Abstract
While previous studies have shown camelina drought tolerance relative to other oilseed crops, drought has been documented to severely influence the productivity of camelina. To date, little information is available on the drought tolerance of camelina genotypes. This study was conducted to evaluate drought tolerance in fifteen camelina genotypes and test the alleviative effect of nanoparticles on PEG-induced water deficit stress (WDS) at the whole-plant level at the Yangzhou University Pratacultural Science Experimental Station in September 2021. Four different degrees of WDS were induced by a range of PEG solution concentrations (0, 16.7, 25.0, 37.5, and 56.3 mM). A petri dish study determined that CamK8 and CamK9 (GR50 = 19.0 and 34.3 mM, respectively) were the most sensitive and tolerant genotypes, respectively, to PEG-induced WDS. Results from the whole-plant test showed that the foliar application of MWCNTs (dose: 50 or 100 mg L−1) or nano-Se (dose: 5 or 10 mg L−1) alleviated the adverse effect of PEG-induced WDS, and increased the camelina plant height (ranges: 51.1–56.3 cm) and crop yield (ranges: 0.11–0.14 g plant−1) compared with untreated control and PEG-treated plants (height: 43.5–56.9 cm; yield: 0.06–0.12 g plant−1) in CamK8 without affecting the principal fatty acid composition and groups in camelina oil. The results of this study demonstrated that applying MWCNTs or nano-Se could alleviate WDS and maintain seed yield in camelina, providing the possibility of using these nanoparticles to manage WDS in agricultural practices.
1. Introduction
Drought is one of the most prominent abiotic stresses constraining the productivity of field crops and resulting in food shortages [1,2]. It can negatively affect the growth, physiological (i.e., photosynthetic performance), and biochemical activities (i.e., antioxidant enzymes activities) of a plant [3,4]. Nanotechnology and its application in agriculture to manage various agricultural stresses have shown the potential for increasing crop yield [5,6]. Results showed that the specific types of nanoparticles in low application doses enabled the plants to counteract the stress by activating the physiological and biochemical processes [7,8].
Multi-walled carbon nanotubes (MWCNTs), carbonaceous nanomaterials with unique physical and chemical properties [9], have been reported as plant-growth regulators to control plant growth and development [10,11]. It has been documented that MWCNTs exaggerated the upregulation of stress-related gene expression in tomato (Solanum lycopersicum L.) germination and further affected the growth of seedlings [11,12]. Studies also revealed that they could enhance the development of tobacco cells by activating water channels and significant gene regulators of cell division and extension [9]. By enhancing the antioxidative system, MWCNTs alleviated salt stress in grape (Vitis Vinifera L.) seedlings [13]. The zero-valent oxidation form, Se0, has attracted interest due to its high nano-selenium (nano-Se) bioavailability to plants [14]. Compared with inorganic and organic Se, nano-Se displayed high bioavailability, low toxicity, and a strong free radical scavenging ability [15]. It has been considered a biostimulant fertilizer to enhance crop resilience under abiotic or biotic stress [14,16]. The foliar application of nano-Se can improve flavonoid and phenolic acid contents in strawberries [17]. It also can enhance wheat (Triticum aestivum L.) resistance to aphids by regulating the biosynthesis of 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one (DIMBOA) and volatile components [18]. These examples demonstrate the potential for using nano-enabled particles to manage/alleviate abiotic (i.e., salt, drought) or biotic (i.e., disease) stress and enhance food production.
With the effects of global warming and climate change on rainfall reduction, the cultivation of crops with drought tolerance would maintain crop production and food security [19]. Camelina (Camelina sativa L.), a re-emerging oilseed crop, has a long cultivation history in northern China [20]. It has been characterized a drought-tolerant crop species relative to other oilseed crops (i.e., oilseed rape) [21,22]. Many rainfed regions with the potential to grow camelina face water deficit situations due to climate change. If farmers have access to drought-tolerant camelina cultivars, they can save water by resorting to low-irrigation methods and devote the amount of water saved to the production of other crops. Variation in drought tolerance in camelina genotypes has been reported [23]. WDS has been documented as a severe factor affecting the seed germination and seedling stand establishment [24,25,26,27], reducing yields [28], and causing crop failure in camelina production [29]. Although genotypes associated with seed size showed the variation in the capacity to tolerate WDS [23,30], to the authors’ knowledge, there is little research on the evaluation of camelina genotypes responding to different levels of WDS. Additionally, under WDS, the growth and productivity of crops is reduced due to the influence of physiological and biochemical functions. Although several agricultural techniques, including the alteration of planting densities [31] and application of bio-stimulant [14], have been used to manage agricultural stress in camelina, there is no study testing nano-enabled particles on the alleviation of WDS in camelina. The present study was the first to evaluate the drought tolerance potential of camelina genotypes to PEG-induced WDS and test using nanoparticles for the alleviation of WDS on the camelina crop. Thus, the objectives of this study were to (I) evaluate the drought tolerance potential of the fifteen camelina genotypes responding to the different levels of WDS induced by PEG and (II) further test the effect of MWCNTs and nano-Se on PEG-induced WDS at the whole-plant level in two predetermined drought-sensitive and -tolerant camelina genotypes. The hypothesis was that the drought tolerance of camelina to PEG-induced WDS could vary among the different camelina genotypes, and the exogenous application of MWCNTs and nano-Se can alleviate the adverse effect of WDS on plants through the alteration of morpho-physiological and biochemical responses, and thus maintain the seed production potential in the camelina crop.
2. Materials and Methods
2.1. Plant Materials and Experimental Procedure
The information on seed origin, seed sources obtained, time of seed harvest, storage condition, and seed accessibility of the fifteen camelina genotypes tested in this study is described in Table 1. Seed germination tests showed > 98% seed germination for those fifteen camelina genotypes. This study simulated WDS by applying polyethylene glycol (PEG 6000, Sangon Biotech Co., Ltd., Shanghai, China) solutions in various PEG concentrations. The present study contains two experiments. In experiment 1, we evaluated the drought tolerance of the fifteen camelina genotypes and determined the most drought-sensitive and -tolerant camelina genotypes based on GR50 (a PEG concentration causing a 50% inhibition of camelina seedling root growth). In experiment 2, we tested the alleviating effect of PEG-induced WDS on the growth of the two determined camelina genotypes (most drought-sensitive and -tolerant camelina genotypes) at the whole-plant level by the exogenous application of MWCNTs (outer diameter: <8 nm; inner diameter: 2–5 nm; Length: 10–30 μm) (Shanghai Macklin Biochemical Co., Ltd., Shanghai, China) and nano-Se (Se0 nanoparticles; mean size: 50–78 nm) (Guilin Jiqi Group Co. Ltd., Guilin, China).

Table 1.
The information of camelina (Camelina sativa L.) genotypes used in this study.
2.1.1. Experiment 1: Evaluation of Drought Tolerance of the Fifteen Camelina Genotypes
The drought tolerance of the fifteen camelina genotypes was evaluated using a petri-dish-based method. Four different degrees of WDS were induced by a range of PEG solution concentrations (0, 16.7, 25.0, 37.5, and 56.3 mM). Before the PEG concentration treatment, seeds of each camelina genotype were sterilized with 75% (v/v) ethanol for 20 min, and 1.5% NaClO for 10 min. Seeds were then rinsed with distilled water. Afterward, each of 50 the seeds from each genotype was evenly placed in petri dishes containing a single layer of filter paper pre-moistened with 5 mL of a range of PEG concentrations, as mentioned before. This experiment was set up as a completely randomized block design with three replications. The petri dishes were incubated in the plant culture chamber (Ningbo Jiangnan Instrument Factory, Ningbo, China) (25 °C with a 10/14 h photoperiod, 70% relative humidity). One mL of the corresponding concentration of PEG solution was added to the petri dishes every two days to maintain the moisture. Among the tested parameters (percent seed germination, seedling root and shoot length, seedling length, seedling chlorophyll content, and fresh weight), a significant dose treatment effect was detected between the camelina seedling root length and PEG concentration; thus, in experiment 1, the inhibition of camelina seedling root growth that resulted from PEG-induced WDS was recorded to determine the drought tolerance of the fifteen camelina genotypes. At 6 d after PEG treatment (DAT), the seedling root length of each camelina genotype was determined from 15 randomly selected seedlings from each petri dish (each petri dish as a replicate, n = 3) using a micrometer (Yongkang Anguanlong Electromechanical Co., Ltd., Yongkang, China). Subsequently, the data were subjected to nonlinear regression analysis (log-logistic dose–response curve) to estimate the GR50 of seedling root lengths of the fifteen camelina genotypes. The most drought-sensitive (CamK8, GR50 = 19.0 mM) and -tolerant (CamK9, GR50 = 34.3 mM) camelina genotypes determined by the GR50 were used in experiment 2 to test the alleviative effect of MWCNTs and nano-Se on PEG-induced WDS in camelina genotypes.
2.1.2. Experiment 2: Alleviative Effect of MWCNTs and Nano-Se on PEG-Induced WDS in Camelina
Seeds of CamK8 and CamK9 determined in experiment 1 were sown in 105-well plastic seedling trays (hole size: 3.5 × 1.5 × 4 cm) with organic horticultural potting soil (Yiyuan Agriculture and Forestry Ltd., Suzhou, China) in September 2021 and grown in a greenhouse at the Yangzhou University Pratacultural Science Experimental Station at 25 °C with a 12/12 h photoperiod supplemented by an overhead sodium lamp. Young camelina seedlings of each genotype with 2–3 leaves were transplanted into the plastic pots (height: 16 cm; diameter: 14 cm) and raised up to 7–8 leaves in the same greenhouse before treatment. Fifty mL of PEG solution concentration (19.0 mM, GR50 value of CamK8) was root-applied to the camelina plants, followed by foliar application of MWCNTs (two doses: 50 and 100 mg L−1) or nano-Se (two doses: 5 and 10 mg L−1) to PEG-treated camelina plants. Daily water supplementary (100 mL) was conducted after 2 DAT. All camelina plants were carefully raised and maintained in the same greenhouse under the conditions described above. Control groups included untreated and only-PEG-treated camelina plants. This experiment layout followed a completely randomized block design, with three replications for each treatment (each pot containing three plants as a replicate, n = 3).
Data on photosynthetic performance, including leaf chlorophyll fluorescence and chlorophyll content, and antioxidant enzymes activities, including superoxide dismutase (SOD), malondialdehyde (MDA) concentration, and soluble protein of the two camelina genotypes under each treatment were determined at 3 DAT. Nine healthy leaves of each camelina genotype from each pot (three leaves plant−1) under each treatment were selected to determine the leaf chlorophyll fluorescence parameter Fv/Fm (Fv/Fm = (Fm − Fo)/Fm, where Fo = minimum fluorescence of dark-adapted leaves and Fm = maximum fluorescence) and chlorophyll content, respectively. Fv/Fm was recorded from those selected leaves with a chlorophyll fluorescence meter (FP110-LM/D, Czech Republic) using a detection time of 2 s and emitting light of 650 nm wavelength with an intensity of 3500 μmol photons m−2 s−1. The measurements were conducted on dark-adapted leaves using leaf clips for 30 min under dark conditions. The chlorophyll content of each camelina genotype under each treatment was also determined from the same leaves with a portable chlorophyll meter (SPAD-502PLUS, Konica Minolta Sensing Korea Co., Ltd., Seoul, Korea) in the morning between 8:00 and 10:00 a.m. For photosynthetic parameters, a total of 27 point measurements, each replicated in a pot with nine measurements, were made on each camelina genotype for each treatment. Regarding the determination of SOD, MDA, and soluble protein concentrations, 0.5 g of camelina leaf samples collected from each treatment were ground into a powder in a mortar with liquid nitrogen and homogenized in 5 mL of 0.2 mM phosphate buffer (pH 7.8). The homogenate was centrifuged at 10,000× g for 20 min at 4 °C. The SOD, MDA, and soluble protein concentrations were determined accordingly based on the experimental guide of plant physiology edited by Zou (2000).
At maturity, all camelina plants were carefully harvested and assessed for plant height and seed weight. The mean plant height (cm) and seed weight plant−1 (g) of each two camelina genotypes under each treatment was computed accordingly. Seed quality, including seed oil content and oil fatty acid profiles, was also analyzed. The oil content of the resulting two camelina genotype seeds under each treatment was determined using a Soxhlet extraction method at the Laboratory of Grass Germplasm Resources Research and Utilization. The fatty acid composition was analyzed by gas chromatography (GC) at the Laboratory of Animal Nutrition and Feed Engineering Technology Research, Yangzhou University, China. The detailed information on sample preparation, GC conditions, fatty acid methyl esters (FAMEs) sorting, and determination of the seed quality parameters were described in a previous study [32].
2.2. Statistical Analysis
Initially, the Shapiro–Wilk test on the normality of all data obtained from this study showed a normal distribution of residuals. For the analysis of experiment 1, among the tested parameters (percent seed germination, seedling root and shoot length, seedling length, seedling chlorophyll content, and fresh weight), analysis of variance (ANOVA) showed the significant dose treatment effect between PEG concentration and camelina seedling root length. Therefore, to estimate the GR50 values of seedling root length of each camelina genotype, those seedling root length data were fitted to the three-parameter log-logistic dose–response model [33,34] (Equation (1)):
where y is an estimate of seedling root length expressed as a percentage of the untreated control, c is the upper limit, and b is the slope of the curve through GR50 (a PEG concentration causing a 50% inhibition of camelina seedling root length). A lack-of-fit was checked for the fitted model. R2 was used to indicate the goodness of fit for the models. The drought tolerance of the fifteen camelina genotypes was then compared and determined based on the GR50 values of seedling root length.
Regarding the study of experiment 2, the data were represented as mean ± SE (standard error) of three replications. When ANOVA revealed the statistically different means, the Tukey post hoc test was conducted to separate means (p ≤ 0.05) for evaluating the alleviative effect of MWCNTs or nano-Se on PEG-induced WDS in camelina genotypes. All statistical analyses were conducted using R 3.2.4 (R Core Team 2016).
3. Results
3.1. PEG Dose Responses and Drought Tolerance of Camelina Genotypes
Overall, the seedling root length of each camelina genotype tested consistently decreased with increasing the PEG solution concentrations (range: 0–56.3 mM), and a three-parameter log-logistic dose–response curve well described the relationship between them (R2: 0.74–0.99; p values of the fitted model < 0.05 for all camelina genotypes) (Figure 1; Table S1). Additionally, no evidence of a lack-of-fit of the fitted model was detected (p values of lack-of-fit > 0.05 for all camelina genotypes), indicating the suitability of the chosen dose–response model. These findings could be used to estimate the reliable GR50 values for those tested camelina genotypes.
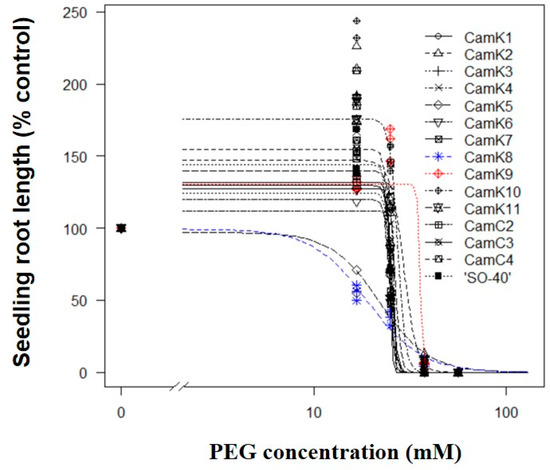
Figure 1.
Dose responses in seedling root length of the fifteen camelina genotypes to various PEG solution concentrations. The blue and red dotted lines indicate the most drought–sensitive (CamK8, GR50 = 19.0 mM) and –tolerant (CamK9, GR50 = 34.3 mM) camelina genotypes determined based on the GR50 values estimated using the seedling root length from the model (Equation (1)) and parameter estimates in Table S1, respectively, among the fifteen camelina genotypes.
The drought tolerance of the fifteen camelina genotypes tested was characterized and compared based on the GR50 values estimated using the seedling root length from the model (Table S1). The results demonstrated that among the tested camelina genotypes, CamK8 and CamK9 were the most drought-sensitive and -tolerant genotypes to PEG-induced WDS, with GR50 values of 19.0 and 34.3 mM, respectively (Figure 1; Table S1).
3.2. Alleviation of PEG-Induced WDS Using MWCNTs or Nano-Se in Camelina
The alleviative effect of MWCNTs or nano-Se on WDS in camelina was tested using CamK8 and CamK9 (determined in experiment 1) at the whole-plant level. Regarding the photosynthetic performance of the two camelina genotypes, compared to CamK8, CamK9 was more tolerant to PEG-induced WDS, showing the statistically similar values of leaf chlorophyll content and fluorescence as untreated control (Figure 2). For example, the PEG concentration (19.0 mM) significantly (p < 0.01) decreased the values of leaf chlorophyll content (31.7 mg g−1) in CamK8 compared with the untreated control (chlorophyll content: 35.8 mg g−1) (Figure 2a). In contrast, CamK9 could endure this degree of WDS, showing a similar chlorophyll content value to the control (38.9 versus 37.7 mg g−1) (Figure 2b). The foliar application of CamK8 with MWCNTs or nano-Se (regardless of concentration) increased the chlorophyll content levels (range: 32.9–37.2 mg g−1), which were statistically greater than in the PEG-treated plants (31.7 mg g−1) (Figure 2a). Additionally, the result showed that the leaf chlorophyll fluorescence was not affected by the present PEG concentration in the two camelina genotypes (Figure 2c,d).
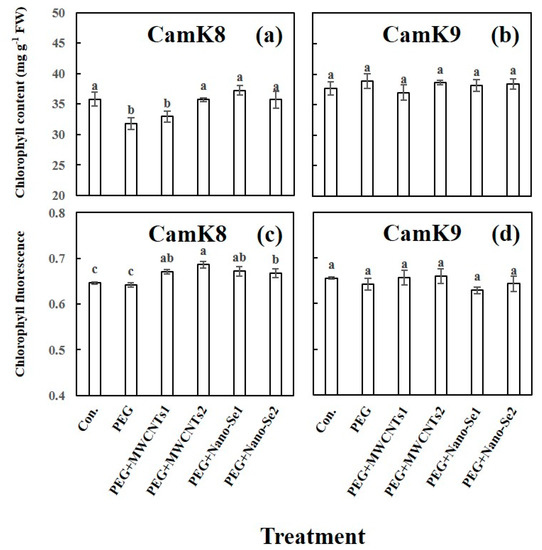
Figure 2.
Effect of various treatments on the chlorophyll content (mg g−1) (a,b) and Fv/Fm (c,d) of CamK8 (a,c) and CamK9 (b,d) under PEG-induced WDS at the third day after treatment. Con.: un–treated control; PEG + MWCNTs 1 or 2: foliar application of MWCNTs with dose 1 (50 mg L−1) or 2 (100 mg L−1) following root application of PEG solution concentration (19.0 mM); PEG + nano-Se 1 or 2: foliar application of nano-Se with dose 1 (5 mg L−1) or 2 (10 mg L−1) following root application of PEG solution concentration (19.0 mM). Values are means (n = 3) and bars denote the standard error of the mean. Different letters represent the significant different values determined by the Tukey post hoc test (p ≤ 0.05).
Concerning the SOD and MDA, both antioxidant enzyme activities were significantly increased in the drought-sensitive genotype (CamK8) under PEG treatment (303.4 U g−1 and 88.4 µmol g−1 for SOD and MDA, respectively) compared with the untreated control (249.6 U g−1 and 61.2 μmol g−1 for SOD and MDA, respectively). Subsequently, those values decreased to the normal level when MWCNTs (applied at 50 and 100 mg L−1) or nano-Se (applied at 5.0 mg L−1) was co-applied (231.7–264.8 U g−1 and 47.6–60.2 μmol g−1 for SOD and MDA, respectively) (Figure 3a,c). However, this is not the case for the drought-tolerant genotype of CamK9. While PEG treatment significantly increased the SOD value in CamK9 (280.7 U g−1) compared to the untreated control (244.8 U g−1), the MDA values between the two treatments were not statistically different (53.8 and 47.6 μmol g−1, respectively). PEG co-applied with MWCNTs at the present doses or nano-Se at a low dose effectively decreased the SOD values (223–269.5 U g−1); however, the higher SOD value was observed (285.6 U g−1) when PEG was co-applied with nano-Se at high dose (10.0 mg L−1). Regarding MDA, the values among the treatments were statistically the same in CamK9 (Figure 3b,d). For the soluble protein, the PEG treatment significantly (p < 0.01) decreased its content in both camelina genotypes (17.6 and 18.9 mg g−1 in CamK8 and CamK9, respectively) compared with the untreated control (20.4 and 21.9 mg g−1 in CamK8 and CamK9, respectively). The exogenous application of MWCNTs or nano-Se (regardless of applied doses) could alleviate the PEG-induced WDS in camelina genotypes by increasing the values of the soluble protein (CamK8: range of 19.8–22.2 mg g−1 vs. PEG treated plant: 17.6 mg g−1; CamK9: range of 19.5–21.7 mg g−1 vs. PEG treated plant: 18.9 mg g−1) (Figure 3e,f).
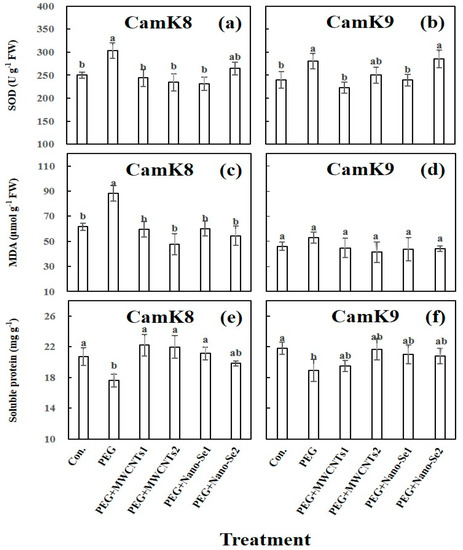
Figure 3.
Effect of various treatments on SOD (U g−1) (a,b), MDA (µmol g−1) (c,d) concentration, and soluble protein (mg g−1) (e,f) of CamK8 (a,c,e) and CamK9 (b,d,f) under PEG-induced WDS at the third day after treatment. Con.: untreated control; PEG + MWCNTs 1 or 2: foliar application of MWCNTs with dose 1 (50 mg L−1) or 2 (100 mg L−1) following root application of PEG solution concentration (19.0 mM); PEG + nano-Se 1 or 2: foliar application of nano-Se with dose 1 (5 mg L−1) or 2 (10 mg L−1) following root application of PEG solution concentration (19.0 mM). Values are means (n = 3) and bars denote the standard error of the mean. Different letters represent the significant different values determined by the Tukey post hoc test (p ≤ 0.05).
At maturity, the mean plant height and seed weight plant−1 for each camelina genotype under each treatment was determined (Figure 4). Overall, the CamK9 showed a greater degree of drought tolerance to the present PEG concentration, with a mean plant height at harvest similar to that of the untreated control (60.2 vs. 60.3 cm) (Figure 4b). By contrast, this PEG concentration significantly decreased the plant height of CamK8 (46.7 cm; untreated control: 56.9 cm) (Figure 4a). Except for the treatment with PEG and MWCNTs1 (50 mg L−1), the exogenous application of MWCNTs2 (100 mg L−1) or nano-Se (5 or 10 mg L−1) increased the plant height of CamK8 stressed by PEG (Figure 4a). It is worth noting that PEG co-applied with nano-Se at a high dose (10 mg L−1) reduced the plant height of CamK9 (Figure 4b). A similar trend in seed yield plant−1 as plant height for the two camelina genotypes was also observed (Figure 4c,d). The seed yield plant−1 of CamK8 treated with PEG (seed yield: 0.06 g plant−1) dramatically dropped to about 50% of the untreated control (0.12 g plant−1). This adverse effect was reversed by the application of MWCNTs or nano-Se (regardless of dose) to the seed yield plant−1 (range: 0.11−0.14 g plant−1), improving it to the control level (Figure 4c). In the case of CamK9, although significant differences among the treatments were not detected, the values of seed yield plant−1 produced under the treatment of PEG co-applied with the lower dose of MWCNTs (0.11 g plant−1) or nano-Se (0.1 g plant−1) were greater than other treatments (0.07–0.08 g plant−1) (Figure 4d). We would like to point out that the relatively lower seed yield plant−1 for the two genotypes in this study might be related to the greenhouse conditions, as the plants grown in the field resulted in a higher yield.
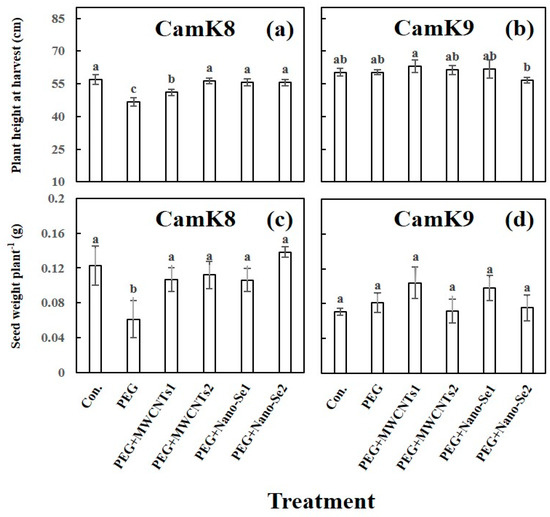
Figure 4.
Effect of various treatments on plant height (cm) (a,b) and seed weight plant−1 (g) (c,d) of CamK8 (a,c) and CamK9 (b,d) under PEG-induced WDS at harvest. Con.: un–treated control; PEG + MWCNTs 1 or 2: foliar application of MWCNTs with dose 1 (50 mg L−1) or 2 (100 mg L−1) following root application of PEG solution concentration (19.0 mM); PEG + nano-Se 1 or 2: foliar application of nano-Se with dose 1 (5 mg L−1) or 2 (10 mg L−1) following root application of PEG solution concentration (19.0 mM). Values are means (n = 3) and bars denote the standard error of the mean. Different letters represent the significant different values determined by the Tukey post hoc test (p ≤ 0.05).
The principal fatty acid composition and fatty acid groups of the two camelina genotypes under various treatments were analyzed, as shown in Table 2. While the PEG concentration affected the plant photosynthetic (i.e., chlorophyll content) and growth-related parameters (i.e., plant height) as described above, the principal fatty acid composition (i.e., C18:1, C18:2, C18:3) and fatty acid groups (i.e., saturated fatty acid (SFA), monounsaturated fatty acid (MUFA), polyunsaturated fatty acid (PUFA)) in CamK8 and CamK9 seed oils among different treatments were not affected by the present application concentration (Table 2). The unique nervonic acid (C24:1) was detected in both camelina genotypes; however, it was not detected when PEG was single- or co-applied with nano-Se in CamK8.

Table 2.
Principal fatty acid composition and fatty acid groups (% of total fatty acids) of CamK8 and CamK9 under various treatments.
4. Discussion
The present study was the first to evaluate the drought tolerance potential of the fifteen camelina genotypes to the various degrees of WDS induced by PEG and to test the foliar application of two nanoparticles, MWCNTs and nano-Se, for the alleviation of WDS on camelina. This study showed that the drought negatively affected the photosynthetic performance, plant growth, and seed yield of camelina genotypes; however, the foliar application of MWCNTs or nano-Se can alleviate the adverse effect of PEG-induced WDS on camelina by producing a relatively consistent seed yield and quality.
The reduction in chlorophyll content under WDS has been reported in several oilseed crops (i.e., sunflower) [35] and other crops (i.e., wheat and chickpea) [36,37]. WDS can severely affect the membrane of the plant by inducing ROS production, subsequently damaging the chloroplasts and reducing the chlorophyll production, decreasing photosynthetic activity [36,38]. This study revealed that the application of MWCNTs (50 or 100 mg L−1) or nano-Se (5 or 10 mg L−1) caused a higher chlorophyll content in the drought-sensitive genotype of CamK8 leave and retained normal photosynthesis under WDS. Concerning Fv/Fm, while it was reported to be an effective indicator of abiotic stresses in several plant species [39], the results in this study did not show a significant response of Fv/Fm to WDS, indicating a certain degree of drought tolerance of camelina compared to other crops [21].
The production of antioxidants is a stress strategy for plants under various stresses to counteract unfavorable conditions. SOD has been documented for its antioxidant capability to scavenge ROS and protect plants against peroxide damage [40,41]. Results of this study showed that the SOD activity of the camelina plant increased, which is in line with the previous studies of licorice (Glycyrrhiza uralensis Fisch) [42], triticale (Triticosecale Wittmack) [43], and barley (Hordeum vulgare L.) [44] under WDS. While an increase in SOD activities was not observed in the foliar application of MWCNTs or Se as in previous studies on several oilseed crops and horticultural plants [13,40], in this study, we found that the application of MWCNTs or nano-Se on the camelina plants that were pre-treated with PEG retained SOD activity at a similar or higher levels compared with the untreated control. As the plants have various defense mechanisms (including the alteration of antioxidant enzyme activities), such as defensive pigment production (i.e., carotenoid) and excess energy dissipation against ROS [45], the conclusions from the previous studies may simplify the changing process of SOD activities under WDS. Therefore, a more comprehensive investigation on the mechanism of the alleviative effect of MWCNTs or nano-Se on WDS is still needed. MDA has been considered an indicator of the damage of plant cell membrane systems [46]. The results of the accumulation of MDA contents in camelina indicated the occurrence of damage due to WDS. Through the application of MWCNTs or nano-Se, the MDA contents significantly decreased and retained a normal level in the drought-sensitive CamK8. Regarding the soluble protein content, a significant decline (p < 0.001) was observed in camelina under PEG-induced WDS, which was similar to the previous findings in the same species of camelina [47]. The decline in soluble protein might be associated with the higher degradation of proteins under WDS for generating low-molecular-weight osmolytes for osmotic adjustment [48]. The exogenous application of MWCNTs or nano-Se alleviated the adverse effects of WDS in camelina by regulating the soluble protein content.
Compared with untreated control, a substantial decline in camelina seed yield was linked to the WDS induced by PEG. These results showing reductions in seed yield under WDS agree with Raza et al. (2015) and Bukhari et al. (2022). Reduced plant height associated with PEG-induced WDS could be attributed to the lower turgidity, cell division, and elongation of plant cells, which is supported by Mesbah et al. (2009). The camelina plants under WDS during flowering and silicles formation periods caused a relatively shorter flowering and growth duration (data not shown), which was closely-related to the subsequent infertility and abscission of some flowers, and eventually reduced the seed yield. This study showed that the foliar application of MWCNTs or nano-Se with the present doses could maintain the basic growth parameters, as well as the crop yield and oil quality in CamK8, compared to untreated control. In the present study, it is worth mentioning that compared to drought-tolerant CamK9, the nanoparticle treatments showed a stronger effect on the drought-sensitive CamK8. This could explain the relatively stable morpho-physiological and biochemical parameters in CamK9 when PEG was co-applied with the nanoparticles.
MWCNTs act as effective plant-growth regulators that affect plant morpho-physiological and biochemistry [5]. They can penetrate the cell wall, enter the cytoplasm, and promote cell elongation in the root system, resulting in faster root growth and higher biomass production [49]. The study also reported that MWCNT-treated tomatoes produced twice as many flowers and fruits compared with the untreated controls [12]. Nano-Se can also be used as a bio-stimulant or fertilizer to improve the crop resilience under abiotic or biotic stress through enhancing antioxidant capacity and photosynthesis-related parameters [14,16]. Studies have shown that nano-Se increased plant photosynthesis via PSII, the absorption of short-wave light, and influenced carbohydrate metabolism. Nano-Se-treated plants also increased the total soluble sugar content [50], sucrose, and metabolic enzyme activities [14], which are closely related to the plant cells’ osmotic adjustment and photosynthesis. All these examples and the results from the current study demonstrate that the adverse effect of PEG-induced WDS on camelina growth could be alleviated by MWCNTs or nano-Se, achieving a satisfactory seed yield. Thus, applying these nanoparticles as growth regulators provides new perspectives on managing WDS on camelina crop production in agricultural practices.
It is worth mentioning that both nanoparticles showed a hormetic effect, characterized by stimulation at low doses and inhibition at high doses [5,40]. This study determined that the effective doses for the alleviation of PEG-induced WDS on camelina were 50–100 mg L−1 for MWCNTs and 5–10 mg L−1 for nano-Se, respectively. These application doses fell within the ranges of 50–200 mg L−1 in sugarcane (Saccharum spp.) [5] and tomato [12], 40−160 mg L−1 in wheat [49], and 90 mg L−1 in grape [13] for MWCNTs, and 2.5–10.0 mg L−1 in melon (Cucumis melo L.) [14] and 5.0 mg L−1 in celery (Apium graveolens L.) [51] for nano-Se. However, when the concentration of MWCNTs is higher than 250 mg L−1, it shows toxicity to tomato plants [52]. Nano-Se applied at 50 mg L−1 to honeybee flowers (Melissa officinalis) significantly reduced the plant growth and caused severe toxicity [53]. Based on above discussion, although the exogenous application of MWCNTs or nano-Se is generally beneficial in managing WDS, a high concentration should be avoided since it can destroy the plant’s metabolism and reduce its growth and production [40]. In addition, accumulating these nanoparticles in reproductive organs and the residual in soil raises questions about the potential hazard to the ecological environment [54]. Therefore, further studies on the impact and evaluation of nanoparticles’ toxicity to the agroecosystem are still required.
5. Conclusions
The foliar application of MWCNTs and nano-Se can alleviate WDS, which could be a biologically beneficial strategy for improving the water-stress tolerance of camelina plants and decreasing the grain yield loss. Additionally, the relative drought tolerance and better adaptability of the camelina crop under WDS makes it more proficient as an oilseed crop in the changing climate. The results in this study provide the possibility of using nanoparticles for the management of WDS in agricultural practices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13040979/s1, Table S1: Summary of parameters estimated from the log-logistic dose–response model (Equation (1)) fitted to the seedling root length of the fifteen camelina genotypes. The values in parentheses are the standard error of each parameter.
Author Contributions
Conceptualization, H.-Z.W. and C.-J.Z.; methodology, H.-Z.W. and C.-J.Z.; validation, H.-Z.W., Y.G. and C.-J.Z.; formal analysis, H.-Z.W. and C.-J.Z.; investigation, H.-Z.W., Y.G. and Y.Z.; resources, C.-J.Z.; data curation, H.-Z.W. and C.-J.Z.; writing—original draft preparation, H.-Z.W. and C.-J.Z.; writing—review and editing, H.-Z.W., Y.G., Y.Z., J.Y., D.-S.K., M.C., Y.W., Y.F., H.Z., X.Y. and C.-J.Z.; supervision, C.-J.Z.; project administration, C.-J.Z.; funding acquisition, C.-J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant no. 32171670), the College Students Innovation Project of Yangzhou University to Hui-Zhen Wu (X20210646), the High-level Talents program of Yangzhou University to Chuan-Jie Zhang, the Project of Forestry Science and Technology Innovation and Promotion of Jiangsu (Grant no. LYKJ-2021-09), and the Major Focus Projects of Henan Academy of Sciences (Grant no. 190113004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- El Sabagh, A.; Hossain, A.; Barutcular, C.; Gormus, O.; Ahmad, Z.; Hussain, S.; Islam, M.S.; Alharby, H.; Bamagoos, A.; Kumar, N.; et al. Effects of drought stress on the quality of major oilseed crops: Implications and possible mitigation strategies—A review. Appl. Ecol. Environ. Res. 2019, 17, 4019–4043. [Google Scholar] [CrossRef]
- Valliyodan, B.; Nguyen, H.T. Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Curr. Opin. Plant Biol. 2006, 9, 189–195. [Google Scholar] [CrossRef]
- Al-Yasi, H.; Attia, H.; Alamer, K.; Hassan, F.; Ali, E.; Elshazly, S.; Siddique, K.H.M.; Hessini, K. Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in damask rose. Plant Physiol. Bioch. 2020, 150, 133–139. [Google Scholar] [CrossRef]
- Miao, Y.Y.; Zhu, Z.B.; Guo, Q.S.; Ma, H.L.; Zhu, L.F. Alternate wetting and drying irrigation-mediated changes in the growth, photosynthesis and yield of the medicinal plant Tulipa edulis. Ind. Crop. Prod. 2015, 66, 81–88. [Google Scholar] [CrossRef]
- Sorcia-Morales, M.; Gomez-Merino, F.C.; Sanchez-Segura, L.; Spinoso-Castillo, J.L.; Bello-Bello, J.J. Multi-walled carbon nanotubes improved development during in vitro multiplication of sugarcane (Saccharum spp.) in a semi-automated bioreactor. Plants 2021, 10, 2015. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.U.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef] [PubMed]
- Adisa, I.O.; Pullagurala, V.L.R.; Peralta-Videa, J.R.; Dimkpa, C.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci.-Nano. 2019, 6, 2002–2030. [Google Scholar] [CrossRef]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; de Silva, K.; Nedosekin, D.A.; Dervishi, E.; Biris, A.S.; Shashkov, E.V.; Galanzha, E.I.; Zharov, V.P. Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 1028–1033. [Google Scholar] [CrossRef]
- El-Moneim, D.A.; Dawood, M.F.A.; Moursi, Y.S.; Farghaly, A.A.; Afifi, M.; Sallam, A. Positive and negative effects of nanoparticles on agricultural crops. Nanotechnol. Environ. Eng. 2021, 6, 21. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, D.K.; Chauhan, D.K. Effects of nano-materials on seed germination and seedling growth: Striking the slight balance between the concepts and controversies. Mater. Focus 2016, 5, 1–6. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; Kim, B.S.; Kim, J.N.; Alimohammadi, M.; Dervishi, E.; Mustafa, T.; Cernigla, C.E. Carbon nanotubes as plant growth regulators: Effects on tomato growth, reproductive system, and soil microbial community. Small 2013, 9, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, M.; Yang, X.L.; Zhang, Y.; Hui, H.T.; Zhang, D.Q.; Shu, J. Multi-walled carbon nanotubes enhanced the antioxidative system and alleviated salt stress in grape seedlings. Sci. Hortic. 2022, 293, 110698. [Google Scholar] [CrossRef]
- Kang, L.; Wu, Y.L.; Zhang, J.B.; An, Q.S.; Zhou, C.R.; Li, D.; Pan, C.P. Nano-selenium enhances the antioxidant capacity, organic acids and cucurbitacin B in melon (Cucumis melo L.) plants. Ecotoxicol. Environ. Saf. 2022, 241, 113777. [Google Scholar] [CrossRef]
- Li, Y.X.; Zhu, N.L.; Liang, X.J.; Zheng, L.R.; Zhang, C.X.; Li, Y.F.; Zhang, Z.Y.; Gao, Y.X.; Zhao, J.T. A comparative study on the accumulation, translocation and transformation of selenite, selenate, and SeNPs in a hydroponic-plant system. Ecotox. Environ. Saf. 2020, 189, 109955. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.Q.; Qi, Z.Y.; Li, M.Q.; Ahammed, G.J.; Chu, X.Y.; Zhou, J. Selenium forms and methods of application differentially modulate plant growth, photosynthesis, stress tolerance, selenium content and speciation in Oryza sativa L. Ecotox. Environ. Saf. 2019, 169, 911–917. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Abdelrahman, M.; Hosseini, M.S.; Hoveizeh, N.F.; Tran, L.S.P. Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ. Pollut. 2019, 253, 246–258. [Google Scholar] [CrossRef]
- Zhou, C.R.; Li, D.; Shi, X.L.; Zhang, J.B.; An, Q.S.; Wu, Y.L.; Kang, L.; Li, J.Q.; Pan, C.P. Nanoselenium enhanced wheat resistance to aphids by regulating biosynthesis of DIMBOA and volatile components. J. Agr. Food Chem. 2021, 69, 14103–14114. [Google Scholar] [CrossRef]
- Fatima, Z.; Ahmed, M.; Hussain, M.; Abbas, G.; Ul-Allah, S.; Ahmad, S.; Ahmed, N.; Ali, M.A.; Sarwar, G.; Haque, E.; et al. The fingerprints of climate warming on cereal crops phenology and adaptation options. Sci. Rep. 2020, 10, 18013. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, C.J.; Zhang, Y.X.; Liu, L.; Wang, Y.W.; Kim, D.; Yu, J.L.; Diao, J.X.; Wu, N.; Chen, M.; et al. Agronomic performance of camelina genotypes selected for seed yield and quality characteristics in eastern China. Ind. Crop. Prod. 2022, 184, 115077. [Google Scholar] [CrossRef]
- Bansal, S.; Durrett, T.P. Camelina sativa: An ideal platform for the metabolic engineering and field production of industrial lipids. Biochimie 2016, 120, 9–16. [Google Scholar] [CrossRef]
- Yang, J.; Caldwell, C.; Corscadden, K.; He, Q.S.; Li, J.L. An evaluation of biodiesel production from Camelina sativa grown in Nova Scotia. Ind. Crops Prod. 2016, 81, 162–168. [Google Scholar] [CrossRef]
- Čanak, P.; Jeromela, A.M.; Vujošević, B.; Kiprovski, B.; Mitrović, B.; Alberghini, B.; Facciolla, E.; Monti, A.; Zanetti, F. Is drought stress tolerance affected by biotypes and seed size in the emerging oilseed crop camelina? Agronomy 2020, 10, 1856. [Google Scholar] [CrossRef]
- Eberle, C.A.; Thom, M.D.; Nemec, K.T.; Forcella, F.; Lundgren, J.G.; Gesch, R.W.; Riedell, W.E.; Papiernik, S.K.; Wagner, A.; Peterson, D.H.; et al. Using pennycress, camelina, and canola cash cover crops to provision pollinators. Ind. Crop. Prod. 2015, 75, 20–25. [Google Scholar] [CrossRef]
- Zanetti, F.; Eynck, C.; Christou, M.; Krzyzaniak, M.; Righini, D.; Alexopoulou, E.; Stolarski, M.J.; Van Loo, E.N.; Puttick, D.; Monti, A. Agronomic performance and seed quality attributes of camelina (Camelina sativa L. crantz) in multi-environment trials across Europe and Canada. Ind. Crop. Prod. 2017, 107, 602–608. [Google Scholar] [CrossRef]
- Zhang, C.J.; Auer, C. Overwintering assessment of camelina (Camelina sativa) cultivars and congeneric species in the northeastern US. Ind. Crop. Prod. 2019, 139, 111532. [Google Scholar] [CrossRef]
- Zhang, C.J.; Wu, N.; Diao, J.X.; Kim, D.S.; Wang, Y.W.; Gao, Y.; Zhang, Y.X.; Chen, M.; Yu, J.L.; Zhang, H.X.; et al. Agronomic evaluation of a Chinese camelina [Camelina sativa (L.) Crantz] cultivar in multiple semi-arid locations of northern China. Ital. J. Agron. 2022, 17, 2034. [Google Scholar] [CrossRef]
- Gugel, R.K.; Falk, K.C. Agronomic and seed quality evaluation of Camelina sativa in western Canada. Can. J. Plant Sci. 2006, 86, 1047–1058. [Google Scholar] [CrossRef]
- Rizzitello, R.; Zhang, C.J.; Auer, C. Camelina (Camelina sativa L. Crantz) crop performance, insect pollinators, and pollen dispersal in the Northeastern US. BioRxiv 2019. [Google Scholar] [CrossRef]
- Karim, A.N.M.A.; Sarker, U.K.; Hasan, A.K.; Islam, N.; Uddin, M.R. Selection of drought tolerant high yielding chickpea genotypes based on field performance and genetic variation in Bangladesh. Legume Res. 2021, 44, 1131–1137. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmed, Z.; Ahmad, Z.; Ahmad, R.; Erman, M.; Cig, F.; El Sabagh, A. Alterations in growth and yield of camelina induced by different planting densities under water deficit stress. Phyton-Int. J. Exp. Bot. 2020, 89, 587–597. [Google Scholar] [CrossRef]
- Wang, Y.W.; Yu, J.L.; Gao, Y.; Li, Z.W.; Kim, D.; Chen, M.; Fan, Y.; Zhang, H.X.; Yan, X.B.; Zhang, C.J. Agronomic evaluation of shade tolerance of 16 spring Camelina sativa (L.) Crantz genotypes under different artificial shade levels using a modified membership function. Front. Plant Sci. 2022, 13, 978932. [Google Scholar] [CrossRef] [PubMed]
- Streibig, J.C. Models for curve-fitting herbicide dose response data. Acta Agric. Scand. 1980, 30, 59–64. [Google Scholar] [CrossRef]
- Knezevic, S.Z.; Streibig, J.C.; Ritz, C. Utilizing R software package for dose-response studies: The concept and data analysis. Weed Technol. 2007, 21, 840–848. [Google Scholar] [CrossRef]
- Manivannan, P.; Jaleel, C.A.; Somasundaram, R.; Panneerselvam, R. Osmoregulation and antioxidant metabolism in drought-stressed Helianthus annuus under triadimefon drenching. Comptes Rendus Biol. 2008, 331, 418–425. [Google Scholar] [CrossRef]
- Chen, Y.L.; Ghanem, M.E.; Siddique, K.H.M. Characterising root trait variability in chickpea (Cicer arietinum L.) germplasm. J. Exp. Bot. 2017, 68, 1987–1999. [Google Scholar] [CrossRef] [PubMed]
- Fotovat, R.; Valizadeh, M.; Toorchi, M. Association between water-use efficiency components and total chlorophyll content (SPAD) in wheat (Triticum aestivum L.) under well-watered and drought stress conditions. J. Food Agric. Environ. 2007, 5, 225–227. [Google Scholar]
- Li, X.Y.; Liu, X.; Yao, Y.; Li, Y.H.; Liu, S.; He, C.Y.; Li, J.M.; Lin, Y.Y.; Li, L. Overexpression of Arachis hypogaea AREB1 gene enhances drought tolerance by modulating ROS scavenging and maintaining endogenous ABA content. Int. J. Mol. Sci. 2013, 14, 12827–12842. [Google Scholar] [CrossRef]
- Shao, Q.S.; Wang, H.Z.; Guo, H.P.; Zhou, A.C.; Huang, Y.Q.; Sun, Y.L.; Li, M.Y. Effects of shade treatments on photosynthetic characteristics, chloroplast ultrastructure, and physiology of Anoectochilus roxburghii. PLoS ONE 2014, 9, e85996. [Google Scholar] [CrossRef]
- Ahmad, Z.; Anjum, S.; Skalicky, M.; Waraich, E.A.; Tariq, R.M.S.; Ayub, M.A.; Hossain, A.; Hassan, M.M.; Brestic, M.; Islam, M.S.; et al. Selenium alleviates the adverse effect of drought in oilseed crops camelina (Camelina sativa L.) and canola (Brassica napus L.). Molecules 2021, 26, 1699. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, L.J.; Yu, Z.L. Effect of salt and drought stress on antioxidant enzymes activities and SOD isoenzymes of liquorice (Glycyrrhiza uralensis Fisch). Plant Growth Regul. 2006, 49, 157–165. [Google Scholar] [CrossRef]
- Saed-Moucheshi, A.; Sohrabi, F.; Fasihfar, E.; Baniasadi, F.; Riasat, M.; Mozafari, A.A. Superoxide dismutase (SOD) as a selection criterion for triticale grain yield under drought stress: A comprehensive study on genomics and expression profiling, bioinformatics, heritability, and phenotypic variability. BMC Plant Biol. 2021, 21, 148. [Google Scholar] [CrossRef] [PubMed]
- Acar, O.; Turkan, I.; Ozdemir, F. Superoxide dismutase and peroxidase activities in drought sensitive and resistant barley (Hordeum vulgare L.) varieties. Acta Physiol. Plant. 2001, 23, 351–356. [Google Scholar] [CrossRef]
- Efeoglu, B.; Ekmekci, Y.; Cicek, N. Physiological responses of three maize cultivars to drought stress and recovery. S. Afr. J. Bot. 2009, 75, 34–42. [Google Scholar] [CrossRef]
- Güneş, A.; Kordali, S.; Turan, M.; Bozhüyük, A.U. Determination of antioxidant enzyme activity and phenolic contents of some species of the Asteraceae family from medicanal plants. Ind. Crops Prod. 2019, 137, 208–213. [Google Scholar] [CrossRef]
- Ahmed, Z.; Waraich, E.A.; Ahmad, R.; Shahbaz, M. Morpho-physiological and biochemical responses of camelina (Camelina sativa crantz) genotypes under drought stress. Int. J. Agric. Biol. 2017, 19, 1–7. [Google Scholar] [CrossRef]
- Nayyar, H.; Walia, D.P. Water stress induced proline accumulation in contrasting wheat genotypes as affected by calcium and abscisic acid. Biol. Plantarum. 2003, 46, 275–279. [Google Scholar] [CrossRef]
- Wang, X.P.; Han, H.Y.; Liu, X.Q.; Gu, X.X.; Chen, K.; Lu, D.L. Multi-walled carbon nanotubes can enhance root elongation of wheat (Triticum aestivum) plants. J. Nanopart. Res. 2012, 14, 814. [Google Scholar] [CrossRef]
- Nawaz, F.; Ahmad, R.; Ashraf, M.Y.; Waraich, E.A.; Khan, S.Z. Effect of selenium foliar spray on physiological and biochemical processes and chemical constituents of wheat under drought stress. Ecotox. Environ. Saf. 2015, 113, 191–200. [Google Scholar] [CrossRef]
- Li, D.; An, Q.S.; Wu, Y.L.; Li, J.Q.; Pan, C.P. Foliar application of selenium nanoparticles on celery stimulates several nutrient component levels by regulating the alpha-linolenic acid pathway. Acs Sustain. Chem. Eng. 2020, 8, 10502–10510. [Google Scholar] [CrossRef]
- González-García, Y.; López-Vargas, E.R.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; González-Morales, S.; Robledo-Olivo, A.; Alpuche-Solís, Á.G.; Juárez-Maldonado, A. Impact of carbon nanomaterials on the antioxidant system of tomato seedlings. Int. J. Mol. Sci. 2019, 20, 5858. [Google Scholar] [CrossRef] [PubMed]
- Babajani, A.; Iranbakhsh, A.; Ardebili, Z.O.; Eslami, B. Differential growth, nutrition, physiology, and gene expression in Melissa officinalis mediated by zinc oxide and elemental selenium nanoparticles. Environ. Sci. Pollut. Res. 2019, 26, 24430–24444. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Majumdar, S.; Servin, A.D.; Pagano, L.; Dhankher, O.P.; White, J.C. Carbon nanomaterials in agriculture: A critical review. Front. Plant Sci. 2016, 7, 172. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).