Abstract
Legumes have an important role in European agriculture. They assimilate N2 to sustainably support maximum crop growth, in turn providing high-protein food for human consumption and livestock feed. However, the extent of the area for legume cultivation in Europe has declined due to the lower economic competitiveness of legumes in relation to other crops, particularly of cereals and oilseed. To increase yields, there is a need to increase the genetic diversity of legumes in terms of adaptation to environmental stresses. We attempted to address this by conducting field and controlled experiments under drought vs. nondrought and different photoperiod conditions. The current study identified the physiological and agronomic traits correlated with productivity and quality performance in five economically important grain legume species (Pisum sativum, Phaseolus vulgaris, Cicer arietinum, Lupinus spp., and Vicia faba). In all species, the days to flowering and seed yield were affected by temperature and photoperiod. For cool-season legume species, long-day photoperiods were favorable and days to flowering was negatively correlated with the average air temperature. For the warm-season legumes, short-day photoperiods and warm temperatures were favorable. Under drought stress, the C/N balance, leaf nutrient (Ca, Fe, and K) concentrations, and yield were significantly reduced, contrary to Zn accumulation, and this information may contribute to improving our understanding and ability to develop sustainable growth. Based on our results, we conclude that the drought-tolerant and photoperiod-insensitive legume genotypes identified in this study constitute valuable starting materials for future programs aimed at improvement of legume productivity at a global/regional scale, which helps to strengthen the competitiveness and economic growth of legumes for European farmers.
1. Introduction
Grain legumes (family Leguminosae) are represented by some of the most numerous species belonging to diverse angiosperm families, which are used either for human consumption or for animal feed as a major source of plant protein. Apart from protein, grain legumes support various nutritional components, such as carbohydrates, sugars, vitamins, essential mineral elements, and mono- and polyunsaturated fatty acids []; they are a rich source of dietary fibers, folic acids, and non-nutritional bioactive components, including phenolics, lectins, phytate, and trypsin inhibitors []. Legume crops could play an important role in achieving sustainability, contributing to reducing the emission of greenhouse gases (GHGs), given they release 5–7 times less GHG per unit area compared with other crops. They facilitate the sequestration of carbon in soils, with estimated values of 2.19–23.6 C kg−1 per year derived from 7.21 g kg−1 of dry matter, inducing savings on fossil energy inputs due to the reduction in N fertilizer compared with cereal crops, which corresponds to 277 kg ha−1 of CO2 per year []. Legumes are versatile high-value protein crops; their higher production in agricultural systems would help to increase crop diversity and greatly reduce the use of external inputs. However, genetic improvement of important grain legumes, and, therefore, their greater use in agriculture, has lagged behind other crops such as cereals.
Legumes have adapted to a wide range of environmental conditions, ranging from cool to warm seasons. Pea (Pisum sativum), chickpea (Cicer arietinum), broad bean (Vicia faba), and lupin (Lupinus spp.) are the major cool-season grain legume crops produced worldwide; they come from temperate regions and, with respect to flowering time, are vernalization-responsive long-day plants (LDPs) [,,,,,,,,,,]. In contrast, those such as the common bean (Phaseolus vulgaris) are warm-season legumes, generally originate from lower latitudes, and are short-day plants (SDPs) []. Grain legume species have different cultivation requirements, such as specific photoperiods and/or temperatures, and flowering may be delayed or even not occur at all, if these requirements are not met. For this reason, a greater adaptation of these crops to the new agronomic conditions resulting from a changing climate is necessary, which is only possible with the incorporation of new genetic resources linked with climate adaptation patterns.
Over the last few years, the importance of the photoperiod for plant responses to abiotic and biotic stresses has received increasing attention []. In general, the adaptability and productivity of cool-season and warm-season legumes are limited by climate change conditions, ranging from various climate-induced biotic (pests and pathogens) and abiotic (solar radiation, drought, temperature, salinity, and photoperiod) stresses [,] and depending upon internal physiological adjustment and crop husbandry practices. The yield loss of legumes varies between species (and varieties) depending on the stress level and timing. Yield losses of more than 25% are expected for several legume crops depending on the stress factor: for example, in the case of lupin and soybean in Western Europe; lupin, bean, and broad bean in Northern Europe; chickpea, soybean, and bean in Southern Europe; and all legume species in Eastern Europe []. The productivity of grain legumes is being harmed by rapidly changing climatic conditions, especially the rise in temperature and water stress [,,,,,]. Drought and heat stress tend to decrease the concentration of most plant nutrients [,,]. Studies show that higher carbon dioxide concentrations will lead to lower protein, zinc, and iron contents in crops [,]. It is estimated that by 2050, approximately 175 million people will develop zinc deficiencies, and 122 million may be protein-deficient. Additionally, light levels during the winter months are a limiting factor for crop production in northern regions [,], and a good strategy to improve yield would be switching to an earlier sowing date, which requires varieties with a phenology well adapted to such a cropping cycle. Over the last few decades, the effects of single abiotic stress on legume crops have been widely studied, but several stresses tend to co-occur under field conditions. The severity of these stresses is unpredictable in field experiments, so controlled prescreening is needed to develop stress combination-resilient crops.
The traditional varieties of legumes represent local adaptations of domesticated species, and, therefore, constitute genetic resources suitable for facing the current challenges expected in agriculture, especially in stressful environments. There is a need to explore genetic resources to identify promising materials that can be used directly in breeding and to further understand the mechanisms of adaptation. In our study, we sought to identify legume germplasms adapted to either local challenges or climate change stresses by investigating the effects of drought and photoperiod (different sowing seasons) on the flowering time, yield, and concentration of nutrient uptake, specifically focusing on the C/N balance gain and Fe, Zn, Ca, and K accumulation. The results of these studies confirmed that different legume species exhibit genotypic variation in sensitivity to a combination of both temperature and photoperiod in addition to the drought level, which can explain most of the variability in flowering behavior and plant growth. Specifically, we identified promising legume accessions and suitable growing conditions for their integration into Atlantic farming environments.
2. Materials and Methods
2.1. Plant Material and Experimental Sites
Sixty-seven genotypes, originally selected for their diverse agronomic traits of different grain legume species, were used in this study (Table S1; Figure 1). These included pea (Pisum sativum, PSM = 20), chickpea (Cicer arietinum, CIC = 6), broad bean (Vicia faba, VIF = 9), lupins (Lupinus angustifolius, L. luteus, L. gredensis, LUP = 17), and common bean (Phaseolus vulgaris, PHA = 15). These accessions are maintained in the MBG legume germplasm collection, and were sampled in different areas of the Iberian Peninsula.

Figure 1.
Images of crops in different environments. (A–E) broad bean, lupin, pea, chickpea, and common bean genotypes growing in Environment 1. (F,G) Pea and common bean genotypes growing in Environment 2.
Four environments (ENVs) with different photoperiods and drought scenarios were studied in two locations (Pontevedra and Orense) and two years (2021 and 2022). Before sowing, soil samples were taken at a depth of 0–60 cm to analyze their chemical and physical properties (Table 1). The first environment received regular irrigation (ENV1; well irrigated) from planting until maturity in high humid conditions and a short-day (SD) photoperiod. To limit the influence of high rainfall and low temperature under the SD photoperiod (from November to June), the ENV1 experiment was conducted under greenhouse conditions with 9 h of natural light. The second environment received regular irrigation (ENV2; well irrigated) with planting in dry environmental conditions and a long-day (LD) photoperiod. Superior genotypes identified during the phenotypic evaluation were tagged and separately harvested for additional field evaluations. The third environment received irrigation from planting to maturity with one treatment and no irrigation with the other treatment (ENV3; severe drought) in moderately humid and LD photoperiod conditions. The fourth environment received regular irrigation from planting to maturity with one treatment and no irrigation with the other treatment (ENV4; terminal drought) in low humid and LD photoperiod conditions. Drought was imposed by withholding water in ENV 3 and ENV4, and the rate and the severity of the drought stress was controlled by daily monitoring of the plants and soil in order to expose plants to gradually-increasing drought stress. Drought conditions were induced beginning from seedling establishment to maturity. The water irrigation regime conditions were: well-watered, 4000 m3 ha−1; severe drought, 2000 m3 ha−1. ENV2 to ENV4 experiments were carried out during the post-rainy season in field conditions (from March to September).

Table 1.
Environmental characteristics of the locations during evaluations in 2021 and 2022 under winter and spring sowing conditions.
The layout of the experiments was a randomized complete design (RCD) with two replications for ENV1 and ENV2, and the design applied to the ENV3 and ENV4 experiments was a split-plot with two replicates. The main plots were randomly designated as the irrigation regimes, and in the sub-plots, the tested genotypes were randomly applied. The experimental unit was a plot consisting of a single row, 13 m long, and sown with 28 seeds. The inter- and intra-row spacings were 0.4 and 0.8 m, respectively, resulting in a plant population of 36,000 plants/ha−1, except for ENV4, with spacings of 0.2 and 0.8 m and 90,000 plants ha−1. Conventional agronomy practices carried out by farmers in the area for fertilization, weeding, and pest management were applied.
2.2. Data Collection
Meteorological data (average daily temperature and photoperiod; Table 2) were recorded for each environment during the vegetative (VE–R1), early reproductive (R1–R5), and late reproductive (R5–R7) developmental phases of each legume species. The key phases were assigned according to [] as VE = emergence, R1 = beginning flowering, R5 = beginning seed, and R7 = physiological maturity.

Table 2.
Average daily temperature and photoperiod in each environment during the vegetative (VE–R1), early reproductive (R1–R5), and late reproductive (R5–R7) developmental phases. Developmental phases were measured according to [].
The following agronomic traits were recorded: days to 50% flowering (DTF) (when 50% of plants started flowering) was recorded for five plants in each plot. At physiological maturity, the plant height (cm) was assessed for five plants in each plot, and measurements were taken from the ground level to the top of the plant. The growth habit was determined according to a scale of 1–7, where 1 = determinate growth, 2 = indeterminate growth with erect branches, 3 = indeterminate growth with prostrate branches, 4 = indeterminate growth with semi-climbing main stem and branches, 5 = indeterminate moderately climbing growth and pods distributed evenly on top of the plant, 6 = strongly climbing indeterminate growth with pods mainly on the top node of the plant, and 7 = other. The number of pods per plant (PP) was recorded as the average number of pods counted on five randomly selected and tagged plants per plot at harvest. The number of seeds per pod (SP) was recorded as the total number of seeds divided by the number of pods from five randomly selected plants in a plot at harvest. The 100-seed weight (SW) was recorded as the weight in each plot by taking 100 randomly selected fully matured and undamaged seeds in triplicate measurements and averaging their weights. The total seed yield (YIELD) was measured as the weight of shelled grain harvested from all plants in a plot and converted to kilogram per hectare after adjusting for 12% moisture content. The relative chlorophyll content (FM) was determined from three fresh leaves sampled from the tops of the plants on five plants in each plot using a chlorophyll meter (SPAD-502 PLUS, Konica Minolta Sensing, Osaka, Japan). Before FM measurement, the SPAD-502 meter was calibrated using the reading checker supplied by the manufacturer. Each leaf SPAD value obtained was the average of ten readings, five on each side of each leaf midrib.
To obtain nutrient concentration measurements in the leaves, the plant biomass was sampled for each genotype at the peak of vegetative development (approximately 100 days from sowing). The samples were divided into shoots and roots, and then washed with deionized water. The samples were immediately frozen in liquid nitrogen and stored at −80 °C for subsequent analysis. The samples were lyophilized in a freeze dryer ( Gamma 2-16 LSC plus, Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany), and the lyophilized material was milled (Fritsch 14, Idar Oberstein, Germany) to a fine powder. The calcium (Ca; g/100g), iron (Fe; mg/kg), zinc (Zn; mg/kg), and potassium (K; g/100g) were determined by mass spectrometry using a plasma ionization source, after mineralization or wet digestion in an acid medium of the sample using a microwave digester. Plant [C] and total [N] were determined using an elemental analyzer (2400 II CHNS/O Elemental Analyzer, Perkin-Elmer, Waltham, MA, USA) at a combustion temperature of 950 °C and a reduction temperature of 640 °C, and the C:N ratio was calculated.
2.3. Data Analysis
One way-analysis of variance (ANOVA) was performed according to a random block (ENV1-ENV2) and split plot (ENV3-ENV4) design using statistics version 9.4 SAS statistical software (SAS Institute, Cary, NC, USA). A PROC GLM was performed to determine the effects of three factors (environment (fixed), treatment or main plot (fixed, control vs. drought), and genotype within species or subplot (random)) on each response variable. Significant differences in each response variable between the environments, treatments, and differences across genotypes within species were compared by lsmeans (least squares means estimates) and adjusted by Tukey’s adjustment to identify pairwise differences at a p < 0.05 level of probability. Pearson correlation was used to estimate the coefficient of the relationship between the traits and the environments. Principal component analysis (PCA) was performed to verify the assay data variation in order to identify the factors that could explain the genotype performance per species and, thus, the factors controlling climatic tolerance. Biplot analysis, represented by principal component analyses (PCAs) that were computed based on a rank correlation matrix using data from six traits (DTF, SW, SP, PP, YIELD, and FM), was conducted using XLSTAT 2021 (Addinsoft, New York, NY, USA).
3. Results
3.1. Comparison of Photoperiod Adaptation Processes of Different Legume Species Based on Seed Yield and Flowering Time
A combined analysis of variance for seed yield under two contrasting photoperiod environments, SD-ENV1 and LD-ENV2, is presented in Table 3. A highly significant difference (p-value < 0.0001) was found for all sources of variation, indicating that the studied genotypes within species behaved differently under the contrasting tested environments. Genotype was found to be the main cause of variation, explaining 74% of the model sum of squares. The GEI (genotype by environment interaction) was higher than the environmental effect, accounting for 14% and 7%, respectively, suggesting that the legume accessions behaved differently under LD and SD environments. Environmental variation usually has a major effect on the variation of yield in genotypes with a narrow genetic base. However, for the evaluation and selection of germplasm (more heterogeneous), the genotypes and GEI are the most relevant, and both must be taken into account when selecting the most adapted genotypes.

Table 3.
Combined analysis of variance among genotypes of the five grain legume species growing under two environments (ENV1 and ENV2) for seed yield (kg ha−1).
The seeds of Environments 1 and 2 were planted in November and June 2021 in SD- and LD-natural photoperiods, respectively. The photoperiod, or day length, which was calculated for the site locations as the time interval between sunrise and sunset in the different developmental phases, was 4 h shorter from VE to R1 in ENV1 compared with ENV2. The accumulative photoperiod from VE to R1 was significantly longer in ENV1 than in ENV2. The average daily temperature from VE to R1 and R1 to R5 was higher in ENV2 compared with ENV1. Thus, during both phases, the photothermal units, which were obtained by multiplying the light hours by the day degrees of temperature, were higher in ENV2 compared with ENV1 (Table 2).
Principal component analysis (PCA) was used with these environmental factors to define both environments. The first two PCA scores accounted for 85.26% of the variation in the environments (Figure 2A). The first PCA score (52.64% variation) allowed us to discriminate the environments on the basis of temperature, with positive loadings for mean temperature (T, from VE to R7), day length/mean photoperiod (MEAN PHOTO, from VE to R5), and photothermal units (PT, from VE to R7). All these vectors had the same direction, indicating that they discriminated between both environments in a similar manner. Accordingly, ENV1 was characterized by lower temperatures and shorter daylight hours until the R5 phase. The second PCA score allowed us to discriminate environments on the basis of the accumulative photoperiod (AC, from VE to R7). The parameter AC largely discriminated the genotypes inside ENV1, in which noninductive conditions (SD) were associated with cool-season legumes. This indicates that within SD-ENV1, the genotypes were separated by their different degrees of sensitivity to the photoperiod, resulting from including the days to flowering and maturity, which did not manifest in LD-ENV2. Based on the cluster analysis, both groups of environments (at the 85% level) were identified, and these influenced the species, as found in the corresponding analysis.
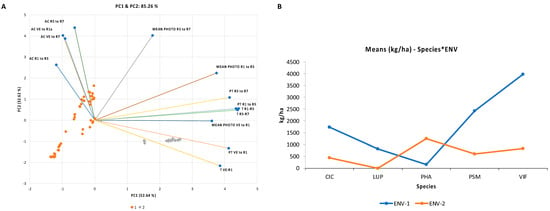
Figure 2.
Variability of the climatic parameters and yield performance of the grain legume species between ENV1 and ENV2. (A) First (PC1) and second (PC2) principal component analysis (PCA) scores for the characterization of the climatic parameters of both environments. The direction of the vectors is shown by the lines (T = average daily temperature (°C); P = mean photoperiod (hours); AC = accumulated (hours); PT = photothermal units). Environmental groups are identified by color. (B) Multiple Tukey’s HSD at p < 0.05 comparison among the genotypes of the five grain legume species growing under two environments (ENV1 and ENV2) for seed yield (kg ha−1). Significant differences among species, and significant interactions between species according to environment were recorded at p < 0.0001.
For all species except PHA, the lower temperatures and shorter photoperiod of ENV1 (average daily Tª of 12 °C and mean photoperiod of 11 h, from VE to R1) significantly enhanced yield in comparison with ENV2 (average daily Tª of 22 °C and mean photoperiod of 15 h, from VE to R1), 1822 vs. 627 kg ha−1, respectively (Table S2; Figure 2B). In addition, the consistency of the seed yield performance, considering species in both environments, was different, from 407 for LUP to 2408 kg ha−1 for VIF (Table S2). Most species responded positively to the shorter daylight hours and temperatures in ENV1 compared with ENV2 (Figure 3A), with a yield increase from 74 to 100% for the CIC, PSM, VIF, and LUP species, with the exception of PHA (decrease of 87%). This could be explained by cool-season grain legumes (chickpea, broad bean, pea, and lupin) reportedly being more sensitive than warm-season grain legumes (common bean) to high temperature stress [,,,]. Most of these species showed later days to flowering times in ENV1 than ENV2, except for lupins, which were nonflowering in ENV2 (Figure 3B). These results further demonstrate that for most of these species, as long as the photoperiod requirements were met in LD-ENV2, reproductive development was dominated by temperature only; within the optimal range, reproductive development was accelerated as the temperatures increase, which may partly explain the reduced yield in this environment.
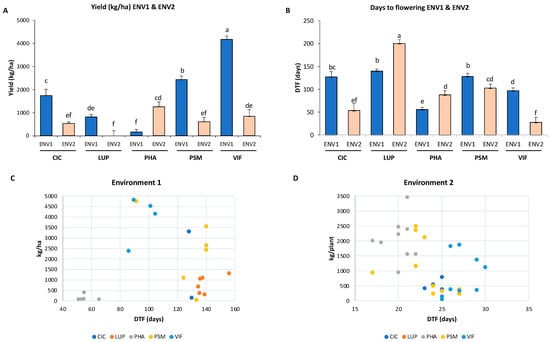
Figure 3.
Mean comparison and standard error for (A) seed yield and (B) days to flowering among the genotypes of the five grain legume species in ENV1 and ENV2. Scatter plot matrix ranking of the genotypes of the five grain legume species for days to flowering (DTF) versus seed yield (kg ha−1) in (C) ENV1 and (D) ENV2. Data for the latest flowering genotypes (DTF = >120) or no flowering (DTF = 200) in Environment 2 were removed in order to more clearly show the dispersion. Different letters indicate significant differences between means among the genotypes of the five grain legume species and environments, ENV1 and ENV2, at p < 0.05 by Tukey’s HSD test. Each bar represents mean + SE (standard error). Significant differences among species, and significant interactions between species by environment, were recorded at p < 0.05. VIF (Vicia faba), PSM (Pisum sativum), CIC (Cicer arietinum), PHA (Phaseolus vulgaris), and LUP (Lupinus spp.).
The correlation coefficients show that the pod number per plant and 100-seed weight potentially contributed to the grain yield (0.71 and 0.67, respectively; p < 0.05). Despite the differences in time to flowering in both environments, DTF is positively correlated between both environments (r = 0.58; p < 0.05), which may be explained by differential expression of the same genes in different environments. However, DTF only showed a negative correlation with grain yield under the LD-ENV2 photoperiodic conditions (−0.72; p < 0.05). The relationships between daily temperature, photoperiod (hours and accumulative photoperiod in hours), and thermal time and duration of the phenological phases are summarized in Figure S1 and Table S3. These results for ENV2, which has a LD photoperiod, show that an extended photoperiod stimulates earlier flowering, but ENV2’s higher temperatures in the summer season were unfavorable for grain yield in the cool-season legumes. In general, the earliest flowering times were 25–50 DTF for common bean in both ENV1 and -2, and for chickpea, pea, and broad bean, in ENV2. An intermediate flowering time of 80 to 100 DTF was observed for broad bean in ENV1; and the latest flowering time was >120 DTF for chickpea, lupin, and pea in ENV1 (Figure 3B). This classification divides the legume species into three groups, namely, early-, medium-, and late-flowering time in SD-ENV1, while all species, with the exception of the nonflowering lupin species, were early-flowering in LD-ENV2. This is evidence of significant variation among species in time to flower initiation, expressed as days to flowering after sowing, and its relationship with seed yield in ENV1 compared with ENV2 (Figure 3C,D). The earliest flowering species in ENV2, common bean, was also found to be associated with the highest seed yield (1254 kg ha−1), whereas the intermediate- and late-flowering species in ENV1, namely, pea, broad bean, and chickpea, also had higher yields (2429, 3977, and 1733 kg ha−1, respectively). These results are evidence of a much larger main effect of genotype compared with the effect of genotype by environment interaction, as well as of differences in the specific photoperiod and/or temperature requirements of the lupin (LUP), pea (PSM), broad bean (VIF), and chickpea (CIC), which are facultative cool long-day (LD) species, while common bean (PHA) is a warm short-day (SD) species.
Discriminant PCA was carried out on the climatic parameters (Table S3) in order to examine the effects of temperature and photoperiod on the genotype per species variation. The PCA scores and latent vectors for axes PC1 and PC2 are plotted in Figure 4. The first PCA score (53.50% variation) discriminates species largely on the basis of most of the photothermal variables and, therefore, separates genotypes on the basis of whether they are relatively sensitive to the photoperiod (positive loading), i.e., have a high value for the mean photoperiod and late flowering in all development phases, or are insensitive to the photoperiod (negative loading). Accordingly, most of the genotypes of chickpea, pea, and lupin were found to be sensitive to the photoperiod, while all genotypes of bean and broad beans were insensitive to the photoperiod. The second PCA score (27.56% variation) had positive loadings for temperature and photothermal unit at the VE to R1 developmental phases, and, therefore, discriminated between the genotypes of those species with a high temperature requirement at the VE to R1 phase (e.g., genotypes from species of broad bean and chickpea). The results for genotypes for the species responses identified in the PCA of photothermal parameters were largely in accord with the previously identified environmental groupings (Figure 2A). This is due to the fact that under the conditions of these experiments, plants—for example, the lupin species—flowered late in an 11 h day when the temperatures in which they were growing were approximately 11 °C (125 photothermal units), but failed to bloom when growing at a 22 °C temperature in a 15 h day (300 photothermal units). It should be noted that the plants of the common bean species were early-flowering in a 9–10 h day when the temperature was lowered from 12 °C. This suggests that there may be two major factors controlling the time to flowering and, therefore, adaptation in the evaluated environments: first, whether the genotypes are sensitive or insensitive to the photoperiod; and second, whether the genotypes are inherently early/late flowering or have low/high temperature requirements.
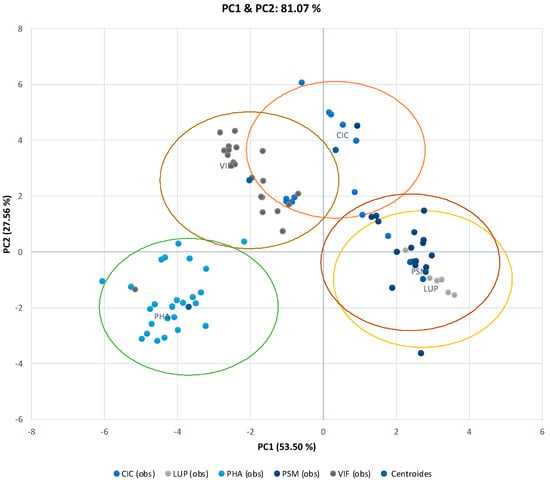
Figure 4.
Discriminants of the first (PC1) and second (PC2) principal component analysis (DPCA) scores for the characterization of photothermal parameters among the five grain legume species in both environments. The five groups of species are identified by color. VIF (Vicia faba), PSM (Pisum sativum), CIC (Cicer arietinum), PHA (Phaseolus vulgaris), and LUP (Lupinus spp.).
According to these experiments, the combination of changing the sowing date or photoperiod together with the use of adapted legume material would increase the potential average yield. There is a clear genotype × environment effect on yield and flowering time. The best photoperiod-adapted legume genotypes for sowing in SD and LD conditions were selected (VIF-0003, VIF-0005, PSM-0112, PSM-0096, PHA-0620, PHA-1160, CIC-0003, CIC-0006, LUP-0023, and LUP-0028) as shown in Table S4. The selected genotypes were well adapted to SD and LD conditions, with the exception of LUP genotypes, which did not flower in LD-ENV2.
3.2. Effects of the Different Climate Manipulation Stress Levels on Agronomical Performance and Leaf Nutrient Status
The analysis of variance (ANOVA) results show that there were significant effects of genotypes (G) and irrigation treatments (T), and no significant effect for the environment variable (ENV3 vs. ENV4). This two-location testing is characterized by a low replication level; thus, maximizing of the number of test locations or increasing the number of replications per genotype would allow for an increase in the repeatability and power of the analysis. The GE and GT interactions (genotype by environment and genotype by treatment interaction) were significant, which indicates a differential response of the genotypes in the different evaluated environments to the different irrigation conditions (Table 4). The change in this variable relative to the species is presented according to treatment type in Figure 5. Averaged across the two environments, the seed yield increased by 31.36% with the administered watering treatment. The seed yield was higher with the watering treatment for all species, except pea, with the highest increase for the lupin species. The water deficit significantly affected the number of pods per plant in all legume species, but not the days to flowering nor the seed weight. The number of seeds per pod was only affected for CIC and VIF, resulting in a lower number of seeds only in VIF. Similarly, moderate drought stress and other physiological stresses generally reduced the pod number rather than the seed number per pod or seed size []. In this sense, our results suggest that drought induces sink reduction, causing a lower number of seeds in some genotypes, although these seeds retain their proper weight for reproductive success (Figure S2). On the other hand, the measurements of the light absorbance on the leaves indicated that drought conditions did not produce a decrease in the chlorophyll content of most of the legumes (with the exception of PSM and VIF), providing full leaf cover in the water-deficient plots comparable to in the irrigated treatment. This is consistent with the fact that when plants are exposed to moderate- or slow-onset drought stress, this generally does not result in chlorophyll depletion [] or limitations to photosynthesis [].

Table 4.
Analysis of variance and Tukey’s HSD test among genotype varieties of the five grain legume species growing under the two environments (ENV3 and ENV4) and two water treatments for seed yield (kg ha−1). The water treatments and their interactions were considered as fixed effects in the model.
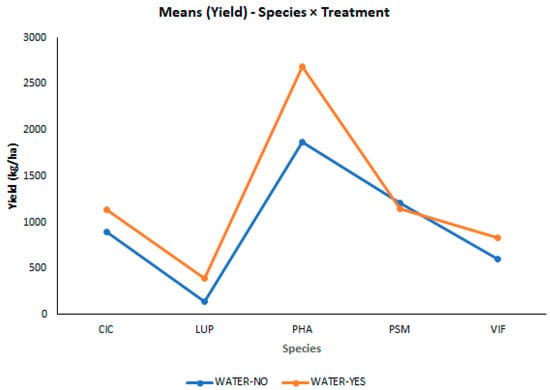
Figure 5.
Multiple Tukey’s HSD comparisons among the five grain legume species growing under two water deficit treatments (normal irrigation control WATER YES vs. drought WATER NO) for seed yield (kg ha−1).
The main components were estimated in order to explore the correlation between the evaluated traits and to examine the genotypes on a two-dimensional map to identify trends. The first dimension (PC1) of the variable factor map included yield and other important traits, such as number of pods per plant and seeds per pod, which could be used to select for high yield under drought conditions (Figure 6A–C). The seed yield, pods per plant, and seeds per pod had the highest contribution (35%, 25%, and 20%, respectively) and were significantly positively correlated with PC1 (r = 0.91, 0.77, and 0.69, p < 0.05, respectively), whereas the fluorometer measurements and seed weight had the highest contribution (46 and 38%, respectively) and were positively correlated with PC2 (r = 0.84 and 0.76, p < 0.05, respectively). By examining the third dimension, we can see that the days to flowering trends were clearer on a PC1 and PC3 map, with a 50% contribution and a positive correlation of r = 0.71 with the factor. In the individual PC1 and PC2 factor maps, the most productive genotypes, with high values for seed weight and chlorophyll fluorescence content, were included in the upper right quadrant, explaining 65% of the variation for the water deficit treatment group and showing the greatest variation for pea genotypes (Figure 6A). The variation in yield due to the flowering time was quite large (PC3; Figure 6B). Finally, PC4 shows the inverse relationship between seed weight and chlorophyll fluorescence content. The PC1 and PC2 factor map for the nonwater deficit conditions explains 57% of the variation (Figure 6D–F). Seed yield and seed weight had the highest contribution (31% and 27%, respectively) and were positively correlated with PC1 (r = 0.80 and 0.74, p < 0.05, respectively). Other traits, such as pods per plant and seeds per pod, had significant contributions (46% and 37%, respectively) and associations between the two extremes with PC2 (r = 0.79 and −0.71, p < 0.05, respectively). Under this watering condition, the chlorophyll fluorescence and days to flowering traits had significant contributions to and associations with PC3 and PC4 (39% and 58%, r = 0.63 and 0.68, p < 0.05, respectively). The upper right quadrants in Figure 6A–F include, as the most productive genotypes in both conditions, PHA-0620, PHA-0683, PHA-1160, PSM-0061, PSM-0112, VIF-0003, VIF-0006, CIC-0004, and CIC-0006. Regarding the genotypes of lupin, they had the lowest yields in comparison with the other legumes, highlighting the good performance of LUP-0023 and LUP-0032 under both conditions (Table S5). All of these genotypes had similar mean fitness in normal and drought conditions.
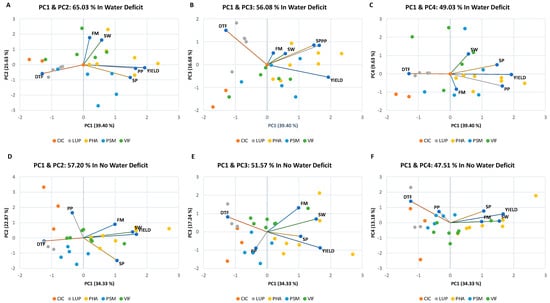
Figure 6.
Biplot for the first (PC1), second (PC2), third (PC3) and fourth (PC4) principal component analysis (PCA) scores for the most important physiological traits associated with seed yield (kg ha−1) under (A–C) drought conditions (water deficit) and (D–F) normal irrigation control conditions (no water deficit). The percentage of variation accounted for by each PC is displayed on the axes. The factor loading values for variables are indicated by arrows radiating from the center showing the direction (angle) and magnitude (length). DTF = days to flowering; FM = chlorophyll fluorometer measurement; SW = seed weight; PP = number of pods per plant; SP = number of seeds per pod.
We investigated the effects of irrigation and drought on the C/N balance and leaf nutrient accumulations (Ca, Fe, K, and Zn) in all five grain legumes. Drought significantly decreased the C:N ratio (32%), as well as the Ca (49%), Fe (31%), and K (97%) accumulation. In contrast, the concentration of Zn was significantly upregulated (97%) (Figure 7). Additionally, a significant and negative correlation between days to flowering; C:N; and Ca, Fe, and K accumulation (from r = −0.47 to −0.53, p < 0.05), and a positive correlation with Zn concentration (r = 0.46, p < 0.05), were observed for drought environments, where the yield was negatively correlated with days to flowering. Similar results were found under the watering control environments (Table 5). These results are in accordance with the drought effect causing reduced root growth and, as a result, restricting the uptake of mobile nutrients [,], thereby causing slow plant growth and leaf senescence []. Furthermore, different studies have provided support for the leaf ionome being finely tuned prior to leaf senescence to ensure plant growth [,,]. As a consequence, there are decreases in leaf nutrient contents (Ca, Fe, and K) and increased concentrations of transition metals such as Zn. For example, in cereals, Mn, Cu, and Zn increase in the early phase of drought [,]; in soybean, small increases in Zn availability promote growth under drought conditions [].
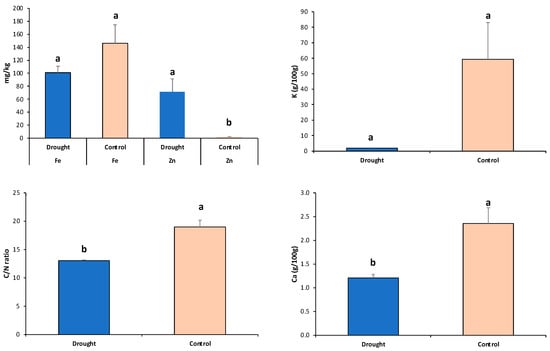
Figure 7.
Effects of normal irrigation control vs. drought treatments on the C/N balance and leaf nutrient concentrations (Ca, Fe, K, and Zn) across the five grain legumes. Different letters indicate significant differences between treatments for the genotypes of the five grain legume species at p < 0.05 by Tukey’s HSD test. Each bar represents mean + SE (standard error).

Table 5.
Correlation analysis between days to flowering and yield, C and N concentration balance, and leaf nutrient accumulations (Ca, Fe, K, and Zn) in the different grain legumes under the different treatments (normal irrigation control vs. drought).
4. Discussion
The flowering times of legume species are a major concern for the security of food worldwide, and represent a key contributing factor to environmental adaptation and productivity. Species classification by days to flowering after sowing is very important, although it depends on the time of year and the region of study. Hence, flowering in response to short days is valuable in plants that flower in the late summer as day length shortens, and flowering in response to long days is valuable in plants that flower in late spring as day lengths lengthen in advance of summer. In addition to day length, temperature can also influence flowering induction under a wide range of environments. Plants need to reach a threshold of a minimum photoperiod and a minimum accumulated thermal time for days to flowering after sowing. Thus, the days to flowering of the five examined short-day and long-day legume species could be predicted based on effects due to the photoperiod and temperature requirements. This will synchronize their developmental processes with a specific time of the year and could, in turn, influence the impact of environmental stresses; for example, it has been shown that the photoperiod influences plants’ resistance to drought stress [,].
The temperatures and hours of light preceding a phenological phase (e.g., vegetative, early-reproductive, and late-reproductive developmental phases) are important drivers of phenology []. The responses to temperature differ among the crop species throughout their life cycles. Crossover genotype × environment (day lengths and temperatures) interactions occur, necessitating the evaluation and selection of numerous genotypes to identify those with optimal behavior for target environments. For all cool legume species, the duration from crop sowing to the vegetative, early-flowering, and late-flowering stages was found to be negatively correlated with the average air temperature. Exposure to temperatures above 20 °C had negative impacts on reproduction and ultimately manifested as reduced yield. Given the negative yield impact of high temperatures, our observations suggest that flowering during cooler seasons is beneficial to cool legumes grown in warm environments. Reductions in yield due to heat stress have previously been reported in these crops (pea [], common bean [,], and chickpea [,,]). High temperatures more negatively affect the yield of broad beans, which were found to be a later-flowering legume in evaluation. This supports the hypothesis that early-flowering varieties are less exposed to unfavorable environmental conditions and, hence, their yields are less influenced by variations in climatic variables. However, higher temperatures were benign for all evaluated common bean varieties, which is in accordance with their classification as a warm-season crop. The mean temperatures and photoperiods varied substantially across the experiments, resulting in a considerable variation in times from sowing to flowering. Quantification of the responses to temperature and photoperiod and the separation of the five legume species was carried out based on the consideration of parameters such as temperature, photoperiod, and thermal time, which are specific to each species and developmental stage. This analysis suggests that there are two main mechanisms controlling yield and, hence, adaptation: first, whether the genotypes are sensitive or insensitive to the photoperiod (54% variation); second, whether the genotypes have a requirement of high temperature from the VE to R1 phase (28% variation).
In the coming decades, a reduction in rainfall and an increase in evapotranspiration rates are expected due to global change in many regions of the world, which will result in increased drought. Our results show that there are significant differences among the short-day and long-day legume species with regard to their adaptability to drought, as measured by their ability to maintain a high yield following a period of water stress. Cool-season food legumes, such as PSM, exhibited no yield reduction (0%), while LUP had the highest yield reduction (63%). Moreover, there was variability in the drought sensitivity within legume species. This is important because cool-season legumes are more susceptible to temperature variations than warm-season food legumes []. These results will allow us to select and develop legume varieties, which are better adapted to heat and drought stress.
Although there are many works evaluating the impact of drought on the seed nutrient content, there are far fewer studies focusing on the effect of drought specifically on the remobilization of nutrients from leaves. In this study, we show that drought decreases the C:N ratio and the concentration (%) of Ca, Fe, and K in plant tissues, in accordance with previous works [,], indicating that drought reduces nutrient acquisition and, hence, plant growth. The decrease in the rate of nutrient uptake by plants under drought conditions is widely established []. Consistent with this, drought decreases the concentration of accumulated nutrients and increases the concentration of Zn. Many plant nutrients are reported to alleviate heat stress. In general, Zn increases the concentration of antioxidant enzymes, thereby improving the response to oxidative stress, helping to activate defense mechanisms, and enhancing the metabolic processes by which plants adapt to various adverse stresses [,]. This is in agreement with the observation that Zn application can contribute to significant expansion in leaf area and increases in photosynthetic pigments such as chlorophyll, stomatal conductance, relative leaf water content, and osmolyte accumulation, thus resulting in improved growth, yield, and protection of leaf tissues from the destructive impacts of moisture deficiencies via prevention []. Thus, it is important to devote attention to ensuring the proper balance of all plant nutrients, which might contribute to plant growth and reductions in stress.
The use of traditional varieties to combat climatic challenges can meet specific climatic needs and represent a source of germplasm for plant breeders []. However, the use of traditional varieties requires greater knowledge of their adaptive, agronomic, and quality characteristics in order to allow for the selection of those best suited to sustainable agriculture. Therefore, the characterization of germplasms can add value to what farmers have pursued for centuries through the maintenance of varieties adapted to the expected agroecological conditions. The legume genetic resources evaluated in this work might be opportune as tools for ensuring resilience against climate change effects and boosting sustainable agricultural practice. The genetic diversity detected in our legume germplasm highlights its potential economic importance for the discovery of varieties adapted to stressful environments and to specific photothermal conditions.
5. Conclusions
The results of this work confirm that the differential genotypic sensitivity to temperature and photoperiod can explain most of the variability in the flowering behavior of the cool- and warm-season grain legume species studied. This is particularly important for the management of these grain legume plants under environmental stresses that occur at the same time every year, as their specific photoperiodic development interacts with the resistance to drought stress, affecting important agronomic features and the leaf nutrient balance. Thus, developing a variety is an essential step for adaptation, and using the correct plant nutrients is a low-cost and sustainable way of managing abiotic stresses, although there is still much that needs to be further explored.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13041025/s1. Figure S1: Average daily temperature (T, ºC), mean photoperiod (P, hours), accumulated hours (AC) and photothermal units (PT) in the Environment 1 (A, B, C, D), and Environment 2 (E, F, G, H). Vegetative (VE-R1), early reproductive (R1-R5), and late reproductive (R5-R7) developmental phases. VE, emergence; R1, beginning flowering; R5, beginning seed; R7, physiological maturity. LUP genotypes did not flower in Environment 2. VIF (Vicia faba), PSM (Pisum sativum), CIC (Cicer arietinum), PHA (Phaseolus vulgaris), and LUP (Lupinus spp.); Figure S2: Multiple Tukey’s HSD comparison among genotypes of five grain legume species growing under two water deficit treatments (normal irrigation control = WATER YES vs. drought = WATER NO) for DTF = days to flowering, FM = chlorophyll fluorometer measurement, SW = seed weight, PP = number of pods per plant, SP = number of seeds per pod. DTF: Pr > F (Species) <0.0001**; Pr > F (Water treatment) 0.672; Pr > F (Species*Treatment) 0.093. PP = Pr > F (Species) <0.0001**; Pr > F (Water treatment) 0.001; Pr > F (Species*Treatment) 0.003. SP = Pr > F (Species) <0.0001**; Pr > F (Water treatment) 0.714; Pr > F (Species*Treatment) 0.001. SW = Pr > F (Species) <0.0001**; Pr > F (Water treatment) 0.661; Pr > F (Species*Treatment) 0.710. FM = Pr > F (Species) <0.029**; Pr > F (Water treatment) 0.615; Pr > F (Species*Treatment) 0.989; Table S1: Description of varieties used in this study; Table S2: Combined analysis of variance and mean comparison among the five grain legume species growing under two environments (ENV1 and ENV2) for seed yield (kg ha-1).; Table S3: Values of the correlations (r) based on the mean temperature, photoperiod (mean and accumulative hours), and photothermal units derived from the vegetative (VE-R1), early reproductive (R1-R5), and late reproductive (R5-R7) developmental phases and coefficients of determination (R2) for each of the five grain legumes; Table S4: Ranking for yield of the best genotypes according to Genotype by Environment interaction comparison means under two environments (ENV1-SD and ENV2-SD); Table S5: Ranking for yield of the best genotypes according to Treatment by Genotype interaction comparison means under two environments (ENV3 and ENV4).
Author Contributions
Conceptualization: M.S. Experiments and data analysis: A.M.G., A.M.P. and L.G. Writing: A.M.G. and M.S. Funding acquisition: M.S. and L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the innovation project PROTEINLEG, with 80% co-funded by the European Agricultural Fund for Rural Development (EAFRD) of the European Union and 20% by the Ministry of Agriculture, Fisheries, and Food, within the framework of the National Rural Development Programme 2014–2020. The General Directorate of Rural Development, Innovation and Agrifood Training (GDRDIAT) is the authority in charge of the application of this aid. Total project budget: EUR 556,240.12; total grant: EUR 551,440.12. A consolidate research unit, GENECROP, was provided by Xunta de Galicia and the project BEANFLOW (MICIU/FEDER 2021–2024, PID2020-114115RB-I00).
Data Availability Statement
The raw data that support the findings of this study are available from the corresponding author, [M.S.], upon reasonable request.
Acknowledgments
We gratefully acknowledge the support and facilities of the Misión Biológica de Galicia (Consejo Superior de Investigaciones Científicas), Centro Tecnolóxico da Carne (CETECA, Xunta de Galicia) and Centro Nacional de Tecnología y Seguridad Alimentaria (CNTA).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maphosa, Y.; Jideani, V.A. The Role of Legumes in Human Nutrition. In Functional Food—Improve Health through Adequate Food; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Burstin, J.; Gallardo, K.; Mir, R.R.; Varshney, R.K.; Duc, G. Improving protein content and nutrition quality. In Biology and Breeding of Food Legumes; CABI: Wallingford, UK, 2011; pp. 314–328. [Google Scholar] [CrossRef]
- Jensen, E.S.; Peoples, M.B.; Boddey, R.M.; Gresshoff, P.M.; Hauggaard-Nielsen, H.; Alves, B., Jr.; Morrison, M.J. Legumes for mitigation of climate change and the provision of feedstock for biofuels and biorefineries. A review. Agron. Sustain. Dev. 2012, 32, 329–364. [Google Scholar] [CrossRef]
- Soltani, A.; Torabi, B.; Zeinali, E.; Sarparast, R. Response of chickpea to photoperiod as a qualitative long-day plant. Asian J. Plant Sci. 2004, 6, 705–708. [Google Scholar] [CrossRef]
- Stefanova, K.T.; Buirchell, B. Multiplicative mixed models for genetic gain assessment in lupin breeding. Crop Sci. 2010, 50, 880–891. [Google Scholar] [CrossRef]
- Duc, G.; Bao, S.; Baum, M.; Redden, B.; Sadiki, M.; Suso, M.J.; Vishniakova, M.; Zong, X. Diversity maintenance and use of Vicia faba L. genetic resources. Field Crops Res. 2010, 115, 270–278. [Google Scholar] [CrossRef]
- Adhikari, K.N.; Buirchell, B.J.; Sweetingham, M.W. Length of vernalization period affects flowering time in three lupin species. Plant Breed. 2012, 131, 631–636. [Google Scholar] [CrossRef]
- Berger, J.D.; Shrestha, D.; Ludwig, C. Reproductive strategies in Mediterranean legumes: Trade-offs between phenology, seed size and vigor within and between wild and domesticated Lupinus species collected along aridity gradients. Front. Plant Sci. 2017, 8, 548. [Google Scholar] [CrossRef] [PubMed]
- Flores, F.; Hybl, M.; Knudsen, J.; Marget, P.; Muel, F.; Nadal, S.; Narits, L.; Raffiot, B.; Sass, O.; Solis, I.; et al. Adaptation of spring faba bean types across European climates. Field Crops Res. 2013, 145, 1–9. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Barrett, B.; Brummer, E.C.; Julier, B.; Marshall, A.H. Achievements and challenges in improving temperate perennial forage legumes. Crit. Rev. Plant Sci. 2015, 34, 327–380. [Google Scholar] [CrossRef]
- Daba, K.; Warkentin, T.D.; Bueckert, R.; Todd, C.D.; Tar’an, B. Determination of Photoperiod-Sensitive Phase in Chickpea (Cicer arietinum L.). Front. Plant Sci. 2016, 7, 478. [Google Scholar] [CrossRef]
- Książkiewicz, M.; Nazzicari, N.; Yang, H.; Nelson, M.N.; Renshaw, D.; Rychel-Bielska, S.; Ferrari, B.; Carelli, M.; Tomaszewska, M.; Stawiński, S.; et al. A high-density consensus linkage map of white lupin highlights synteny with narrow-leafed lupin and provides markers tagging key agronomic traits. Sci. Rep. 2017, 7, 15335. [Google Scholar] [CrossRef]
- Cowling, W.A. Genetic diversity in narrow-leafed lupin breeding after the domestication bottleneck. In The Lupin Genome; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–17. [Google Scholar] [CrossRef]
- Dutta, A.; Trivedi, A.; Nath, C.P.; Gupta, D.S.; Hazra, K.K. A comprehensive review on grain legumes as climate-smart crops: Challenges and prospects. Environ. Chall. 2022, 7, 100479. [Google Scholar] [CrossRef]
- White, J.W.; Laing, D.R. Photoperiod response of flowering in diverse genotypes of common bean (Phaseolus vulgaris). Field Crops Res. 1989, 22, 113–128. [Google Scholar] [CrossRef]
- Roeber, V.M.; Schmülling, T.; Cortleven, A. The photoperiod: Handling and causing stress in plants. Front. Plant Sci. 2022, 12, 781988. [Google Scholar] [CrossRef]
- Sita, K.; Sehgal, A.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.V.; Kumar, S.; Gaur, P.M.; Farooq, M.; Siddique, K.H.M.; Varshney, R.K.; et al. Food Legumes and Rising Temperatures: Effects, Adaptive Functional Mechanisms Specific to Reproductive Growth Stage and Strategies to Improve Heat Tolerance. Front. Plant Sci. 2017, 8, 1658. [Google Scholar] [CrossRef] [PubMed]
- Vadez, V.; Berger, J.D.; Warkentin, T.; Asseng, S.; Ratnakumar, P.; Rao, K.P.C.; Gaur, P.M.; Munier-Jolain, N.; Larmure, A.; Voisin, A.-S.; et al. Adapting grain legumes to climatic changes: A review. Agron. Sustain. Dev. 2012, 32, 31–44. [Google Scholar] [CrossRef]
- Cernay, C.; Ben-Ari, T.; Pelzer, E.; Meynard, J.M.; Makowski, D. Estimating variability in grain legume yields across Europe and the Americas. Sci. Rep. 2015, 5, 11171. [Google Scholar] [CrossRef]
- Amede, T.; Schubert, S.; Stahr, K. Mechanisms of drought resistance in grain legumes I: Osmotic adjustment. SINET Ethiop. J. Sci. 2003, 26, 37–46. [Google Scholar] [CrossRef]
- Ashraf, M.F.M.R.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Alessandri, A.; De Felice, M.; Zeng, N.; Mariotti, A.; Pan, Y.; Cherchi, A.; Lee, J.-Y.; Wang, B.; Ha, K.-J.; Ruti, P.; et al. Robust assessment of the expansion and retreat of Mediterranean climate in the 21st century. Sci. Rep. 2014, 4, 7211. [Google Scholar] [CrossRef]
- Ergon, A.; Seddaiu, G.; Korhonen, P.; Virkajärvi, P.; Bellocchi, G.; Jørgensen, M.; Østrem, L.; Reheul, D.; Volaire, F. How can forage production in Nordic and Mediterranean Europe adapt to the challenges and opportunities arising from climate change. Eur. J. Agron. 2018, 92, 97–106. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Dijkstra, F.A. Drought effect on plant nitrogen and phosphorus: A meta-analysis. New Phytol. 2014, 204, 924–931. [Google Scholar] [CrossRef]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.B.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Iqbal, M.A.; Li, C.; Iqbal, A.; Abbas, R.N. Overviewing Drought and Heat Stress Amelioration—From Plant Responses to Microbe-Mediated Mitigation. Sustainability 2023, 15, 1671. [Google Scholar] [CrossRef]
- Smith, M.R.; Myers, S.S. Impact of anthropogenic CO2 emissions on global human nutrition. Nat. Clim. Chang. 2018, 8, 834–839. [Google Scholar] [CrossRef]
- Weyant, C.; Brandeau, M.L.; Burke, M.; Lobell, D.B.; Bendavid, E.; Basu, S. Anticipated burden and mitigation of carbon-dioxide-induced nutritional deficiencies and related diseases: A simulation modeling study. PLoS Med. 2018, 15, e1002586. [Google Scholar] [CrossRef] [PubMed]
- Demers, D.A.; Gosselin, A. Growing greenhouse tomato and sweet pepper under supplemental lighting: Optimal photoperiod, negative effects of long photoperiod and their causes. In Proceedings of the IV International ISHS Symposium on Artificial Lighting 580, Quebec, QC, Canada, 7–9 November 2000; pp. 83–88. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Response of growth, yield, and quality of edible-podded snow peas to supplemental LED lighting during winter greenhouse production. Can. J. Plant Sci. 2018, 99, 676–687. [Google Scholar] [CrossRef]
- Fehr, W.R.; Caviness, C.E. Stages of soybean development. Iowa Coop. Ext. Serv. Iowa Agric. Home Econ. Exp. Stn. Spec. Rep. 1977, 80, 11. [Google Scholar]
- Guilioni, L.; Wery, J.; Tardieu, F. Heat stress-induced abortion of buds and flowers in pea: Is sensitivity linked to organ age or to relations between reproductive organs? Ann. Bot. 1997, 80, 159–168. [Google Scholar] [CrossRef]
- Kaushal, N.; Awasthi, R.; Gupta, K.; Gaur, P.; Siddique, K.H.; Nayyar, H. Heat-stress-induced reproductive failures in chickpea (Cicer arietinum) are associated with impaired sucrose metabolism in leaves and anthers. Funct. Plant Biol. 2013, 40, 1334–1349. [Google Scholar] [CrossRef]
- Kumari, V.; Roy, A.; Vijayan, R.; Banerjee, P.; Verma, V.; Nalia, A.; Pramanik, M.; Mukherjee, B.; Ghosh, A.; Reja, H.; et al. Drought and Heat Stress in Cool-Season Food Legumes in Sub-Tropical Regions: Consequences, Adaptation, and Mitigation Strategies. Plants 2021, 10, 1038. [Google Scholar] [CrossRef] [PubMed]
- El Nadi, A.H. Water relations of beans I. Effects of water stress on growth and flowering. Exp. Agric. 1969, 5, 195–207. [Google Scholar] [CrossRef]
- Hailemichael, G.; Catalina, A.; González, M.R.; Martin, P. Relationships between water status, leaf chlorophyll content and photosynthetic performance in Tempranillo vineyards. South Afr. J. Enol. Vitic. 2016, 37, 149–156. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Balatova, Z.; Drevenakova, P.; Olsovska, K.; Kalaji, H.M.; Yang, X.; Allakhverdiev, S.I. Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. Photosynth. Res. 2013, 117, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.K. Nutrient uptake and management under drought: Nutrient-moisture interaction. Curr. Agric. 2003, 27, 1–8. [Google Scholar]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Barber, S.A. Soil Nutrient Bioavailability: A Mechanistic Approach; John Wiley & Sons: Hoboken, NJ, USA, 1995. [Google Scholar]
- Himelblau, E.; Amasino, R.M. Nutrients mobilized from leaves of Arabidopsis thaliana during leaf senescence. J. Plant Physiol. 2001, 158, 1317–1323. [Google Scholar] [CrossRef]
- Sankaran, R.P.; Grusak, M.A. Whole shoot mineral partitioning and accumulation in pea (Pisum sativum). Front. Plant Sci. 2014, 5, 149. [Google Scholar] [CrossRef]
- Maillard, A.; Diquélou, S.; Billard, V.; Laîné, P.; Garnica, M.; Eprudent, M.; Garcia-Mina, J.-M.; Eyvin, J.-C.; Ourry, A. Leaf mineral nutrient remobilization during leaf senescence and modulation by nutrient deficiency. Front. Plant Sci. 2015, 6, 317. [Google Scholar] [CrossRef]
- Price, A.H.; Hendry, G.A.F. Iron-catalysed oxygen radical formation and its possible contribution to drought damage in nine native grasses and three cereals. Plant Cell Environ. 1991, 14, 477–484. [Google Scholar] [CrossRef]
- Gadallah MAA Effects of indole-3-acetic acid and zinc on the growth, osmotic potential and soluble carbon and nitrogen components of soybean plants growing under water deficit. J. Arid. Environ. 2000, 44, 451–467. [CrossRef]
- Iuchi, S.; Kobayashi, M.; Taji, T.; Naramoto, M.; Seki, M.; Kato, T.; Tabata, S.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001, 27, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, X.; Wang, W.; Wang, Y.; Ming, F. The suppression of WRKY44 by GIGANTEA-miR172 pathway is involved in drought response of Arabidopsis thaliana. PLoS ONE 2013, 8, e73541. [Google Scholar] [CrossRef] [PubMed]
- Fitter, A.H.; Fitter, R.S.R.; Harris, I.T.B.; Williamson, M.H. Relationships between first flowering date and temperature in the flora of a locality in central England. Funct. Ecol. 1995, 9, 55–60. [Google Scholar] [CrossRef]
- Prasad, P.V.; Boote, K.J.; Allen, L.H., Jr.; Thomas, J.M. Effects of elevated temperature and carbon dioxide on seed-set and yield of kidney bean (Phaseolus vulgaris L.). Glob. Change Biol. 2002, 8, 710–721. [Google Scholar] [CrossRef]
- Rainey, K.M.; Griffiths, P.D. Inheritance of heat tolerance during reproductive development in snap bean (Phaseolus vulgaris L.). J. Am. Soc. Hortic. Sci. 2005, 130, 700–706. [Google Scholar] [CrossRef]
- Krishnamurthy, L.; Gaur, P.M.; Basu, P.S.; Chaturvedi, S.K.; Tripathi, S.; Vadez, V.; Rathore, A.; Varshney, R.K.; Gowda, C.L.L. Large genetic variation for heat tolerance in the reference collection of chickpea (Cicer arietinum L.) germplasm. Plant Genet. Resour. 2011, 9, 59–69. [Google Scholar] [CrossRef]
- Kumar, S.; Thakur, P.; Kaushal, N.; Malik, J.A.; Gaur, P.; Nayyar, H. Effect of varying high temperatures during reproductive growth on reproductive function, oxidative stress and seed yield in chickpea genotypes differing in heat sensitivity. Arch. Agron. Soil Sci. 2013, 59, 823–843. [Google Scholar] [CrossRef]
- Kumari, V.V.; Banerjee, P.; Verma, V.C.; Sukumaran, S.; Chandran, M.A.S.; Gopinath, K.A.; Venkatesh, G.; Yadav, S.K.; Singh, V.K.; Awasthi, N.K. Plant nutrition: An effective way to alleviate abiotic stress in agricultural crops. Int. J. Mol. Sci. 2022, 23, 8519. [Google Scholar] [CrossRef]
- Bista, D.R.; Heckathorn, S.A.; Jayawardena, D.M.; Mishra, S.; Boldt, J.K. Effects of drought on nutrient uptake and the levels of nutrient-uptake proteins in roots of drought-sensitive and-tolerant grasses. Plants 2018, 7, 28. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Schwarz, D.; Franken, P.; Colla, G. Effects of drought on nutrient uptake and assimilation in vegetable crops. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Springer: Berlin/Heidelberg, Germany, 2012; pp. 171–195. [Google Scholar] [CrossRef]
- Peck, A.W.; McDonald, G.K. Adequate zinc nutrition alleviates the adverse effects of heat stress in bread wheat. Plant Soil 2010, 337, 355–374. [Google Scholar] [CrossRef]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Galluzzi, G.; Seyoum, A.; Halewood, M.; López Noriega, I.; Welch, E.W. The role of genetic resources in breeding for climate change: The case of public breeding programmes in eighteen developing countries. Plants 2020, 9, 1129. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).