The Production of Oxalate by Aspergillus niger under Different Lead Concentrations
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Media
2.2. Strain Preparation
2.3. Experimental Design
2.4. Instrumentation
2.5. Enzyme Activity Determination
2.6. Data Analysis
3. Results
3.1. Fungal Dry Biomass and pH Value
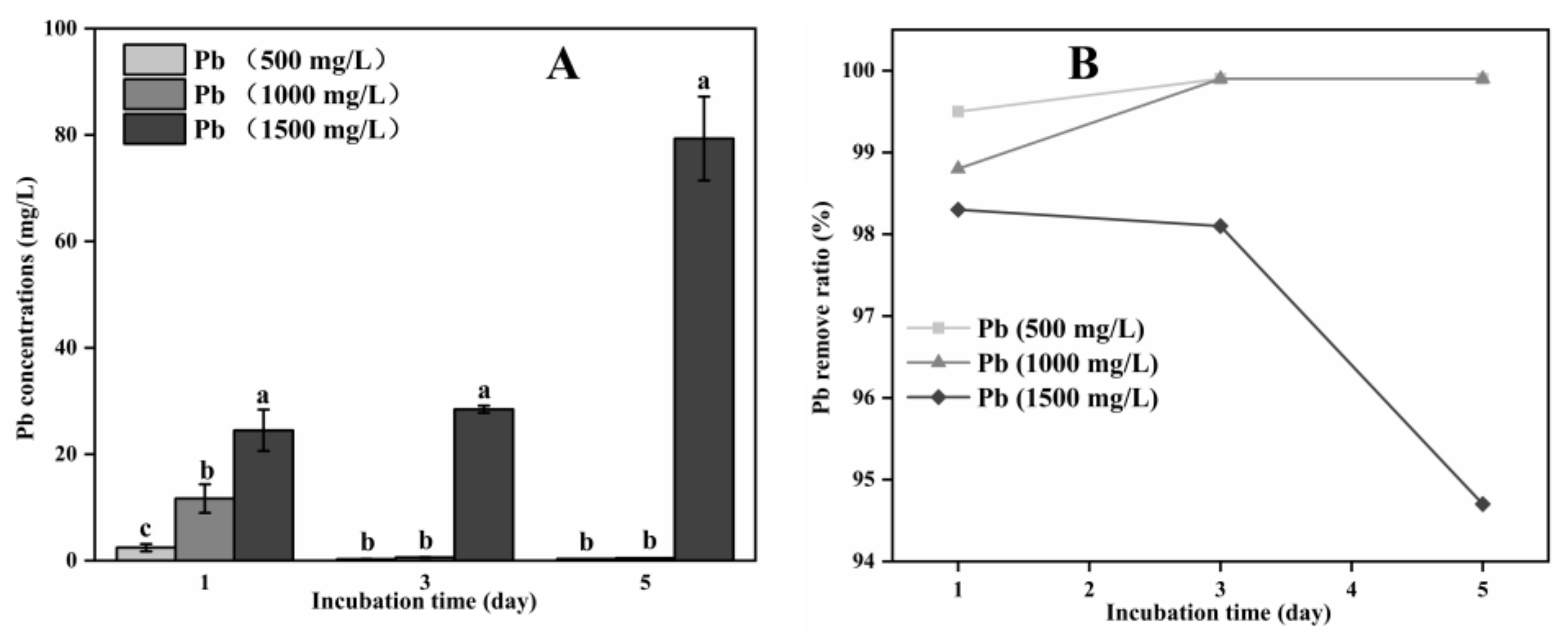
3.2. Organic Acid Production and Pb Remove by A. niger
3.3. TCA Cycle Enzyme Activity in A. niger
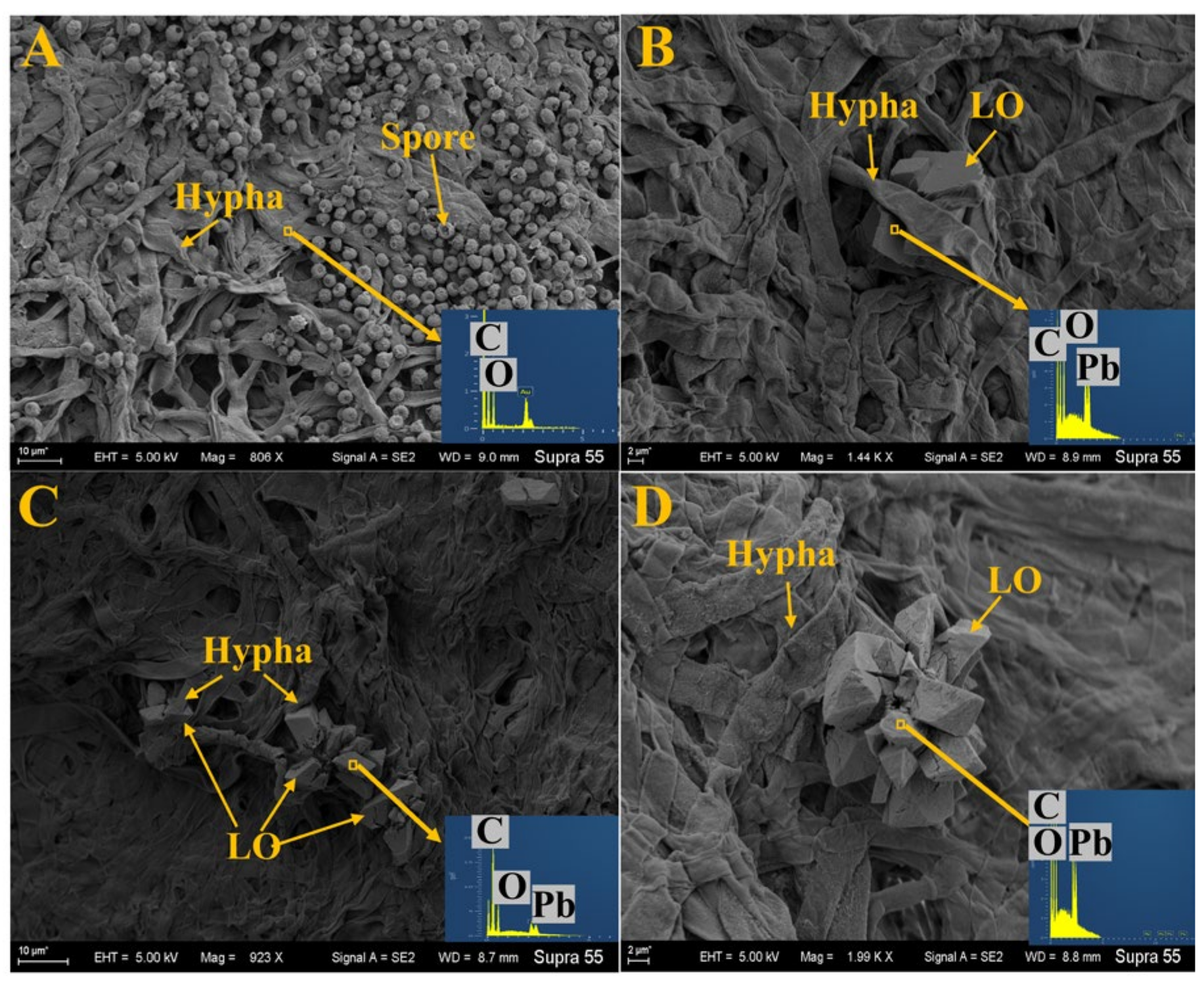
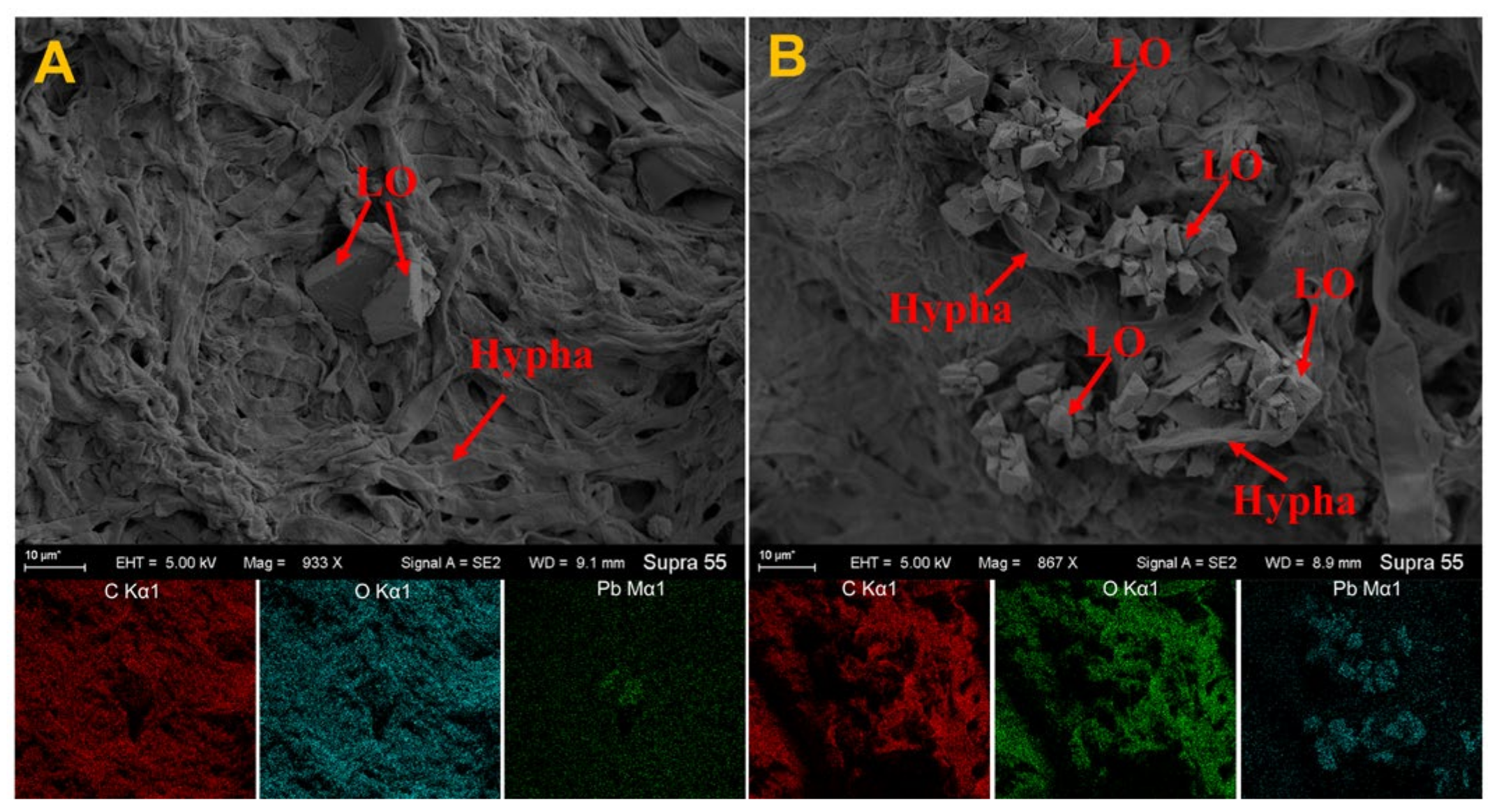
3.4. Analysis of SEM and EDS
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, G.M.; Wan, J.; Huang, D.L.; Hu, L.; Huang, C.; Cheng, M.; Jiang, D.N. Precipitation, adsorption and rhizosphere effect: The mechanisms for Phosphate-induced Pb immobilization in soils—A review. J. Hazard. Mater. 2017, 339, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Geomycology: Biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol. Res. 2007, 111, 3–49. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Ding, Z.; Li, G.; Wu, L.; Hu, P.; Guo, G.; Wang, X. A sustainability assessment framework for agricultural land remediation in China. Land Degrad. Dev. 2018, 29, 1005–1018. [Google Scholar] [CrossRef]
- Shen, Z.; Tian, D.; Zhang, X.; Tang, L.; Su, M.; Zhang, L.; Hou, D. Mechanisms of biochar assisted immobilization of Pb2+ by bioapatite in aqueous solution. Chemosphere 2018, 190, 260–266. [Google Scholar] [CrossRef]
- Tian, D.; Cheng, X.; Wang, L.; Hu, J.; Zhou, N.; Xia, J.; Zhang, C. Remediation of lead-contaminated water by red yeast and different types of phosphate. Front. Bioeng. Biotech. 2022, 10, 775058. [Google Scholar] [CrossRef]
- Gadd, G.M. The geomycology of elemental cycling and transformations in the environment. Microbiol. Spectr. 2017, 5, 1. [Google Scholar] [CrossRef]
- Hou, D.Y.; Qi, S.Q.; Zhao, B.; Rigby, M.; O’Connor, D. Incorporating life cycle assessment with health risk assessment to select the ‘greenest’ cleanup level for Pb contaminated soil. J. Clean. Prod. 2017, 162, 1157–1168. [Google Scholar] [CrossRef]
- Meng, L.; Pan, S.; Zhou, L.; Santasup, C.; Su, M.; Tian, D.; Li, Z. Evaluating the survival of Aspergillus niger in a highly polluted red soil with addition of phosphogypsum and bioorganic fertilizer. Environ. Sci. Pollut. R. 2022, 29, 76446–76455. [Google Scholar] [CrossRef]
- Guo, C.M.; Tian, W.T.; Wang, Z.J.; Han, F.Y.; Su, M.; Wu, Y.L.; Hu, S.J. Reduction of Pb availability during surficial leaching in different types of soils with addition of apatite and oxalic acid. J. Soils Sediment. 2019, 19, 741–749. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, L.; Li, X.; Wang, L.; Yusef, K.K.; Gao, H.; Tian, D. Remediation of lead contamination by Aspergillus niger and phosphate rocks under different nitrogen sources. Agronomy 2022, 12, 1639. [Google Scholar] [CrossRef]
- Li, Z.; Wang, F.W.; Bai, T.S.; Tao, J.J.; Guo, J.Y.; Yang, M.Y.; Hu, S.J. Lead immobilization by geological fluorapatite and fungus Aspergillus niger. J. Hazard. Mater. 2016, 320, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Viraraghavan, T.; Cullimore, D.R. Removal of heavy metals using the fungus Aspergillus niger. Bioresour. Technol. 1999, 70, 95–104. [Google Scholar] [CrossRef]
- Li, X.; Luo, L.; Yang, J.; Li, B.; Yuan, H. Mechanisms for solubilization of various insoluble phosphates and activation of immobilized phosphates in different soils by an efficient and salinity-tolerant Aspergillus niger strain An2. Appl. Biochem. Biotechnol. 2015, 175, 2755–2768. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Interactions of fungi with toxic metals. New Phytol. 1993, 124, 25–60. [Google Scholar] [CrossRef]
- Tian, D.; Wang, W.; Su, M.; Zheng, J.; Wu, Y.; Wang, S.; Hu, S. Remediation of lead-contaminated water by geological fluorapatite and fungus Penicillium oxalicum. Environ. Sci. Pollut. Res. 2018, 25, 21118–21126. [Google Scholar] [CrossRef]
- Tian, D.; Lai, Z.; Zou, X.; Guo, C.; Tang, L.; Su, M.; Hu, S. A contrast of lead immobilization via bioapatite under elevated CO2 between acidic and alkaline soils. Soil Use Manag. 2018, 34, 542–544. [Google Scholar] [CrossRef]
- Tian, D.; Li, Z.; O’Connor, D.; Shen, Z.; Hou, D. The need to prioritize sustainable phosphate-based fertilizers. Soil Use Manag. 2020, 36, 351–354. [Google Scholar] [CrossRef]
- Mendes, G.D.O.; Murta, H.M.; Valadares, R.V.; Silveira, W.B.; da Silva, I.R.; Costa, M.D. Oxalic acid is more efficient than sulfuric acid for rock phosphate solubilization. Miner. Eng. 2020, 155, 106458. [Google Scholar] [CrossRef]
- Tian, D.; Jiang, Z.; Jiang, L.; Su, M.; Feng, Z.; Zhang, L.; Hu, S. A new insight into lead (II) tolerance of environmental fungi based on a study of Aspergillus niger and Penicillium oxalicum. Environ. Microbiol. 2019, 21, 471–479. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Kirkes, L.; Westfall, T.; Roselle, R. Experimental determination of solubilities of lead oxalate (PbC2O4(cr)) in a NaCl medium to high ionic strengths, and the importance of lead oxalate in low temperature environments. Chem. Geol. 2013, 342, 128–137. [Google Scholar] [CrossRef]
- Palmieri, F.; Estoppey, A.; House, G.L.; Lohberger, A.; Bindschedler, S.; Chain, P.S.G.; Junier, P. Oxalic acid, a molecule at the crossroads of bacterial-fungal interactions. Adv. Appl. Microbiol. 2019, 106, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Wang, L.; Hu, J.; Zhang, L.; Zhou, N.; Xia, J.; Gao, H. A study of P release from Fe-P and Ca-P via the organic acids secreted by Aspergillus niger. J. Microbiol. 2021, 59, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, M.R.; Hildén, K.; Lundell, T.K. Oxalate decarboxylase: Biotechnological update and prevalence of the enzyme in filamentous fungi. Appl. Microbiol. Biot. 2010, 87, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.K.; Banerjee, P.C. Submerged production of oxalic acid from glucose by immobilized Aspergillus niger. Process. Biochem. 2005, 40, 1605–1610. [Google Scholar] [CrossRef]
- Gadd, G.M.; Bahri-Esfahani, J.; Li, Q.; Rhee, Y.J.; Wei, Z.; Fomina, M.; Liang, X. Oxalate production by fungi: Significance in geomycology, biodeterioration and bioremediation. Fungal. Biol. Rev. 2014, 28, 36–55. [Google Scholar] [CrossRef]
- Li, Z.; Bai, T.; Dai, L.; Wang, F.; Tao, J.; Meng, S.; Hu, S. A study of organic acid production in contrasts between two phosphate solubilizing fungi: Penicillium oxalicum and Aspergillus niger. Sci. Rep. 2016, 6, 25313. [Google Scholar] [CrossRef]
- Behera, B.C. Citric acid from Aspergillus niger: A comprehensive overview. Crit. Rev. Microbiol. 2020, 46, 727–749. [Google Scholar] [CrossRef]
- Dutton, M.V.; Evans, C.S. Oxalate production by fungi: Its role in pathogenicity and ecology in the soil environment. Can. J. Microbiol. 1996, 42, 881–895. [Google Scholar] [CrossRef]
- Mattevi, A.; Obmolova, G.; Schulze, E.; Kalk, K.H.; Westphal, A.H.; de Kok, A.; Hol, W.G. Atomic structure of the cubic core of the pyruvate dehydrogenase multienzyme complex. Science 1992, 255, 1544–1550. [Google Scholar] [CrossRef]
- Amary, M.F.; Bacsi, K.; Maggiani, F.; Damato, S.; Halai, D.; Berisha, F.; Flanagan, A.M. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J. Pathol. 2011, 224, 334–343. [Google Scholar] [CrossRef]
- Wang, L.; Guan, H.; Hu, J.; Feng, Y.; Li, X.; Yusef, K.K.; Tian, D. Aspergillus niger enhances organic and inorganic phosphorus release from wheat straw by secretion of degrading enzymes and oxalic acid. J. Agric. Food. Chem. 2022, 70, 10738–10746. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Xia, J.; Zhou, N.; Xu, M.; Li, X.; Zhang, L.; Gao, H. The utilization of phosphogypsum as a sustainable phosphate-based fertilizer by Aspergillus niger. Agronomy 2022, 12, 646. [Google Scholar] [CrossRef]
- Geng, Y.; Pan, S.; Zhang, L.; Qiu, J.; He, K.; Gao, H.; Tian, D. Phosphorus biogeochemistry regulated by carbonates in soil. Environ. Res. 2022, 214, 113894. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.Y.; Traina, S.J.; Logan, T.J.; Ryan, J.A. In-situ lead immobilization by apatite. Environ. Sci. Technol. 1993, 27, 1803–1810. [Google Scholar] [CrossRef]
- Ruby, M.V.; Davis, A.; Nicholson, A. In-situ formation of lead phosphates in soils as a method to immobilize lead. Environ. Sci. Technol. 1994, 28, 646–654. [Google Scholar] [CrossRef]
- Liang, X.J.; Kierans, M.; Ceci, A.; Hillier, S.; Gadd, G.M. Phosphatase-mediated bioprecipitation of lead by soil fungi. Environ. Microbiol. 2016, 18, 219–231. [Google Scholar] [CrossRef]
- Purchase, D.; Scholes, L.N.L.; Revitt, D.M.; Shutes, R.B.E. Effects of temperature on metal tolerance and the accumulation of Zn and Pb by metal-tolerant fungi isolated from urban runoff treatment wetlands. J. Appl. Microbiol. 2009, 106, 1163–1174. [Google Scholar] [CrossRef]
- Fomina, M.; Hillier, S.; Charnock, J.M.; Melville, K.; Alexander, I.J.; Gadd, G.M. Role of oxalic acid overexcretion in transformations of toxic metal minerals by Beauveria caledonica. Appl. Environ. Microb. 2005, 71, 371. [Google Scholar] [CrossRef]
- Su, M.; Meng, L.; Zhao, L.; Tang, Y.; Qiu, J.; Tian, D.; Li, Z. Phosphorus deficiency in soils with red color: Insights from the interactions between minerals and microorganisms. Geoderma 2021, 404, 115311. [Google Scholar] [CrossRef]
- Tian, D.; Su, M.; Zou, X.; Zhang, L.; Tang, L.; Geng, Y.; Li, Z. Influences of phosphate addition on fungal weathering of carbonate in the red soil from karst region. Sci. Total Environ. 2021, 755, 142570. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Li, S.; Tang, L.; Chen, T.; Gu, T.; Chen, G.; Gadd, G.M.; Li, Z. A contrast of Pb(II), Cd(II), and Cu(II) toxicities to Aspergillus niger through biochemical, morphological, and genetic investigations. J. Hazard. Mater. 2023, 446, 130691. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Hattori, T.; Honda, Y.; Kirimura, K. Oxalic acid production by citric acid-producing Aspergillus niger overexpressing the oxaloacetate hydrolase gene oahA. J. Ind. Microbiol. Biot. 2014, 41, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Barrington, S.; Sheppard, J.; Lee, B. Nutrient optimization for the production of citric acid by Aspergillus niger NRRL 567 grown on peat moss enriched with glucose. Process. Biochem. 2006, 41, 1253–1260. [Google Scholar] [CrossRef]
- Knox, K. Le Châtelier’s Principle. J. Chem. Educ. 1985, 62, 863. [Google Scholar] [CrossRef]
- Gautam, A.K.; Bhadauria, R. Diversity of fungi and mycotoxins associated with stored triphala churn and its ingredients. J. Biol. Sci. 2011, 11, 226–235. [Google Scholar] [CrossRef]
- Susca, A.; Proctor, R.H.; Morelli, M.; Haidukowski, M.; Gallo, A.; Logrieco, A.F.; Moretti, A. Variation in fumonisin and ochratoxin production associated with differences in biosynthetic gene content in Aspergillus niger and A. welwitschiae isolates from multiple crop and geographic origins. Front. Microbiol. 2016, 7, 1412. [Google Scholar] [CrossRef]
- Han, X.; Jiang, H.; Li, F. Dynamic ochratoxin A production by strains of Aspergillus niger intended used in food industry of china. Toxins 2019, 11, 122. [Google Scholar] [CrossRef]
- Fiema, M.; Wlodarczyk, A.; Wojkowska-Mach, J.; Garlicki, J.; Gregorczyk-Maga, I. Atypical presentation of Aspergillus niger infection in the oral cavity as a prediction of invasive pulmonary Aspergillosis in a patient with COVID-19: Case report and literature review. Microorganisms 2022, 10, 1630. [Google Scholar] [CrossRef]


| Incubation Time (day) | Organic Acid (mg/L) | Pb Concentration (mg/L) | |||
|---|---|---|---|---|---|
| 0 | 500 | 1000 | 1500 | ||
| One day | Oxalic acid | 73.9 ± 5.4 c | 1101.7 ± 22.6 a | 959.9 ± 163.6 ab | 753.2 ± 30.7 b |
| Tartaric acid | 2.51 ± 0.2 c | 117.9 ± 11.4 b | 251.2 ± 44.7 a | 193.1 ± 10.5 a | |
| Formic acid | 63.9 ± 7.2 b | 214.1 ± 14.3 a | N.A. | N.A. | |
| Malic acid | N.A. | 33.5 ± 2.7 a | 32.9 ± 4.6 a | 25.4 ± 3.8 b | |
| Citric acid | 365.4 ± 33.7 a | 205.4 ± 12.4 b | 79.2 ± 4.6 c | 40.2 ± 4.8 d | |
| Three days | Oxalic acid | 1147.1 ± 56.8 a | 1130.5 ± 33.7 a | 1059.1 ± 35.5 a | 705.1 ± 163.6 b |
| Tartaric acid | N.A. | 53.6 ± 2.7 a | N.A. | N.A. | |
| Formic acid | 19.4 ± 1.9 c | 14.1 ± 0.9 d | 71 ± 5.9 b | 522.8 ± 36.6 a | |
| Malic acid | N.A. | 71.2 ± 9.6 b | 283.4 ± 16.5 a | 66.4 ± 7.9 b | |
| Citric acid | 233 ± 1.33 a | 159.7 ± 5.5 b | 65.2 ± 7.8 c | 14.5 ± 1.4 d | |
| Five days | Oxalic acid | 1133.9 ± 67.9 a | 1002.3 ± 31.9 b | 1024.3 ± 165.3 ab | 632.5 ± 56.6 c |
| Tartaric acid | N.A. | N.A. | N.A. | N.A. | |
| Formic acid | N.A. | N.A. | N.A. | 591.2 ± 22.2 a | |
| Malic acid | N.A. | N.A. | N.A. | 143.4 ± 15.7 a | |
| Citric acid | N.A. | N.A. | N.A. | 182.4 ± 11.8 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Zhang, L.; Yuan, S.; Liu, W.; Zhang, C.; Tian, D.; Ye, X. The Production of Oxalate by Aspergillus niger under Different Lead Concentrations. Agronomy 2023, 13, 1182. https://doi.org/10.3390/agronomy13041182
Huang Y, Zhang L, Yuan S, Liu W, Zhang C, Tian D, Ye X. The Production of Oxalate by Aspergillus niger under Different Lead Concentrations. Agronomy. 2023; 13(4):1182. https://doi.org/10.3390/agronomy13041182
Chicago/Turabian StyleHuang, Yijun, Liangliang Zhang, Shijia Yuan, Wenpei Liu, Chaochun Zhang, Da Tian, and Xinxin Ye. 2023. "The Production of Oxalate by Aspergillus niger under Different Lead Concentrations" Agronomy 13, no. 4: 1182. https://doi.org/10.3390/agronomy13041182
APA StyleHuang, Y., Zhang, L., Yuan, S., Liu, W., Zhang, C., Tian, D., & Ye, X. (2023). The Production of Oxalate by Aspergillus niger under Different Lead Concentrations. Agronomy, 13(4), 1182. https://doi.org/10.3390/agronomy13041182








