H2O2 Participates in the Induction and Formation of Potato Tubers by Activating Tuberization-Related Signal Transduction Pathways
Abstract
1. Introduction
2. Materials and Methods
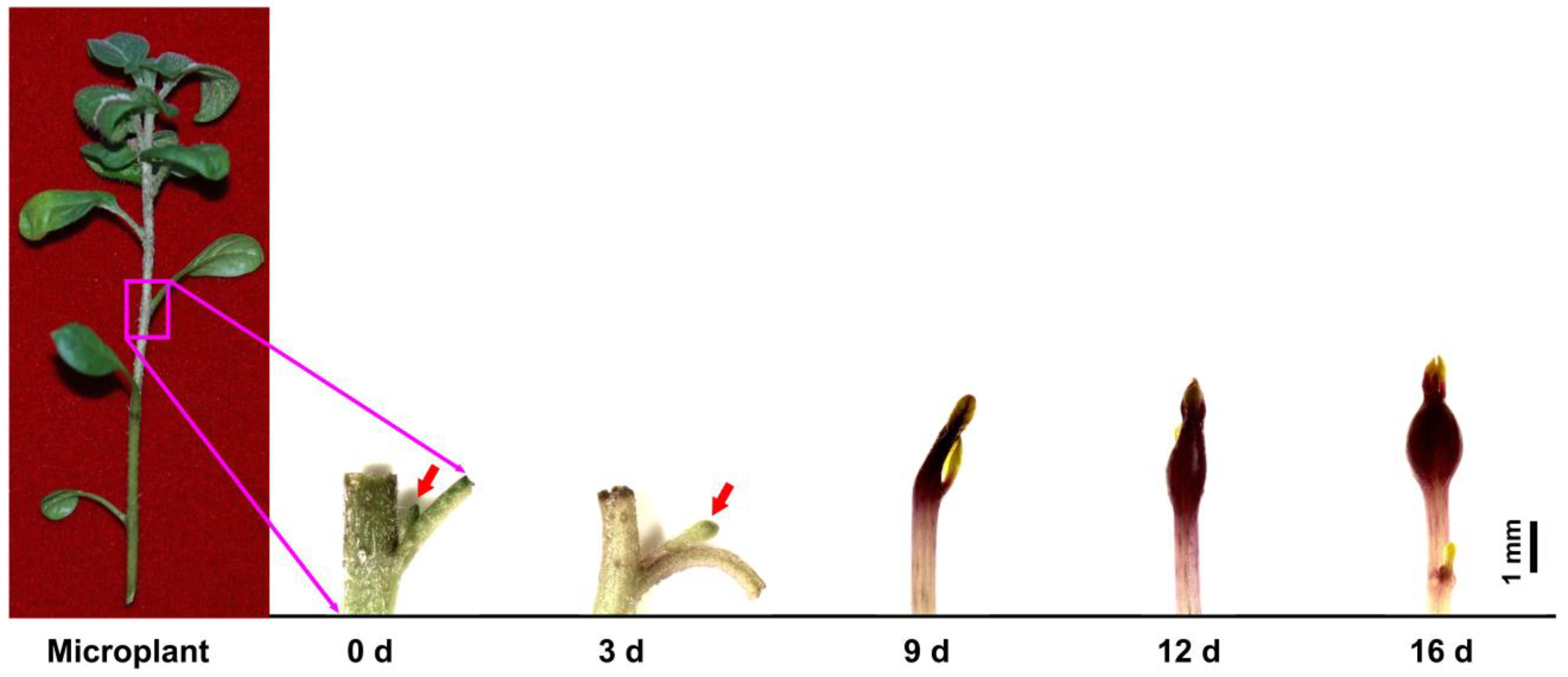
2.1. Plant Materials
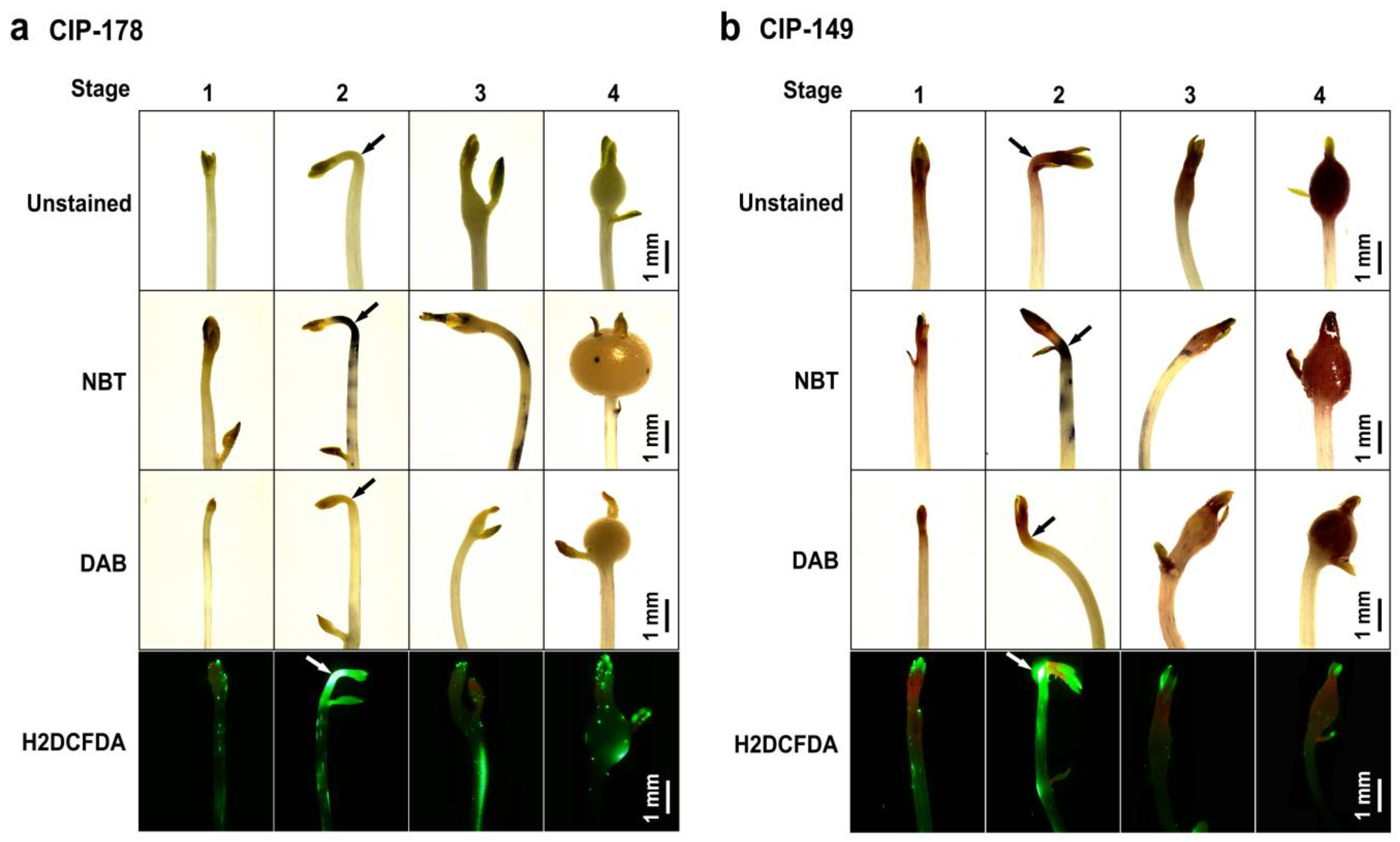
2.2. Observation of the Accumulation of O2−, H2O2, and Total ROS in Stolons/Tubers during Four Stages of Tuber Formation by Histochemical Staining
2.3. Quantitative Determination of O2− Production Rate and H2O2 Levels in Stolons/Tubers
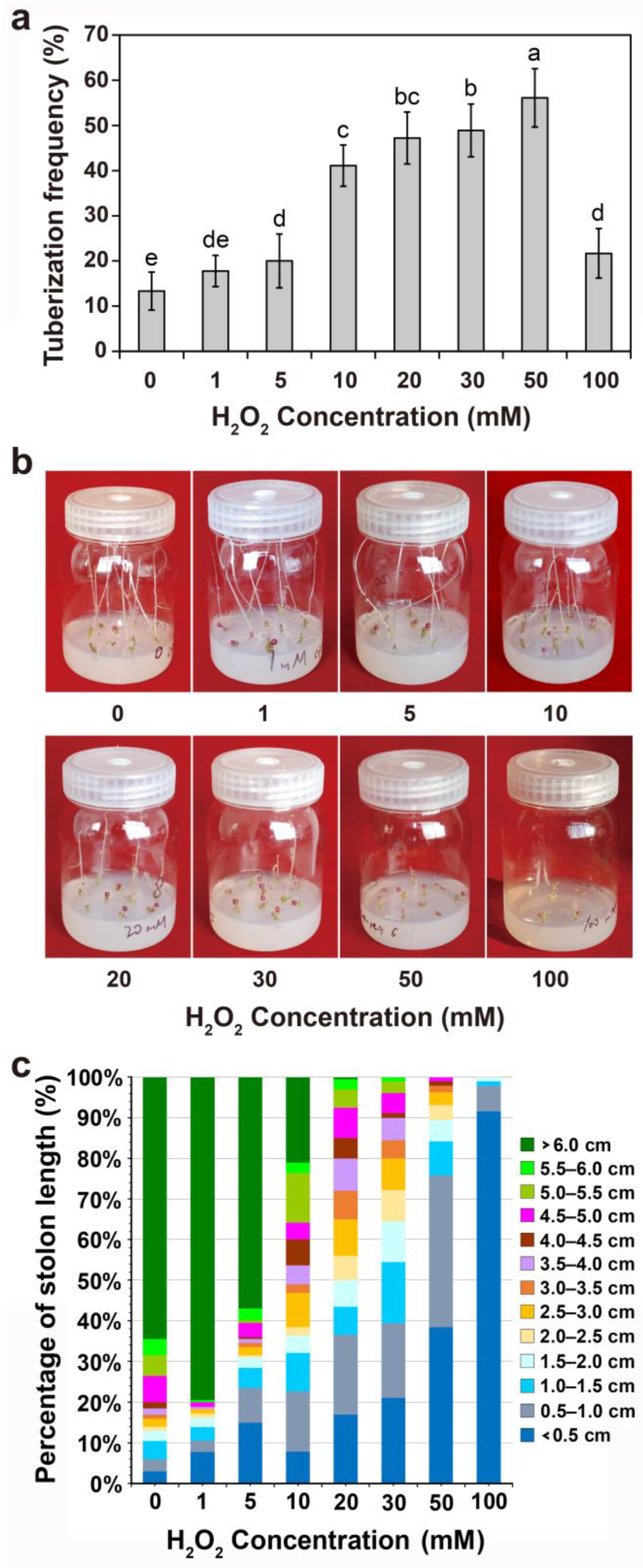
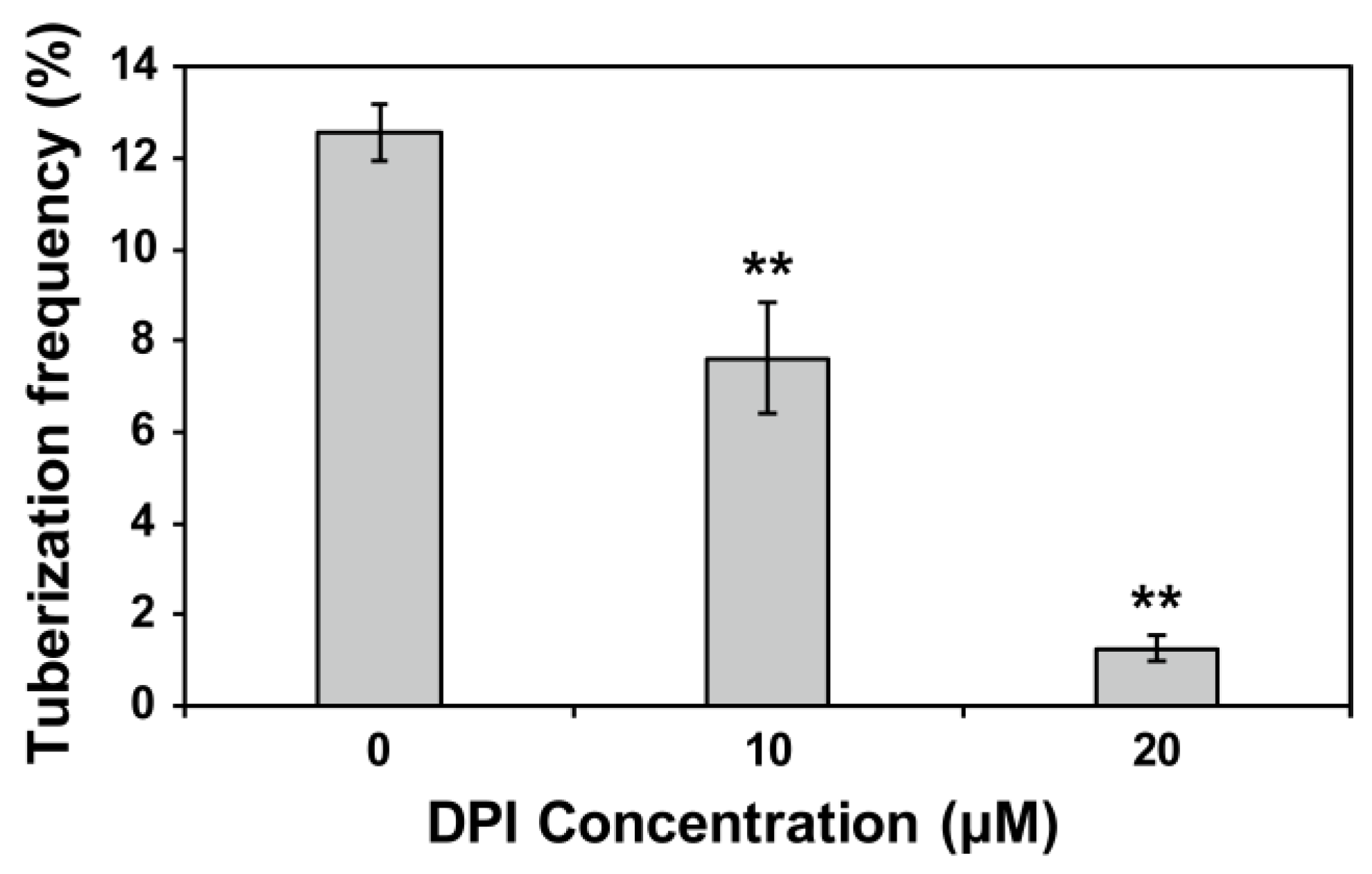
2.4. Effects of Exogenous H2O2 and the ROS Inhibitor DPI or H2O2 Scavenger CAT Treatments on Tuber Formation
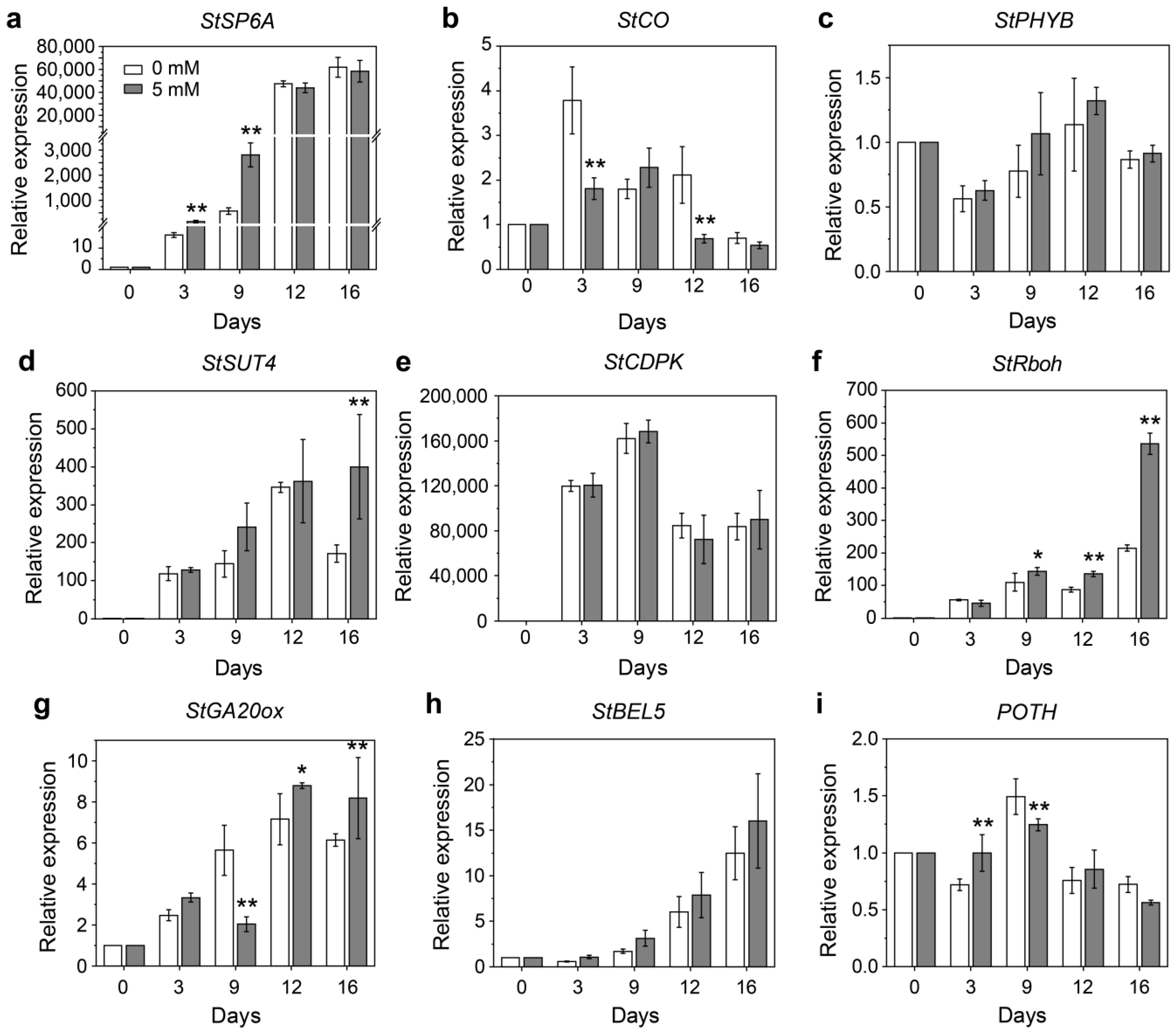
2.5. Analysis of Key Tuberization-Related Genes Using RT-qPCR
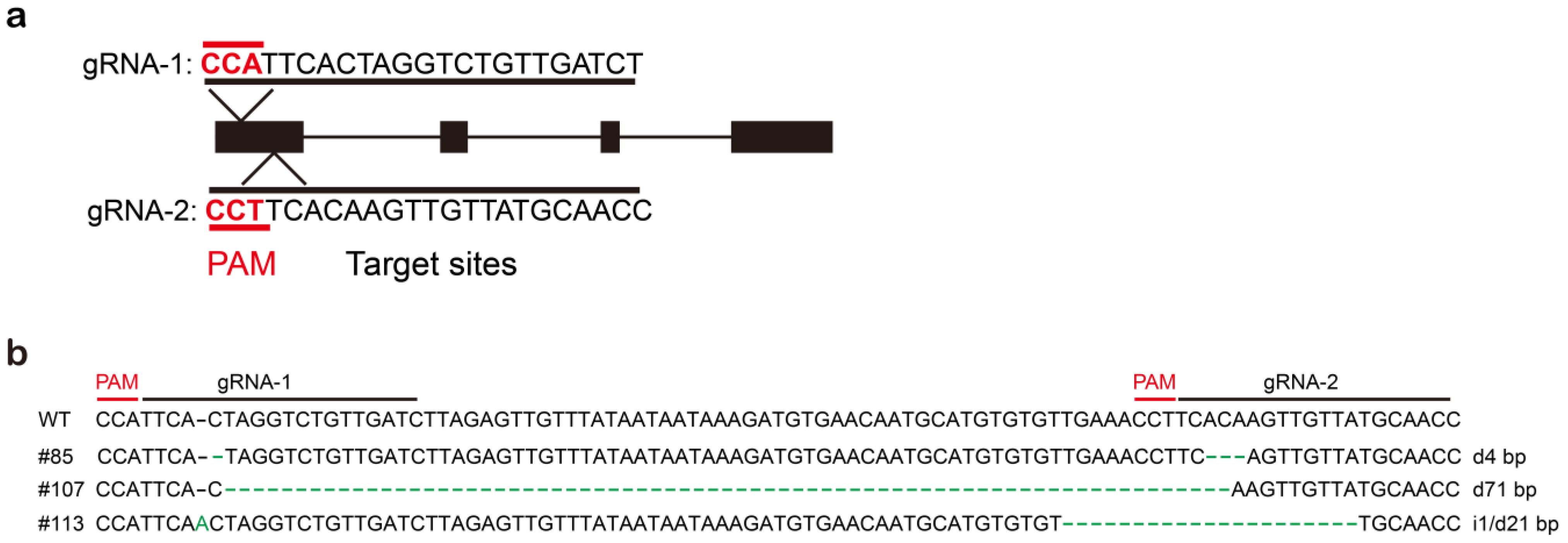
2.6. Construction of StSP6A Knockout Vector for Null-Mutants
2.6.1. Plasmid Construct
2.6.2. A. Tumefaciens Transformation of Potato
2.6.3. Homozygous StSP6A Null-Mutants Were Obtained
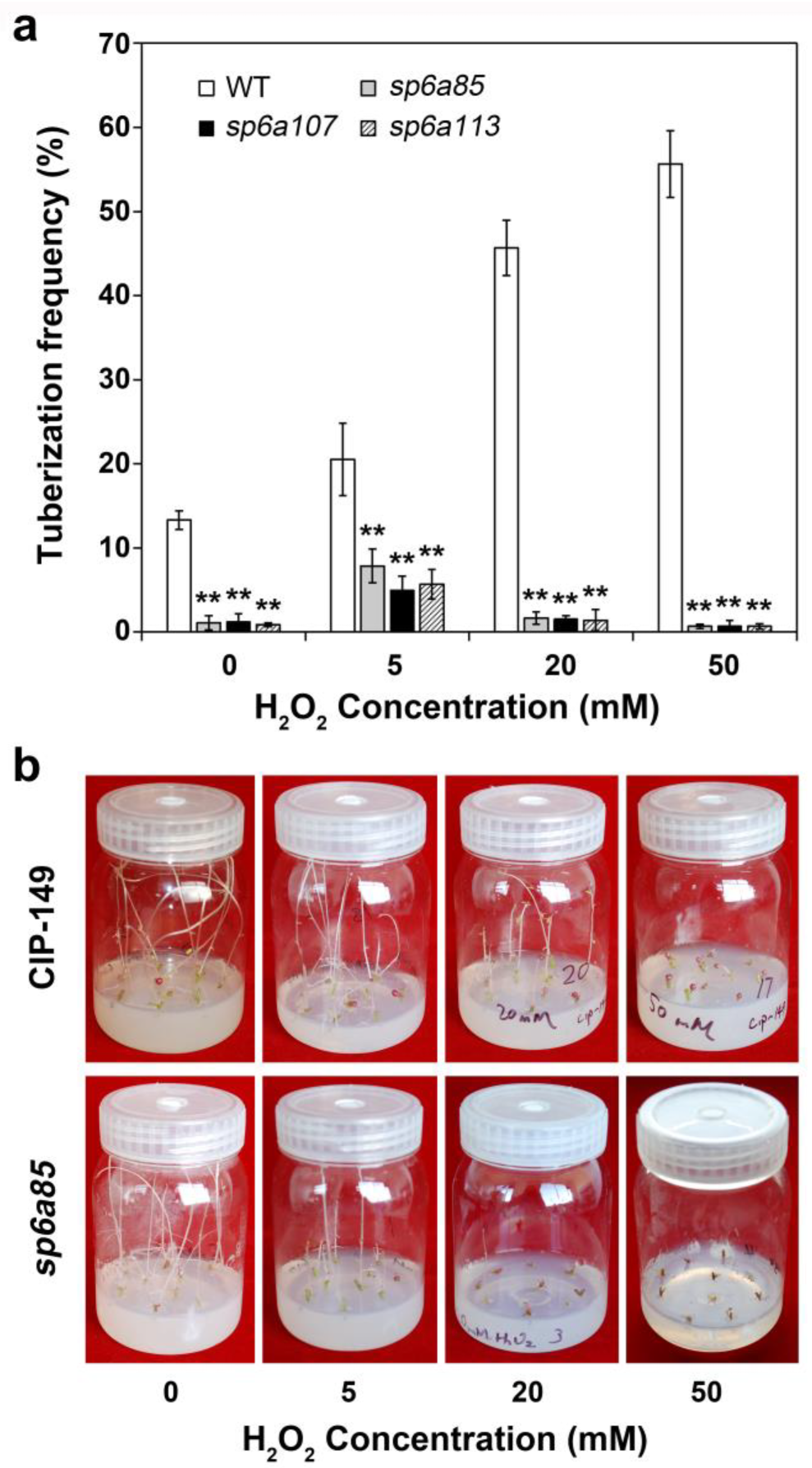
2.7. H2O2 Treatments of the StSP6A Null-Mutants sp6a85, sp6a107 and sp6a113
2.8. Statistical Analysis
3. Results
3.1. Accumulation of O2−, H2O2, and Total ROS in Stolons/Tubers during Tuber Formation
3.2. Effects of Exogenous H2O2 and the ROS Inhibitor DPI or H2O2 Scavenger CAT Treatments on Tuber Formation
3.3. Expression Analysis of Key Tuberization-Related Genes during H2O2-Induced Tuber Formation
3.4. Effects of H2O2 Treatment on Tuber Formation of StSP6A Null-Mutants
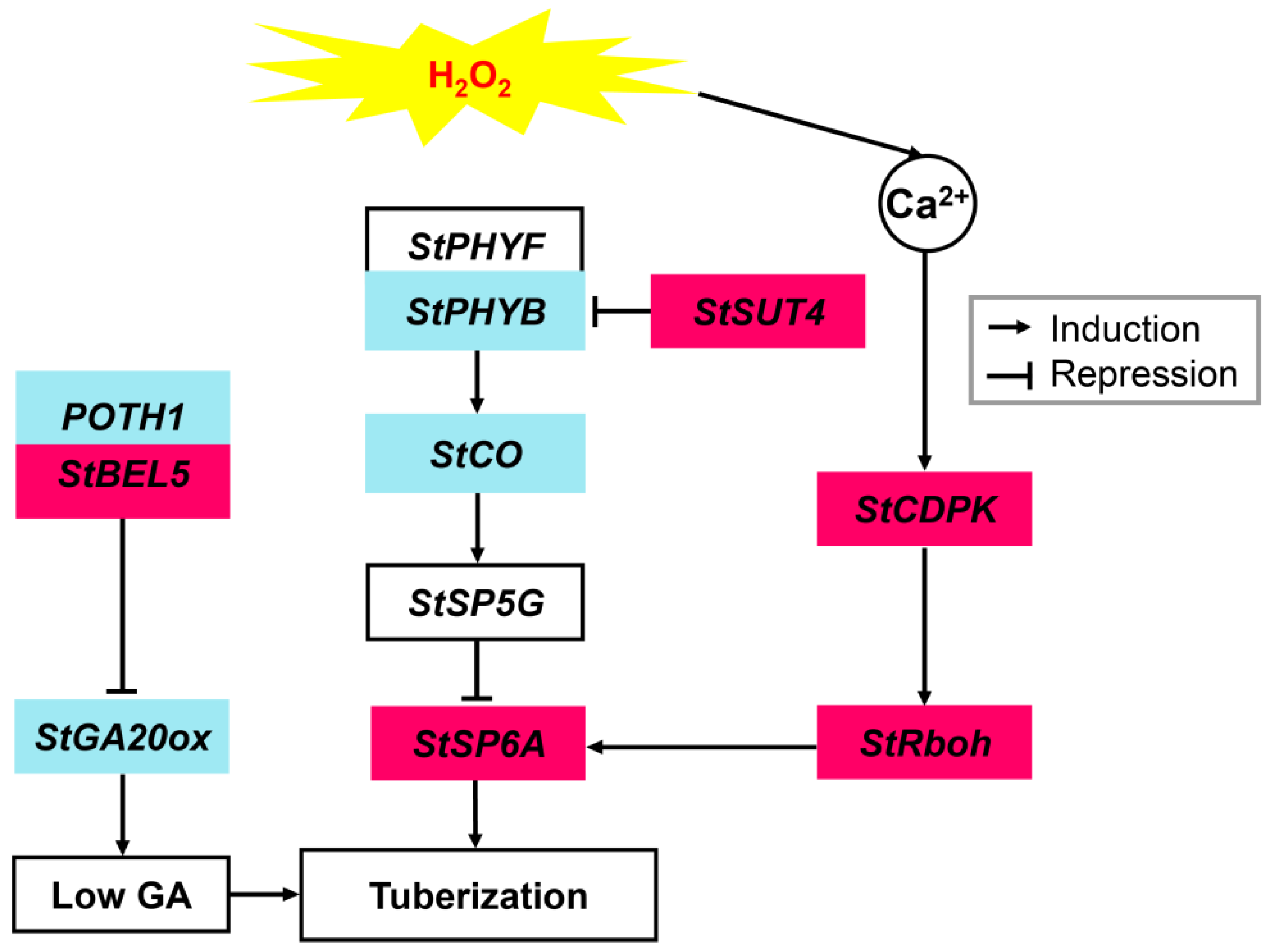
4. Discussion
4.1. ROS Accumulation Is Involved in Induction and Formation of Potato Tubers
4.2. H2O2-Induced Tuberization Could Be Associated with H2O2-Controlled Regulation of These Tuberization- and Signaling-Pathway-Related Genes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, M.; Li, C.; Gong, M. Applications and prospect of genome editing techniques in precise potato molecular breeding. Biotechnol. Bull. 2020, 36, 9–17. [Google Scholar]
- Lei, C.; Li, C.; Chen, Y.; Gong, M. Physiological and biochemical basis and molecular mechanism of solanum tuberosum tuberization. Biotechnol. Bull. 2022, 38, 44–57. [Google Scholar]
- Zierer, W.; Rüscher, D.; Sonnewald, U.; Sonnewald, S. Tuber and tuberous root development. Annu. Rev. Plant Biol. 2021, 72, 551–580. [Google Scholar] [CrossRef] [PubMed]
- Aksenova, N.; Konstantinova, T.; Golyanovskaya, S.; Sergeeva, L.; Romanov, G. Hormonal regulation of tuber formation in potato plants. Russ. J. Plant Physiol. 2012, 59, 451–466. [Google Scholar] [CrossRef]
- Dutt, S.; Manjul, A.; Raigond, P.; Singh, B.; Siddappa, S.; Bhardwaj, V.; Kawar, P.; Patil, V.; Kardile, H. Key players associated with tuberization in potato: Potential candidates for genetic engineering. Crit. Rev. Biotechnol. 2017, 37, 942–957. [Google Scholar] [CrossRef]
- Teo, C.; Takahashi, K.; Shimizu, K.; Shimamoto, K.; Taoka, K. Potato tuber induction is regulated by interactions between components of a tuberigen complex. Plant Cell Physiol. 2017, 58, 365–374. [Google Scholar] [CrossRef]
- Ševčíková, H.; Mašková, P.; Tarkowská, D.; Mašek, T.; Lipavská, H. Carbohydrates and gibberellins relationship in potato tuberization. J. Plant Physiol. 2017, 214, 53–63. [Google Scholar] [CrossRef]
- Castro, B.; Citterico, M.; Kimura, S.; Stevens, D.; Wrzaczek, M.; Coaker, G. Stress-induced reactive oxygen species compartmentalization, perception and signalling. Nat. Plants 2021, 7, 403–412. [Google Scholar] [CrossRef]
- Niu, L.; Liao, W. Hydrogen peroxide signaling in plant development and abiotic responses: Crosstalk with nitric oxide and calcium. Front. Plant Sci. 2016, 7, 230. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 745–748. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Kim, M.; Kim, H.; Kim, Y.; Baek, K.; Oh, H.; Hahn, K.; Bae, R.; Lee, I.; Joung, H.; Jeon, J. Superoxide anion regulates plant growth and tuber development of potato. Plant Cell Rep. 2007, 26, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Yusuf, M.A.; Singh, P.; Sardar, M.; Sarin, N. Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio-Protocol 2014, 4, e1108. [Google Scholar] [CrossRef]
- Guan, B.; Lin, Z.; Liu, D.; Li, C.; Zhou, Z.; Mei, F.; Li, J.; Deng, X. Effect of waterlogging-induced autophagy on programmed cell death in Arabidopsis roots. Front. Plant Sci. 2019, 10, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gong, M. Observation on heat tolerance of maize seedlings induced by heat shock and its physiological and biochemical mechanisms. In Comprehensive and Designed Experimental Course in Plant Physiology; Huazhong University of Science and Technology Publishing: Wuhan, China, 2014; pp. 81–82, 85–86. [Google Scholar]
- Wei, J.; Li, D.; Zhang, J.; Shan, C.; Rengel, Z.; Song, Z.; Chen, Q. Phytomelatonin receptor PMTR1—Mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef]
- Nicolas, M.; Torres-Pérez, R.; Wahl, V.; Cruz-Oró, E.; Rodríguez-Buey, M.; Zamarreño, A.; Martín-Jouve, B.; García-Mina, J.; Oliveros, J.; Prat, S.; et al. Spatial control of potato tuberization by the TCP transcription factor BRANCHED1b. Nat. Plants 2022, 8, 281–294. [Google Scholar] [CrossRef]
- Park, J.; Park, S.; Kwon, S.; Shin, A.; Moon, K.; Park, J.; Cho, H.; Park, S.; Jeon, J.; Kim, H.; et al. Temporally distinct regulatory pathways coordinate thermo-responsive storage organ formation in potato. Cell Rep. 2022, 38, 110579. [Google Scholar] [CrossRef]
- Nicot, N.; Hausman, J.; Hoffmann, L.; Evers, D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 2005, 56, 2907–2914. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Peng, Z.; Tang, D.; Yang, Z.; Li, D.; Xu, Y.; Zhang, C.; Huang, S. Generation of self-compatible diploid potato by knockout of S-RNase. Nat. Plants 2018, 4, 651–654. [Google Scholar] [CrossRef]
- Xing, H.; Dong, L.; Wang, Z.; Zhang, H.; Han, C.; Liu, B.; Wang, X.; Chen, Q. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 2014, 14, 327. [Google Scholar] [CrossRef]
- Yang, Z.; Feng, S.; Tang, D.; Zhang, L.; Zhang, C. The mutation of a PECTATE LYASE-LIKE gene is responsible for the Yellow Margin phenotype in potato. Theor. Appl. Genet. 2020, 133, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kawakita, K.; Maeshima, M.; Doke, N.; Yoshioka. Hirofumi. Subcellular localization of Strboh proteins and NADPH-dependent O2−-generating activity in potato tuber tissues. J. Exp. Bot. 2006, 57, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Rejeb, K.B.; Vos, D.L.-D.; Disquet, I.L.; Leprince, A.-S.; Bordenave, M.; Maldiney, R.; Jdey, A.; Abdelly, C.; Savouré, A. Hydrogen peroxide produced by NADPH oxidases increases proline accumulation during salt or mannitol stress in Arabidopsis thaliana. New Phytol. 2015, 208, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.H.; Yu, C.W.; Lin, C.H. Hydrogen peroxide functions as a stress signal in plants. Bot. Bull. Acad. Sin. 2005, 46, 1–10. [Google Scholar]
- Navarro, C.; Cruz-Oró, E.; Prat, S. Conserved function of FLOWERING LOCUS T (FT) homologues as signals for storage organ differentiation. Curr. Opin. Plant Biol. 2015, 23, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Navarro, C.; Abelenda, J.A.; Cruz-Oró, E.; Cuéllar, C.A.; Tamaki, S.; Silva, J.; Shimamoto, K.; Prat, S. Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature 2011, 478, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Chincinska, I.; Gier, K.; Krügel, U.; Liesche, J.; He, H.; Grimm, B.; Harren, F.; Cristescu, S.; Kühn, C. Photoperiodic regulation of the sucrose transporter StSUT4 affects the expression of circadian-regulated genes and ethylene production. Front. Plant Sci. 2013, 4, 26. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ohura, I.; Kawakita, K.; Yokota, N.; Fujiwara, M.; Shimamoto, K.; Doke, N.; Yoshioka, H. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell 2007, 19, 1065–1080. [Google Scholar] [CrossRef]
- Kloosterman, B.; Navarro, C.; Bijsterbosch, G.; Lange, T.; Prat, S.; Visser, R.; Bachem, C. StGA2ox1 is induced prior to stolon swelling and controls GA levels during potato tuber development. Plant J. 2007, 52, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Banerjee, A.K.; Hannapel, D.J. The tandem complex of BEL and KNOX partners is required for transcriptional repression of ga20ox1. Plant J. 2004, 38, 276–284. [Google Scholar] [CrossRef]
- Raices, M.; Ulloa, R.; Macintosh, G.; Crespi, M.; Téllez-Iñón, M. StCDPK1 is expressed in potato stolon tips and is induced by high sucrose concentration. J. Exp. Bot. 2003, 54, 2589–2591. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Panda, P.; Sahoo, L.; Panda, S. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 2013, 8, e23681. [Google Scholar] [CrossRef] [PubMed]
- Abelenda, J.; Cruz-Oró, E.; Franco-Zorrilla, J.; Prat, S. Potato StCONSTANS-like1 suppresses storage organ formation by directly activating the FT-like StSP5G repressor. Curr. Biol. 2016, 26, 872–881. [Google Scholar] [CrossRef]
- Abelenda, J.A.; Bergonzi, S.; Oortwijn, M.; Sonnewald, S.; Du, M.; Visser, R.G.F.; Sonnewald, U.; Bachem, C.W.B. Source-sink regulation is mediated by interaction of an FT homolog with a sweet protein in potato. Curr. Biol. 2019, 29, 1178–1186. [Google Scholar] [CrossRef]
- Schreiber, M.; Stein, N.; Mascher, M. Genomic approaches for studying cropevolution. Genome Biol. 2018, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D. The signal transduction pathways controlling in planta tuberization in potato: An emerging synthesis. Plant Cell Rep. 2008, 27, 1–8. [Google Scholar] [CrossRef]
- Kloosterman, B.; Vorst, O.; Hall, R.; Visser, R.; Bachem, C. Tuber on a chip: Differential gene expression during potato tuber development. Plant Biotechnol. J. 2005, 3, 505–519. [Google Scholar] [CrossRef]
- Wu, F.; Chi, Y.; Jiang, Z.; Xu, Y.; Xie, L.; Huang, F.; Wan, D.; Ni, J.; Yuan, F.; Wu, X.; et al. Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature 2020, 578, 577–581. [Google Scholar] [CrossRef]


| Gene Name | Gene ID | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|---|
| StSP6A | Soltu.DM.05G026370.1 | AGGGTTCATATTGGAGGGGAC | TGCTGGGATATCTGTGACCA |
| StSUT4 | Soltu.DM.04G031670.2 | TGCTGCGCTGGTTGTATTTT | GGGAACACAATTGCCAGGTT |
| POTH | Soltu.DM.05G009240.1 | AGCTTTGATGTCACCGGAGA | CGGATCCAAACATCATCGGA |
| StCDPK1 | Soltu.DM.03G021780.1 | GCTTTGAAGGCAACAGAT | TTGAGCAGCAGAAATACG |
| StPHYB | Soltu.DM.01G019510.1 | CCCAATCCTCTGATCCCTCC | TCTCCCCTCTAGACCAACCA |
| StBEL5 | Soltu.DM.06G029500.1 | TGGTGGTGGTGAAAGTAGCA | ACCTTTGCTCCACCTCTTCA |
| StCO | Soltu.DM.02G030260.1 | CCAACCGCAACAACAACAAC | ACACTGACATCCATCGACGA |
| Stga20ox1 | Soltu.DM.03G016400.1 | CACCATGTCAGAAACCGGAG | AACTTGAAGTCCGCCAACAC |
| Strboh | Soltu.DM.08G028440.1 | TGGCTTAGAATATGGGAGGG | GCCATGATTGTCTGTCCTTT |
| L2 | 39816659 | GGCGAAATGGGTCGTGTTAT | CATTTCTCTCGCCGAAATCG |
| Gene Name | Function | Effect on Tuberization | References |
|---|---|---|---|
| StSP6A | Triggering tuberization | Induction | [27] |
| StCO | Represses StSP6A gene expression in LDs | Repression | [28] |
| StPHYB | Perception of external cues | Repression | [5] |
| StSUT4 | Inhibits sucrose export from leaves | Repression | [29] |
| StCDPK | Highly expressed in the swelling part of stolon | Induction | [30] |
| StRboh | Regulates intracellular ROS production | Unknown | [24] |
| StGA20ox1 | Encodes a key enzyme in the GA biosynthetic pathway | Repression | [31] |
| StBEL5 | StBEL5-POTH1 heterodimer can lower endogenous GAs in potato | Induction | [32] |
| POTH | Induction | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, C.; Ye, M.; Li, C.; Gong, M. H2O2 Participates in the Induction and Formation of Potato Tubers by Activating Tuberization-Related Signal Transduction Pathways. Agronomy 2023, 13, 1398. https://doi.org/10.3390/agronomy13051398
Lei C, Ye M, Li C, Gong M. H2O2 Participates in the Induction and Formation of Potato Tubers by Activating Tuberization-Related Signal Transduction Pathways. Agronomy. 2023; 13(5):1398. https://doi.org/10.3390/agronomy13051398
Chicago/Turabian StyleLei, Chunxia, Mingwang Ye, Canhui Li, and Ming Gong. 2023. "H2O2 Participates in the Induction and Formation of Potato Tubers by Activating Tuberization-Related Signal Transduction Pathways" Agronomy 13, no. 5: 1398. https://doi.org/10.3390/agronomy13051398
APA StyleLei, C., Ye, M., Li, C., & Gong, M. (2023). H2O2 Participates in the Induction and Formation of Potato Tubers by Activating Tuberization-Related Signal Transduction Pathways. Agronomy, 13(5), 1398. https://doi.org/10.3390/agronomy13051398






