Comparison of Biochar- and Lime-Adjusted pH Changes in N2O Emissions and Associated Microbial Communities in a Tropical Tea Plantation Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Biochar Preparation
2.2. Microcosm Incubation Experiment
2.3. Nucleic Acid Extraction and Quantification of Functional Gene Abundance
2.4. Amplicon Sequencing of Fungal Denitrifier nirK and Bioinformatics Analysis
2.5. Statistical Analysis
3. Results
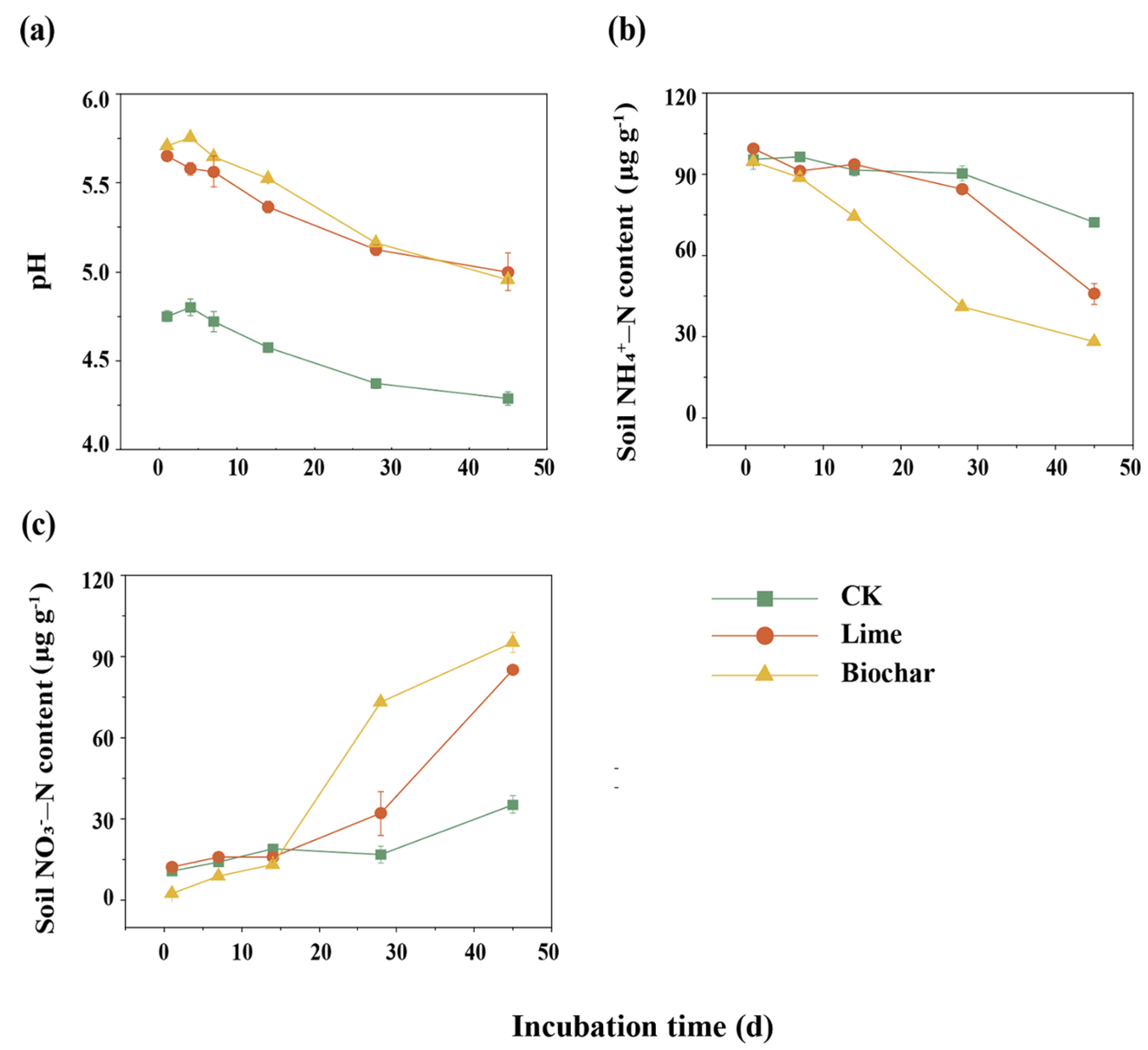
3.1. Soil pH and Dynamics of Mineral N Concentrations
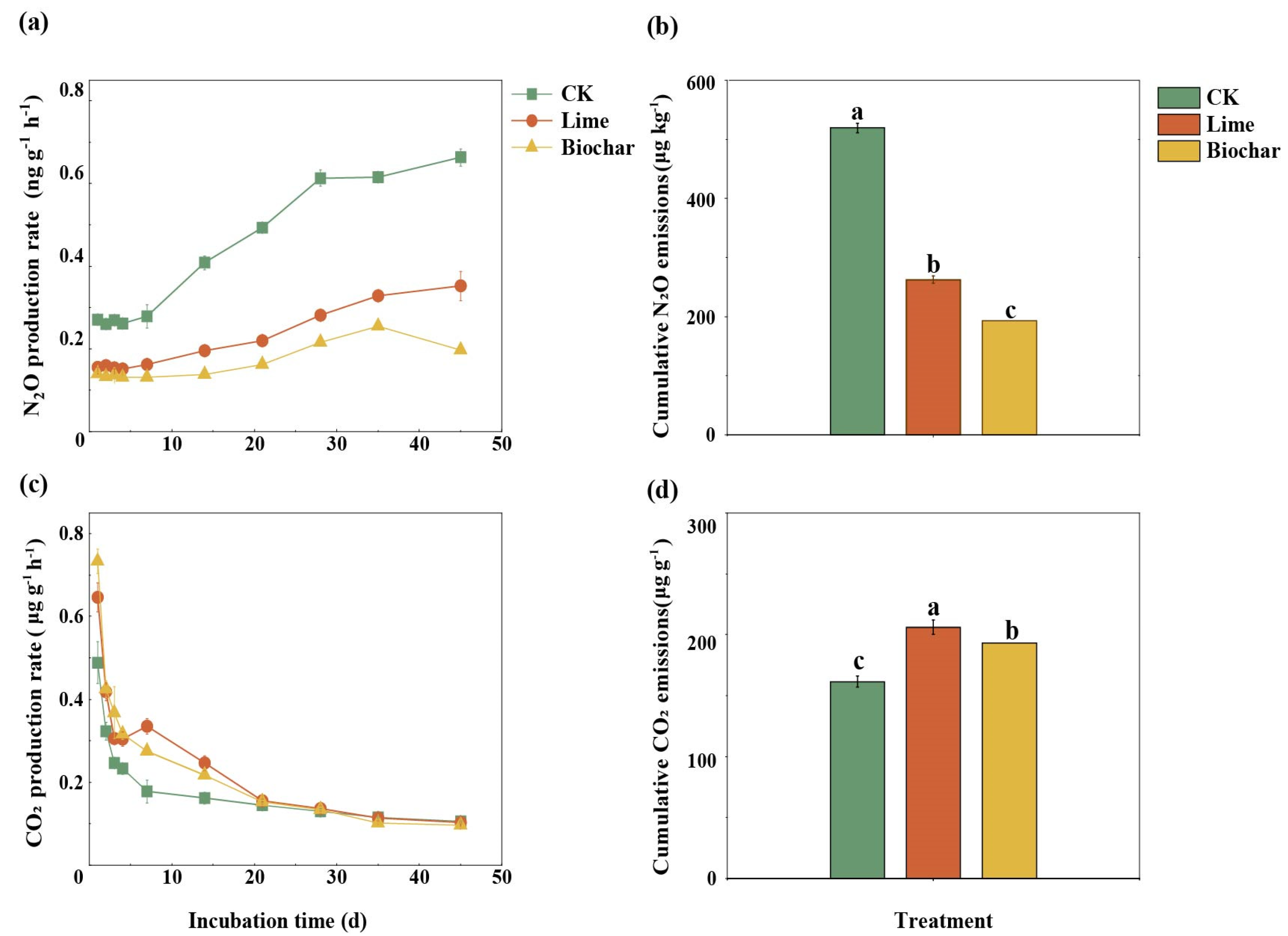
3.2. Soil N2O and CO2 Emissions
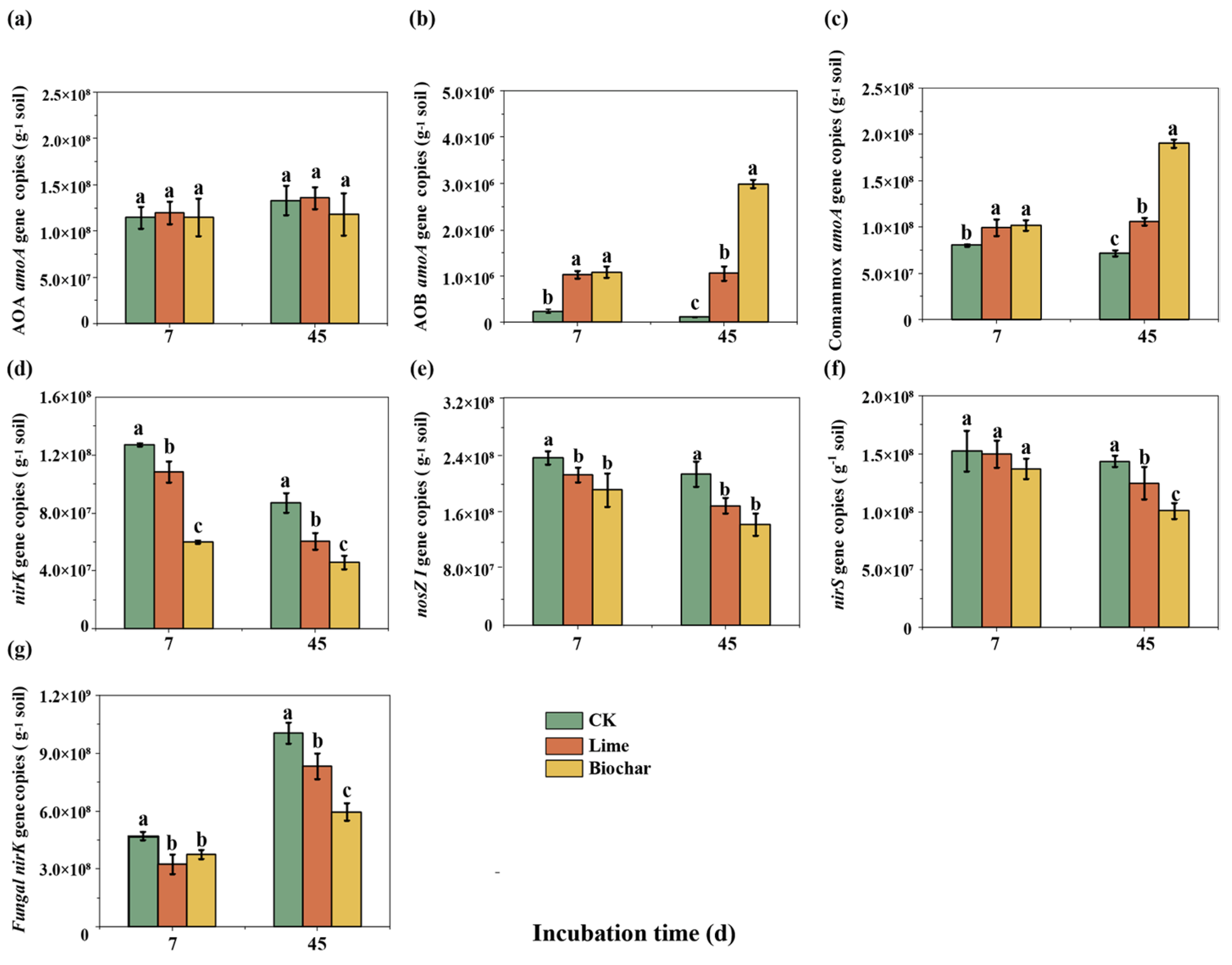
3.3. Abundances of Ammonia-Oxidizers, Bacterial Denitrifiers and Fungal Denitrifiers
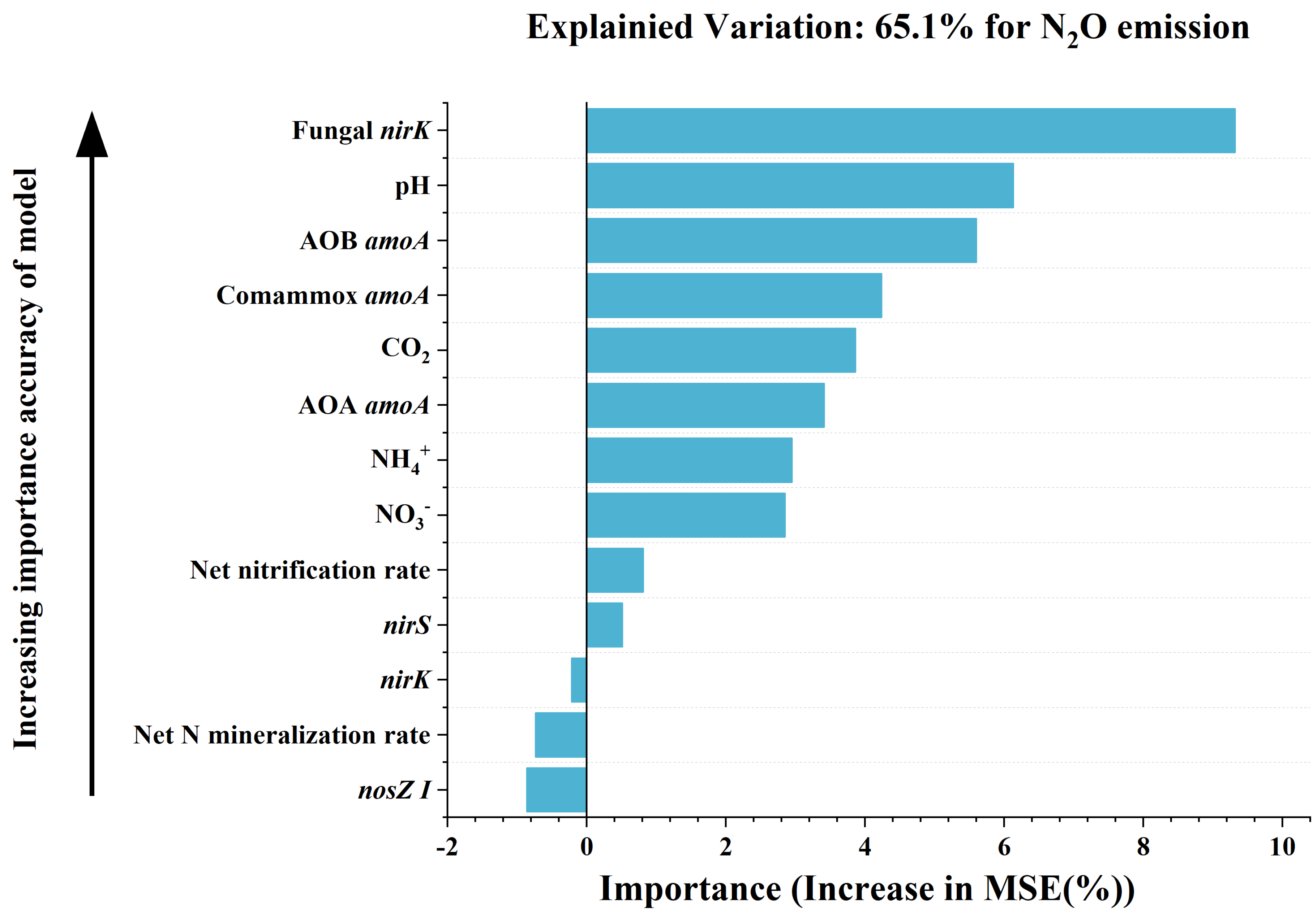
3.4. Correlations between Soil Chemical Properties, the Abundances of N-Cycling Genes and N2O Emissions
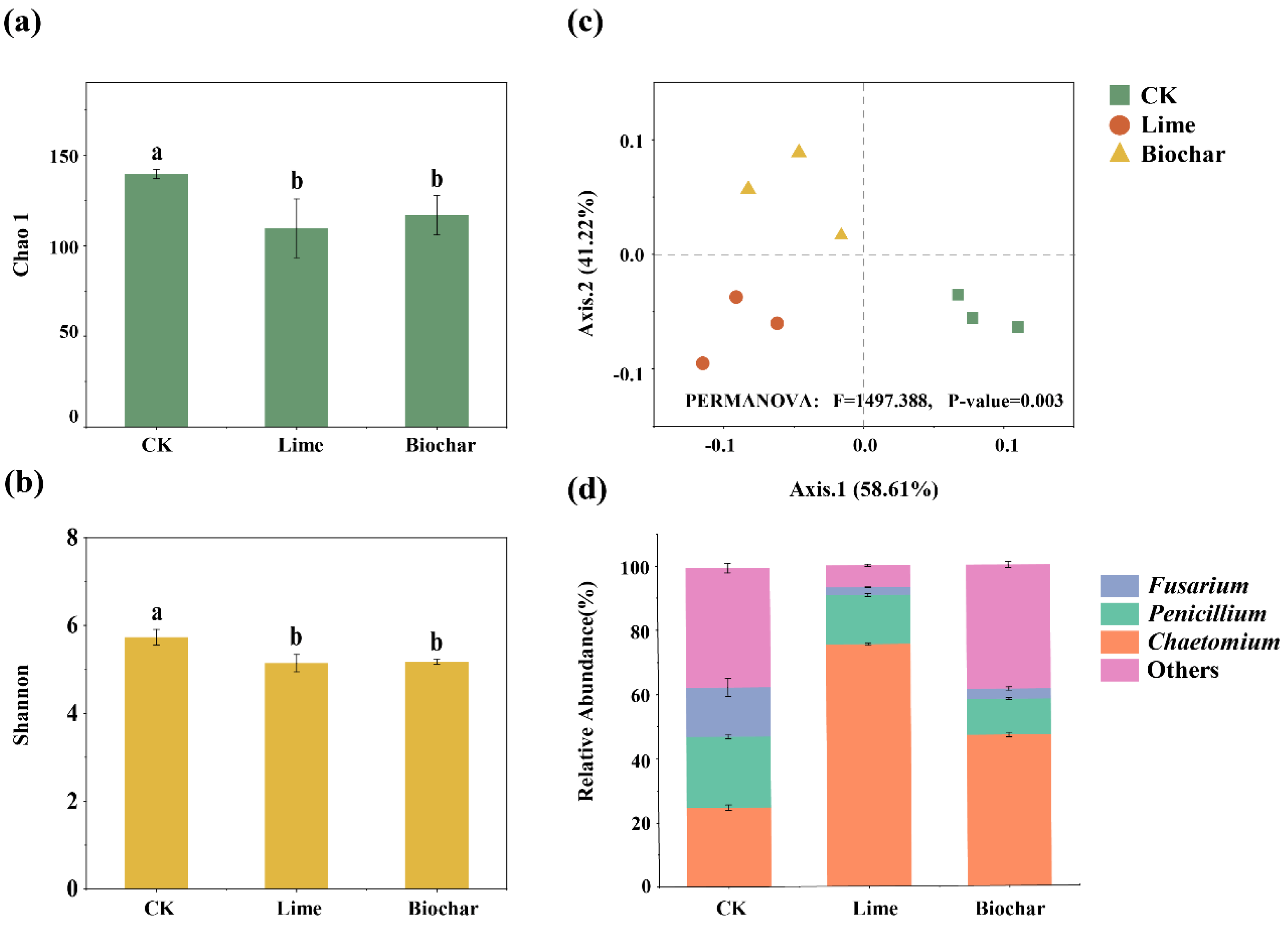
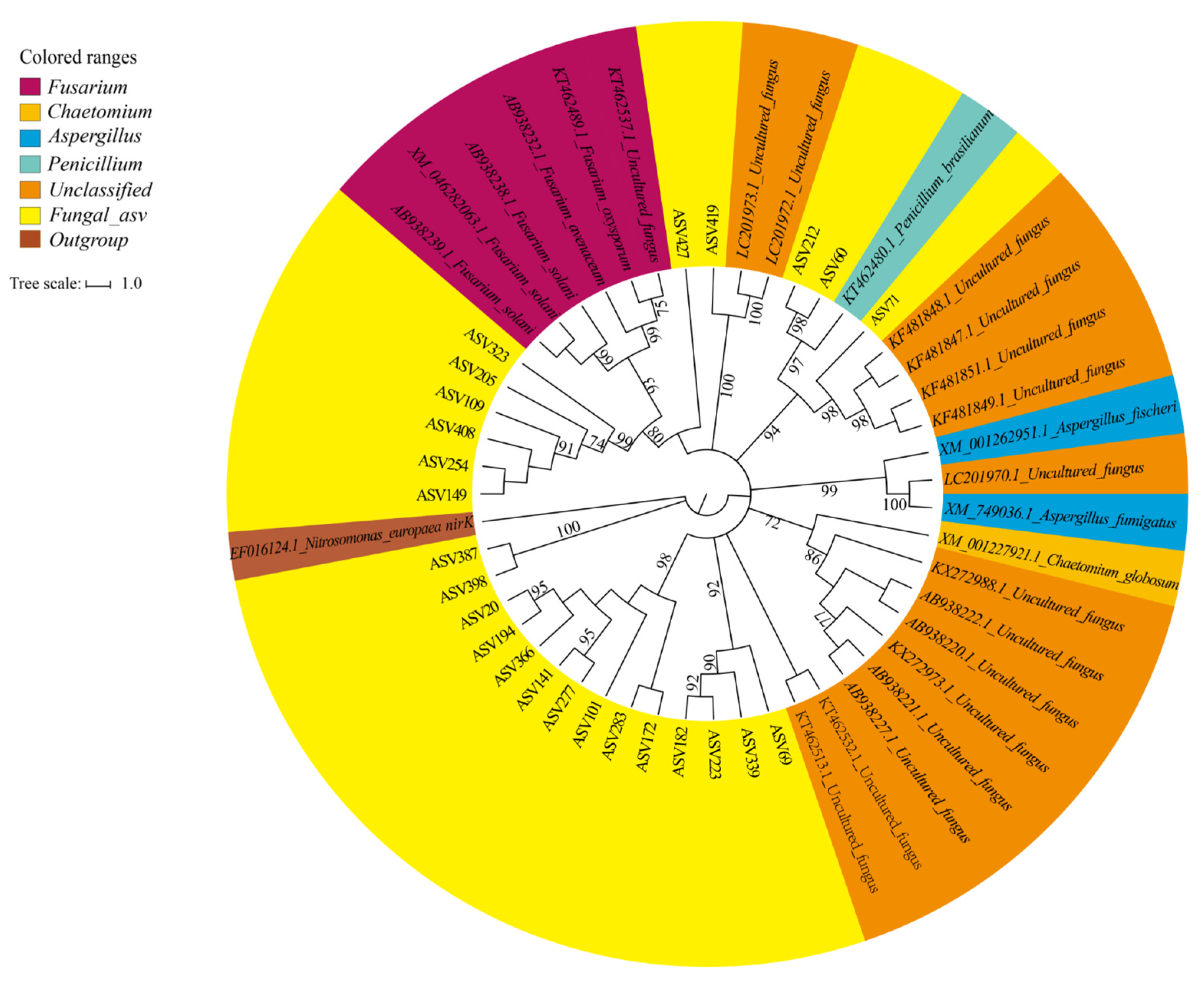
3.5. Diversity and Composition of Fungal Denitrifiers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yang, J.; Xu, R.; Lu, C.; Canadell, J.G.; Davidson, E.A.; Jackson, R.B.; Arneth, A.; Chang, J.; Ciais, P.; et al. Global soil nitrous oxide emissions since the preindustrial era estimated by an ensemble of terrestrial biosphere models: Magnitude, attribution, and uncertainty. Glob. Chang. Biol. 2019, 25, 640–659. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, B.; Tian, Y.; Zhang, H.; Cheng, Y.; Zhang, J. A soil management strategy for ameliorating soil acidification and reducing nitrification in tea plantations. Eur. J. Soil Biol. 2018, 88, 36–40. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Wang, H.; Wang, Z.; Fu, X.; Shen, J.; Wang, Y.; Liu, X.; Meng, L.; Wu, J. Response of N2O emissions to biochar amendment on a tea field soil in subtropical central China: A three-year field experiment. Agric. Ecosyst. Environ. 2021, 318, 107473. [Google Scholar] [CrossRef]
- Liu, S.; Lin, F.; Wu, S.; Ji, C.; Sun, Y.; Jin, Y.; Li, S.; Li, Z.; Zou, J. A meta-analysis of fertilizer-induced soil NO and combined NO+N2O emissions. Glob. Chang. Biol. 2017, 23, 2520–2532. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? (Review). Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palat-inszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete nitrification by Nitrospira bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef]
- Prosser, J.I.; Hink, L.; Gubry-Rangin, C.; Nicol, G.W. Nitrous oxide production by ammonia oxidizers: Physiological diversity, niche differentiation and potential mitigation strategies. Glob. Chang. Biol. 2020, 26, 103–118. [Google Scholar] [CrossRef]
- Shaw, L.J.; Nicol, G.W.; Smith, Z.; Fear, J.; Prosser, J.I.; Baggs, E.M. Nitrosospira spp. can produce nitrous oxide via a nitrifier denitrification pathway. Environ. Microbiol. 2006, 8, 214–222. [Google Scholar] [CrossRef]
- Hink, L.; Nicol, G.W.; Prosser, J.I. Archaea produce lower yields of N2O than bacteria during aerobic ammonia oxidation in soil. Environ. Microbiol. 2017, 19, 4829–4837. [Google Scholar] [CrossRef]
- Kits, K.D.; Jung, M.Y.; Vierheilig, J.; Pjevac, P.; Sedlacek, C.J.; Liu, S.; Herbold, C.; Stein, L.Y.; Richter, A.; Wissel, H.; et al. Low yield and abiotic origin of N2O formed by the complete nitrifier Nitrospira inopinata. Nat. Commun. 2019, 10, 1836. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.W.; Chen, D.L.; He, J.Z. Microbial regulation of terrestrial nitrous oxide formation: Understanding the biological pathways for prediction of emission rates. FEMS Microbiol. Rev. 2015, 39, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.E.; Boehm, A.B.; Francis, C.A. Denitrifier community composition along a nitrate and salinity gradient in a coastal aquifer. Appl. Environ. Microbiol. 2006, 72, 2102–2109. [Google Scholar] [CrossRef]
- Shoun, H.; Fushinobu, S.; Jiang, L.; Kim, S.W.; Wakagi, T. Fungal denitrification and nitric oxide reductase cytochrome P450nor. Phil. Trans. R. Soc. B Biol. Sci. 2012, 367, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Isobe, K.; Shiratori, Y.; Nishizawa, T.; Ohte, N.; Otsuka, S.; Senoo, K. N2O emission from cropland field soil through fungal denitrification after surface applications of organic fertilizer. Soil Biol. Biochem. 2014, 69, 157–167. [Google Scholar] [CrossRef]
- Wankel, S.D.; Ziebis, W.; Buchwald, C.; Charoenpong, C.; Beer, D.; Dentinger, J.; Xu, Z.; Zengler, K. Evidence for fungal and chemodenitrification based N2O flux from nitrogen impacted coastal sediments. Nat. Commun. 2017, 8, 15595. [Google Scholar] [CrossRef]
- Lourenço, K.S.; Costa, O.Y.A.; Cantarella, H.; Kuramae, E.E. Ammonia-oxidizing bacteria and fungal denitrifier diversity are associated with N2O production in tropical soils. Soil Biol. Biochem. 2022, 166, 108563. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, S.; Xu, X.; Wang, Y.; Rui, Y.; Zhou, X.; Shen, Q.; Wang, J.; Jiang, L.; Luo, C.; et al. Fungi regulate the response of the N2O production process to warming and grazing in a Tibetan grassland. Biogeosciences 2018, 15, 4447–4457. [Google Scholar] [CrossRef]
- Zheng, N.; Yu, Y.; Wang, J.; Chapman, S.J.; Yao, H.; Zhang, Y. The conversion of subtropical forest to tea plantation changes the fungal community and the contribution of fungi to N2O production. Environ. Pollut. 2020, 265, 115106. [Google Scholar] [CrossRef]
- Baggs, E.M.; Smales, C.L.; Bateman, E.J. Changing pH shifts the microbial source as well as the magnitude of N2O emission from soil. Biol. Fertil. Soils 2010, 46, 793–805. [Google Scholar] [CrossRef]
- Blum, J.M.; Su, Q.X.; Ma, Y.J.; Valverde-Perez, B.; Domingo-Felez, C.; Jensen, M.M.; Smets, B.F. The pH dependency of N-converting enzymatic processes, pathways and microbes: Effect on net N2O production. Environ. Microbiol. 2018, 20, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Mothapo, N.V.; Shi, W. Soil moisture and pH control relative contributions of fungi and bacteria to N2O production. Microb. Ecol. 2015, 69, 180–191. [Google Scholar] [CrossRef]
- Wang, J.; Cui, W.; Che, Z.; Liang, F.; Wen, Y.; Zhan, M.; Dong, X.; Jin, W.; Dong, Z.; Song, H. Effects of synthetic nitrogen fertilizer and manure on fungal and bacterial contributions to N2O production along a soil acidity gradient. Sci. Total Environ. 2021, 753, 142011. [Google Scholar] [CrossRef]
- Dai, Z.M.; Zhang, X.; Tang, C.; Muhammad, N.; Wu, J.; Brookes, P.C.; Xu, J.M. Potential role of biochars in decreasing soil acidification—A critical review. Sci. Total Environ. 2017, 581, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.; Wu, Y.; Khalid, M.S.; Peng, Q.A.; Xu, X.Y.; Wu, L.; Younas, A.; Bashir, S.; Mo, Y.L.; Lin, S.; et al. Reduction in soil N2O emissions by pH manipulation and enhanced nosZ gene transcription under different water regimes. Environ. Pollut. 2018, 235, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Li, S.; Geng, Y.; Yuan, Y.; Zhi, J.; Yu, K.; Han, Z.; Wu, S.; Liu, S.; Zou, J. Decreased N2O and NO emissions associated with stimulated denitrification following biochar amendment in subtropical tea plantations. Geoderma 2020, 365, 114223. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, J.; Almoy, T.; Bakken, L.R. Excessive use of nitrogen in Chinese agriculture results in high N2O/(N2O+N2) product ratio of denitrification, primarily due to acidification of the soils. Glob. Chang. Biol. 2014, 20, 1685–1698. [Google Scholar] [CrossRef]
- Jha, N.; Palmada, T.; Berben, P.; Saggar, S.; Luo, J.; McMillan, A.M.S. Influence of liming-induced pH changes on nitrous oxide emission, nirS, nirK and nosZ gene abundance from applied cattle urine in allophanic and fluvial grazed pasture soils. Biol. Fertil. Soils 2020, 56, 811–824. [Google Scholar] [CrossRef]
- Nicol, G.W.; Leininger, S.; Schleper, C.; Prosser, J.I. The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ. Microbiol. 2008, 10, 2966–2978. [Google Scholar] [CrossRef]
- Cuhel, J.; Simek, M.; Laughlin, R.J.; Bru, D.; Cheneby, D.; Watson, C.J.; Philippot, L. Insights into the effect of soil pH on N2O and N2 emissions and denitrifier community size and activity. Appl. Environ. Microbiol. 2010, 76, 1870–1878. [Google Scholar] [CrossRef]
- Che, J.; Zhao, X.Q.; Zhou, X.; Jia, Z.J.; Shen, R.F. High pH-enhanced soil nitrification was associated with ammonia-oxidizing bacteria rather than archaea in acidic soils. Appl. Soil Ecol. 2015, 85, 21–29. [Google Scholar] [CrossRef]
- Zhang, M.M.; Alves, R.J.E.; Zhang, D.D.; Han, L.L.; He, J.Z.; Zhang, L.M. Time-dependent shifts in populations and activity of bacterial and archaeal ammonia oxidizers in response to liming in acidic soils. Soil Biol. Biochem. 2017, 112, 77–89. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, H.; Muhammad, A.; Huang, G. Emission mechanism and reduction counter measures of agricultural greenhouse gases—A review. Greenh. Gas. Sci. Technol. 2019, 9, 160–174. [Google Scholar] [CrossRef]
- Harter, J.; Krause, H.M.; Schuettler, S.; Ruser, R.; Fromme, M.; Scholten, T.; Kappler, A.; Behrens, S. Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. ISME J. 2014, 8, 660–674. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yin, C.; Fan, X.; Ye, M.; Peng, H.; Li, T.; Zhao, Y.; Wakelin, S.A.; Chu, G.; Liang, Y. Reduction of N2O emission by biochar and/or 3,4-dimethylpyrazole phosphate (DMPP) is closely linked to soil ammonia oxidizing bacteria and nosZI-N2O reducer populations. Sci. Total Environ. 2019, 694, 133658. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ding, W.; Liu, D.; He, T.; Yoo, G.; Yuan, J.; Chen, Z.; Fan, J. Wheat straw-derived biochar amendment stimulated N2O emissions from rice paddy soils by regulating the amoA genes of ammonia-oxidizing bacteria. Soil Biol. Biochem. 2017, 113, 89–98. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sanchez-Monedero, M.A. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Aamer, M.; Shaaban, M.; Hassan, M.U.; Huang, G.; Liu, Y.; Tang, H.Y.; Rasul, F.; Ma, Q.; Li, Z.; Rasheed, A.; et al. Biochar mitigates the N2O emissions from acidic soil by increasing the nosZ and nirK gene abundance and soil pH. J. Environ. Manag. 2020, 255, 109891. [Google Scholar] [CrossRef]
- Sánchez-García, M.; Roig, A.; Sánchez-Monedero, M.; Cayuela, M.L. Biochar increases N2O emissions produced by nitrification-mediated pathways. Front. Environ. Sci. 2014, 2, 25. [Google Scholar]
- Cheng, Y.; Zhang, H.; Chen, Z.; Wang, J.; Cai, Z.; Sun, N.; Wang, S.; Zhang, J.B.; Chang, S.X.; Xu, M.; et al. Contrasting effects of different pH-raising materials on N2O emissions in acidic upland soils. Eur. J. Soil Sci. 2021, 72, 432–445. [Google Scholar] [CrossRef]
- Higgins, S.; Laughlin, R.J.; Watson, C.J. Antecedent effect of lime on nitrous oxide and dinitrogen emissions from grassland soils. Nutr. Cycl. Agroecosyst. 2013, 95, 219–229. [Google Scholar] [CrossRef]
- Page, K.L.; Allen, D.E.; Dalal, R.C.; Slattery, W. Processes and magnitude of CO2, CH4, and N2O fluxes from liming of Australian acidic soils: A review. Aust. J. Soil Res. 2009, 47, 747–762. [Google Scholar] [CrossRef]
- Xu, H.J.; Wang, X.H.; Li, H.; Yao, H.Y.; Su, J.Q.; Caporaso, Y.G. Biochar impacts soil microbial community composition and nitrogen cycling in an acidic soil planted with rape. Environ. Sci. Technol. 2014, 48, 9391–9399. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Cai, Y.; Fu, S.; Luo, Y.; Wang, H.; Liang, C.; Lin, Z.; Hu, S.; Li, Y.; et al. Biochar decreases soil N2O emissions in Moso bamboo plantations through decreasing labile N concentrations, N-cycling enzyme activities and nitrification/denitrification rates. Geoderma 2019, 348, 135–145. [Google Scholar] [CrossRef]
- Li, S.; Chen, D.W.; Wang, C.; Chen, D.; Wang, Q. Reduced nitrification by biochar and/or nitrification inhibitor is closely linked with the abundance of comammox Nitrospira in a highly acidic sugarcane soil. Biol. Fertil. Soils 2020, 56, 1219–1228. [Google Scholar] [CrossRef]
- Mothapo, N.V.; Chen, H.; Cubeta, M.A.; Shi, W. Nitrous oxide producing activity of diverse fungi from distinct agroecosystems. Soil Biol. Biochem. 2013, 66, 94–101. [Google Scholar] [CrossRef]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef]
- Tourna, M.; Freitag, T.E.; Nicol, G.W.; Prosser, J.I. Growth, activity and temperature responses of ammonia-oxidizing archaea and bacteria in soil microcosms. Environ. Microbiol. 2008, 10, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.J.; Palomo, A.; Dechesne, A.; Mines, P.D.; Smets, B.F. Comammox Nitrospira are abundant ammonia oxidizers in diverse groundwater-fed rapid sand filter communities. Environ. Microbiol. 2018, 20, 1002–1015. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.; Baudoin, E.; López-Gutiérrez, J.C.; Martin-Laurent, F.; Brauman, A.; Philippot, L. Quantification of denitrifying bacteria in soils by nirK gene targeted real-time PCR. J. Microbiol. Methods 2004, 59, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Throbäck, I.N.; Enwall, K.; Jarvis, A.; Hallin, H. Reassesing PCR primers targeting nirS, nirK and nosZ genes for community surveys of ammonia oxidizer bacteria with DGGE. FEMS Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.; Bru, D.; Stres, B.; Hallet, S.; Philippot, L. Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl. Environ. Microbiol. 2006, 72, 5181–5189. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Liu, S.; Ruan, Y.; Wang, Q.; Zhang, Z. Comparison of Biochar- and Lime-Adjusted pH Changes in N2O Emissions and Associated Microbial Communities in a Tropical Tea Plantation Soil. Agronomy 2023, 13, 1144. https://doi.org/10.3390/agronomy13041144
Wang Z, Liu S, Ruan Y, Wang Q, Zhang Z. Comparison of Biochar- and Lime-Adjusted pH Changes in N2O Emissions and Associated Microbial Communities in a Tropical Tea Plantation Soil. Agronomy. 2023; 13(4):1144. https://doi.org/10.3390/agronomy13041144
Chicago/Turabian StyleWang, Ziwei, Shuoran Liu, Yunze Ruan, Qing Wang, and Zhijun Zhang. 2023. "Comparison of Biochar- and Lime-Adjusted pH Changes in N2O Emissions and Associated Microbial Communities in a Tropical Tea Plantation Soil" Agronomy 13, no. 4: 1144. https://doi.org/10.3390/agronomy13041144
APA StyleWang, Z., Liu, S., Ruan, Y., Wang, Q., & Zhang, Z. (2023). Comparison of Biochar- and Lime-Adjusted pH Changes in N2O Emissions and Associated Microbial Communities in a Tropical Tea Plantation Soil. Agronomy, 13(4), 1144. https://doi.org/10.3390/agronomy13041144





