A New SNP in AGPL2, Associated with Floury Endosperm in Rice, Is Identified Using a Modified MutMap Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Microscopy Observation
2.3. Characterization of Starch and Soluble Sugar Contents and Grain Hardness
2.4. Whole-Genome Sequencing of Bulked DNA
2.5. Re-Sequencing Analysis and Calculation of Δ (SNP Index)
2.6. Mutation Site Sequencing and Allele-Specific PCR (AS-PCR)
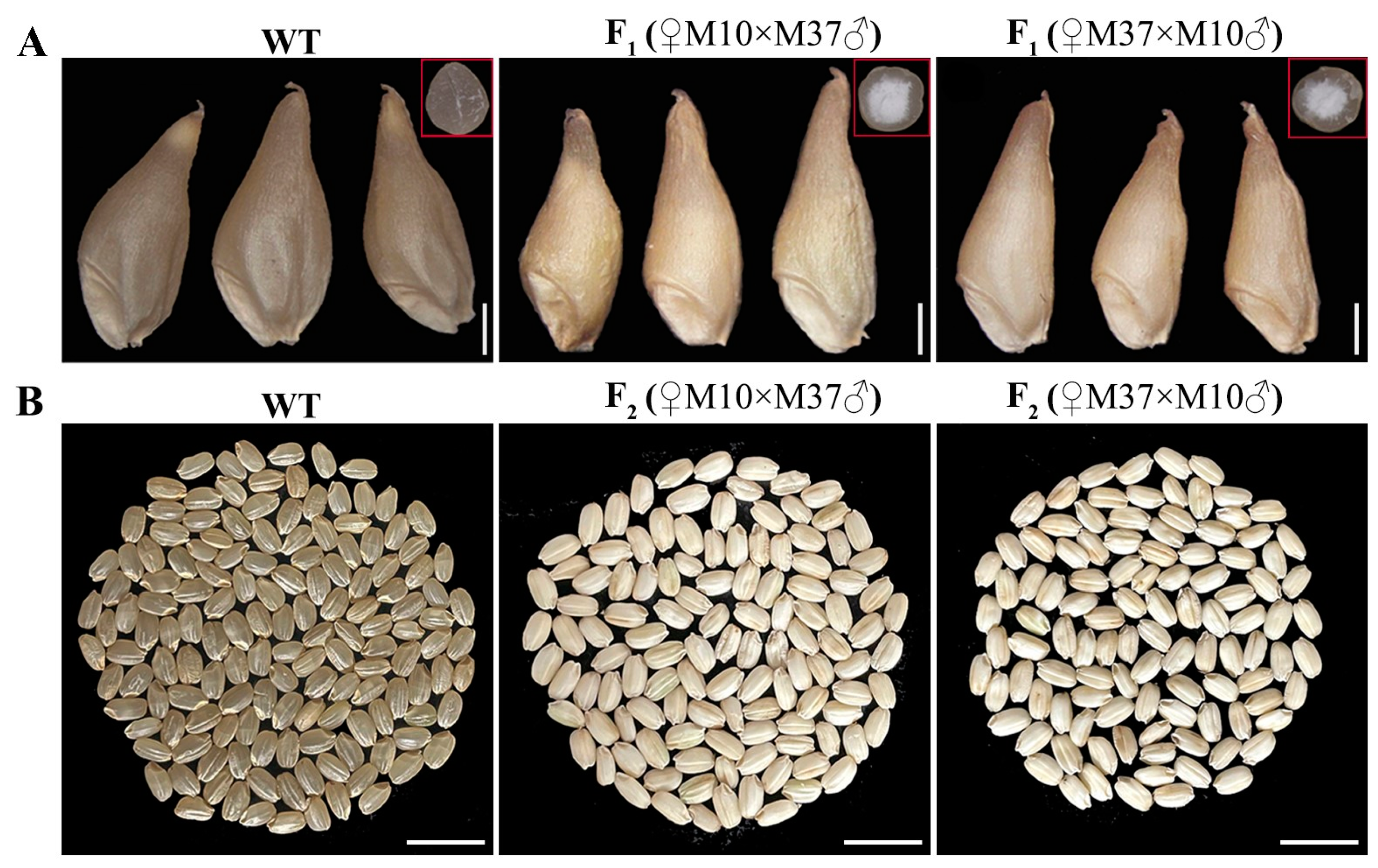
2.7. Allelic Test of M10 and M37
2.8. RNA Isolation and qRT-PCR
2.9. Protein Extraction and Western Blot Analysis
3. Results
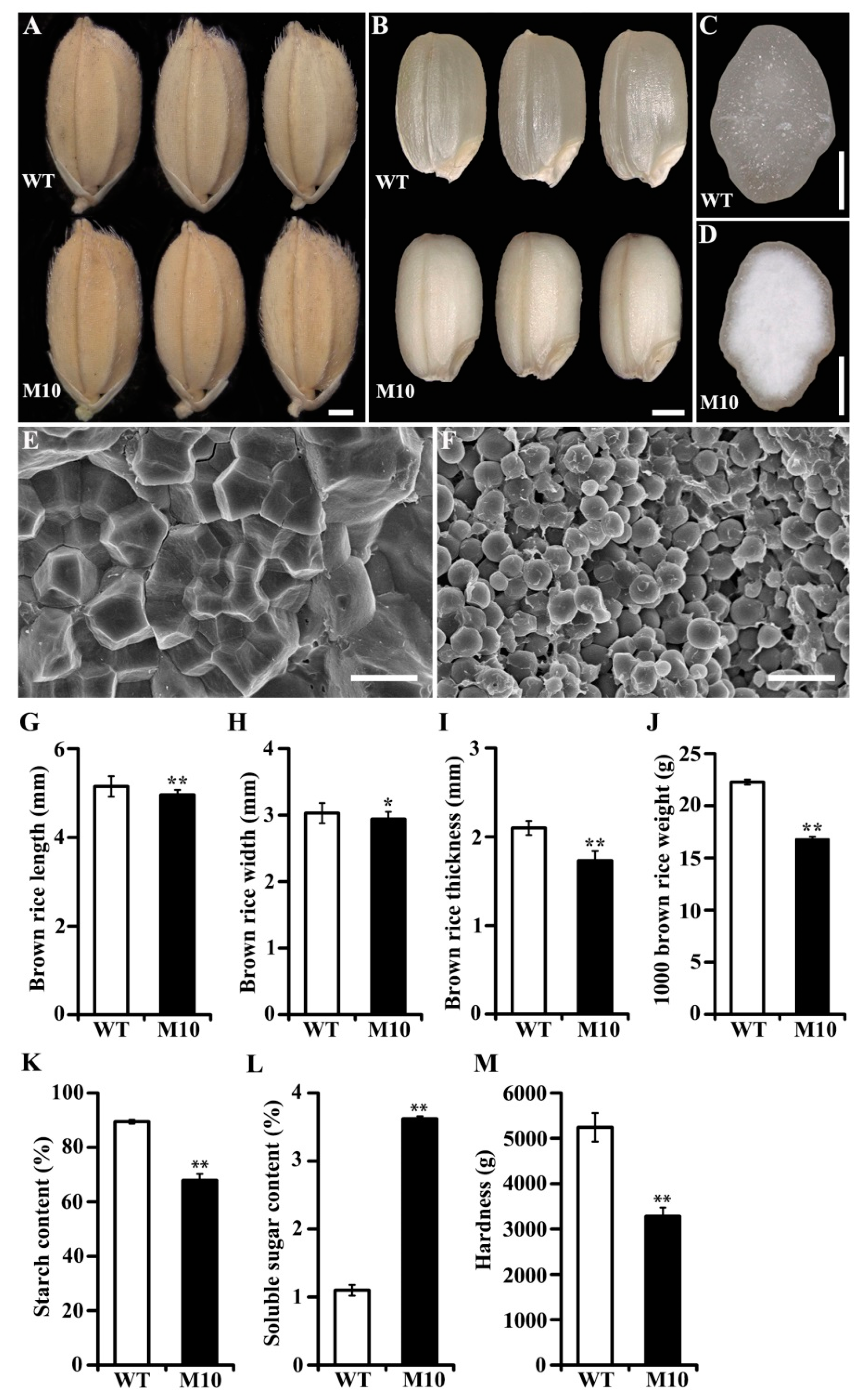
3.1. Phenotypes of the M10 Mutant
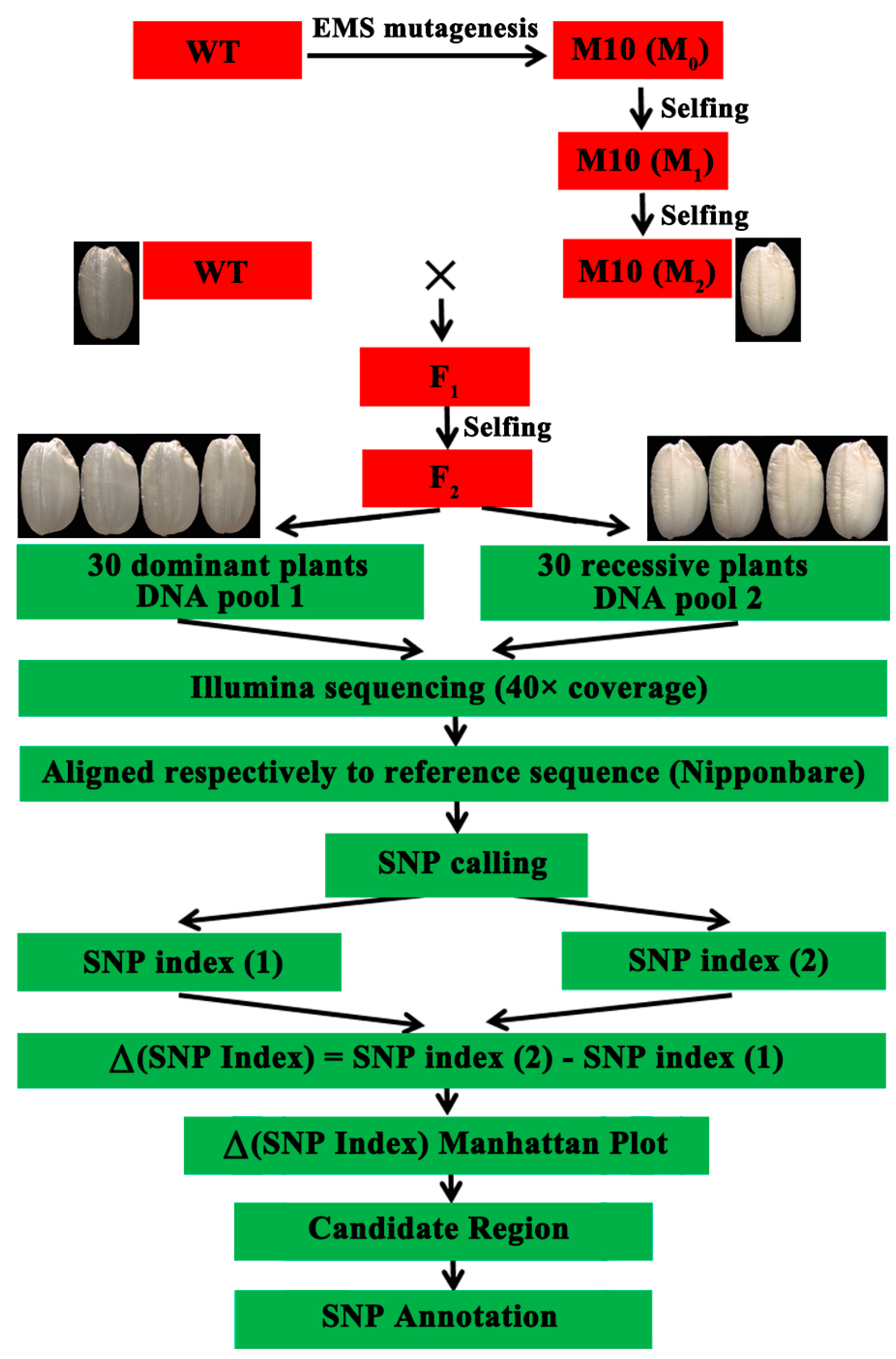
3.2. Genetic Mapping of the Floury Endosperm Locus Using the Modified MutMap Method
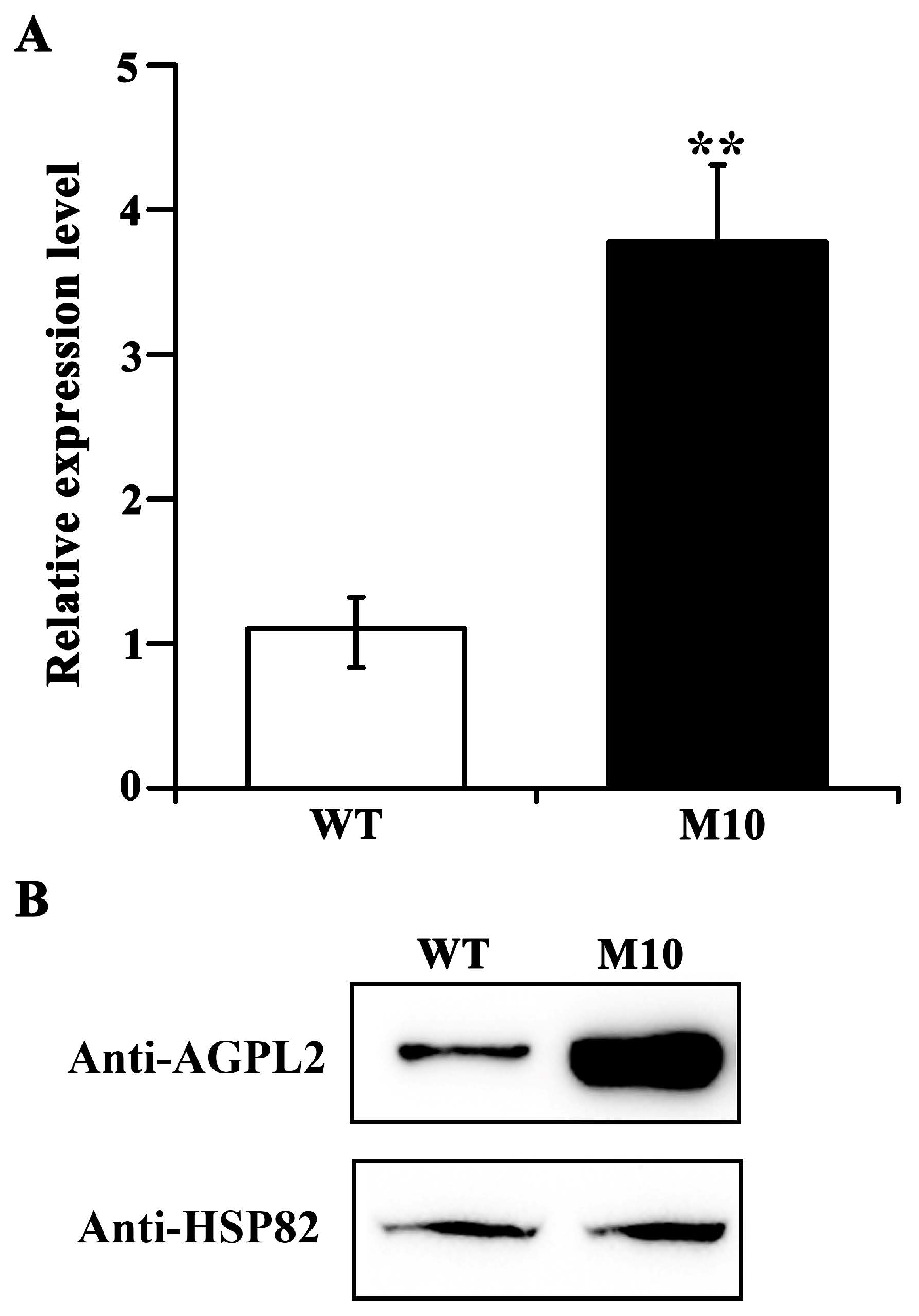
3.3. AGPL2 Is the Causative Gene for Floury Endosperm in M10
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, N.; Kaur, L.; Sodhi, N.; Sekhon, K. Physicochemical, cooking and textural properties of milled rice from different Indian rice cultivars. Food Chem. 2005, 89, 253–259. [Google Scholar] [CrossRef]
- Zhou, Z.; Robards, K.; Helliwell, S.; Blanchard, C. Composition and functional properties of rice. Int. J. Food Sci. Technol. 2002, 37, 849–868. [Google Scholar] [CrossRef]
- Heo, S.; Lee, S.; Shim, J.; Yoo, S.; Lee, S. Effect of dry- and wet-milled rice flours on the quality attributes of gluten-free dough and noodles. J. Food Eng. 2013, 116, 213–217. [Google Scholar] [CrossRef]
- Mo, Y.; Jeung, J.; Shin, Y.; Park, C.; Kang, K.; Kim, B. Agronomic and genetic analysis of Suweon 542, a rice floury mutant line suitable for dry milling. Rice 2013, 6, 37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, H.; Mo, Y.; Im, D.; Jang, S.; Ham, T.; Lee, J.; Jeung, J.; Kwon, S. A new SNP in cyOsPPDK gene is associated with floury endosperm in Suweon 542. Mol. Gen. Genet. 2018, 293, 1151–1158. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N.; Zhang, J.; Zhao, L.; Qiu, J.; Wei, C. The CBM48 domain-containing protein FLO6 regulates starch synthesis by interacting with SSIVb and GBSS in rice. Plant Mol. Biol. 2022, 108, 343–361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ren, Y.; Lu, B.; Yang, C.; Feng, Z.; Liu, Z.; Chen, J.; Ma, W.; Wang, Y.; Yu, X.; et al. FLOURY ENDOSPERM7 encodes a regulator of starch synthesis and amyloplast development essential for peripheral endosperm development in rice. J. Exp. Bot. 2016, 67, 633–647. [Google Scholar] [CrossRef][Green Version]
- Lee, S.; Hwang, S.; Han, M.; Eom, J.; Kang, H.; Han, Y.; Choi, S.; Cho, M.; Bhoo, S.; An, G.; et al. Identification of the ADP-glucose pyrophosphorylase isoforms essential for starch synthesis in the leaf and seed endosperm of rice (Oryza sativa L.). Plant Mol. Biol. 2007, 65, 531–546. [Google Scholar] [CrossRef]
- Naoko, F.; Mayumi, Y.; Tomonori, K.; Kaori, S.; Yoshinori, U.; Takashi, T.; Aiko, N.; Hikaru, S.; Park, J.; Jane, J.; et al. Characterization of SSIIIa-deficient mutants of rice: The function of SSIIIa and pleiotropic effects by SSIIIa deficiency in the rice endosperm. Plant Physiol. 2007, 144, 2009–2023. [Google Scholar]
- Aiko, N.; Yasunori, N.; Naoki, T.; Hikaru, S. Biochemical and genetic analysis of the effects of amylose-extender mutation in rice endosperm. Plant Physiol. 2001, 127, 459–472. [Google Scholar]
- Long, W.; Dong, B.; Wang, Y.; Pan, P.; Wang, Y.; Liu, L.; Chen, X.; Liu, X.; Liu, S.; Tian, Y.; et al. FLOURY ENDOSPERM8, encoding the UDP-glucose pyrophosphorylase 1, affects the synthesis and structure of starch in rice endosperm. J. Integr. Plant Biol. 2017, 60, 513–522. [Google Scholar] [CrossRef]
- Wu, M.; Ren, Y.; Cai, M.; Wang, Y.; Zhu, S.; Zhu, J.; Hao, Y.; Teng, X.; Zhu, X.; Jing, R.; et al. Rice FLOURY ENDOSPERM10 encodes a pentatricopeptide repeat protein that is essential for the trans-splicing of mitochondrial nad1 intron 1 and endosperm development. New Phytol. 2019, 223, 736–750. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Liu, X.; Liu, F.; Ren, Y.; Wang, Y.; Zhu, J.; Teng, X.; Duan, E.; Wang, F.; Zhang, H.; et al. FLOURY ENDOSPERM12 encoding alanine aminotransferase 1 regulates carbon and nitrogen metabolism in rice. J. Plant Biol. 2019, 62, 61–73. [Google Scholar] [CrossRef]
- Hu, T.; Tian, Y.; Zhu, J.; Wang, Y.; Jing, R.; Lei, J.; Sun, Y.; Yu, Y.; Li, J.; Chen, X.; et al. OsNDUFA9 encoding a mitochondrial complex I subunit is essential for embryo development and starch synthesis in rice. Plant Cell Rep. 2018, 37, 1667–1679. [Google Scholar] [CrossRef]
- Xue, M.; Liu, L.; Yu, Y.; Zhu, J.; Gao, H.; Wang, Y.; Wan, J. Lose-of-function of a rice nucleolus-localized pentatricopeptide repeat protein is responsible for the floury endosperm14 mutant phenotypes. Rice 2019, 12, 100. [Google Scholar] [CrossRef][Green Version]
- You, X.; Zhang, W.; Hu, J.; Jing, R.; Cai, Y.; Feng, Z.; Kong, F.; Zhang, J.; Yan, H.; Chen, W.; et al. FLOURY ENDOSPERM15 encodes a glyoxalase I involved in compound granule formation and starch synthesis in rice endosperm. Plant Cell Rep. 2019, 38, 345–359. [Google Scholar] [CrossRef]
- Teng, X.; Zhong, M.; Zhu, X.; Wang, C.; Ren, Y.; Wang, Y.; Zhang, H.; Jiang, L.; Wang, D.; Hao, Y.; et al. FLOURY ENDOSPERM16 encoding a NAD-dependent cytosolic malate dehydrogenase plays an important role in starch synthesis and seed development in rice. Plant Biotechnol. J. 2019, 17, 1914–1927. [Google Scholar] [CrossRef][Green Version]
- Yu, M.; Wu, M.; Ren, Y.; Wang, Y.; Li, J.; Lei, C.; Sun, Y.; Bao, X.; Wu, H.; Yang, H.; et al. Rice FLOURY ENDOSPERM 18 encodes a pentatricopeptide repeat protein required for 5′ processing of mitochondrial nad5 messenger RNA and endosperm development. J. Integr. Plant Biol. 2021, 63, 834–847. [Google Scholar] [CrossRef]
- Lei, J.; Teng, X.; Wang, Y.; Jiang, X.; Zhao, H.; Zheng, X.; Ren, Y.; Dong, H.; Wang, Y.; Duan, E.; et al. Plastidic pyruvate dehydrogenase complex E1 component subunit Alpha1 is involved in galactolipid biosynthesis required for amyloplast development in rice. Plant Biotechnol. J. 2022, 20, 437–453. [Google Scholar] [CrossRef]
- Yan, M.; Tian, P.; Zhu, Y.; Jiang, X.; Yu, M.; Wang, R.; Zhang, F.; Luo, S.; Bao, X.; Chen, Y.; et al. FLOURY ENDOSPERM20 encoding SHMT4 is required for rice endosperm development. Plant Biotechnol. J. 2022, 20, 1438–1440. [Google Scholar] [CrossRef]
- Akira, A.; Shunichi, K.; Kentaro, Y.; Satoshi, N.; Hiroki, T.; Hiroyuki, K.; Hideo, M.; Kakoto, Y.; Chikako, M.; Muluneh, T.; et al. Genome sequencing reveals agronomically important loci in rice using MutMap. Nat. Biotechnol. 2012, 30, 174–178. [Google Scholar]
- Fekih, R.; Takagi, H.; Tamiru, M.; Abe, A.; Natsume, S.; Yaegashi, H.; Sharma, S.; Sharma, S.; Kanzaki, H.; Matsumura, H.; et al. MutMap+: Genetic mapping and mutant identification without crossing in rice. PLoS ONE 2013, 8, e68529. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Uemura, A.; Yaegashi, H.; Tamiru, M.; Abe, A.; Mitsuoka, C.; Utsushi, H.; Natsume, S.; Kanzaki, H.; Matsumura, H.; et al. MutMap-Gap: Whole-genome resequencing of mutant F2 progeny bulk combined with de novo assembly of gap regions identifies the rice blast resistance gene Pii. New Phytol. 2013, 200, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Wang, D.; Duan, P.; Liu, Y.; Huang, K.; Zeng, D.; Zhang, L.; Dong, G.; Li, Y.; Xu, R.; et al. Control of grain size and weight by the GSK2-LARGE1/OML4 pathway in rice. Plant Cell. 2020, 32, 1905–1918. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Wang, H.; Yin, W.; Meng, W.; Xiao, Y.; Liu, D.; Zhang, X.; Dong, N.; Liu, J.; Yang, Y.; et al. Rice DWARF AND LOW-TILLERING and the homeodomain protein OSH15 interact to regulate internode elongation via orchestrating brassinosteroid signaling and metabolism. Plant Cell 2022, 34, 3754–3772. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, H.; Xiao, Y.; Zhang, G.; Cao, S.; Yin, W.; Qian, Y.; Yin, Y.; Zhang, J.; Chen, S.; et al. A cryptic inhibitor of cytokinin phosphorelay controls rice grain size. Mol. Plant 2022, 15, 293–307. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, H.; Mou, C.; Wang, P.; Hao, Q.; Zhang, M.; Wu, H.; Zhang, F.; Ma, T.; Miao, R.; et al. Ribonuclease H-like gene SMALL GRAIN2 regulates grain size in rice through brassinosteroid signaling pathway. J. Integr. Plant Biol. 2022, 64, 1883–1900. [Google Scholar] [CrossRef]
- Takagi, H.; Tamiru, M.; Abe, A.; Yoshida, K.; Uemura, A.; Yaeqashi, H.; Obara, T.; Oikawa, K.; Utsushi, H.; Kanzaki, E.; et al. MutMap accelerates breeding of a salt-tolerant rice cultivar. Nat. Biotechnol. 2015, 33, 445–449. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Sun, L.; Xu, P.; Tu, R.; Meng, S.; Wu, W.; Galal, B.; Kashif, H.; Aamiar, R.; et al. WB1, a regulator of endosperm development in rice, is identified by a modified MutMap method. Int. J. Mol. Sci. 2018, 19, 2159. [Google Scholar] [CrossRef][Green Version]
- Man, J.; Lin, L.; Wang, Z.; Wang, Y.; Liu, Q.; Wei, C. Different structures of heterogeneous starch granules from high-amylose rice. J. Agric. Food Chem. 2014, 62, 11254–11263. [Google Scholar] [CrossRef]
- Lin, L.; Pan, T.; Liu, Q.; Wei, C. Cooking, morphological, mechanical and digestion properties of cooked rice with suppression of starch branching enzymes. Int. J. Biol. Macromol. 2019, 137, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Ewing, B.; Hillier, L.; Wendl, M.; Green, P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 1988, 8, 175–185. [Google Scholar] [CrossRef][Green Version]
- Cox, M.; Peterson, D.; Biggs, P. SolexaQA: At-a-glance quality assessment of Illumina second-generation sequencing data. BMC Bioinform 2010, 11, 485. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, D.; Ronald, P. A rapid DNA minipreparation method suitable for AFLP and other PCR applications. Plant Mol. Biol. Rep. 1999, 17, 53–57. [Google Scholar] [CrossRef]
- Jiang, Y.; Ren, Y.; Xu, X.; Wang, H.; Wei, C. Application of allele specific PCR in identifying offspring genotypes of bi-allelic sbeIIb mutant lines in rice. Plants 2022, 11, 524. [Google Scholar] [CrossRef]
- Han, X.; Wang, Y.; Liu, X.; Jiang, L.; Ren, Y.; Liu, F.; Peng, C.; Li, J.; Jin, X.; Wu, F.; et al. The failure to express a protein disulphide isomerase-like protein results in a floury endosperm and an endoplasmic reticulum stress response in rice. J. Exp. Bot. 2012, 63, 121–130. [Google Scholar] [CrossRef]
- Pienkowska, M.; Glickman, B.; Ferreira, A.; Anderson, M.; Zielenska, M. Large-scale mutational analysis of EMS-induced mutation in the lacI gene of Escherichia coli. Mutat. Res. 1993, 288, 123–131. [Google Scholar] [CrossRef]
- Wei, X.; Jiao, G.; Lin, H.; Sheng, Z.; Shao, G.; Xie, L.; Tang, S.; Xu, Q.; Hu, P. GRAIN INCOMPLETE FILLING 2 regulates grain filling and starch synthesis during rice caryopsis development. J. Integr. Plant Biol. 2016, 59, 134–153. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Zhao, L.; Ren, Y.; Zhang, J.; Xu, A.; Wei, C. Gene mapping and mutation analysis of floury endosperm mutant M37 in rice. J. Yangzhou Univ. 2019, 40, 10–17. [Google Scholar]
- Zhang, L.; Zhao, L.; Lin, L.; Zhao, L.; Liu, Q.; Wei, C. A novel mutation of OsPPDKB, encoding pyruvate orthophosphate dikinase, affects metabolism and structure of starch in the rice endosperm. Int. J. Mol. Sci. 2018, 19, 2268. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Lu, F.; Lü, Y.; Luo, R.; Jiao, G.; Wu, Y.; Tang, S.; Hu, P.; Wei, X. Identification and gene mapping-based clone of two chalkiness mutants in rice. Chin. J. Rice Sci. 2017, 31, 568–579. [Google Scholar]
- Tang, X.; Peng, C.; Zhang, J.; Cai, Y.; You, X.; Kong, F.; Yan, H.; Wang, G.; Wang, L.; Jin, J.; et al. ADP-glucose pyrophosphorylase large subunit 2 is essential for storage substance accumulation and subunit interactions in rice endosperm. Plant Sci. 2016, 249, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Homma, N.; Morohashi, K.; Yoshii, Y.; Hosokawa, H.; Miura, K. Properties and components of the floury and sugary mutant rice cultivars developed in the Hokuriku region. Food Sci. Technol. Res. 2017, 13, 422–426. [Google Scholar] [CrossRef][Green Version]
- Giroux, M.; Boyer, C.; Feix, G.; Hannah, L. Coordinated transcriptional regulation of storage product genes in the maize endosperm. Plant Physiol. 1994, 106, 713–722. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Akihiro, T.; Mizuno, K.; Fujimura, T. Gene expression of ADP-glucose pyrophosphorylase and starch contents in rice cultured cells are cooperatively regulated by sucrose and ABA. Plant Cell Physiol. 2005, 46, 937–946. [Google Scholar] [CrossRef]
- Yu, J.; Hu, S.; Wang, J.; Wong, G.; Li, S.; Liu, B.; Deng, Y.; Dai, L.; Zhou, Y.; Zhang, X.; et al. A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 2002, 296, 79–92. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Xing, F.; Kudrna, D.; Yao, W.; Copetti, D.; Mu, T.; Li, W.; Song, J.; Xie, W.; et al. Extensive sequence divergence between the reference genomes of two elite indica rice varieties Zhenshan 97 and Minghui 63. Proc. Natl. Acad. Sci. USA. 2016, 113, E5163–E5171. [Google Scholar] [CrossRef][Green Version]
- Du, H.; Yu, Y.; Ma, Y.; Gao, Q.; Cao, Y.; Chen, Z.; Ma, B.; Qi, M.; Li, Y.; Zhao, X.; et al. Sequencing and de novo assembly of a near complete indica rice genome. Nat. Commun. 2017, 8, 15324. [Google Scholar] [CrossRef][Green Version]

| Total | No. of Normal Seeds | No. of Floury Endosperm Seeds | X23:1 a | |
|---|---|---|---|---|
| M10(♀) × WT(♂) | 242 | 185 | 57 | 0.270 |
| WT(♀) × M10(♂) | 226 | 173 | 53 | 0.289 |
| Δ (SNP Index) | Gene ID | Nucleotide Location (bp) | Reference Base (WT) | Altered Base (M10) | Type of Mutation | Gene Annotation |
|---|---|---|---|---|---|---|
| 0.744 | LOC_Os01g43820 | 25109152 | G | A | Missense (Pro451Ser) | Retrotransposon protein |
| 0.789 | LOC_Os01g44220 | 25359718 | G | A | Missense Gly251Glu | Glucose-1-phosphate adenylyltransferase large subunit |
| 0.833 | LOC_Os01g47150 | 26950694 | G | A | Missense (Val1093Ile) | Retrotransposon protein |
| 0.827 | LOC_Os01g48620 | 27875985 | G | A | Missense (Ser116Asn) | Expressed protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; You, R.; Chen, H.; Zhu, J.; Lin, L.; Wei, C. A New SNP in AGPL2, Associated with Floury Endosperm in Rice, Is Identified Using a Modified MutMap Method. Agronomy 2023, 13, 1381. https://doi.org/10.3390/agronomy13051381
Zhang L, You R, Chen H, Zhu J, Lin L, Wei C. A New SNP in AGPL2, Associated with Floury Endosperm in Rice, Is Identified Using a Modified MutMap Method. Agronomy. 2023; 13(5):1381. https://doi.org/10.3390/agronomy13051381
Chicago/Turabian StyleZhang, Long, Ran You, Hualan Chen, Jun Zhu, Lingshang Lin, and Cunxu Wei. 2023. "A New SNP in AGPL2, Associated with Floury Endosperm in Rice, Is Identified Using a Modified MutMap Method" Agronomy 13, no. 5: 1381. https://doi.org/10.3390/agronomy13051381
APA StyleZhang, L., You, R., Chen, H., Zhu, J., Lin, L., & Wei, C. (2023). A New SNP in AGPL2, Associated with Floury Endosperm in Rice, Is Identified Using a Modified MutMap Method. Agronomy, 13(5), 1381. https://doi.org/10.3390/agronomy13051381







