Mealworm Frass as a Potential Organic Fertilizer in Synergy with PGP-Based Biostimulant for Lettuce Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Frass and Biostimulant
2.2. Pot Experimental Design
2.3. Plant Sampling and Analyses
2.4. Determination of Rhizosphere Cultivable Microorganism Determination
2.5. Statistical Analysis
3. Results
3.1. Chemical Characteristics of the Substrate
3.2. Lettuce Plant Biometrical Parameters
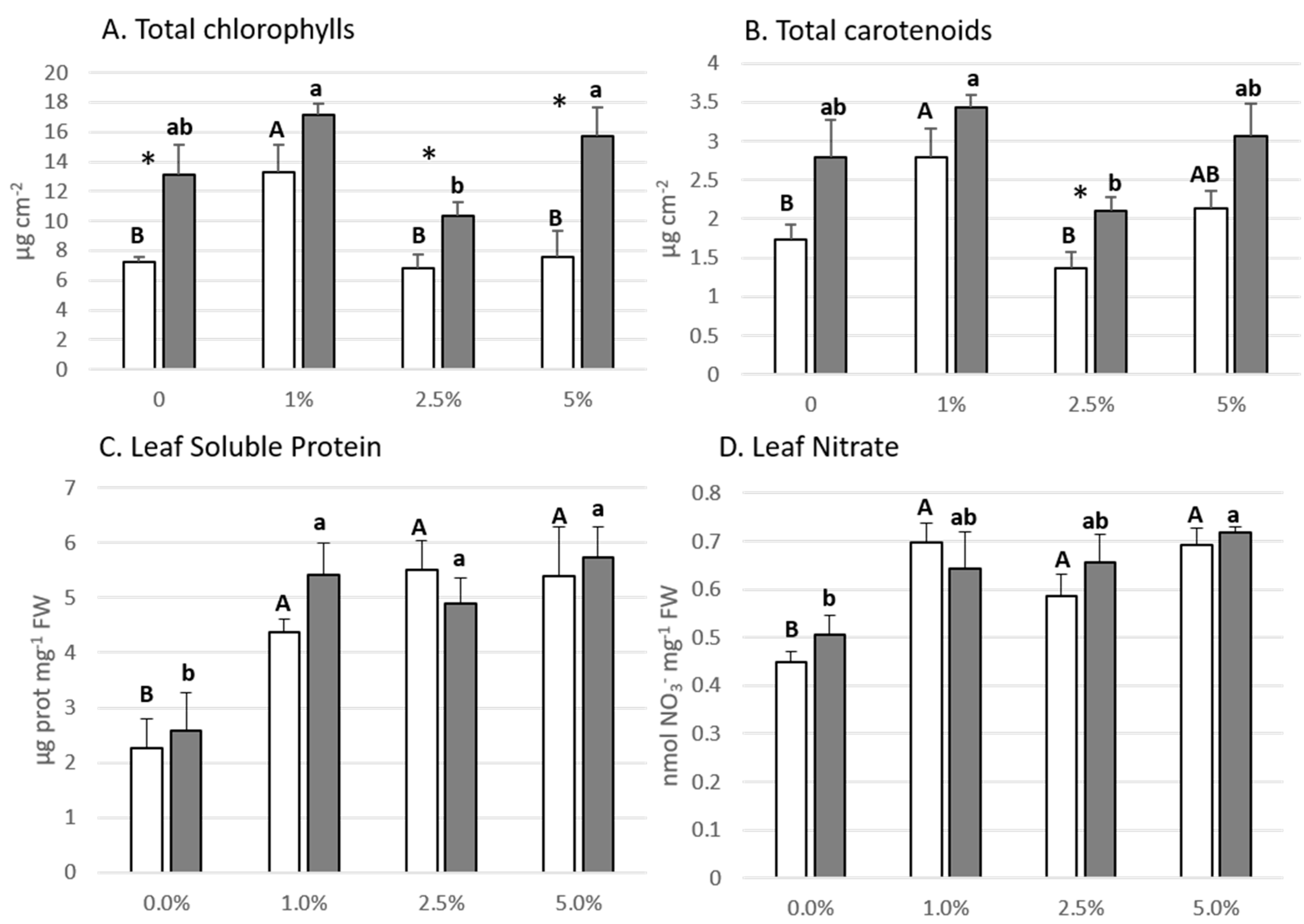
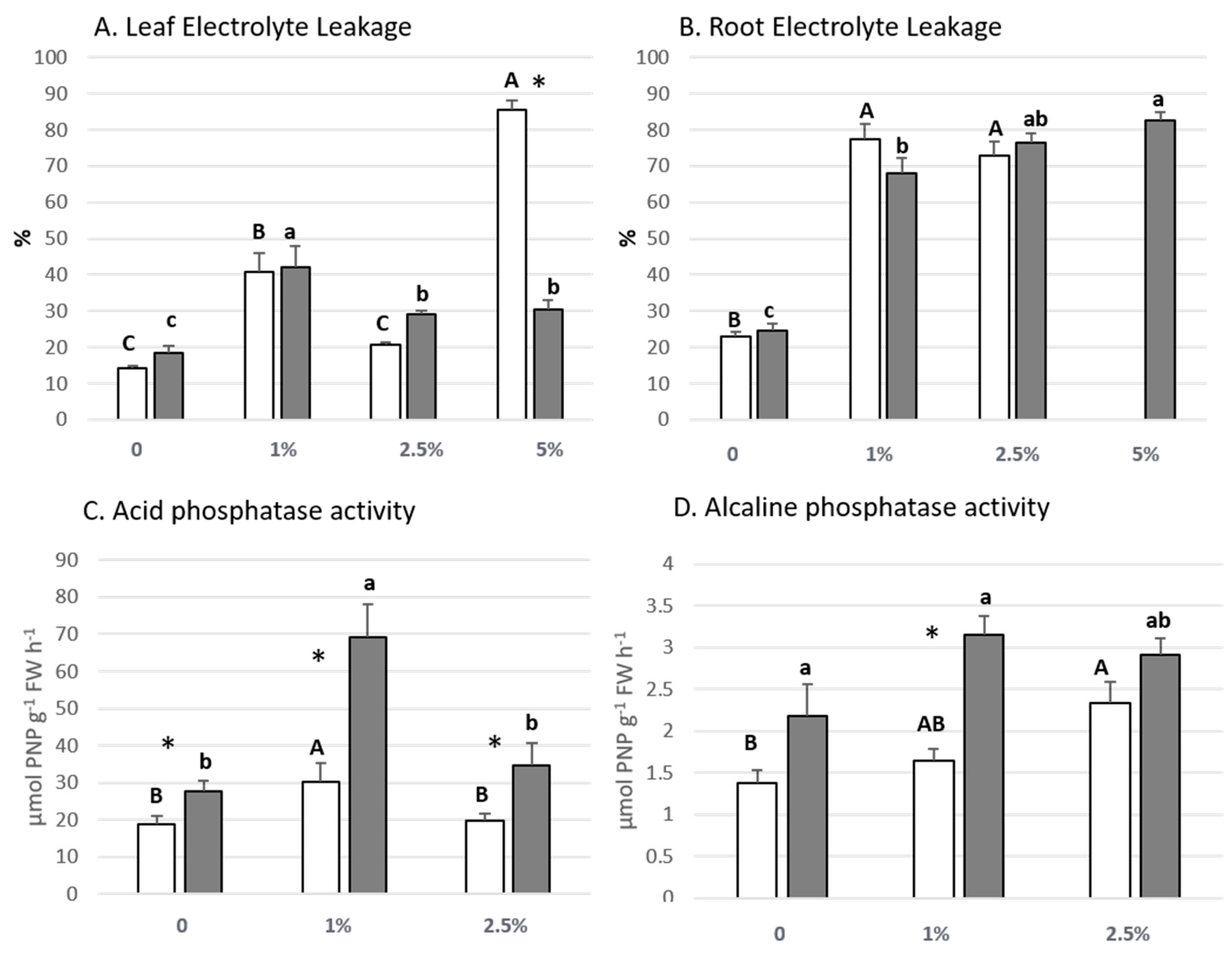
3.3. Lettuce Plant Biochemical Parameters
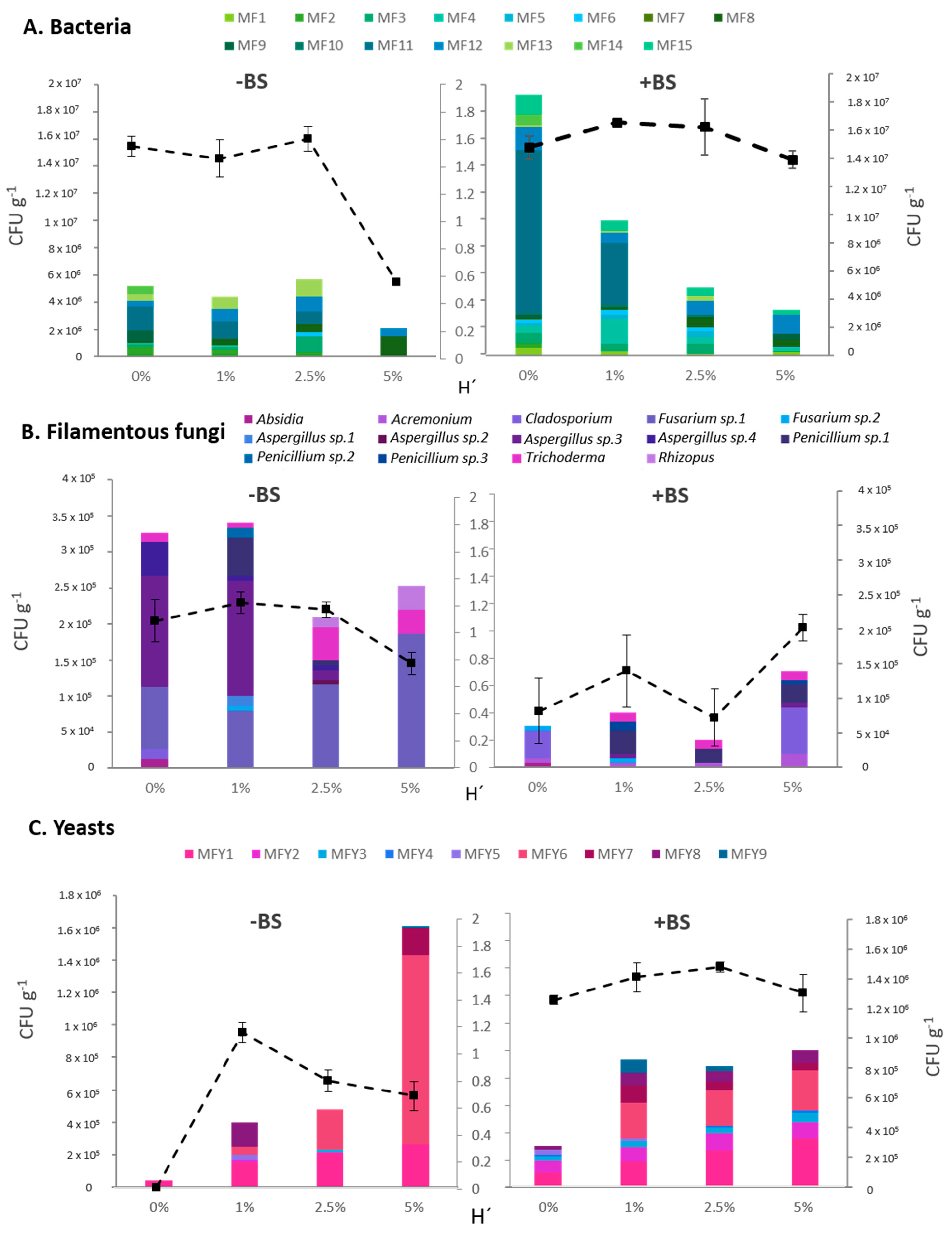
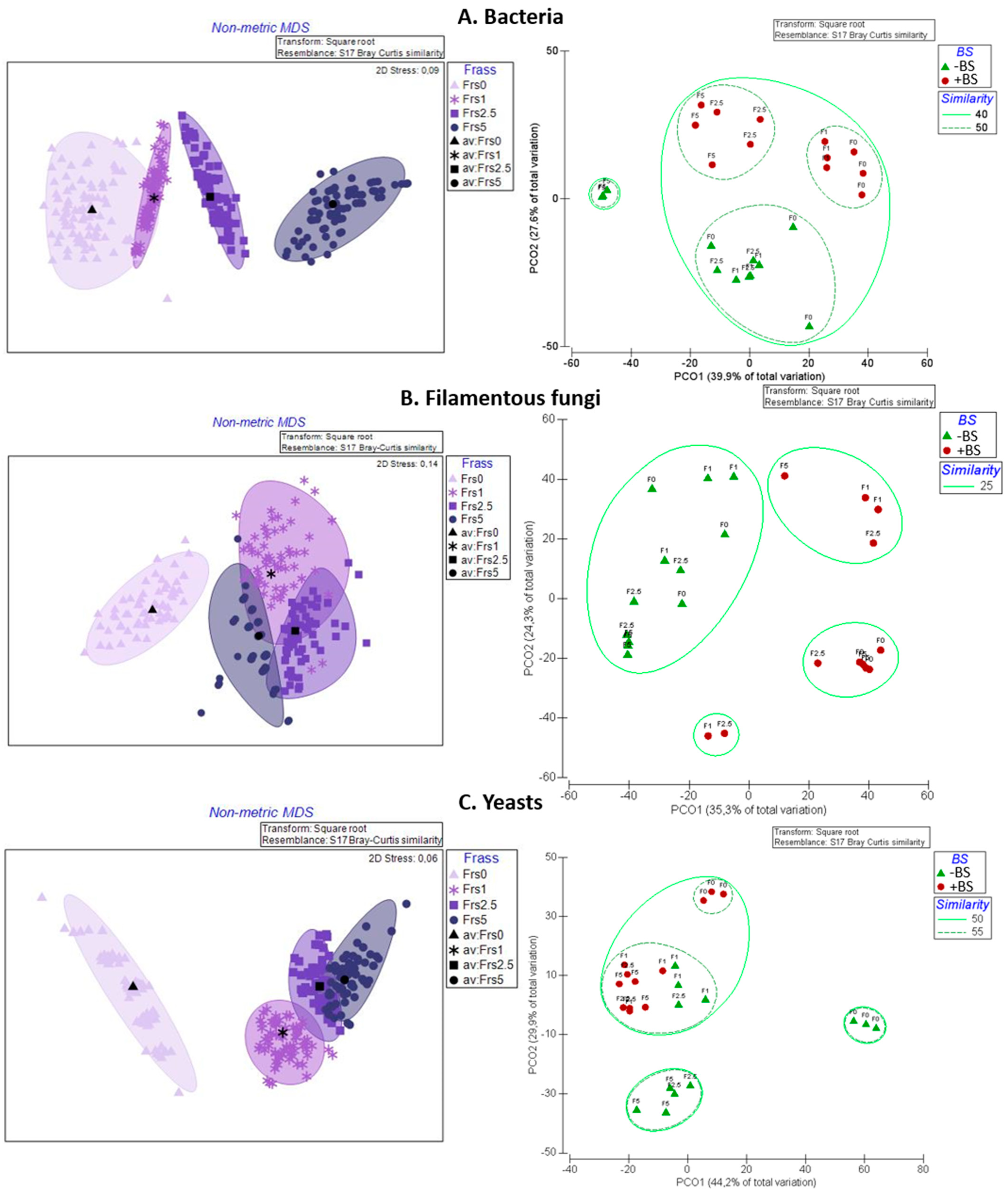
3.4. Rhizosphere Cultivable Microbiota Analysis
4. Discussion
4.1. Use of Frass as an Alternative Organic Amendment
4.2. Use of a PGP-Based Biostimulant to Improve Plant Yield and Quality
4.3. Use of the Combination of Frass and PGP-Based Biostimulant as an Alternative to Improve Plant Growth and Nutritional Quality
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mijangos, I.; Epelde, L.; Garbisu, C.; González-Oreja, J.A. Modification of soil enzyme activities as a consequence of replacing meadows by pine plantations under temperate climate. Pedobiologia 2014, 57, 61–66. [Google Scholar] [CrossRef]
- Kesavan, P.C.; Swaminathan, M.S. Strategies and models for agricultural sustainability in developing Asian countries. Philos. Trans. R. Soc. B Biol. Sc. 2008, 363, 877–891. [Google Scholar] [CrossRef] [PubMed]
- Gomiero, T.; Pimentel, D.; Paoletti, M.G. Environmental impact of different agricultural management practices: Conventional vs. Organic agriculture. Crit. Rev. Plant Sci. 2011, 30, 95–124. [Google Scholar] [CrossRef]
- Dicke, M. Insects on the menu. C. R. Biol. 2019, 342, 275–276. [Google Scholar] [CrossRef]
- Watson, C.; Preißing, T.; Wichern, F. Plant nitrogen uptake from insect frass is affected by the nitrification rate as revealed by urease and nitrification inhibitors. Front. Sust. Food Sys. 2021, 5, 1–14. [Google Scholar] [CrossRef]
- Frost, C.J.; Hunter, M.D. Insect canopy herbivory and frass deposition affect soil nutrient dynamics and export in oak mesocosms. Ecology 2004, 85, 3335–3347. [Google Scholar] [CrossRef]
- Kagata, H.; Ohgushi, T. Positive and negative impacts of insect frass quality on soil nitrogen availability and plant growth. Pop. Ecol. 2012, 54, 75–82. [Google Scholar] [CrossRef]
- Chavez, M.; Uchanski, M. Insect left-over substrate as plant fertiliser. J. Insects Food Feed 2021, 7, 683–694. [Google Scholar] [CrossRef]
- Houben, D.; Daoulas, G.; Dulaurent, A.M. Assessment of the Short-Term Fertilizer Potential of Mealworm Frass Using a Pot Experiment. Front. Sust. Food Sys. 2021, 5, 714596. [Google Scholar] [CrossRef]
- Poveda, J. Insect frass in the development of sustainable agriculture. A review. Agron. Sustain. Dev. 2021, 41, 1–10. [Google Scholar] [CrossRef]
- Houben, D.; Daoulas, G.; Faucon, M.P.; Dulaurent, A.M. Potential use of mealworm frass as a fertilizer: Impact on crop growth and soil properties. Sci. Rep. 2020, 10, 4659. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Jiménez-Gómez, A.; Saati-Santamaría, Z.; Usategui-Martín, R.; Rivas, R.; García-Fraile, P. Mealworm frass as a potential biofertilizer and abiotic stress tolerance-inductor in plants. Appl. Soil. Ecol. 2019, 142, 110–122. [Google Scholar] [CrossRef]
- Watson, C.; Schlösser, C.; Vögerl, J.; Wichern, F. Excellent excrement? Frass impacts on a soil’s microbial community, processes and metal bioavailability. Appl. Soil Ecol. 2021, 168, 104110. [Google Scholar] [CrossRef]
- Abd–Elrahman, S.H.; Saudy, H.S.; El–Fattah, D.A.A.; Hashem, F.A. Effect of Irrigation Water and Organic Fertilizer on Reducing Nitrate Accumulation and Boosting Lettuce Productivity. J. Soil Sci. Plant Nutr. 2022, 22, 2144–2155. [Google Scholar] [CrossRef]
- Kumar, B.; Jacob, J. Plant growth promoting rhizobacteria as a biological tool for augmenting productivity and controlling disease in agriculturally important crops—A review. J. Spices Arom. Comp. 2019, 28, 77–95. [Google Scholar]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotech. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Sarabia, M.; Cornejo, P.; Azcón, R.; Carreón-Abud, Y.; Larsen, J. Mineral phosphorus fertilization modulates interactions between maize, rhizosphere yeasts and arbuscular mycorrhizal fungi. Rhizosphere 2017, 4, 89–93. [Google Scholar] [CrossRef]
- Yang, J.; Kloepper, J.W.; Ryu, C.M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef]
- Sandhya, V.Z.; Grover, M.; Reddy, G.; Venkateswarlu, B. Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-p45. Biol. Fert. Soils 2009, 46, 17–26. [Google Scholar] [CrossRef]
- Liu, F.; Xing, S.; Ma, H.; Du, Z.; Ma, B. Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl. Microbiol. Biotech. 2013, 97, 9155–9164. [Google Scholar] [CrossRef] [PubMed]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Front. Plant. Sci. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fuertes-Mendizábal, T.; Huérfano, X.; Ortega, U.; González-Murua, C.; Estavillo, J.M.; Salcedo, I.; Duñabeitia, M.K. Compost and PGP-based biostimulant as alternative to peat and NPK fertilization in chestnut (Castanea sativa Mill.) nursery production. Forests 2021, 12, 850. [Google Scholar] [CrossRef]
- Kenneth, O.C.; Nwadibe, E.C.; Kalu, A.U.; Unah, U.V. Plant Growth Promoting Rhizobacteria (PGPR): A Novel Agent for Sustainable Food Production. Am. J. Agric. Biol. Sci. 2019, 14, 35–54. [Google Scholar] [CrossRef]
- Slabbert, M.M.; Krüger, G.H.J. Antioxidant enzyme activity, proline accumulation, leaf area and cell membrane stability in water stressed Amaranthus leaves. S. Afr. J. Bot. 2014, 95, 123–128. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantification of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, D.A.; Schrader, L.E.; Youngs, V.L. Analysis by digestion and colorimetric assay of total nitrogen in plant tissues high in nitrate. Crop Sci. 1974, 14, 854–856. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial; PRIMER-Eplymouth: Devon, UK, 2015. [Google Scholar]
- Cabrera, M.L.; Kissel, D.E.; Vigil, M.F. Nitrogen mineralization from organic residues. J. Environ. Qual. 2005, 34, 75–79. [Google Scholar] [CrossRef]
- Bowden, C.; Spargo, J.; Evanylo, G. Mineralization and n fertilizer equivalent value of composts as assessed by tall fescue (Festuca arundinacea). Compost Sci. Util. 2007, 15, 111–118. [Google Scholar] [CrossRef]
- Musyoka, M.W.; Adamtey, N.; Bünemann, E.K.; Muriuki, A.W.; Karanja, E.N.; Mucheru-Muna, M.; Fiaboe, K.K.M.; Cadisch, G. Nitrogen release and synchrony in organic and conventional farming systems of the Central Highlands of Kenya. Nutr. Cycl. Agroecosyst. 2019, 113, 283–305. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R.; Joergensen, R.G.; Ludwig, B. Pathways of nitrogen utilization by soil microorganisms—A review. Soil Biol. Biochem. 2010, 42, 2058–2067. [Google Scholar] [CrossRef]
- Barragán-Fonseca, K.Y.; Nurfikari, A.; van de Zande, E.M.; Wantulla, M.; van Loon, J.J.A.; de Boer, W.; Dicke, M. Insect frass and exuviae to promote plant growth and health. Trends Plant Sci. 2022, 27, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Nabel, M.; Schrey, S.D.; Poorter, H.; Koller, R.; Nagel, K.A.; Temperton, V.M.; Jablonowski, N.D. Coming late for dinner: Localized digestate depot fertilization for extensive cultivation of marginal soil with Sida hermaphrodita. Front. Plant Sci. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Gebremikael, M.T.; Wickeren, N.; Hosseini, P.S.; De Neve, S. The impacts of black soldier fly frass on nitrogen availability, microbial activities, C sequestration and plant growth. Front. Sust. Food Sys. 2022, 6, 795050. [Google Scholar] [CrossRef]
- Kawasaki, K.; Kawasaki, T.; Hirayasu, H.; Matsumoto, Y. Evaluation of fertilizer value of residues obtained after processing household organic waste with black soldier fly larvae (Hermetia illucens). Sustainability 2020, 12, 4920. [Google Scholar] [CrossRef]
- Wichern, J.; Wichern, F.; Joergensen, R.G. Impact of salinity on soil microbial communities and the decomposition of maize in acidic soils. Geoderma 2006, 137, 100–108. [Google Scholar] [CrossRef]
- Rath, K.M.; Rousk, J. Salt effects on the soil microbial decomposer community and their role in organic carbon cycling: A review. Soil Biol. Biochem. 2015, 81, 108–123. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Cardinali, F.; Garofalo, C.; Clementi, F.; Pasquini, M.; Aquilanti, L. The bacterial biota of laboratory-reared edible mealworms (Tenebrio molitor L.): From feed to frass. Int. J. Food Microbiol. 2018, 272, 49–60. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-solubilizing microorganisms: Mechanism and their role in phosphate solubilization and uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Ali, A.M.; Awad, M.Y.M.; Hegab, S.A.; Abd El Gawad, A.M.; Eissa, M.A. Effects of potassium solubilizing bacteria (Bacillus cereus) on growth and yield of potato. J. Plant Nutr. 2021, 44, 411–420. [Google Scholar] [CrossRef]
- Talboys, P.J.; Owen, D.W.; Healey, J.R.; Withers, P.J.A.; Jones, D.L. Auxin secretion by Bacillus amyloliquefaciens FZB42 both stimulates root exudation and limits phosphorus uptake in Triticum aestivum. BMC Plant Biol. 2014, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Sivasakthi, S.D.; Kanchana, G.; Usharani, P. Production of plant growth promoting substance by Pseudomonas fluorescens and Bacillus subtilis isolates from paddy rhizosphere soil of Cuddalore District, Tamil Nadu, India. Int. J. Microbiol. Res. 2013, 4, 227–233. [Google Scholar]
- Gómez-Lama Cabanás, C.G.; Legarda, G.; Ruano-rosa, D.; Pizarro-Tobías, P.; Valverde-Corredor, A.; Niqui, J.L.; Triviño, J.C. Indigenous Pseudomonas spp. strains from the olive (Olea europaea L.) rhizosphere as effective biocontrol agents against Verticillium dahliae: From the host roots to the bacterial genomes. Front. Microbiol. 2018, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Yang, J.; Dou, Y.; Chen, M.; Ping, S.; Peng, J.; Lu, W.; Zhang, W.; Yao, Z.; Li, H.; et al. Nitrogen fixation island and rhizosphere competence traits in the genome of root-associated Pseudomonas stutzeri A1501. Proc. Natl. Acad. Sci. USA 2008, 105, 7564–7569. [Google Scholar] [CrossRef]
- Iqbal, S.; Hameed, S.; Shahid, M.; Hussain, K.; Ahmad, N.; Niaz, M. In vitro characterization of bacterial endophytes from tomato (Solanum lycopersicum L.) for phytobeneficial traits. Appl. Ecol. Environ. Res. 2018, 16, 1037–1051. [Google Scholar] [CrossRef]
- Hamayun, M.; Lee, I. Gibberellin production and plant growth promotion from pure cultures of Cladosporium sp MH-6 isolated from cucumber (Cucumis sativus L). Mycologia 2010, 102, 980–995. [Google Scholar] [CrossRef]
- Li, Z.T.; Janisiewicz, W.J.; Liu, Z.; Callahan, A.M.; Evans, B.E.; Ii, W.M.J.; Dardick, C. Exposure in vitro to an environmentally isolated strain TC09 of Cladosporium sphaerospermum triggers plant growth promotion, early flowering, and fruit yield. Front. Plant Sci. 2019, 9, 1–19. [Google Scholar] [CrossRef]
- Albiach, R.; Canet, R.; Pomares, F.; Ingelmo, F. Microbial biomass content and enzymatic activities after the application of organic amendments to a horticultural soil. Biores. Technol. 2000, 75, 43–48. [Google Scholar] [CrossRef]
- Condron, L.; Stark, C.; O’Callaghan, M.; Clinton, P.; Huang, Z. The role of microbial communities in the formation and decomposition of soil organic matter. In Soil Microbiology and Sustainable Crop Production; Dixon, G., Tilston, E., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 81–118. [Google Scholar]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef]
- Gutiérrez-Mañero, F.J.; Ramos-Solano, B.; Probanza, A.; Mehouachi, J.; Tadeo, F.R.; Talon, M. The plant-growth-promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberellins. Physiol. Plantarum 2001, 111, 206–211. [Google Scholar] [CrossRef]
- Sah, S.; Krishnani, S.; Singh, R. Pseudomonas mediated nutritional and growth promotional activities for sustainable food security. Current Res. Microb. Sci. 2021, 2, 100084. [Google Scholar] [CrossRef] [PubMed]
- Caporale, A.G.; Sommella, A.; Lorito, M.; Lombardi, N.; Azam, S.M.G.G.; Pigna, M.; Ruocco, M. Trichoderma spp. Alleviate phytotoxicity in lettuce plants (Lactuca sativa L.) irrigated with arsenic-contaminated water. J. Plant Physiol. 2014, 171, 1378–1384. [Google Scholar]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Rouphael, Y. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Da Silva, L.R.; Valadares-Inglis, M.C.; Peixoto, G.H.S.; de Luccas, B.E.G.; Muniz, P.H.P.C.; Magalhães, D.M.; de Mello, S.C.M. Volatile organic compounds emitted by Trichoderma azevedoi promote the growth of lettuce plants and delay the symptoms of white mold. Biol. Control 2021, 152, 104447. [Google Scholar] [CrossRef]
- Lim, H.B.; Chung, Y.J.; Bae, J.Y.; Kim, D.; Kwon, H.J.; Lee, I.H.; Suh, J.W. Plant Growth-promoting activity of Acremonium strictum MJN1 isolated from roots of Panax ginseng. Agric. Chem. Biotech. 2000, 13, 104–108. [Google Scholar]
- Khan, M.S.; Gao, J.; Munir, I.; Zhang, M.; Liu, Y.; Moe, T.S.; Xue, J.; Zhang, X. Characterization of endophytic fungi, Acremonium sp., from Lilium davidii and analysis of its antifungal and plant growth-promoting effects. Biomed. Res. Int. 2021, 3, 9930210. [Google Scholar] [CrossRef]
- Choi, G.J.; Kim, J.C.; Jang, K.S.; Nam, M.H.; Lee, S.W.; Kim, H.T. Biocontrol activity of Acremonium strictum BCP against Botrytis diseases. Plant Pathol. J. 2009, 25, 165–171. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; De Colstoun, E.B.; McMurtrey, J.E. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Liu, C.W.; Sung, Y.; Chen, B.C.; Lai, H.Y. Effects of nitrogen fertilizers on the growth and nitrate content of lettuce (Lactuca sativa L.). Int. J. Environ. Res. Public. Health 2014, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Gashas, E.A.; Osman, N.A.; Alshali, A.A.; Hewait, H.M.; Ashmawi, A.E.; Alshallash, K.S.; El Taher, A.M.; Azab, E.S.; El-Raouf, H.S.; Ibrahim, M.F.M. Effects of plant-growth-promoting rhizobacteria (PGPR) and Cyanobacteria on botanical characteristics of tomato (Solanum lycopersicon L.) plants. Plants 2020, 11, 2732. [Google Scholar] [CrossRef] [PubMed]
- Azarmi-Atajan, F.; Sayyari-Zohan, M.H. Alleviation of salt stress in lettuce (Lactuca sativa L.) by plant growth-promoting rhizobacteria. J. Hortic. Postharvest Res. 2020, 3, 67–78. [Google Scholar]
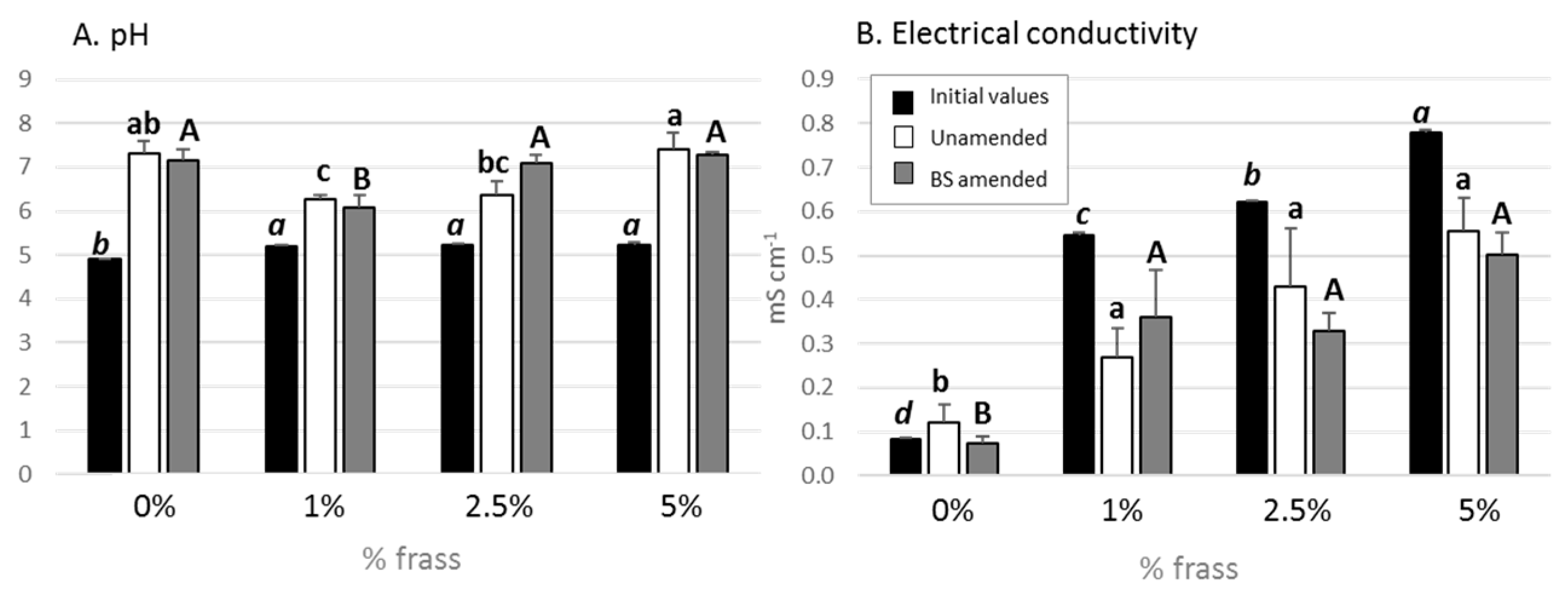


| Dry matter (%) | 89.95 | Total calcium (mg/L) | 105 |
| Organic matter (%) | 86.4 | Sulphate (mg/L) | 338 |
| pH (1/5 v/v) | 5.8 | Phosphate (mg/L) | 5780 |
| Organic C (%) | 50.2 | Magnesium (mg/L) | 460 |
| Total N (%) | 3.64 | Carbonate (mg/L) | <5 |
| C/N | 13.8 | Bicarbonate (mg/L) | <5 |
| Ammonium (mg/L) | 192 | Chloride (mg/L) | 314 |
| Nitrate (mg/L) | 27.9 | Potassium (mg/L) | 3440 |
| Conductivity (mS/cm) (1/5 v/v) | 18.2 | Sodium (mg/L) | 804 |
| Density (g/cm3) | 1.59 | Boron (mg/L) | 2.08 |
| Humic acids (%) | 7.52 | Manganese (µg/L) | 2760 |
| Fulvic acids (%) | 19.6 | Iron (µg/L) | 4750 |
| Total humic extract (%) | 27.1 | Zinc (µg/L) | 1200 |
| Escherichia coli (MPN/g) | n.d. | Copper (µg/L) | 2730 |
| Salmonella (25 g) | <1 MPN/g |
| Frass | BS | BS x Frass | Frass | BS | BS x Frass | ||||
|---|---|---|---|---|---|---|---|---|---|
| Shoot FW (g) | sig | ** | *** | * | Chl a (µg cm−2) | sig | ** | *** | ns |
| η2p | 0.650 | 0.884 | 0.425 | η2p | 0.588 | 0.635 | 0.164 | ||
| Shoot DW (g) | sig | ns | ** | ns | Chl b (µg cm−2) | sig | ** | *** | ns |
| η2p | 0.127 | 0.535 | 0.131 | η2p | 0.587 | 0.554 | 0.361 | ||
| Root FW (g) | sig | *** | ns | ns | Chl tot (µg cm−2) | sig | ** | *** | ns |
| η2p | 0.772 | 0.202 | 0.024 | η2p | 0.591 | 0.631 | 0.174 | ||
| Root DW (g) | sig | ns | ns | ns | Chl a/b (µg cm−2) | sig | * | ** | * |
| η2p | 0.205 | 0.054 | 0.081 | η2p | 0.435 | 0.42 | 0.444 | ||
| Height (cm) | sig | ns | ** | * | Carot (µg cm−2) | sig | ** | *** | ns |
| η2p | 0.282 | 0.513 | 0.429 | η2p | 0.579 | 0.495 | 0.035 | ||
| Leaf DW/FW | sig | ns | ns | ns | Leaf prot (µg mg−1 FW) | sig | *** | ns | ns |
| η2p | 0.285 | 0.022 | 0.035 | η2p | 0.687 | 0.026 | 0.11 | ||
| Root FW/DW | sig | *** | * | ns | Root prot (µg mg−1 FW) | sig | ** | ns | ns |
| η2p | 0.851 | 0.206 | 0.196 | η2p | 0.589 | 0.057 | 0.236 | ||
| Root diameter (cm) | sig | * | ns | ns | Leaf NO3− (nmol mg−1 FW) | sig | ** | ns | * |
| η2p | 0.471 | 0.056 | 0.015 | η2p | 0.6 | 0.079 | 0.455 | ||
| Canopy diameter (cm) | sig | ns | *** | ns | Root NO3− (nmol mg−1FW) | sig | ** | ns | * |
| η2p | 0.231 | 0.566 | 0.298 | η2p | 0.602 | 0.024 | 0.403 | ||
| Leaf N (%) | sig | *** | ns | ns | Leaf EC (%) | sig | *** | *** | *** |
| η2p | 0.82 | 0.029 | 0.225 | η2p | 0.812 | 0.327 | 0.715 | ||
| Root N (%) | sig | * | ns | ns | Root EC (%) | sig | *** | ns | ns |
| η2p | 0.424 | 0.053 | 0.15 | η2p | 0.914 | 0.007 | 0.12 | ||
| Ca (µg g−1 DW) | sig | *** | ns | ns | Acid phosphatase (µmol PNP g−1 FW h−1) | sig | *** | *** | * |
| η2p | 0.819 | 0.162 | 0.241 | η2p | 0.635 | 0.582 | 0.347 | ||
| K (µg g−1 DW) | sig | *** | ns | ns | Alcaline phosphatase (µmol PNP g−1 FW h−1) | sig | ** | *** | ns |
| η2p | 0.925 | 0.043 | 0.332 | η2p | 0.356 | 0.512 | 0.156 | ||
| Mg (µg g−1 DW) | sig | *** | ns | ns | WRC (%) | sig | * | ns | ns |
| η2p | 0.926 | 0.001 | 0.315 | η2p | 0.197 | 0.029 | 0.062 | ||
| Na (µg g−1 DW) | sig | *** | ns | ns | Succulence | sig | ns | *** | * |
| η2p | 0.883 | 0.007 | 0.292 | η2p | 0.029 | 0.355 | 0.183 | ||
| P (µg g−1 DW) | sig | *** | ns | ns | Substrate pH | sig | *** | ns | ns |
| η2p | 0.922 | 0.025 | 0.024 | η2p | 0.632 | 0.007 | 0.211 | ||
| S (µg g−1 DW) | sig | *** | ns | ns | Substrate EC | sig | *** | ns | ns |
| η2p | 0.907 | 0.115 | 0.107 | η2p | 0.685 | 0.016 | 0.105 |
| Bacteria | Filamentous Fungi | Yeasts | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | Df | Sum Sq | Pseudo-F | p (Perm) | Source | Df | Sum Sq | Pseudo-F | p (Perm) | Source | Df | Sum Sq | Pseudo-F | p (Perm) |
| Frass | 3 | 14,569 | 7.721 | 0.001 | Frass | 3 | 16,108 | 3.1693 | 0.002 | Frass | 3 | 14,431 | 13.122 | 0.001 |
| BS | 1 | 10,647 | 16.93 | 0.001 | BS | 1 | 21,498 | 12.689 | 0.001 | BS | 1 | 8077.7 | 22.035 | 0.001 |
| Frass x BS | 3 | 2958.7 | 1.568 | 0.151 | Frass x BS | 3 | 6,642.9 | 1.307 | 0.261 | Frass x BS | 3 | 5439.2 | 4.9458 | 0.001 |
| Residuals | 16 | 10,063 | Residuals | 16 | 27,107 | Residuals | 16 | 5865.4 | ||||||
| Total | 23 | 38,237 | Total | 23 | 71,356 | Total | 23 | 33,813 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuertes-Mendizábal, T.; Salcedo, I.; Huérfano, X.; Riga, P.; Estavillo, J.M.; Ávila Blanco, D.; Duñabeitia, M.K. Mealworm Frass as a Potential Organic Fertilizer in Synergy with PGP-Based Biostimulant for Lettuce Plants. Agronomy 2023, 13, 1258. https://doi.org/10.3390/agronomy13051258
Fuertes-Mendizábal T, Salcedo I, Huérfano X, Riga P, Estavillo JM, Ávila Blanco D, Duñabeitia MK. Mealworm Frass as a Potential Organic Fertilizer in Synergy with PGP-Based Biostimulant for Lettuce Plants. Agronomy. 2023; 13(5):1258. https://doi.org/10.3390/agronomy13051258
Chicago/Turabian StyleFuertes-Mendizábal, Teresa, Isabel Salcedo, Ximena Huérfano, Patrick Riga, José María Estavillo, David Ávila Blanco, and Miren Karmele Duñabeitia. 2023. "Mealworm Frass as a Potential Organic Fertilizer in Synergy with PGP-Based Biostimulant for Lettuce Plants" Agronomy 13, no. 5: 1258. https://doi.org/10.3390/agronomy13051258
APA StyleFuertes-Mendizábal, T., Salcedo, I., Huérfano, X., Riga, P., Estavillo, J. M., Ávila Blanco, D., & Duñabeitia, M. K. (2023). Mealworm Frass as a Potential Organic Fertilizer in Synergy with PGP-Based Biostimulant for Lettuce Plants. Agronomy, 13(5), 1258. https://doi.org/10.3390/agronomy13051258






