Fermentation Quality and Chemical Composition of Industrial Hemp (Cannabis sativa L.) Silage Inoculated with Bacterial Starter Cultures—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
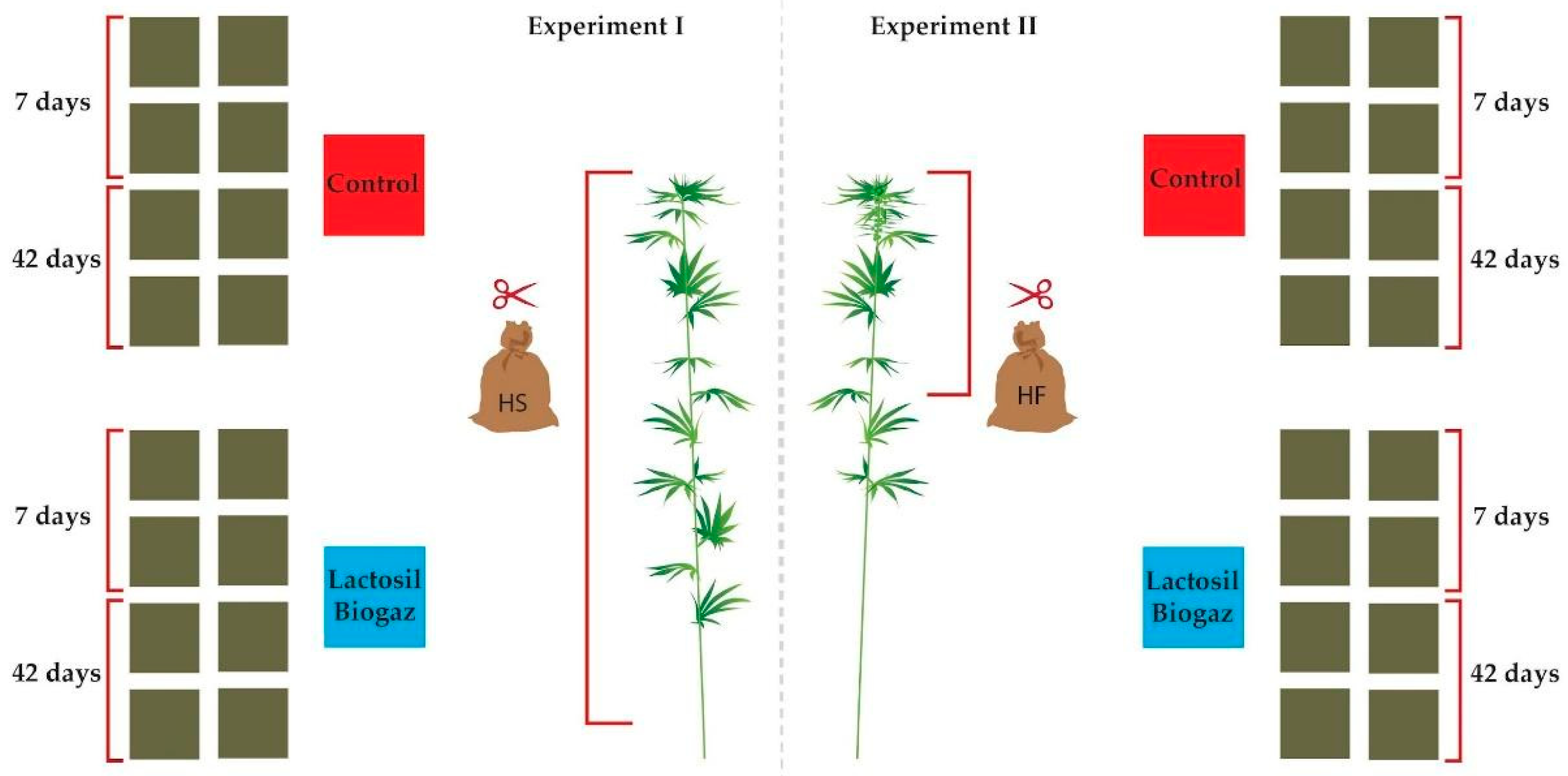
2.1. Experiment Design and Treatments
2.2. Chemical Composition of Raw Material and Silages
2.3. Microbial Analyses
2.4. Statistical Analysis
3. Results
3.1. Fermentation Quality of Industrial Hemp Silage
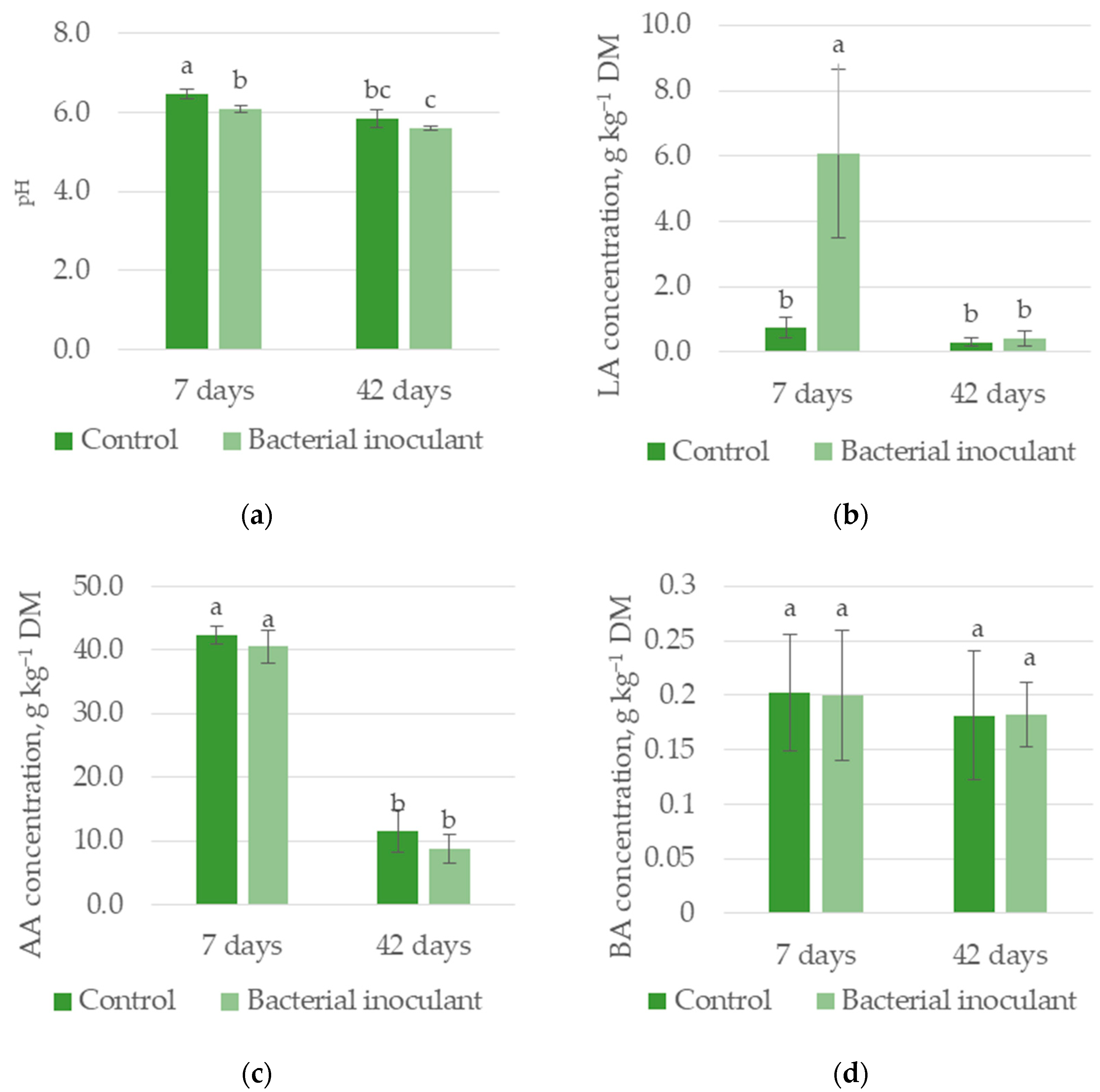
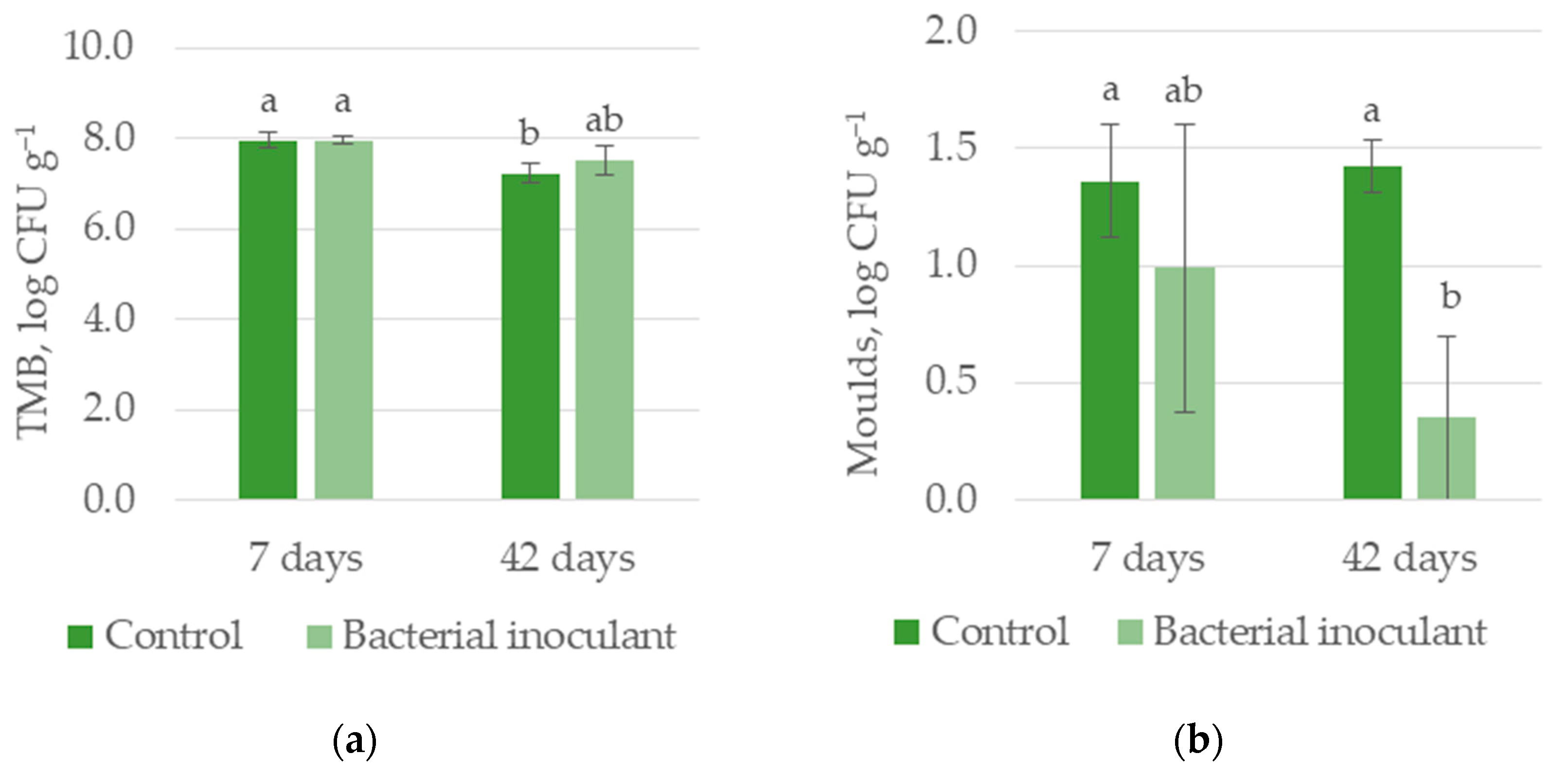
3.1.1. Experiment I—HS Silage
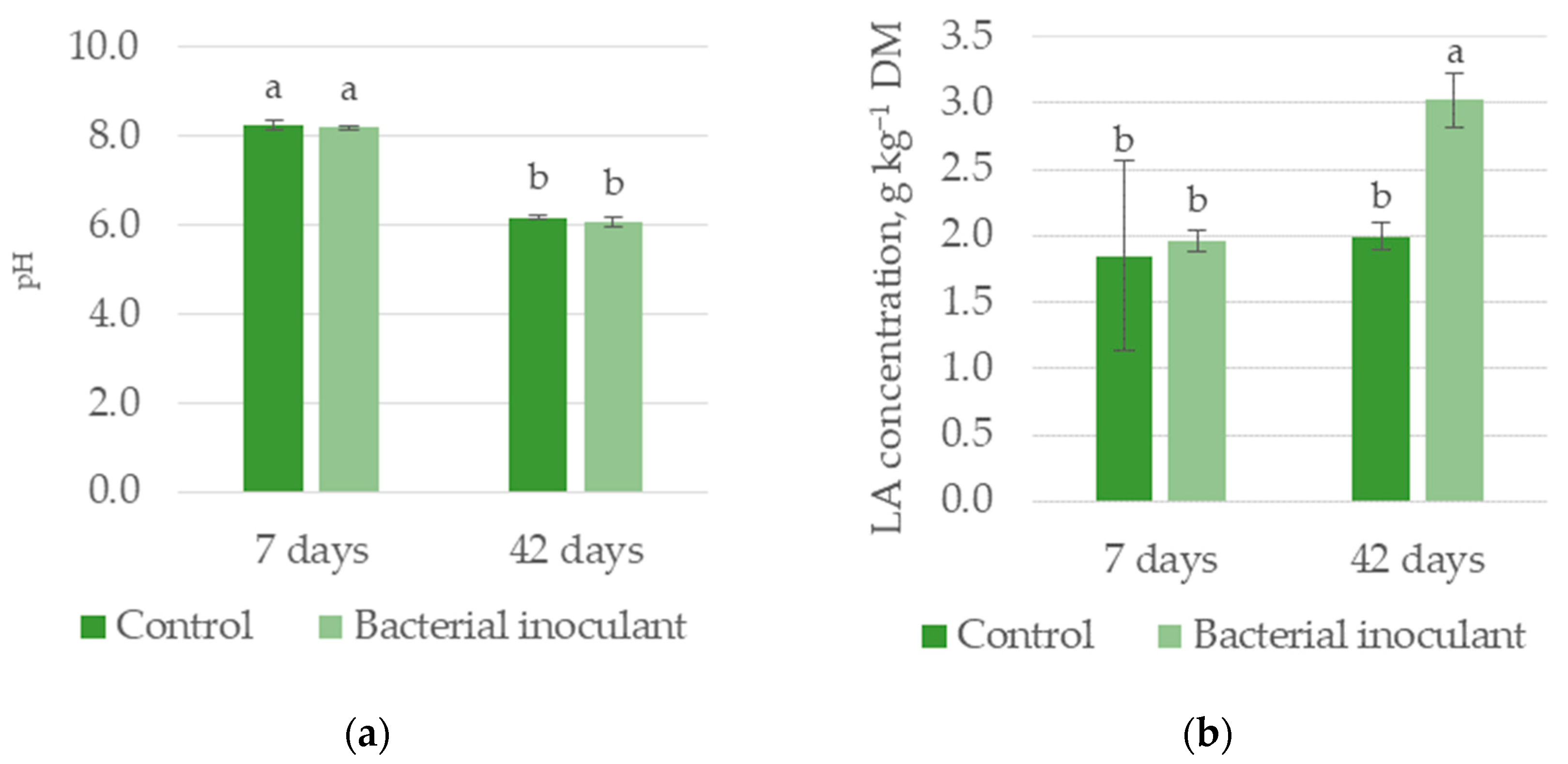
3.1.2. Experiment II—HF Silage
3.2. Chemical Composition of Industrial Hemp Silage
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adesina, I.; Bhowmik, A.; Sharma, H.; Shahbazi, A. A Review on the Current State of Knowledge of Growing Conditions, Agronomic Soil Health Practices and Utilities of Hemp in the United States. Agriculture 2020, 10, 129. [Google Scholar] [CrossRef]
- European Environment Agency. Greenhouse Gas Emissions by Source Sector. Available online: https://agriculture.ec.europa.eu/system/files/2021-03/cdg-arable-crops-2020-02-03-minutes_en_0.pdf (accessed on 14 February 2023).
- Karche, T. The application of hemp (Cannabis sativa L.) for a green economy: A review. Turk. J. Bot. 2019, 43, 710–723. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Baiton, A.; Salami, S.A.; Jones, A.M.P. New insight into ornamental applications of cannabis: Perspectives and challenges. Plants 2022, 11, 2383. [Google Scholar] [CrossRef] [PubMed]
- Krüger, M.; van Eeden, T.; Beswa, D. Cannabis sativa Cannabinoids as Functional Ingredients in Snack Foods—Historical and Developmental Aspects. Plants 2022, 11, 3330. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Baiton, A.; Jones, A.M.P. Current status and future prospects in cannabinoid production through in vitro culture and synthetic biology. Biotechnol. Adv. 2022, 62, 108074. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, I.; Pellino, M.; Rigault, P.; Van Velzen, R.; Ebersbach, J.; Ashnest, J.R.; Mau, M.; Schranz, M.E.; Alcorn, J.; Laprairie, R.B.; et al. The genomics of Cannabis and its close relatives. Annu. Rev. Plant Biol. 2020, 71, 713–739. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Alizadeh, M.; Rakei, A.; Baiton, A.; Jones, A.M.P. Recent advances in cannabis biotechnology. Ind. Crops Prod. 2020, 158, 113026. [Google Scholar] [CrossRef]
- Schluttenhofer, C.; Yuan, L. Challenges towards revitalizing hemp: A multifaceted crop. Trends Plant Sci. 2017, 22, 917–929. [Google Scholar] [CrossRef]
- Brzyski, P.; Barnat-Hunek, D.; Suchorab, Z.; Łagód, G. Composite Materials Based on Hemp and Flax for Low-Energy Buildings. Materials 2017, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Applications of Hemp in Textiles, Paper Industry, Insulation and Building Materials, Horticulture, Animal Nutrition, Food and Beverages, Nutraceuticals, Cosmetics and Hygiene, Medicine, Agrochemistry, Energy Production and Environment: A Review. Environ. Chem. Lett. 2020, 18, 1451–1476. [Google Scholar] [CrossRef]
- Maoduš, N. Razvoj I Primena Biokompozitnog Pločastog Termoizolacionog Materijala na Bazi Biomase I Micelijuma Gljiva. Ph.D. Thesis, University of Novi Sad, Novi Sad, Serbia, 2019. [Google Scholar] [CrossRef]
- Ely, K.; Fike, J. Industrial Hemp and Hemp Byproducts as Sustainable Feedstuffs in Livestock Diets. In Cannabis/Hemp for Sustainable Agriculture and Materials; Agrawal, D.C., Kumar, R., Dhanasekaran, M., Eds.; Springer: Singapore, 2022; pp. 145–162. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, J.; Cheng, C.; Lv, J.; Lambo, M.T.; Zhang, G.; Li, Y.; Zhang, Y. Nutritional Values of Industrial Hemp Byproducts for Dairy Cattle. Animals 2022, 12, 3488. [Google Scholar] [CrossRef]
- Gabriele, I.; Race, M.; Papirio, S.; Papetti, P.; Esposito, G. Phytoremediation of a Pyrene-Contaminated Soil by Cannabis sativa L. at Different Initial Pyrene Concentrations. Chemosphere 2022, 300, 134578. [Google Scholar] [CrossRef]
- Konieczna, A.; Mazur, K.; Koniuszy, A.; Gawlik, A.; Sikorski, I. Thermal Energy and Exhaust Emissions of a Gasifier Stove Feeding Pine and Hemp Pellets. Energies 2022, 15, 9458. [Google Scholar] [CrossRef]
- Todde, G.; Carboni, G.; Marras, S.; Caria, M.; Sirca, C. Industrial Hemp (Cannabis sativa L.) for Phytoremediation: Energy and Environmental Life Cycle Assessment of Using Contaminated Biomass as an Energy Resource. Sustain. Energy Technol. Assess. 2022, 52, 102081. [Google Scholar] [CrossRef]
- Czekała, W.; Pulka, J.; Jasiński, T.; Szewczyk, P.; Bojarski, W.; Jasiński, J. Waste as substrates for agricultural biogas plants: A case study from Poland. J. Water Land Dev. 2023, 56, 45–50. [Google Scholar] [CrossRef]
- Adamovics, A.M.; Ivanovs, S.A.; Dubrovskis, V.S. Methane production from industrial hemp. Agric. Mach. Technol. 2019, 13, 20–26. [Google Scholar] [CrossRef]
- Prade, T.; Svensson, S.-E.; Mattsson, J.E. Energy Balances for Biogas and Solid Biofuel Production from Industrial Hemp. Biomass Bioenergy 2012, 40, 36–52. [Google Scholar] [CrossRef]
- Paul, S.K.; Chakraborty, S. Mixing Effects on the Kinetics of Enzymatic Hydrolysis of Lignocellulosic Sunn Hemp Fibres for Bioethanol Production. Chem. Eng. J. 2019, 377, 120103. [Google Scholar] [CrossRef]
- Heiermann, M.; Ploechl, M.; Linke, B.; Schelle, H.; Herrmann, C. Biogas Crops—Part I: Specifications and Suitability of Field Crops for Anaerobic Digestion. Agric. Eng. Int. CIGR J. 2009, 11, 1–17. [Google Scholar]
- Kim, J.; Yu, Y.; Lee, C. Thermo-Alkaline Pretreatment of Waste Activated Sludge at Low-Temperatures: Effects on Sludge Disintegration, Methane Production, and Methanogen Community Structure. Bioresour. Technol. 2013, 144, 194–201. [Google Scholar] [CrossRef]
- Amon, T.; Amon, B.; Kryvoruchko, V.; Zollitsch, W.; Mayer, K.; Gruber, L. Biogas Production from Maize and Dairy Cattle Manure—Influence of Biomass Composition on the Methane Yield. Agric. Ecosyst. Environ. 2007, 118, 173–182. [Google Scholar] [CrossRef]
- McEniry, J.; Allen, E.; Murphy, J.D.; O’Kiely, P. Grass for Biogas Production: The Impact of Silage Fermentation Characteristics on Methane Yield in Two Contrasting Biomethane Potential Test Systems. Renew. Energy 2014, 63, 524–530. [Google Scholar] [CrossRef]
- Kalač, P. The required characteristics of ensiled crops used as a feedstock for biogas production: A review. J. Agrobiol. 2011, 28, 85–96. [Google Scholar] [CrossRef]
- Herrmann, C.; Idler, C.; Heiermann, M. Improving Aerobic Stability and Biogas Production of Maize Silage Using Silage Additives. Bioresour. Technol. 2015, 197, 393–403. [Google Scholar] [CrossRef]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L. Silage Review: Recent Advances and Future Uses of Silage Additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Oude Elferink, S.J.W.H.; Krooneman, J.; Gottschal, J.C.; Spoelstra, S.F.; Faber, F.; Driehuis, F. Anaerobic Conversion of Lactic Acid to Acetic Acid and 1,2-Propanediol by Lactobacillus buchneri. J. Appl. Environ. Microbiol. 2001, 67, 125–132. [Google Scholar] [CrossRef]
- Kung, L.; Taylor, C.C.; Lynch, M.P.; Neylon, J.M. The Effect of Treating Alfalfa with Lactobacillus buchneri 40788 on Silage Fermentation, Aerobic Stability, and Nutritive Value for Lactating Dairy Cows. J. Dairy Sci. 2003, 86, 336–343. [Google Scholar] [CrossRef]
- Zielińska, K.; Fabiszewska, A.; Świątek, M.; Szymanowska-Powałowska, D. Evaluation of the ability to metabolize 1,2-propanediol by heterofermentative bacteria of the genus Lactobacillus. Electron. J. Biotechnol. 2017, 26, 60–63. [Google Scholar] [CrossRef]
- Zielińska, K.J.; Fabiszewska, A.U. Improvement of the quality of maize grain silage by a synergistic action of selected lactobacilli strains. World J. Microbiol. Biotechnol. 2018, 34, 9. [Google Scholar] [CrossRef]
- Kaya, H.İ.; Şimşek, Ö. Characterization of Pediococcus acidilactici PFC69 and Lactococcus lactis PFC77 Bacteriocins and Their Antimicrobial Activities in Tarhana Fermentation. Microorganisms 2020, 8, 1083. [Google Scholar] [CrossRef]
- Surachat, K.; Kantachote, D.; Deachamag, P.; Wonglapsuwan, M. Genomic Insight into Pediococcus acidilactici HN9, a Potential Probiotic Strain Isolated from the Traditional Thai-Style Fermented Beef Nhang. Microorganisms 2021, 9, 50. [Google Scholar] [CrossRef]
- Kupryś-Caruk, M.; Lisowski, A.; Chomontowski, C. The effect of silage additive on the kinetics of biogas production from lignocellulosic perennial crops. J. Water Land Dev. 2023, 56, 58–66. [Google Scholar] [CrossRef]
- Zielińska, K.; Fabiszewska, A.; Piasecka-Jóźwiak, K.; Choińska, R. Increasing Biogas Yield from Fodder by Microbial Stimulation of Propionic Acid Synthesis in Grass Silages. Energies 2021, 14, 2843. [Google Scholar] [CrossRef]
- PN–ISO 6496:2002; Fodders. Determination of Humidity and Other Flight Substances. Polish Standard: Warszawa, Poland, 2002.
- Idler, C.; Pecenka, R.; Fürll, C.; Gusovius, H.-J. Wet processing of hemp: An overview. J. Nat. Fibers 2011, 8, 59–80. [Google Scholar] [CrossRef]
- Gusovius, H.-J.; Lühr, C.; Hoffmann, T.; Pecenka, R.; Idler, C. An Alternative to Field Retting: Fibrous Materials Based on Wet Preserved Hemp for the Manufacture of Composites. Agriculture 2019, 9, 140. [Google Scholar] [CrossRef]
- Pecenka, R.; Idler, C.; Grundmann, P.; Furll, C.; Gusovius, H.J. Tube ensiling of hemp—Initial practical experience. Agric. Eng. Res. 2007, 13, 15–26. [Google Scholar]
- Iqbal, M.; Sharma, R.K.; Rastogi, A.; Ali, S.; Pathak, A.K. Effect of Silage Prepared from Parthenium hysterophorous (Congress Grass) and Cannabis sps. (Hemp) with Maize on Blood Biochemistry of Goats. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 3256–3265. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage Review: Interpretation of Chemical, Microbial, and Organoleptic Components of Silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Wróbel, B.; Nowak, J.; Fabiszewska, A.; Paszkiewicz-Jasińska, A.; Przystupa, W. Dry Matter Losses in Silages Resulting from Epiphytic Microbiota Activity—A Comprehensive Study. Agronomy 2023, 13, 450. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.R. Buffering capacity of herbage samples as a factor in ensilage. J. Sci. Food Agric. 1962, 13, 395–400. [Google Scholar] [CrossRef]
- Ţiţei, V. The evaluation of the quality of the energy phytomass from industrial hemp Cannabis sativa grown in Moldavia. Agron. Ser. Sci. Res. Lucr. Ştiinţi. Ser. Agron. 2022, 65, 123–128. Available online: https://ibn.idsi.md/vizualizare_articol/167155 (accessed on 10 November 2022).
- Kreuger, E.; Nges, I.A.; Björnsson, L. Ensiling of Crops for Biogas Production: Effects on Methane Yield and Total Solids Determination. Biotechnol. Biofuels 2011, 4, 44. [Google Scholar] [CrossRef]
- Pahlow, G.; Muck, R.E.; Driehuis, F.; Oude Elferink, S.J.W.H.; Spoelstra, S.F. Microbiology of ensiling. In Silage Science and Technology; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; American Society of Agronomy: Madison, WI, USA, 2003; pp. 31–93. ISBN 9780891182344. [Google Scholar]
- Fabiszewska, A.U.; Zielińska, K.J.; Wróbel, B. Trends in Designing Microbial Silage Quality by Biotechnological Methods Using Lactic Acid Bacteria Inoculants: A Minireview. World J. Microbiol. Biotechnol. 2019, 35, 76. [Google Scholar] [CrossRef]
- Guo, X.; Xu, D.; Li, F.; Bai, J.; Su, R. Current Approaches on the Roles of Lactic Acid Bacteria in Crop Silage. Microb. Biotechnol. 2023, 16, 67–87. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, X.; Li, J.; Wang, S.; Dong, Z.; Shao, T. Effect of Lactic Acid Bacteria and Propionic Acid on Conservation Characteristics, Aerobic Stability and In Vitro Gas Production Kinetics and Digestibility of Whole-Crop Corn Based Total Mixed Ration Silage. J. Integr. Agric. 2017, 16, 1592–1600. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Weinberg, Z.G.; Ogunade, I.M.; Cervantes, A.A.P.; Arriola, K.G.; Jiang, Y.; Kim, D.; Li, X.; Gonçalves, M.C.M.; Vyas, D.; et al. Meta-Analysis of Effects of Inoculation with Homofermentative and Facultative Heterofermentative Lactic Acid Bacteria on Silage Fermentation, Aerobic Stability, and the Performance of Dairy Cows. J. Dairy Sci. 2017, 100, 4587–4603. [Google Scholar] [CrossRef]
- Selwet, M. Influence of Inoculation with Lactobacillus on Fermentation, Production of 1,2-Propanediol and 1-Propanol as Well as Maize Silage Aerobic Stability. Open Life Sci. 2020, 15, 373–378. [Google Scholar] [CrossRef]
- Kasinath, A.; Fudala-Ksiazek, S.; Szopinska, M.; Bylinski, H.; Artichowicz, W.; Remiszewska-Skwarek, A.; Luczkiewicz, A. Biomass in Biogas Production: Pretreatment and Codigestion. Renew. Sustain. Energy Rev. 2021, 150, 111509. [Google Scholar] [CrossRef]
- Kleinhenz, M.D.; Magnin, G.; Ensley, S.M.; Griffin, J.J.; Goeser, J.; Lynch, E.; Coetzee, J.F. Nutrient Concentrations, Digestibility, and Cannabinoid Concentrations of Industrial Hemp Plant Components. Appl. Anim. Sci. 2020, 36, 489–494. [Google Scholar] [CrossRef]
- Pu, Y.; Hu, F.; Huang, F.; Davison, B.H.; Ragauskas, A.J. Assessing the Molecular Structure Basis for Biomass Recalcitrance during Dilute Acid and Hydrothermal Pretreatments. Biotechnol. Biofuels 2013, 6, 15. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Ahmed, N.A.; Ogunkunle, O. Optimization of Biogas Yield from Lignocellulosic Materials with Different Pretreatment Methods: A Review. Biotechnol. Biofuels 2021, 14, 159. [Google Scholar] [CrossRef] [PubMed]
- Teixeira Franco, R.; Buffière, P.; Bayard, R. Ensiling for Biogas Production: Critical Parameters. A Review. Biomass Bioenergy 2016, 94, 94–104. [Google Scholar] [CrossRef]
- Schroyen, M.; Van Hulle, S.W.H.; Holemans, S.; Vervaeren, H.; Raes, K. Laccase Enzyme Detoxifies Hydrolysates and Improves Biogas Production from Hemp Straw and Miscanthus. Bioresour. Technol. 2017, 244, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyński, J.; Wróbel, B.; Górska, E.B. Cellulolytic Properties of a Potentially Lignocellulose-Degrading Bacillus sp. 8E1A Strain Isolated from Bulk Soil. Agronomy 2022, 12, 665. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, Z.; Li, J.; Chen, L.; Bai, Y.; Jia, Y.; Shao, T. Ensiling as Pretreatment of Rice Straw: The Effect of Hemicellulase and Lactobacillus plantarum on Hemicellulose Degradation and Cellulose Conversion. Bioresour. Technol. 2018, 266, 158–165. [Google Scholar] [CrossRef]

| Parameter | HS | HF | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| DM, g kg−1 | 283.5 | 6.6 | 310.4 | 72.1 |
| CP, g kg−1 DM | 36.2 | 14.0 | 263.2 | 8.4 |
| CF, g kg−1 DM | 495.9 | 12.1 | 355.5 | 4.8 |
| Ash, g kg−1 DM | 81.6 | 2.5 | 164.9 | 4.9 |
| NDF, g kg−1 DM | 681.6 | 28.7 | 437.2 | 8.5 |
| ADF, g kg−1 DM | 519.6 | 19.9 | 309.3 | 6.2 |
| ADL, g kg−1 DM | 79.1 | 4.4 | 78.5 | 0.7 |
| OMD, % | 111.6 | 61.4 | 606.1 | 19.9 |
| DMD, % | 149.3 | 67.6 | 664.3 | 22.0 |
| WSC, g kg−1 DM | 31.0 | 8.9 | 119.2 | 4.0 |
| WSC/CP | 1.0 | 0.3 | 0.5 | 0.0 |
| Parameters | Treatment | Storage Length | ||
|---|---|---|---|---|
| Control (n = 8) | Bacterial Inoculant (n = 8) | 7 Days (n = 8) | 42 Days (n = 8) | |
| pH | 6.16 a | 5.84 b | 6.27 a | 5.73 b |
| LA, g kg−1 DM | 0.52 b | 3.24 a | 3.41 a | 0.36 b |
| AA, g kg−1 DM | 24.68 a | 26.93 a | 41.46 a | 10.14 b |
| BA, g kg−1 DM | 0.19 a | 0.19 a | 0.20 a | 0.18 a |
| SA, g kg−1 DM | 27.70 a | 28.12 a | 45.07 a | 10.74 b |
| TMB, log CFU g−1 | 7.60 a | 7.74 a | 7.96 a | 7.38 b |
| Molds, log CFU g−1 | 1.39 a | 0.67 b | 1.17 a | 0.89 a |
| Parameters | Treatment | Storage Length | ||
|---|---|---|---|---|
| Control (n = 8) | Bacterial Inoculant (n = 8) | 7 Days (n = 8) | 42 Days (n = 8) | |
| pH | 7.21 a | 7.12 a | 8.21 a | 6.11 b |
| LA, g kg−1 DM | 1.92 b | 2.49 a | 1.90 b | 2.51 a |
| AA, g kg−1 DM | 6.32 a | 6.20 a | 6.36 a | 6.16 a |
| BA, g kg−1 DM | 0.23 a | 0.11 b | 0.18 a | 0.16 a |
| SA, g kg−1 DM | 8.42 a | 8.84 a | 8.44 a | 8.83 a |
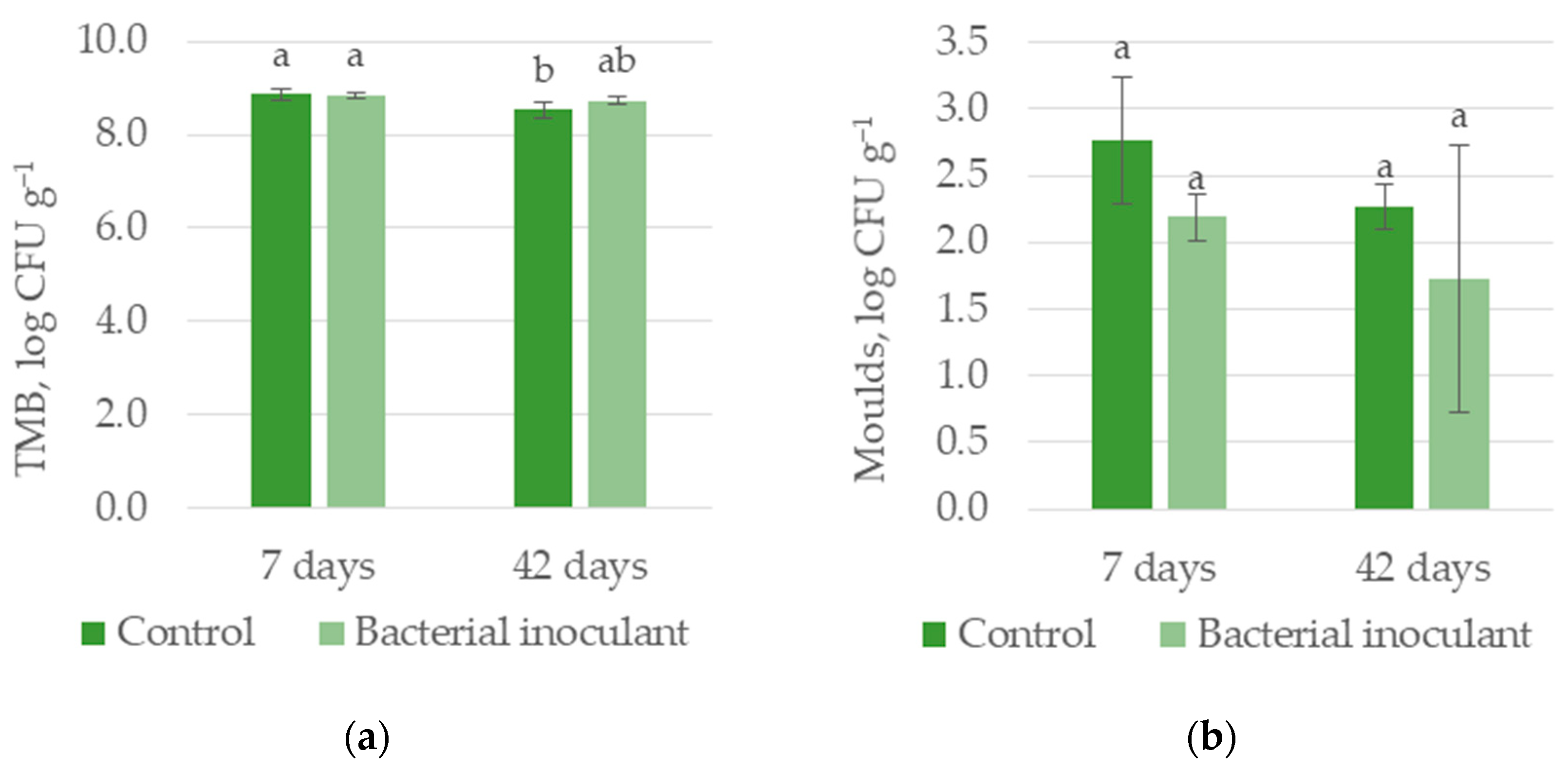
| TMB, log CFU g−1 | 8.69 a | 8.79 a | 8.85 a | 8.63 b |
| Molds, log CFU g−1 | 2.52 a | 1.96 a | 2.48 a | 2.00 a |
| Parameters | Treatment | Storage Length | ||
|---|---|---|---|---|
| Control (n = 8) | Bacterial Inoculant (n = 8) | 7 Days (n = 8) | 42 Days (n = 8) | |
| DM, g kg−1 | 282.0 a | 287.3 a | 285.8 a | 283.5 a |
| CP, g kg−1 DM | 52.9 a | 48.3 a | 33.3 b | 67.9 a |
| CF, g kg−1 DM | 484.6 a | 487.1 a | 492.2 a | 479.4 a |
| Ash, g kg−1 DM | 101.7 a | 105.4 a | 103.3 a | 103.8 a |
| NDF, g kg−1 DM | 649.6 a | 649.7 a | 658.3 a | 641.0 a |
| ADF, g kg−1 DM | 516.0 a | 512.2 a | 522.5 a | 505.7 b |
| Cellulose, g kg−1 DM | 436.6 a | 436.1 a | 446.6 a | 426.1 b |
| Hemicellulose, g kg−1 DM | 133.6 a | 137.5 a | 135.9 a | 135.2 a |
| ADL, g kg−1 DM | 79.4 a | 76.1 a | 75.9 a | 79.6 a |
| OMD, % | 13.15 a | 16.35 a | 15.26 a | 14.25 a |
| DMD, % | 17.63 a | 21.17 a | 20.88 a | 17.91 a |
| Parameters | Treatment | Storage Length | ||
|---|---|---|---|---|
| Control (n = 8) | Bacterial Inoculant (n = 8) | 7 Days (n = 8) | 42 Days (n = 8) | |
| DM, g kg−1 | 335.2 a | 330.5 a | 328.7 a | 337.0 a |
| CP, g kg−1 DM | 244.3 a | 245.5 a | 229.4 b | 260.5 a |
| CF, g kg−1 DM | 341.9 a | 346.0 a | 344.8 a | 343.1 a |
| Ash, g kg−1 DM | 189.8 a | 180.9 b | 180.8 b | 189.9 a |
| NDF, g kg−1 DM | 405.6 a | 407.6 a | 426.3 a | 386.9 b |
| ADF, g kg−1 DM | 331.0 a | 337.1 a | 337.2 a | 331.0 a |
| Cellulose, g kg−1 DM | 246.6 a | 250.2 a | 254.5 a | 242.3 b |
| Hemicellulose, g kg−1 DM | 74.6 a | 70.5 a | 89.1 a | 55.9 b |
| ADL, g kg−1 DM | 84.4 b | 86.9 a | 82.7 b | 88.7 a |
| OMD, % | 54.94 a | 52.48 a | 52.91 a | 54.52 a |
| DMD, % | 61.12 a | 59.22 a | 58.03 b | 62.30 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróbel, B.; Hryniewicz, M.; Kulkova, I.; Mazur, K.; Jakubowska, Z.; Borek, K.; Dobrzyński, J.; Konieczna, A.; Miecznikowski, A.; Piasecka-Jóźwiak, K.; et al. Fermentation Quality and Chemical Composition of Industrial Hemp (Cannabis sativa L.) Silage Inoculated with Bacterial Starter Cultures—A Pilot Study. Agronomy 2023, 13, 1371. https://doi.org/10.3390/agronomy13051371
Wróbel B, Hryniewicz M, Kulkova I, Mazur K, Jakubowska Z, Borek K, Dobrzyński J, Konieczna A, Miecznikowski A, Piasecka-Jóźwiak K, et al. Fermentation Quality and Chemical Composition of Industrial Hemp (Cannabis sativa L.) Silage Inoculated with Bacterial Starter Cultures—A Pilot Study. Agronomy. 2023; 13(5):1371. https://doi.org/10.3390/agronomy13051371
Chicago/Turabian StyleWróbel, Barbara, Marek Hryniewicz, Iryna Kulkova, Kamila Mazur, Zuzanna Jakubowska, Kinga Borek, Jakub Dobrzyński, Anita Konieczna, Antoni Miecznikowski, Katarzyna Piasecka-Jóźwiak, and et al. 2023. "Fermentation Quality and Chemical Composition of Industrial Hemp (Cannabis sativa L.) Silage Inoculated with Bacterial Starter Cultures—A Pilot Study" Agronomy 13, no. 5: 1371. https://doi.org/10.3390/agronomy13051371
APA StyleWróbel, B., Hryniewicz, M., Kulkova, I., Mazur, K., Jakubowska, Z., Borek, K., Dobrzyński, J., Konieczna, A., Miecznikowski, A., Piasecka-Jóźwiak, K., & Fabiszewska, A. (2023). Fermentation Quality and Chemical Composition of Industrial Hemp (Cannabis sativa L.) Silage Inoculated with Bacterial Starter Cultures—A Pilot Study. Agronomy, 13(5), 1371. https://doi.org/10.3390/agronomy13051371









