Impact of Biomass Recycling and Fertilization on Soil Microbiological Characteristics and Wheat Productivity in Semi-Arid Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design and Treatments
2.3. Soil Sampling
2.4. Microbiological Analyses
2.5. Statistical Analysis
3. Results
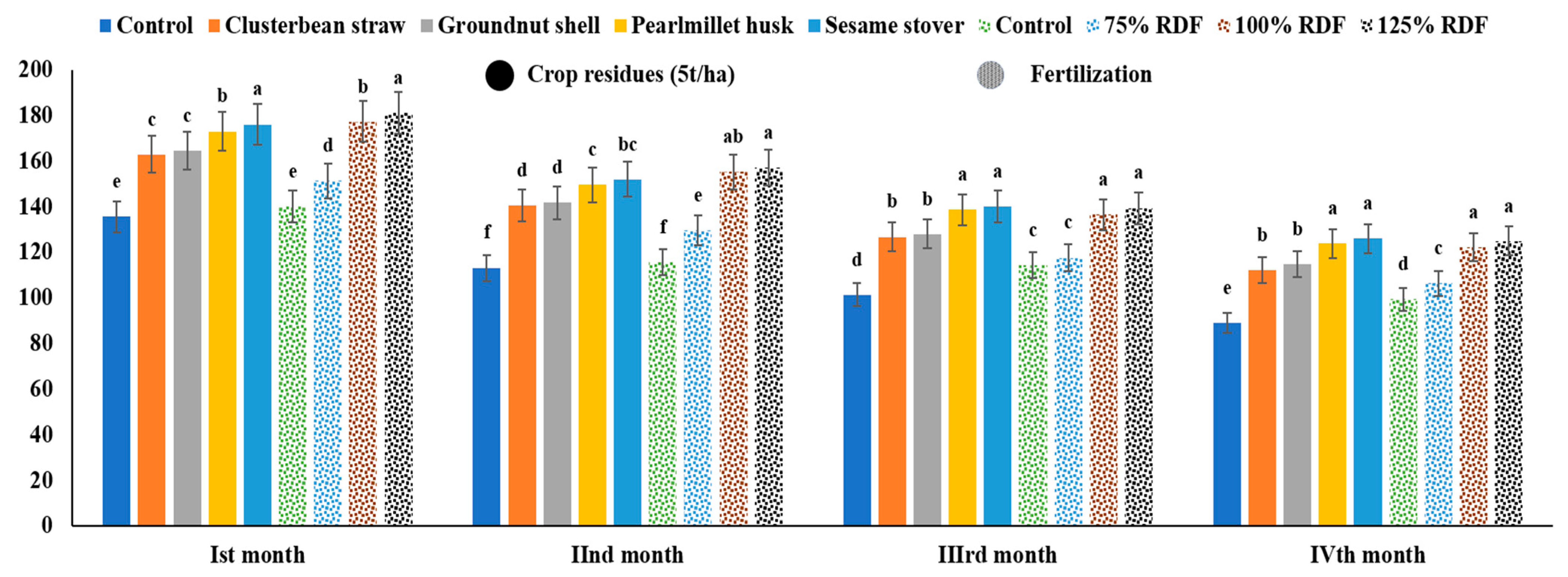
3.1. Soil Microbial Biomass C, Microbial Biomass N, and Microbial Biomass P
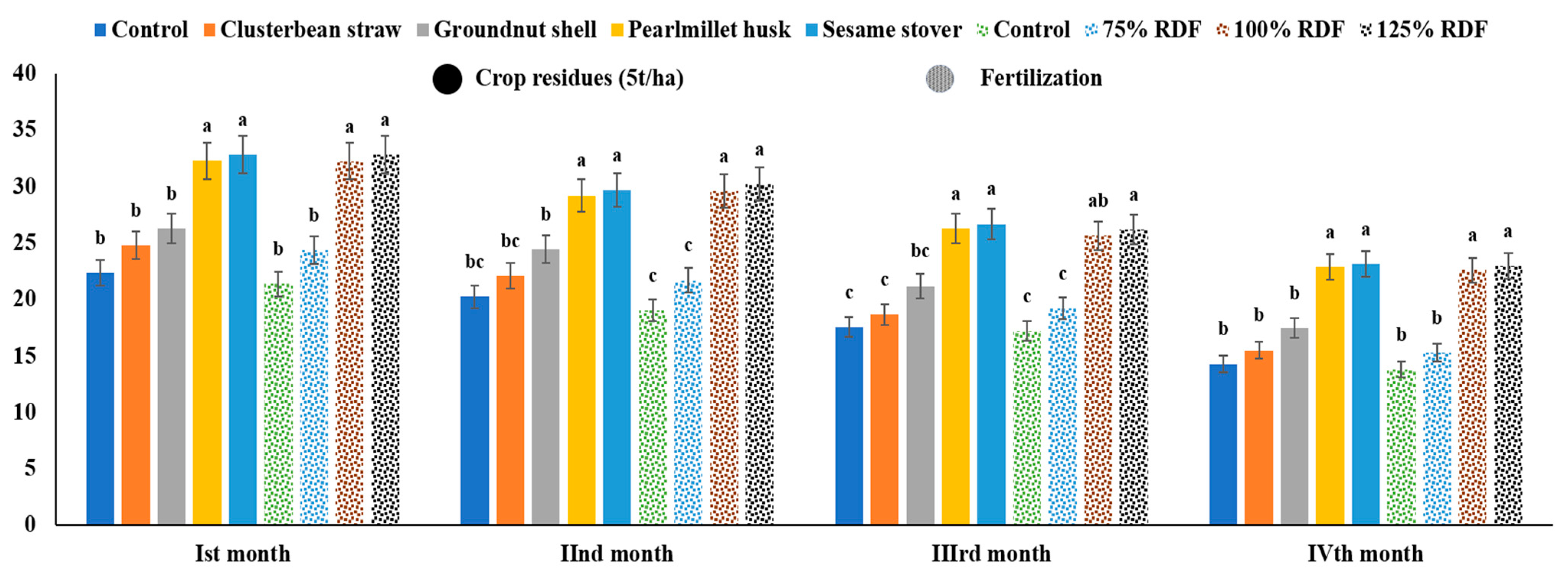
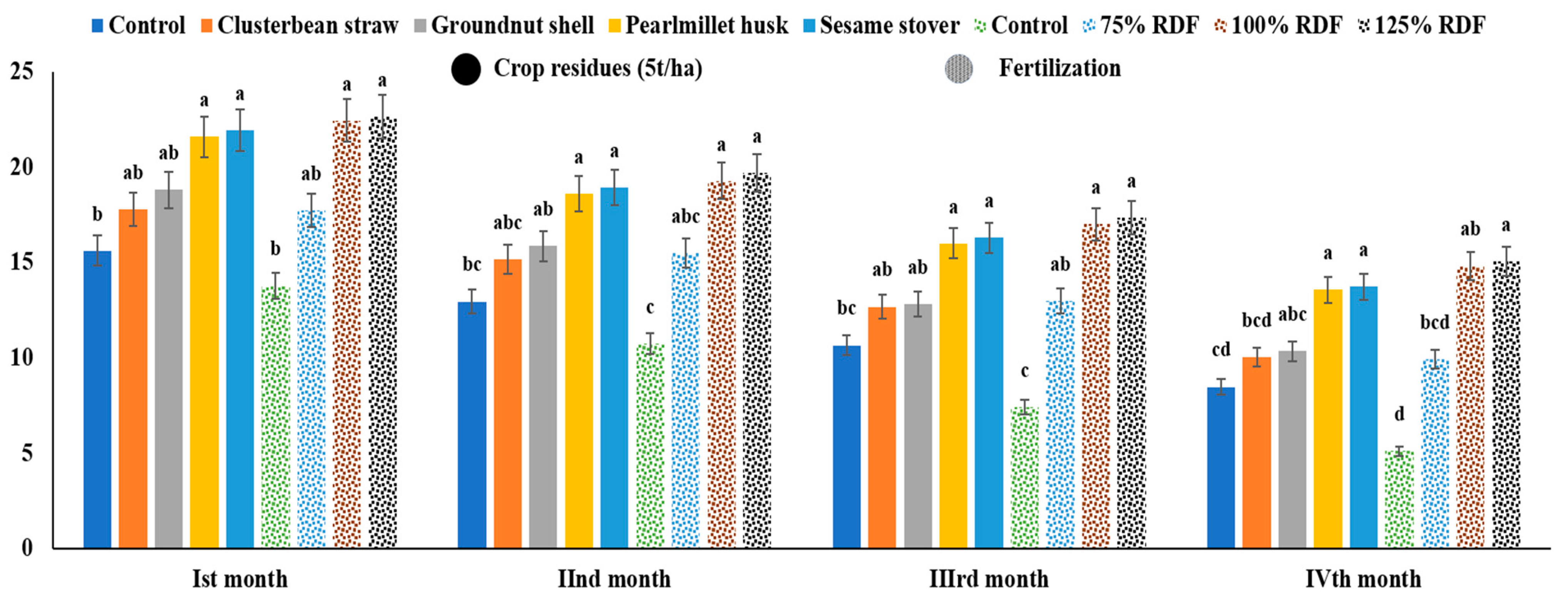
3.2. Soil Enzymes
3.3. Correlation Coefficient between Soil Biological Properties and Grain Yield
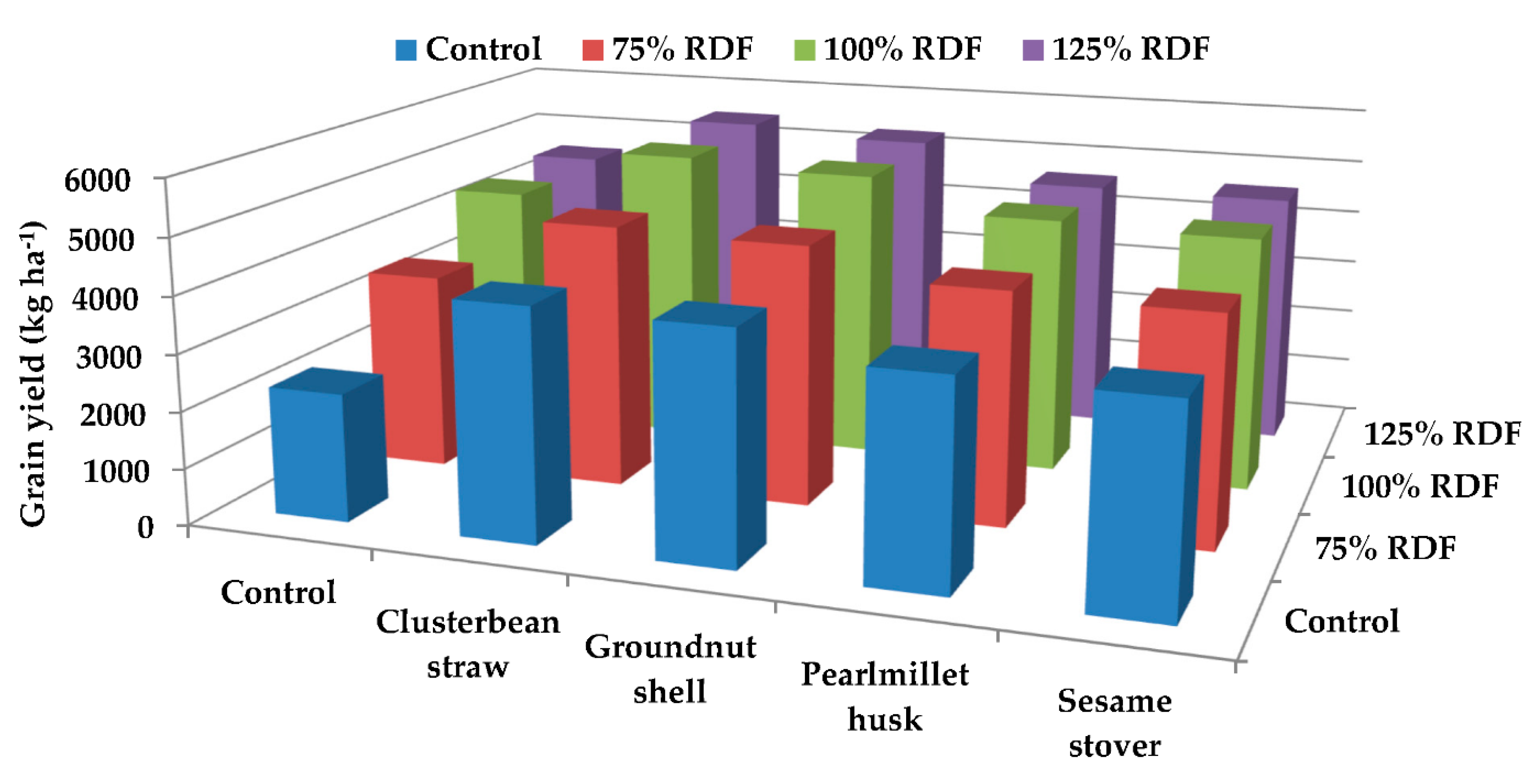
3.4. Grain Yield
4. Discussion
4.1. Soil Microbial Biomass
4.2. Soil Enzymes Activity
4.3. Grain Yield
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, S.; Zhang, S.; Pu, Y.; Li, T.; Xu, X.; Jia, Y.; Gong, G. Dynamics of soil labile organic carbon fractions and C-cycle enzyme activities under straw mulch in Chengdu Plain. Soil Tillage Res. 2016, 155, 289–297. [Google Scholar] [CrossRef]
- Khan, M.A.; Adnan, M.U.; Basir, A.B.; Fahad, S.H.; Hafeez, A.Q.; Saleem, M.H.; Ahmad, M.; Gul, F.; Durrishahwar, F.; Subhan, F.; et al. Impact of tillage and potassium levels and sources on growth, yield and yield attributes of wheat. Pak. J. Bot. 2022, 55, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Bolinder, M.A.; Crotty, F.; Elsen, A.; Frac, M.; Kismányoky, T.; Lipiec, J.; Kätterer. The effect of crop residues, cover crops, manures and nitrogen fertilization on soil organic carbon changes in agroecosystems: A synthesis of reviews. Mitig. Adapt. Strateg. Glob. Chang. 2020, 25, 929–952. [Google Scholar] [CrossRef]
- Saleem, K.; Asghar, M.A.; Saleem, M.H.; Raza, A.; Kocsy, G.; Iqbal, N.; Ali, B.; Albeshr, M.F.; Bhat, E.A. Chrysotile-asbestos-induced damage in Panicum virgatum and Phleum pretense species and its alleviation by organic-soil amendment. Sustainability 2022, 14, 10824. [Google Scholar] [CrossRef]
- Fu, B.; Chen, L.; Huang, H.; Qu, P.; Wei, Z. Impacts of crop residues on soil health: A review. Environ. Pollut. Bioavailab. 2021, 33, 164–173. [Google Scholar] [CrossRef]
- Fahad, S.; Chavan, S.B.; Chichaghare, A.R.; Uthappa, A.R.; Kumar, M.; Kakade, V.; Pradhan, A.; Jinger, D.; Rawale, G.; Yadav, D.K. Agroforestry systems for soil health improvement and maintenance. Sustainability 2022, 14, 14877. [Google Scholar] [CrossRef]
- Salam, A.; Afridi, M.S.; Javed, M.A.; Saleem, A.; Hafeez, A.; Khan, A.R.; Zeeshan, M.; Ali, B.; Azhar, W.; Sumaira. Nano-priming against abiotic stress: A way forward towards sustainable agriculture. Sustainability 2022, 14, 14880. [Google Scholar] [CrossRef]
- Adnan, M.; Fahad, S.; Saleem, M.H.; Ali, B.; Mussart, M.; Ullah, R.; Arif, M.; Ahmad, M.; Shah, W.A.; Romman, M. Comparative efficacy of phosphorous supplements with phosphate solubilizing bacteria for optimizing wheat yield in calcareous soils. Sci. Rep. 2022, 12, 11997. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, J.; Yu, Y.; Karlen, D.L.; Hao, X. Crop residue management and fertilization effects on soil organic matter and associated biological properties. Environ. Sci. Pollut. Res. 2016, 23, 17581–17591. [Google Scholar] [CrossRef]
- Umar, U.d.; Ahmed, N.; Zafar, M.Z.; Rehman, A.; Naqvi, S.A.H.; Zulfiqar, M.A.; Malik, M.T.; Ali, B.; Saleem, M.H.; Marc, R.A. Micronutrients foliar and drench application mitigate mango sudden decline disorder and impact fruit yield. Agronomy 2022, 12, 2449. [Google Scholar] [CrossRef]
- Afridi, M.S.; Javed, M.A.; Ali, S.; De Medeiros, F.H.V.; Ali, B.; Salam, A.; Sumaira; Marc, R.A.; Alkhalifah, D.H.M.; Selim, S.; et al. New opportunities in plant microbiome engineering for increasing agricultural sustainability under stressful conditions. Front. Plant Sci. 2022, 13, 899464. [Google Scholar] [CrossRef]
- Malobane, M.E.; Nciizah, A.D.; Nyambo, P.; Mudau, F.N.; Wakindiki, I.I. Microbial biomass carbon and enzyme activities as influenced by tillage, crop rotation and residue management in a sweet sorghum cropping system in marginal soils of South Africa. Heliyon 2020, 6, e05513. [Google Scholar] [CrossRef]
- Zainab, N.; Amna; Khan, A.A.; Azeem, M.A.; Ali, B.; Wang, T.; Shi, F.; Alghanem, S.M.; Hussain Munis, M.F.; Hashem, M.; et al. PGPR-mediated plant growth attributes and metal extraction ability of Sesbania sesban l. in industrially contaminated soils. Agronomy 2021, 11, 1820. [Google Scholar] [CrossRef]
- Chander, K.; Goyal, S.; Mundra, M.C.; Kapoor, K.K. Organic matter, microbial biomass and enzyme activity of soils under different crop rotations in the tropics. Biol. Fertil. Soils. 1997, 24, 306–310. [Google Scholar] [CrossRef]
- Ali, B.; Hafeez, A.; Ahmad, S.; Javed, M.A.; Sumaira Afridi, M.S.; Dawoud, T.M.; Almaary, K.S.; Muresan, C.C.; Marc, R.A.; Alkhalifah, D.H.M. Bacillus thuringiensis PM25 ameliorates oxidative damage of salinity stress in maize via regulating growth, leaf pigments, antioxidant defense system, and stress responsive gene expression. Front. Plant Sci. 2022, 13, 921668. [Google Scholar] [CrossRef]
- Ali, B.; Wang, X.; Saleem, M.H.; Azeem, M.A.; Afridi, M.S.; Nadeem, M.; Ghazal, M.; Batool, T.; Qayyum, A.; Alatawi, A. Bacillus mycoides PM35 reinforces photosynthetic efficiency, antioxidant defense, expression of stress-responsive genes, and ameliorates the effects of salinity stress in maize. Life 2022, 12, 219. [Google Scholar] [CrossRef]
- Ali, B.; Wang, X.; Saleem, M.H.; Sumaira; Hafeez, A.; Afridi, M.S.; Khan, S.; Zaib-Un-Nisa; Ullah, I.; Amaral Júnior, A.T. PGPR-mediated salt tolerance in maize by modulating plant physiology, antioxidant defense, compatible solutes accumulation and bio-surfactant producing genes. Plants 2022, 11, 345. [Google Scholar] [CrossRef]
- Ali, B.; Hafeez, A.; Javed, M.A.; Afridi, M.S.; Abbasi, H.A.; Qayyum, A.; Batool, T.; Ullah, A.; Marc, R.A.; Al-Jouni, S.K.; et al. Role of endophytic bacteria in salinity stress amelioration by physiological and molecular mechanisms of defense: A comprehensive review. South Afr. J. Bot. 2022, 151, 33–46. [Google Scholar] [CrossRef]
- Wardle, D.A. A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biol. Rev. 1992, 67, 321–358. [Google Scholar] [CrossRef]
- Schloter, M.; Dilly, O.; Munch, J.K. Indicators for evaluating soil quality. Agric. Ecosyst. Environ. 2003, 98, 255–262. [Google Scholar] [CrossRef]
- Kumar, U.; Saqib, H.S.A.; Islam, W.; Prashant, P.; Patel, N.; Chen, W.; Yang, F.; You, M.; He, W. Landscape composition and soil physical–chemical properties drive the assemblages of bacteria and fungi in conventional vegetable fields. Microorganisms 2022, 10, 1202. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Abaidoo, R.C.; Iwuafor, E.N.O.; Olufajo, O.O.; Sanginga, N. Rotation effects of grain legumes and fallow on maize yield, microbial biomass and chemical properties of an Alfisol in the Nigerian savanna. Agric. Ecosyst. Environ. 2009, 129, 325–331. [Google Scholar] [CrossRef]
- Wang, Q.J.; Bai, Y.H.; Gao, H.W.; He, J.; Chen, H.; Chesney, R.C.; Kuhn, N.J.; Li, H.W. Soil chemical properties and microbial biomass after 16 years of no-tillage farming on the Loess Plateau, China. Geoderma 2008, 144, 502–508. [Google Scholar] [CrossRef]
- Liu, E.K.; Zhao, B.Q.; Mei, X.R.; So, H.B.; Li, J.; Li, X.Y. Effects of no-tillage management on soil biochemical characteristics in northern China. J. Agric. Sci. Camb. 2010, 148, 217–223. [Google Scholar] [CrossRef]
- Farooq, T.H.; Kumar, U.; Shakoor, A.; Albasher, G.; Alkahtani, S.; Rizwana, H.; Tayyab, M.; Dobaria, J.; Hussain, M.I.; Wu, P. Influence of intraspecific competition stress on soil fungal diversity and composition in relation to tree growth and soil fertility in sub-tropical soils under chinese fir monoculture. Sustainability 2021, 13, 10688. [Google Scholar] [CrossRef]
- Wang, X.L.; Jia, Y.; Li, X.G.; Long, R.J.; Ma, Q.F.; Li, F.M.; Song, Y.J. Effects of land use on soil total and light fraction organic, and microbial biomass C and N in a semi-arid ecosystem of northwest China. Geoderma 2009, 153, 285–290. [Google Scholar] [CrossRef]
- Bing-Cheng, Y.U.A.N.; Dong-Xia, Y.U.E. Soil microbial and enzymatic activities across a chronosequence of Chinese pine plantation development on the loess plateau of China. Pedosphere 2012, 22, 1–12. [Google Scholar]
- Salazar, S.L.E.; Sánchez, J.; Alvarez, A.; Valverde, P.; Galindo, J.M.; Igual, A.; Peix, I.; Santa-Regina. Correlation among soil enzyme activities under different forest system management practices. Ecol. Eng. 2011, 37, 1123–1131. [Google Scholar] [CrossRef]
- Zhang, N.; He, X.; Gao, Y.; Li, Y.; Wang, H.; Ma, D.; Zhang, R.; Yang, S. 2010 Pedogenic carbonate and soil dehydrogenase activity in response to soil organic matter in Artemisia ordosica community. Pedosphere 2010, 20, 229235. [Google Scholar] [CrossRef]
- Touhami, D.; McDowell, R.W.; Condron, L.M. Role of organic anions and phosphatase enzymes in phosphorus acquisition in the rhizospheres of legumes and grasses grown in a low phosphorus pasture soil. Plants 2020, 9, 1185. [Google Scholar] [CrossRef]
- Masto, R.E.; Chhonkar, P.K.; Singh, D.; Patra, A.K. Changes in soil biological and biochemical characteristics in a long-term field trial on a sub-tropical Inceptisol. Soil Biol. Biochem. 2006, 38, 1577–1582. [Google Scholar] [CrossRef]
- Frankenberger, W.T.; Dick, W.A. Relationships between enzyme activities and microbial growth and activity indices in soil. Soil Sci. Soc. Am. J. 1983, 47, 945–951. [Google Scholar] [CrossRef]
- Roldán, A.; Salinas-García, J.R.; Alguacil, M.M.; Díaz, E.; Caravaca, F. Soil enzyme activities suggest advantages of conservation tillage practices in sorghum cultivation under subtropical conditions. Geoderma 2005, 129, 178–185. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, P.; Kumar, S. Responses of soil carbon pools, enzymatic activity, and crop yields to nitrogen and straw incorporation in a rice-wheat cropping system in north-western India. Front. Sustain. Food Syst. 2020, 4, 532704. [Google Scholar] [CrossRef]
- Solanki, M.K.; Solanki, A.C.; Rai, S.; Srivastava, S.; Kashyap, B.K.; Divvela, P.K.; Kumar, S.; Yandigeri, M.S.; Kashyap, P.L.; Shrivastava, A.K.; et al. Functional interplay between antagonistic bacteria and Rhizoctonia solani in the tomato plant rhizosphere. Front. Microbiol. 2022, 13, 990850. [Google Scholar] [CrossRef]
- Akram, N.A.; Saleem, M.H.; Shafiq, S.; Naz, H.; Farid-ul-Haq, M.; Ali, B.; Shafiq, F.; Iqbal, M.; Jaremko, M.; Qureshi, K.A. Phytoextracts as crop biostimulants and natural protective agents-a critical review. Sustainability 2022, 14, 14498. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R. Short-term dynamics of soil carbon, microbial biomass, and soil enzyme activities as compared to longer-term effects of tillage in irrigated row crops. Biol. Fertil. Soils 2009, 46, 65–72. [Google Scholar] [CrossRef]
- Ali, S.; Ullah, S.; Khan, M.N.; Khan, W.M.; Razak, S.A.; Wahab, S.; Hafeez, A.; Khan Bangash, S.A.; Poczai, P. The effects of osmosis and thermo-priming on salinity stress tolerance in Vigna radiata L. Sustainability 2022, 14, 12924. [Google Scholar] [CrossRef]
- Gupta, R.K.; Hans, H.; Kalia, A.; Kang, J.S.; Kaur, J.; Sraw, P.K.; Mattar, M.A. Long-Term Impact of Different Straw Management Practices on Carbon Fractions and Biological Properties under Rice–Wheat System. Agriculture 2022, 12, 1733. [Google Scholar] [CrossRef]
- Farooq, T.H.; Kumar, U.; Yan, Y.; Arif, M.S.; Shakoor, A.; Tayyab, M.; Rathod, P.H.; Altaf, M.M.; Wu, P. 2022. Receptiveness of soil bacterial diversity in relation to soil nutrient transformation and canopy growth in Chinese fir monoculture influenced by varying stand density. Trees 2022, 36, 1149–1160. [Google Scholar] [CrossRef]
- Cano-Ortiz, A.; Musarella, C.M.; Piñar Fuentes, J.C.; Pinto Gomes, C.J.; Quinto-Canas, R.; del Río, S.; Cano, E. Indicative Value of the Dominant Plant Species for a Rapid Evaluation of the Nutritional Value of Soils. Agronomy 2021, 11, 1. [Google Scholar] [CrossRef]
- Dola, D.B.; Mannan, M.A.; Sarker, U.; Mamun, M.A.A.; Islam, T.; Ercisli, S.; Saleem, M.H.; Ali, B.; Pop, O.L.; Marc, R.A. Nano-iron oxide accelerates growth, yield, and quality of Glycine max seed in water deficits. Front. Plant Sci. 2022, 13, 992535. [Google Scholar] [CrossRef] [PubMed]
- Shafie, S.M. A review on paddy residue-based power generation: Energy, environment and economic perspective. Renew. Sustain. Energy Rev. 2013, 59, 1089–1100. [Google Scholar] [CrossRef]
- Raza, M.H.; Abid, M.; Faisal, M.; Yan, T.; Akhtar, S.; Adnan, K.M.M. Environmental and health impacts of crop residue burning: Scope of sustainable crop residue management practices. Int. J. Environ. Res. Public Health 2022, 19, 4753. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, N.C.; Jat, L.; Tiwari, R.; Yadav, A. Soil organic carbon fractions, soil microbial biomass carbon, and enzyme activities impacted by crop rotational diversity and conservation tillage in North West IGP: A review. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 3573–3600. [Google Scholar]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Plants’ Physio-Biochemical and Phyto-Hormonal Responses to Alleviate the Adverse Effects of Drought Stress: A Comprehensive Review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, H.; Ali, A.; Saleem, M.H.; Ameer, A.; Hafeez, A.; Alharbi, K.; Ezzat, A.; Khan, A.; Jamil, M.; Farid, G. Comparative effectiveness of EDTA and citric acid assisted phytoremediation of Ni contaminated soil by using canola (Brassica napus). Braz. J. Biol. 2022, 82, e261785. [Google Scholar] [CrossRef]
- Perrino, E.V.; Musarella, C.M.; Magazzini, P. Management of grazing Italian river buffalo to preserve habitats defined by Directive 92/43/EEC in a protected wetland area on the Mediterranean coast: PaludeFrattarolo, Apulia, Italy. Euro-Mediterr. J. Environ. Integr. 2021, 6, 32. [Google Scholar] [CrossRef]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. Organic Amendments: Microbial Community Structure, Activity and Abundance of Agriculturally Relevant Microbes are Driven by Long-Term Fertilization Strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef]
- Kamble, P.N.; Rousk, J.; Frey, S.D.; Bååth, E. Bacterial Growth and Growth-Limiting Nutrients Following Chronic Nitrogen Additions to a Hardwood Forest Soil. Soil Boil. Biochem. 2013, 59, 32–37. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Mallaci, C.; Attinà, E.; Muscolo, A. Using Digestate as Fertilizer for a Sustainable Tomato Cultivation. Sustainability 2021, 13, 1574. [Google Scholar] [CrossRef]
- Reicosky, D.C.; Wilts, A.R. Crop-residue management in: Reference module in earth systems and environmental Sciences. In Encyclopaedia of Soils in the Environment; Elsevier: Amsterdam, The Netherlands, 2005; pp. 334–338. [Google Scholar]
- Pathak, H.; Singh, R.; Bhatia, A.; Jain, N. Recycling of rice straw to improve wheat yield and soil fertility and reduce atmospheric pollution. Paddy Water Environ. 2006, 4, 111–117. [Google Scholar] [CrossRef]
- Dadhich, S.K.; Yadav, G.K.; Yadav, K.; Kumawat, C.; Munalia, M.K. Recycling of Crop Residues for Sustainable Soil Health Management: A Review. Int. J. Plant Soil Sci. 2021, 2, 66–75. [Google Scholar] [CrossRef]
- Operational Guidelines on Promotion of Agricultural Mechanization for In-Situ Management of Crop Residue in the States of Punjab, Haryana, Uttar Pradesh and Delhi; Ministry of Agriculture & Farmers Welfare: New Delhi, India, 2020.
- SPSS. IBM knowledge center. Available online: https://www.ibm.com/support/knowledgecenter/en/SS3RA7_15.0.0/com.ibm.spss.modeler.help/dataaudit_displaystatistics.htm (accessed on 11 April 2018).
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass carbon. Soil Biol. and Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, R.V.; Watanabe, F.S.; Lean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; United States Department of Agriculture Circular: Washington, DC, USA, 1954; p. 939. [Google Scholar]
- Brookes, P.C.; Powlson, D.S.; Jenkinson, D.S. Measurement of microbial biomass phosphorus in soil. Soil Biol. Biochem. 1982, 14, 319–329. [Google Scholar] [CrossRef]
- Casida, L.E.; Klein, D.A.; Santora, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of P nitrophenyl phosphate for assay of soil phosphate activity. Soil Biol. and Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Powlson, D.S.; Brooks, P.C. Measurement of soil microbial biomass provides an early indication of changes in total soil organic matter due to straw incorporation. Soil Biol. Biochem. 1987, 19, 159–164. [Google Scholar] [CrossRef]
- Manna, M.C.; Hazara, J.N. 1996. Comparative performance of cowdung slurry, microbial inoculation and inorganic fertilizers on maize. J. Indian Soc. Soil Sci. 1996, 44, 526–528. [Google Scholar]
- Bowen, G.D.; Rovira, A.D. The rhizosphere. The hidden half of the hidden half. In Plant Roots: The Hidden Half; Waisel, Y., Eshel, A., Kafkafi, V., Eds.; Marcel Dekker: New York, NY, USA, 1991; pp. 641–689. [Google Scholar]
- Goyal, S.; Chander, K.; Mundra, M.C.; Kapoor, K.K. Influence of inorganic fertilizers and organic amendments on soil organic matter and soil microbial properties under tropical conditions. Biol. Fertil. Soils 1999, 29, 196–200. [Google Scholar] [CrossRef]
- Dhull, S.K.; Goyal, S.; Krishan, K.K.; Mool, C.M. Microbial biomass carbon and microbial activities of soils receiving chemical fertilizers and organic amendments. Arch. Agron. Soil Sci. 2004, 50, 641–647. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, N.; Yang, M.; Zhan, X.; Zhang, Z. Effects of different tillage and straw return on soil organic carbon in a rice-wheat rotation system. PLoS ONE 2014, 9, e88900. [Google Scholar] [CrossRef]
- Pathak, D.V.; Ram, K.G.; Yadav, P.K.; Yadav, S.S. Effect of green manuring and residue incorporation on soil properties and seed yield of rapeseed mustard. Int. J. Farm Sci. 2015, 5, 70–77. [Google Scholar]
- Kaur, K.; Kapoor, K.K.; Gupta, A.P. Impact of organic manures with and without mineral fertilizers on soil chemical and biological properties under tropical conditions. J. Plant Nutr. Soil Sci. 2005, 168, 117–122. [Google Scholar] [CrossRef]
- Mandal, A.; Patra, A.K.; Singh, D.; Swarup, A.; Masto, R.E. Effect of long-term application of manure and fertilizer on biological and biochemical activities in soil during crop development stages. Bioresour. Technol. 2007, 98, 3585–3592. [Google Scholar] [CrossRef] [PubMed]
- Chand, S.; Somani, L.L.; Bhandari, S.C. Effect of fertilizer, farmyard manure (FYM) and biofertilizers on the population of Azotobacter and PSB in the soil. J. Indian Soc. Soil Sci. 2010, 58, 460–463. [Google Scholar]
- Parewa, H.P.; Yadav, J.; Rakshit, A. Effect of fertilizer levels, FYM and bioinoculants on soil properties in Inceptisol of Varanasi, Uttar Pradesh, India. Int. J. Agric. Environ. Biotech. 2014, 7, 517–525. [Google Scholar] [CrossRef]
- Bhatt, M.K.; Labanya, R.; Joshi, H.C.; Pareek, N.; Chandra, R.; Raverkar, K.P. Long-term effects of inorganic fertilizers and FYM on soil chemical properties and yield of wheat under rice-wheat cropping system. Himal. Ecol. 2017, 25, 28–35. [Google Scholar]
- Kumari, S.; Sharma, M.; Dudi, D.S.; Kaswan, P.K.; Kharra, R.; Purohit, H.S. Effect of fertility levels, organic sources and bio-inoculants on soil biological properties of wheat (Triticum aestivum L.). Pharma Innov. J. 2022, 11, 628–630. [Google Scholar]
- Nannipieri, P.; Grego, S.; Ceccanti, B. Ecological significance of biological activity. In Soil Biochemistry; Bollag, J.-M., Stotzky, G., Eds.; Marcel Dekker: New York, NY, USA, 1990; Volume 6, pp. 293–355. [Google Scholar]
- Speir, T.W. Studies on a clumosequence of soils in tussock grasslands. II. Urease, phosphatase and sulphatase activities of topsoils and their relationships with other properties including plant available sulphur. N. Z. J. Sci. 1977, 20, 159–166. [Google Scholar]
- Nannipieri, P.L.; Muccini, L.; Ciardi, C. Microbial biomass and enzyme activities: Production and persistence. Soil Biol. Biochem. 1993, 15, 679–685. [Google Scholar] [CrossRef]
- Martens, D.A.; Johansen, J.B.; Frankenberger, W.T. Production and persistance of soil enzymes with repeated addition of organic residues. Soil Sci. 1992, 153, 53–61. [Google Scholar] [CrossRef]
- Garg, S.; Bahl, G.S. Phosphorus availability to maize as influenced by organic manures and fertilizer P associated phosphatase activity in soils. Bioresour. Technol. 2008, 99, 5773–5777. [Google Scholar] [CrossRef]
- Kumar, A.; Jat, M.L.; Kumar, A.; Tomar, J.; Kumar, S.; Kushwaha, S.R. Rice residue management in wheat under different tillage practices and nitrogen doses. Ann. Agric. Res. 2016, 37, 49–55. [Google Scholar]
- Bharali, A.; Baruah, K.K.; Bhattacharyya, P.; Gorh, D. Integrated nutrient management in wheat grown in a northeast India soil: Impacts on soil organic carbon fractions in relation to grain yield. Soil Tillage Res. 2017, 168, 81–91. [Google Scholar] [CrossRef]
- Davari, M.R.; Sharma, S.N.; Mirzakhani, M. The effect of combinations of organic materials and biofertilizers on productivity, grain quality, nutrient uptake and economics in organic farming of wheat. J. Org. Syst. 2012, 7, 26–35. [Google Scholar]
- Verma, N.K.; Pandey, B.K. Effect of varying rice residue management practices on growth and yield of wheat and soil organic carbon in rice-wheat sequence. Glob. J. Sci. Front. Res. Agric. Vet. Sci. 2013, 13, 33–38. [Google Scholar]
- Dhar, D.; Datta, A.; Basak, N.; Paul, N.; Badole, S.; Thomas, T. Residual effect of crop residues on growth, yield attributes and soil properties of wheat under rice-wheat cropping system. Indian J. Agric. Res. 2014, 48, 373–378. [Google Scholar] [CrossRef]
- Singh, R.K.; Sharma, G.K.; Kumar, P.; Singh, S.K.; Singh, R. Effect of crop residues management on soil properties and crop productivity of rice-wheat system in Inceptisols of Seemanchal region of Bihar. Curr. J. Appl. Sci. Technol. 2019, 37, 1–6. [Google Scholar] [CrossRef][Green Version]


| S. No. | Crop Residues | TC (%) | TN (%) | TP (%) | TK (%) | C:N Ratio | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | ||
| 1. | Clusterbean straw | 25.3 | 26.9 | 1.36 | 1.40 | 0.32 | 0.34 | 0.87 | 0.84 | 18.6 | 19.2 |
| 2. | Groundnut shell | 26.6 | 25.3 | 1.04 | 1.10 | 0.27 | 0.26 | 0.83 | 0.79 | 25.6 | 23.0 |
| 3. | Pearlmillet husk | 28.1 | 27.5 | 0.71 | 0.70 | 0.22 | 0.19 | 0.70 | 0.73 | 39.6 | 39.3 |
| 4. | Sesame stover | 29.4 | 28.7 | 0.64 | 0.66 | 0.19 | 0.18 | 0.61 | 0.62 | 45.9 | 43.5 |
| Treatments | First Month | Second Month | Third Month | Fourth Month |
|---|---|---|---|---|
| Crop residues (5 t ha−1) | ||||
| Control | 16.3 b | 15.3 b | 14.5 b | 14.3 b |
| Clusterbean straw | 17.9 b | 16.0 b | 14.9 b | 14.6 b |
| Groundnut shell | 18.4 ab | 17.5 ab | 16.3 ab | 16.0 ab |
| Pearlmillet husk | 18.8 a | 18.6 a | 17.8 a | 16.7 a |
| Sesame stover | 19.2 a | 20.0 a | 18.0 a | 17.0 a |
| Fertilization | ||||
| Control | 15.7 b | 15.2 b | 14.5 b | 14.1 b |
| 75% RDF | 17.1 b | 16.8 b | 15.7 b | 15.1 b |
| 100% RDF | 19.2 a | 18.4 a | 17.3 a | 16.6 a |
| 125% RDF | 19.7 a | 18.7 a | 17.7 a | 17.0 a |
| Treatments | First Month | Second Month | Third Month | Fourth Month |
|---|---|---|---|---|
| Crop residues (5 t ha−1) | ||||
| Control | 10.2 b | 10.21 b | 9.3 b | 9.12 b |
| Clusterbean straw | 10.5 b | 10.3 b | 10.0 b | 9.7 b |
| Groundnut shell | 11.6 ab | 11.5 ab | 11.2 ab | 10.8 ab |
| Pearlmillet husk | 12.9 a | 12.9 a | 12.4 a | 12.3 a |
| Sesame stover | 13.1 a | 13.0 a | 12.6 a | 12.5 a |
| Fertilization | ||||
| Control | 10.01 b | 9.8 b | 9.5 b | 9.4 b |
| 75% RDF | 11.0 ab | 10.9 b | 10.5 b | 10.6 ab |
| 100% RDF | 12.7 a | 12.6 a | 12.1 a | 11.8 a |
| 125% RDF | 13.0 a | 12.8 a | 12.3 a | 12.0 a |
| MBC | MBN | MBP | DHA | APA | Grain Yield | |
|---|---|---|---|---|---|---|
| MBC | 1.000 | 0.921 ** | 0.955 ** | 0.950 ** | 0.918 ** | 0.765 * |
| MBN | 1.000 | 0.976 ** | 0.959 ** | 0.984 ** | 0.679 * | |
| MBP | 1.000 | 0.980 ** | 0.966 ** | 0.666 * | ||
| DHA | 1.000 | 0.972 ** | 0.669 * | |||
| APA | 1.000 | 0.661 * | ||||
| Grain yield | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, G.K.; Dadhich, S.K.; Yadav, R.K.; Kumar, R.; Dobaria, J.; Paray, B.A.; Chang, S.W.; Ravindran, B. Impact of Biomass Recycling and Fertilization on Soil Microbiological Characteristics and Wheat Productivity in Semi-Arid Environment. Agronomy 2023, 13, 1054. https://doi.org/10.3390/agronomy13041054
Yadav GK, Dadhich SK, Yadav RK, Kumar R, Dobaria J, Paray BA, Chang SW, Ravindran B. Impact of Biomass Recycling and Fertilization on Soil Microbiological Characteristics and Wheat Productivity in Semi-Arid Environment. Agronomy. 2023; 13(4):1054. https://doi.org/10.3390/agronomy13041054
Chicago/Turabian StyleYadav, Govind Kumar, Sunil Kumar Dadhich, Rajendra Kumar Yadav, Rajesh Kumar, Jalpa Dobaria, Bilal Ahamad Paray, Soon Woong Chang, and Balasubramani Ravindran. 2023. "Impact of Biomass Recycling and Fertilization on Soil Microbiological Characteristics and Wheat Productivity in Semi-Arid Environment" Agronomy 13, no. 4: 1054. https://doi.org/10.3390/agronomy13041054
APA StyleYadav, G. K., Dadhich, S. K., Yadav, R. K., Kumar, R., Dobaria, J., Paray, B. A., Chang, S. W., & Ravindran, B. (2023). Impact of Biomass Recycling and Fertilization on Soil Microbiological Characteristics and Wheat Productivity in Semi-Arid Environment. Agronomy, 13(4), 1054. https://doi.org/10.3390/agronomy13041054








