellite Cells during Diferentiation through RNA Sequencing Analysis. PLoS ONE 2014, 9, e92447. [Google Scholar] [CrossRef]
- Fan, J.; Yang, R.; Li, X.; Zhao, W.; Zhao, F.; Wang, S. The processes of graf union formation in tomato. Hortic. Environ. Biotechnol. 2015, 56, 569–574. [Google Scholar] [CrossRef]
- Trinchera, A.; Pandozy, G.; Rinaldi, S.; Crinò, P.; Temperini, O.; Rea, E. Graf union formation in artichoke grafing onto wild and cultivated cardoon: An anatomical study. J. Plant Physiol. 2013, 170, 1569–1578. [Google Scholar] [CrossRef]
- Xie, L.; Dong, C.; Shang, Q. Gene co-expression network analysis reveals pathways associated with graf healing by asymmetric profling in tomato. BMC Plant Biol. 2019, 19, 373. [Google Scholar] [CrossRef]
- Cui, Q.; Xie, L.; Dong, C.; Gao, L.; Shang, Q. Stage-specifc events in tomato graf formation and the regulatory efects of auxin and cytokinin. Plant Sci. 2021, 304, 110803. [Google Scholar] [CrossRef] [PubMed]
- Overvoorde, P.; Fukaki, H.; Beeckman, T. Auxin control of root development. J. Cold Spring Harb. Perspect. Biol. 2010, 2, a001537. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.K.; Melnyk, C.W. The role of plant hormones during grafing. J. Plant Res. 2018, 131, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.K.; Kim, M.K.; Kwak, H.R.; Choi, H.S.; Nam, M.; Choe, J.; Choia, B.; Hana, S.J.; Kanga, J.H.; Jung, C. Molecular dissection of distinct symptoms induced by tomato chlorosis virus and tomato yellow leaf curl virus based on comparative transcriptome analysis. Virology 2018, 516, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Nookaew, I.; Papini, M.; Pornputtapong, N.; Scalcinati, G.; Fagerberg, L.; Uhlén, M.; Nielsen, J. A comprehensive comparison of RNA-Seq based transcriptome analysis from reads to diferential gene expression and cross-comparison with microarrays: A case study in Saccharomyces cerevisiae. Nucleic Acids Res. 2012, 40, 10084–10097. [Google Scholar] [CrossRef] [PubMed]
- Marsch-Martínez, N.; Franken, J.; Gonzalez-Aguilera, K.L.; Folter, S.D.; Angenent, G.; Alvarez-Buylla, E.R. An efficient flat-surface collar-free grafting method for Arabidopsis thaliana seedlings. Plant Methods 2013, 9, 14. [Google Scholar] [CrossRef]
- Wright, K.M.; Oparka, K.J. The fluorescent probe HPTS as a phloem-mobile, symplastic tracer: An evaluation using confocal laser scanning microscopy. J. Exp. Bot. 1996, 47, 439–445. [Google Scholar] [CrossRef]
- Zhang, W.; Kollwig, G.; Stecyk, E.; Apelt, F.; Dirks, R.; Kragler, F. Graft-transmissible movement of inverted-repeat-induced siRNA signals into flowers. Plant J. 2014, 80, 106–121. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2007, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Pachter, L. Streaming fragment assignment for real-time analysis of sequencing experiments. Nat. Methods 2012, 10, 71–73. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression of RNA-Seq data at the gene level-the DESeq package. Heidelb. Ger. Eur. Mol. Biol. Lab. 2013, 10, f1000research. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Moore, R.; Walker, D.B. Study of vegetative compatibility-incompatibility in higher plants. I. A structural studies of a compatible autograft in Sedum telephoides (Crassulaceae). Am. J. Bot. 1981, 68, 820–830. [Google Scholar] [CrossRef]
- Tiedemann, R. Graft union development and symplastic phloem contact in the heterograft Cucumis sativus on Curcubita ficifolia. J. Plant Physiol. 1989, 134, 427–440. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T.; Geneve, R.L. Plant Propagation. Principles and Practices, 7th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2002; pp. 199–220. [Google Scholar]
- Pina, A.; Errea, P.; Martens, H.J. Graft union formation and cell-to-cell communication via plasmodesmata in compatible and incompatible stem unions of Prunus spp. Sci. Hortic. 2012, 143, 144–150. [Google Scholar] [CrossRef]
- Yousef, A.F.; Ali, M.M.; Rizwan, H.M.; Gad, A.G.; Liang, D.; Binqi, L.; Kalaji, H.M.; Wróbel, J.; Xu, Y.; Chen, F. Light quality and quantity affect graft union formation of tomato plants. Sci. Rep. 2021, 11, 9870. [Google Scholar] [CrossRef]
- Yang, X.; Hu, X.; Zhang, M.; Xu, J.; Ren, R.; Liu, G.; Yao, X.F.; Chen, X.H. Effect of low night temperature on graft union formation in watermelon grafted onto bottle gourd rootstock. Sci. Hortic. 2016, 212, 29–34. [Google Scholar] [CrossRef]
- Miao, L.; Li, Q.; Sun, T.S.; Chai, S.; Wang, C.L.; Bai, L.Q.; Sun, M.T.; Li, Y.S.; Qin, X.; Zhang, Z.H.; et al. Sugars promote graft union development in the heterograft of cucumber onto pumpkin. Hortic. Res. 2021, 8, 146. [Google Scholar] [CrossRef]
- Cookson, S.J.; Clemente Moreno, M.J.; Hevin, C.; Nyamba Mendome, L.Z.; Delrot, S.; Trossat-Magnin, C.; Ollat, N. Graft union formation in grapevine induces transcriptional changes related to cell wall modification, wounding, hormone signalling, and secondary metabolism. J. Exp. Bot. 2013, 64, 2997–3008. [Google Scholar] [CrossRef]
- Mo, Z.H.; Feng, G.; Su, W.C.; Liu, Z.Z.; Peng, F.R. Transcriptomic Analysis Provides Insights into Grafting Union Development in Pecan (Carya illinoinensis). Genes 2018, 9, 71. [Google Scholar] [CrossRef]
- Gerszberg, A.; Hnatuszko-Konka, K. Tomato tolerance to abiotic stress: A review of most often engineered target sequences. Plant Growth Regul. 2017, 83, 175–198. [Google Scholar] [CrossRef]
- Ren, Y.; Xu, Q.; Wang, L.W.; Guo, S.R.; Shu, S.; Lu, N.; Sun, J. Involvement of metabolic, physiological and hormonal responses in the graft-compatible process of cucumber/pumpkin combinations was revealed through the integrative analysis of mRNA and miRNA expression. Plant Physiol. Biochem. 2018, 129, 368–380. [Google Scholar] [CrossRef]
- Kovtun, Y.; Chiu, W.L.; Tena, G.; Sheen, J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc. Natl. Acad. Sci. USA 2000, 97, 2940–2945. [Google Scholar] [CrossRef] [PubMed]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Agrawal, G.K.; Tamogami, S.; Iwahashi, H.; Agrawal, V.P.; Rakwal, R. Transient regulation of jasmonic acid-inducible rice MAP kinase gene (OsBWMK1) by diverse biotic and abiotic stresses. Plant Physiol. Bioch. 2003, 41, 355–361. [Google Scholar] [CrossRef]
- Melnyk, C.W.; Schuster, C.; Leyser, O.; Meyerowitz, E.M. A Developmental Framework for Graft Formation and Vascular Reconnection in Arabidopsis thaliana. Curr. Biol. 2015, 25, 1306–1318. [Google Scholar] [CrossRef]
- Asahina, M.; Azuma, K.; Pitaksaringkarn, W.; Yamazaki, T.; Mitsuda, N.; Ohme-Takagi, M.; Yamaguchi, S.; Kamiya, Y.; Okada, K.; Nishimura, T.; et al. Spatially selective hormonal control of RAP2.6L and ANAC071 transcription factors involved in tissue reunion in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 16128–16132. [Google Scholar] [CrossRef]
- Shen, C.J.; Yue, R.Q.; Sun, T.; Zhang, L.; Yang, Y.J.; Wang, H.Z. OsARF16, a transcription factor regulating auxin redistribution, is required for iron defificiency response in rice (Oryza sativa L.). Plant Sci. 2015, 231, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.L.; Sun, C.D.; Shen, C.J.; Wang, S.K.; Liu, F.; Liu, Y.; Chen, Y.L.; Qian, Q.; Aryal, B.; Geisler, M.; et al. The auxin transporter, OsAUX1, is involved in primary root and root hair elongation and in Cd stress responses in rice (Oryza sativa L.). Plant J. 2015, 83, 818–830. [Google Scholar] [CrossRef]
- Scarpella, E. Control of leaf vascular patterning by polar auxin transport. Genes Dev. 2006, 20, 1015–1027. [Google Scholar] [CrossRef]
- Aloni, R. Differentiation of vascular tissues. Annu. Rev. Plant Physiol. 1987, 38, 179–204. [Google Scholar] [CrossRef]
- Heo, J.O.; Roszak, P.; Furuta, K.M.; Helariutta, Y. Phloem development: Current knowledge and future perspectives. Am. J. Bot. 2014, 101, 1393–1402. [Google Scholar] [CrossRef]
- Iwase, A.; Mitsuda, N.; Koyama, T.; Hiratsu, K.; Kojima, M.; Arai, T.; Inoue, Y.; Seki, M.; Sakakibara, H.; Sugimoto, K.; et al. The AP2/ERF Transcription Factor WIND1 Controls Cell Dedifferentiation in Arabidopsis. Curr. Biol. 2011, 21, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Kumar, J.; Kumar, A.; Chaudhury, A.; Singh, S.P. Grafting triggers difffferential responses between scion and rootstock. PLoS ONE 2015, 10, e0124438. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Guo, S.R.; Shu, S.; Xu, Y.; Sun, J. Isolation and expression pattern analysis of CmRNF5 and CmNPH3L potentially involved in graft compatibility in cucumber/pumpkin graft combinations. Sci. Hortic. 2018, 227, 92–101. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; González-Agüero, M.; Cisneros-Zevallos, L. Cross-talk between signaling pathways: The link between plant secondary metabolite production and wounding stress response. Sci. Rep. 2015, 5, 8608. [Google Scholar] [CrossRef]
- Erpen, L.; Devi, H.S.; Grosser, J.W.; Dutt, M. Potential use of the DREB/ERF, MYB, NAC and WRKY transcription factors to improve abiotic and biotic stress in transgenic plants. Plant Cell Tissue Organ Cult. 2017, 132, 1–25. [Google Scholar] [CrossRef]
- Sakuma, Y.; Liu, Q.; Dubouzet, J.G.; Abe, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration-and cold-inducible gene expression. Biochem. Biophys. Res. Commun. 2002, 290, 998–1009. [Google Scholar] [CrossRef]
- Zarei, A.; Körbes, A.P.; Younessi, P.; Montiel, G.; Champion, A.; Memelink, J. Two GCC boxes and AP2/ERF-domain transcription factor ORA59 in jasmonate/ethylene-mediated activation of the PDF1.2 promoter in Arabidopsis. Plant Mol. Biol. 2011, 75, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.S.; Chen, M.; Li, L.C.; Ma, Y.Z. Functions of the ERF transcription factor family in plants. Botany 2008, 86, 969–977. [Google Scholar] [CrossRef]
- Li, C.W.; Su, R.C.; Cheng, C.P.; You, S.J.; Hsieh, T.H.; Chao, T.C.; Chan, M.T. Tomato RAV transcription factor is a pivotal modulator involved in the AP2/EREBP-mediated defense pathway. Plant Physiol. 2011, 156, 213–227. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Z.; Xie, B.; Chen, Q.; Tian, X.; Zhang, X.; Zhang, H.; Lu, X.; Huang, D.; Huang, R. The ethylene-, jasmonate-, abscisic acid- and NaCl-responsive tomato transcription factor JERF1 modulates expression of GCC box-containing genes and salt tolerance in tobacco. Planta 2004, 220, 262–270. [Google Scholar] [CrossRef]
- Rehman, S.; Mahmood, T. Functional role of DREB and ERF transcription factors: Regulating stress-responsive network in plants. Acta Physiol. Plant. 2015, 37, 178. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, L.N.; Latoszek-Green, M.; Brown, D.; Wu, K.Q. Arabidopsis ERF4 is a transcriptional repressor capable of modulating ethylene and abscisic acid responses. Plant Mol. Biol. 2005, 58, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, O.; Piqueras, R.; Sanchez-Serrano, J.J.; Solano, R. Ethylene response factor 1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell 2003, 15, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, P.T.; Cheng, S.; Zhao, Z.L.; Liu, Y.L.; Wei, Y.Y.; Lu, Q.W.; Han, J.P.; Cai, X.Y.; Zhou, Z.L.; et al. Protoplast Dissociation and Transcriptome Analysis Provides Insights to Salt Stress Response in Cotton. Int. J. Mol. Sci. 2022, 23, 2845. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, L.; Li, D.; Wang, F.; Yu, D. WRKY8 transcription factor functions in the TMV-cg defense response by mediating both abscisic acid and ethylene signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.N.; Liu, D.D.; Hao, L.L.; Wu, C.A.; Guo, X.Q.; Li, H. GhWRKY39, a member of the WRKY transcription factor family in cotton, has a positive role in disease resistance and salt stress tolerance. Plant Cell Tissue Organ Cult. 2014, 118, 17–32. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2006, 58, 221–227. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Stracke, R.; Werber, M.; Weisshaar, B. The R2R3-MYB gene family in Arabidopsis thaliana. Curr. Opin. Plant Biol. 2001, 4, 447–456. [Google Scholar] [CrossRef]
- Bostock, R.M. Signal crosstalk and induced resistance: Straddling the line between cost and benefit. Annu. Rev. Phytopathol. 2005, 43, 545–580. [Google Scholar] [CrossRef]
- Durrant, W.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Udagawa, M.; Nishikubo, N.; Horiguchi, G.; Yamaguchi, M.; Ito, J.; Mimura, T.; Fukuda, H.; Demura, T. Transcription switches for protoxylem and metaxylem vessel formation. Gene. Dev. 2005, 19, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Furuta, K.M.; Hellmann, E.; Helariutta, Y. Molecular control of cell specifification and cell difffferentiation during procambial development. Annu. Rev. Plant Biol. 2014, 65, 607–638. [Google Scholar] [CrossRef]
- Kondo, Y.; Nurani, A.M.; Saito, C.; Ichihashi, Y.; Saito, M.; Yamazaki, K.; Mitsuda, N.; Ohme-Takagi, M.; Fukuda, H. Vascular cell induction culture system using Arabidopsis leaves (VISUAL) reveals the sequential difffferentiation of sieve element-like cells. Plant Cell 2016, 28, 1250–1262. [Google Scholar] [CrossRef] [PubMed]
- Pitaksaringkarn, W.; Matsuoka, K.; Asahina, M.; Miura, K.; Sage-Ono, K.; Ono, M.; Yokoyama, R.; Nishitani, K.; Lshii, H.; Satoh, S. XTH20 and XTH19 regulated by ANAC071 under auxin flflow are involved in cell proliferation in incised Arabidopsis inflflorescence stems. Plant J. 2014, 80, 604–614. [Google Scholar] [CrossRef]
- Matsuoka, K.; Matsuoka, K.; Sato, R.; Matsukura, Y.; Kawajiri, Y.; Iino, H.; Nozawa, N.; Shibata, K.; Kondo, Y.; Satoh, S.; et al. Wound-inducible ANAC071 and ANAC096 transcription factors promote cambial cell formation in incised Arabidopsis flflowering stems. Commun. Biol. 2021, 4, 369. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Deng, K.; Ai, J.; Wang, Y.; Wang, Y.; Ren, Y.; Zhang, N. Integrated Anatomical and Transcriptomic Analysis Revealed the Molecular Mechanism of the Healing Process in Homografted and Heterografted Seedlings of Acanthopanax senticosus. Agronomy 2023, 13, 1527. https://doi.org/10.3390/agronomy13061527
Wang Q, Deng K, Ai J, Wang Y, Wang Y, Ren Y, Zhang N. Integrated Anatomical and Transcriptomic Analysis Revealed the Molecular Mechanism of the Healing Process in Homografted and Heterografted Seedlings of Acanthopanax senticosus. Agronomy. 2023; 13(6):1527. https://doi.org/10.3390/agronomy13061527
Chicago/Turabian StyleWang, Qi, Kedan Deng, Jun Ai, Yingping Wang, Yougui Wang, Yueying Ren, and Nanqi Zhang. 2023. "Integrated Anatomical and Transcriptomic Analysis Revealed the Molecular Mechanism of the Healing Process in Homografted and Heterografted Seedlings of Acanthopanax senticosus" Agronomy 13, no. 6: 1527. https://doi.org/10.3390/agronomy13061527
APA StyleWang, Q., Deng, K., Ai, J., Wang, Y., Wang, Y., Ren, Y., & Zhang, N. (2023). Integrated Anatomical and Transcriptomic Analysis Revealed the Molecular Mechanism of the Healing Process in Homografted and Heterografted Seedlings of Acanthopanax senticosus. Agronomy, 13(6), 1527. https://doi.org/10.3390/agronomy13061527




