Chemical Speciation of Trace Elements in Soil Fertilized with Biomass Combustion Ash and Their Accumulation in Winter Oilseed Rape Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Ash from Biomass Combustion and Field-Experiment Conditions
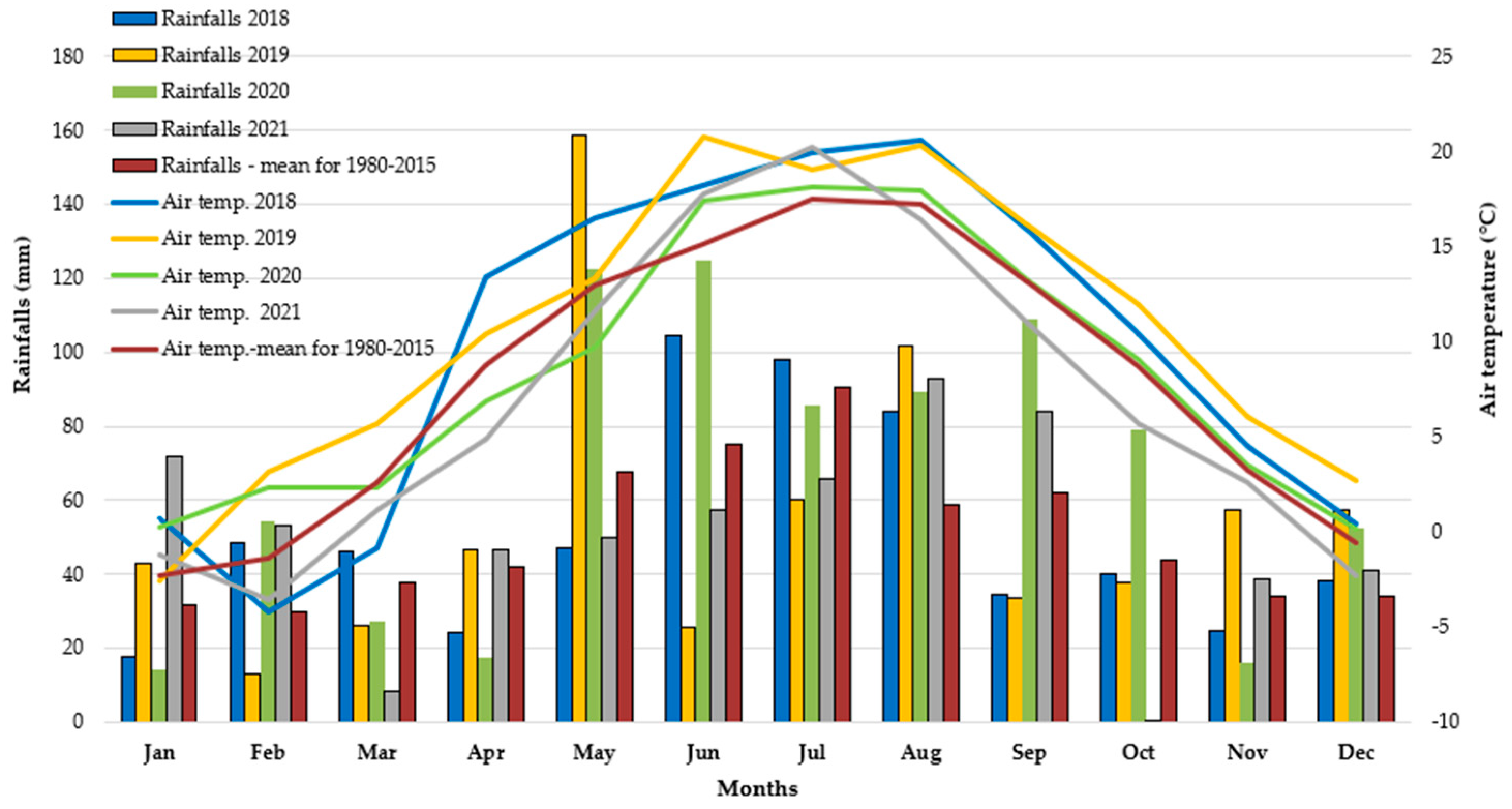
2.2. Weather Conditions during the Study Period (2019–2021)
2.3. BCR Sequential Extraction Procedure Applied to Biomass Combustion Ashes and Soil Samples, and Assessment of Environmental Risk
2.4. Analysis of the Trace Element Content in Different Parts of Winter Oilseed Rape Plants
2.5. Statistical Analysis
3. Results
3.1. Chemical Fraction and Total Concentration of Trace Elements in BAs
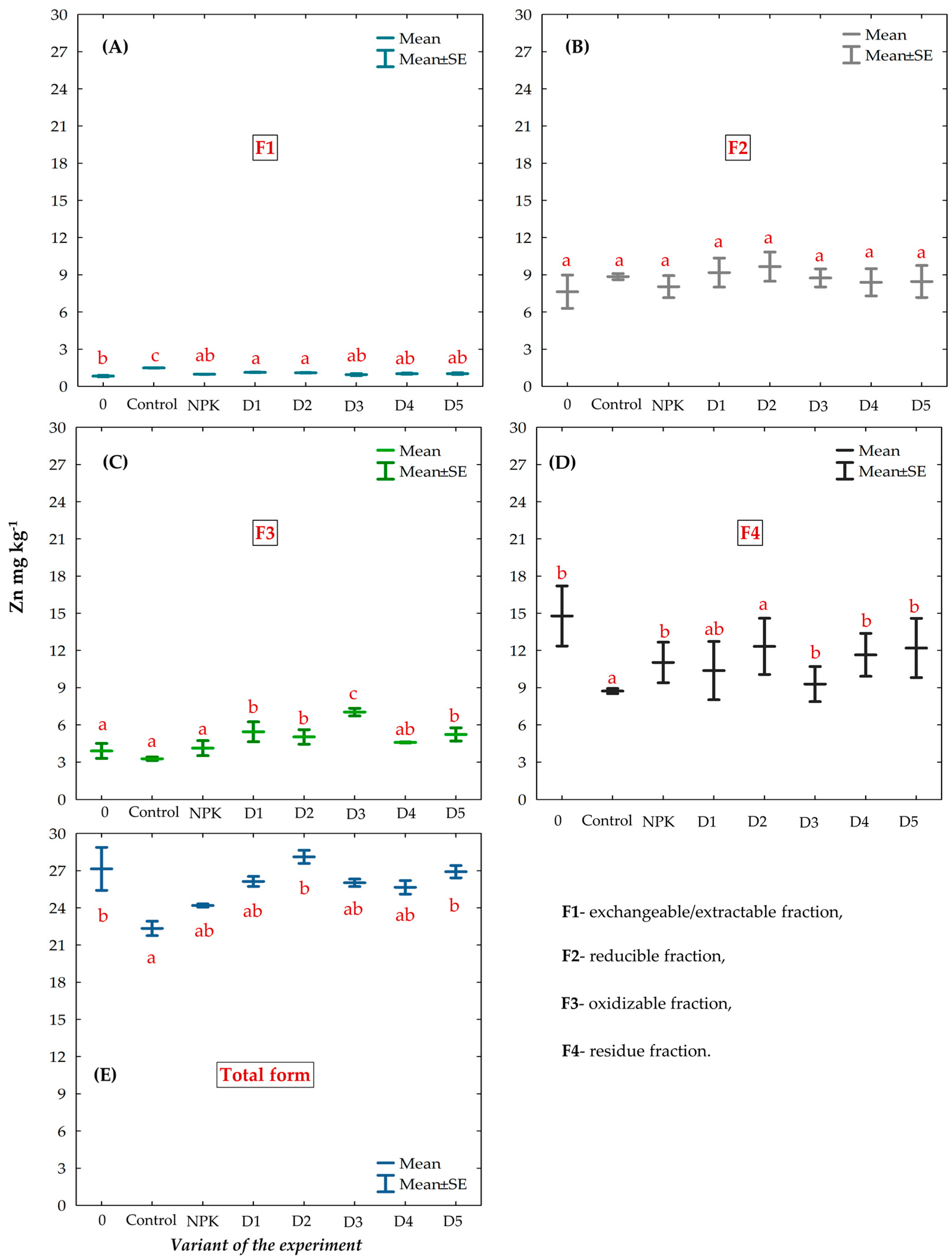
3.2. Chemical Fractions and Total Concentration of Zn in the Gleyic Chernozem Soil Fertilized with BAs
3.3. Chemical Fractions and Total Concentration of Cu in the Gleyic Chernozem Soil Fertilized with BAs
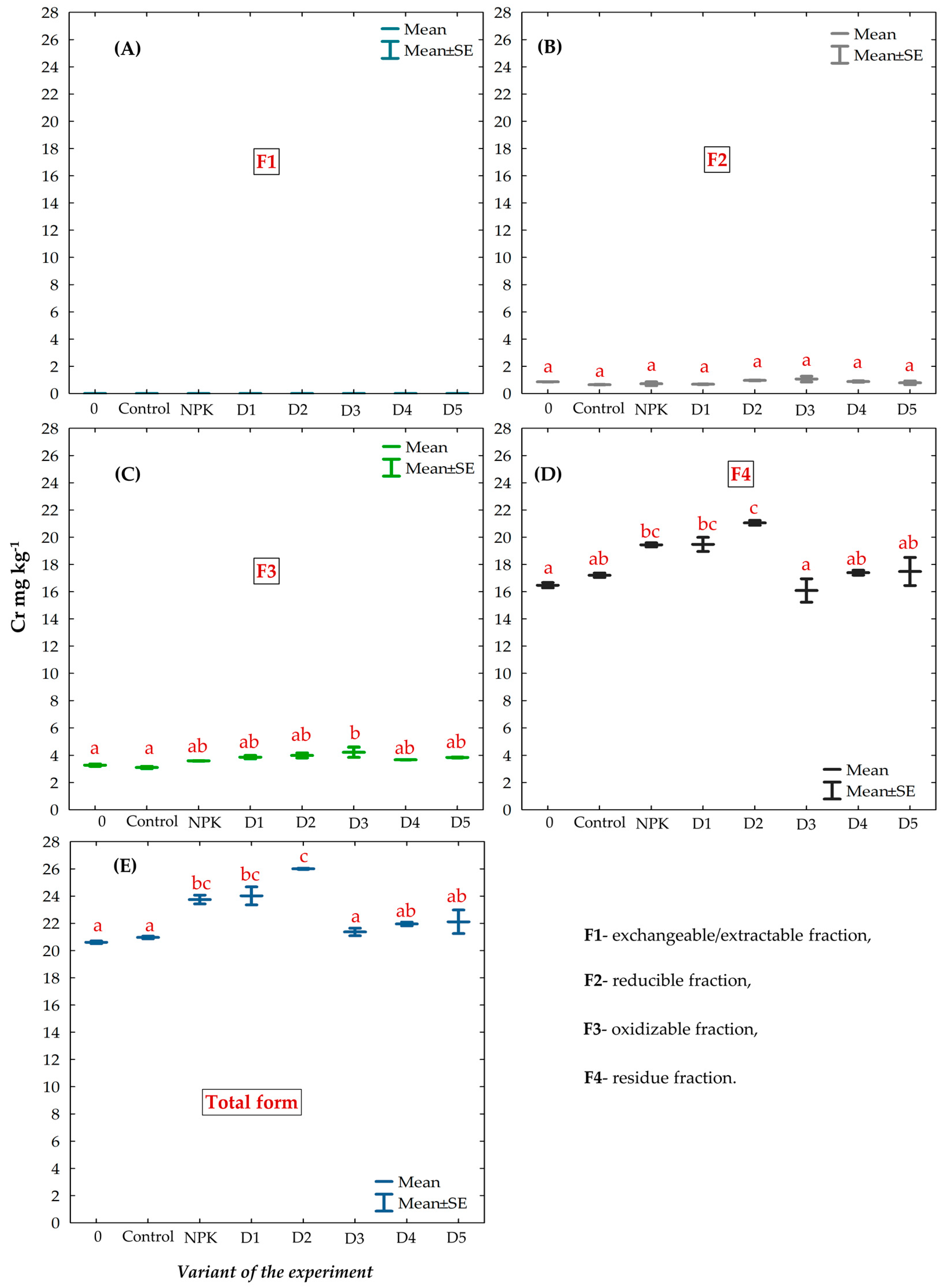
3.4. Chemical Fractions and Total Concentration of Cr in the Gleyic Chernozem Soil Fertilized with BAs
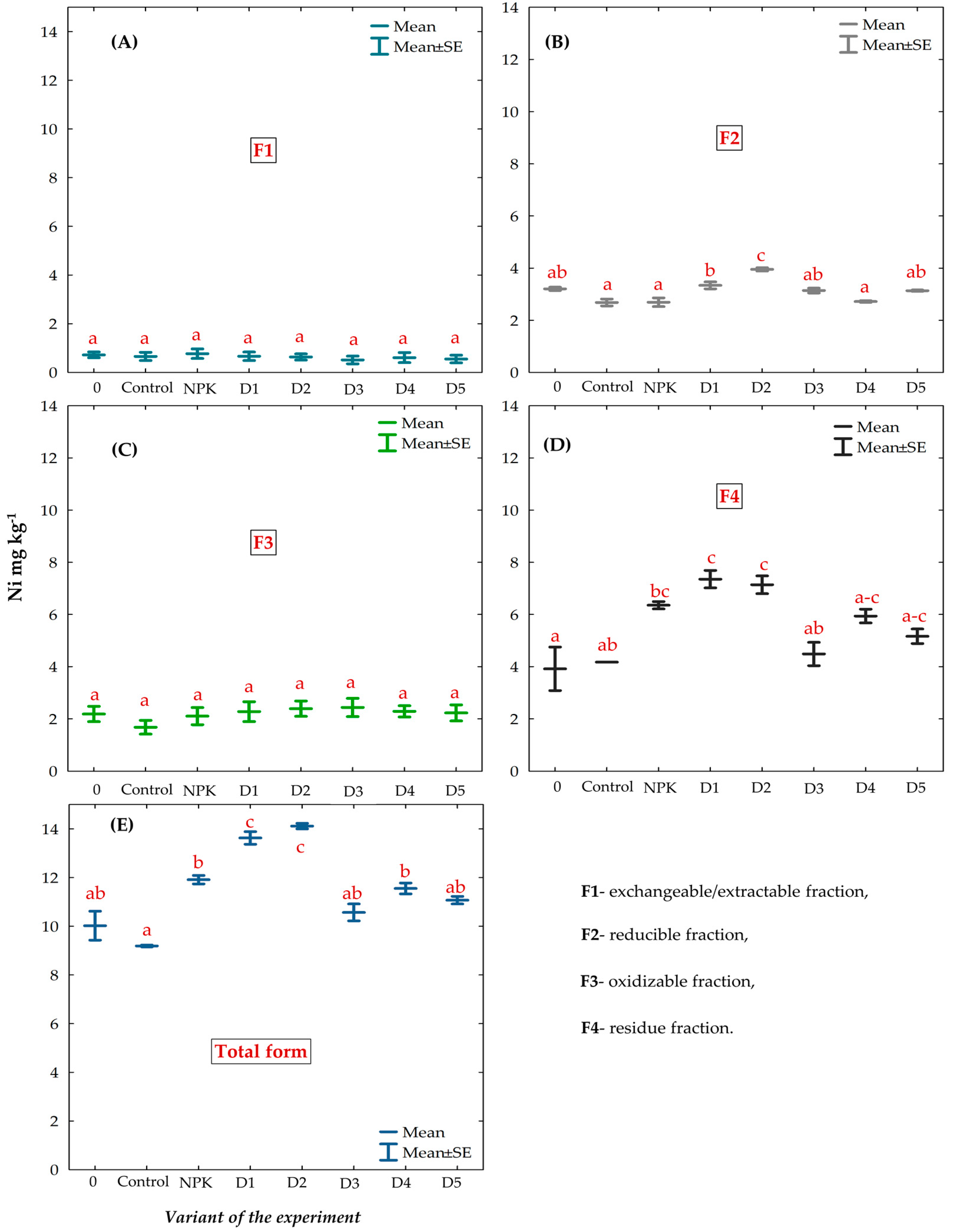
3.5. Chemical Fractions and Total Concentration of Ni in the Gleyic Chernozem Soil Fertilized with BAs
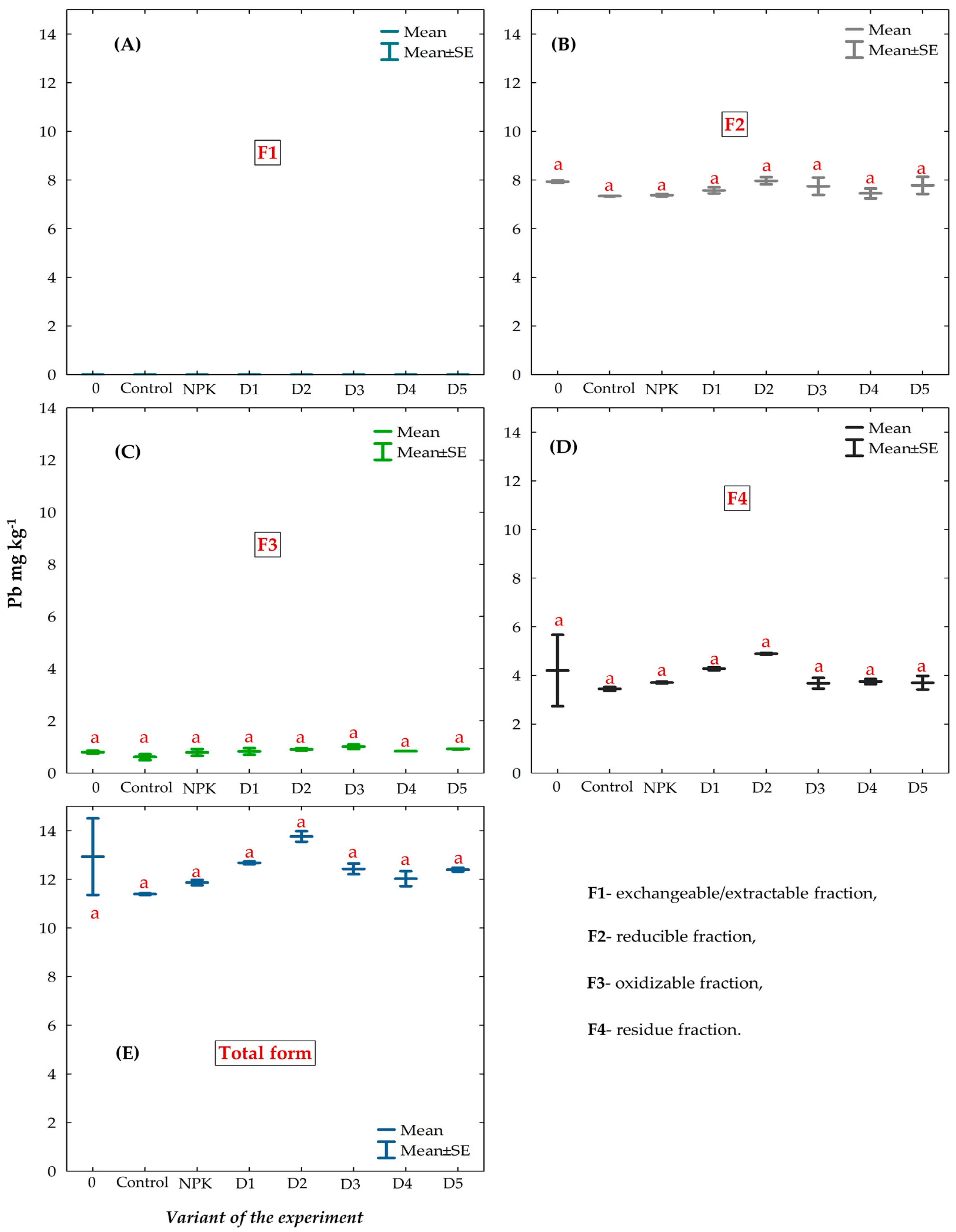
3.6. Chemical Fractions and Total Concentration of Pb in the Gleyic Chernozem Soil Fertilized with BAs
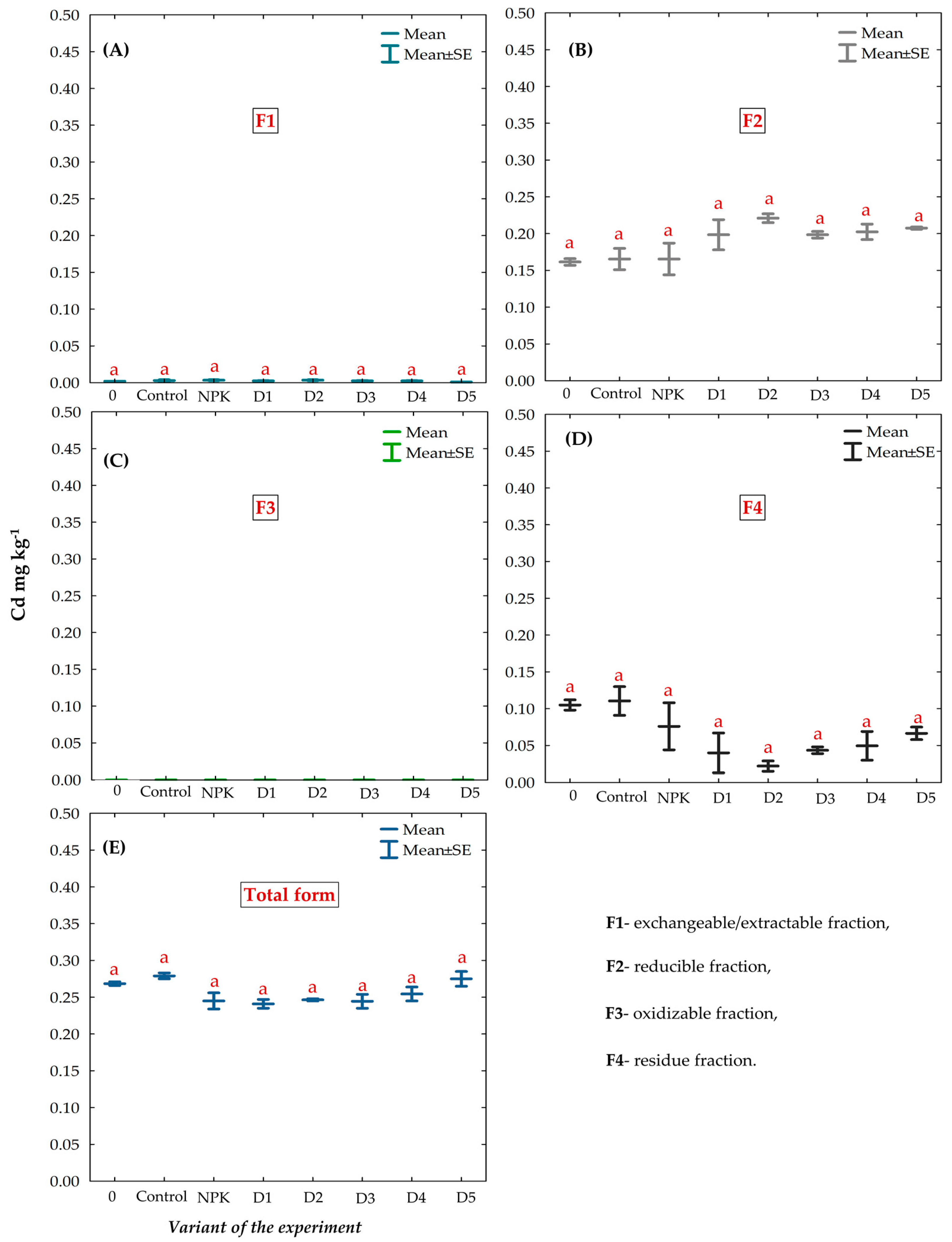
3.7. Chemical Fractions and Total Concentration of Cd in the Gleyic Chernozem Soil Fertilized with BAs
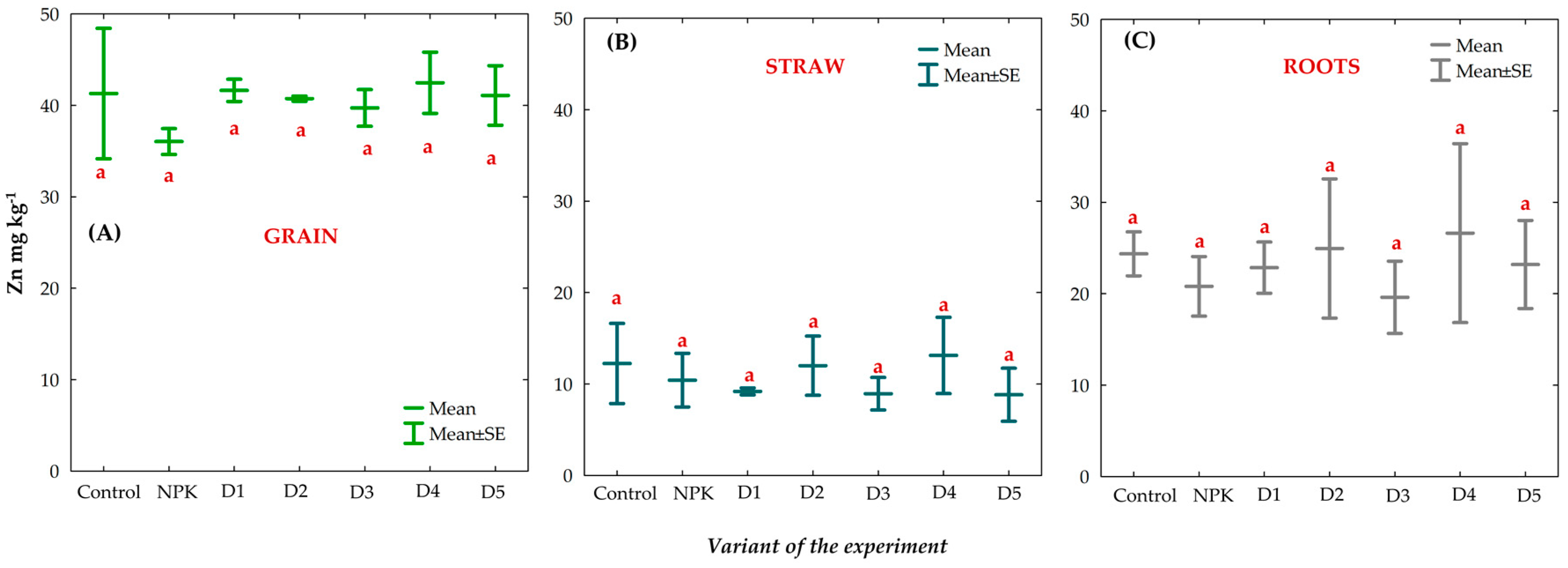
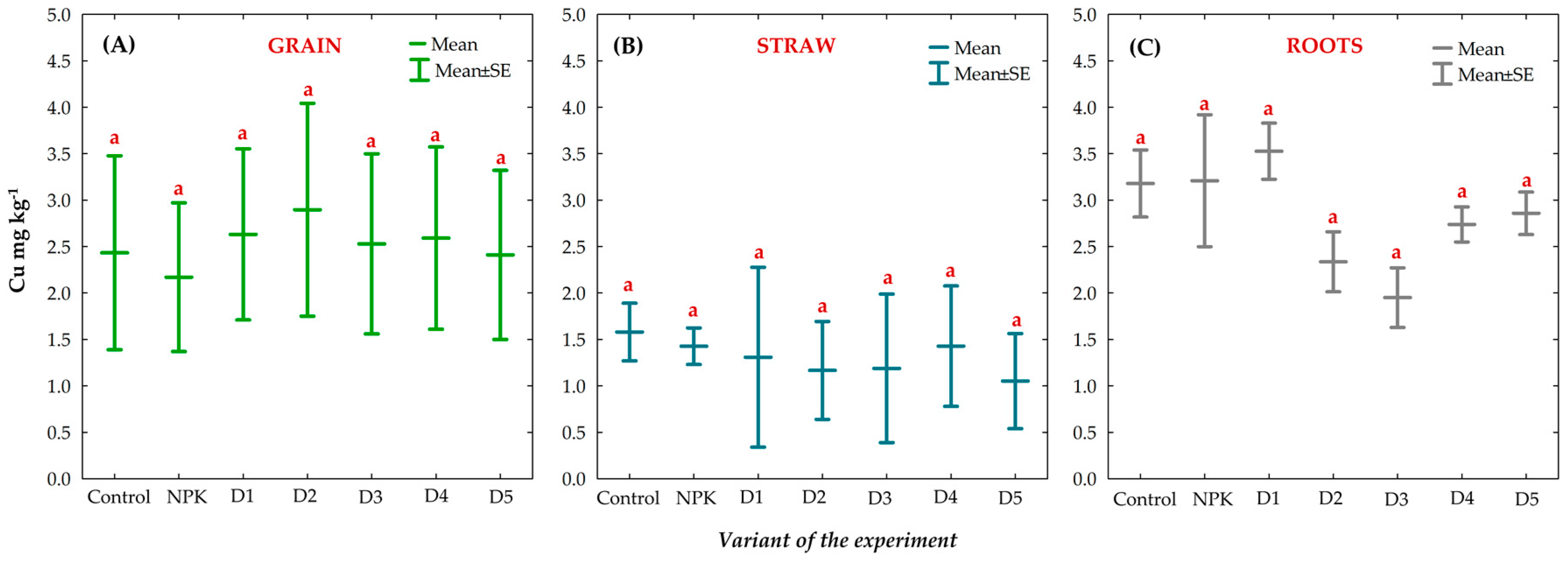
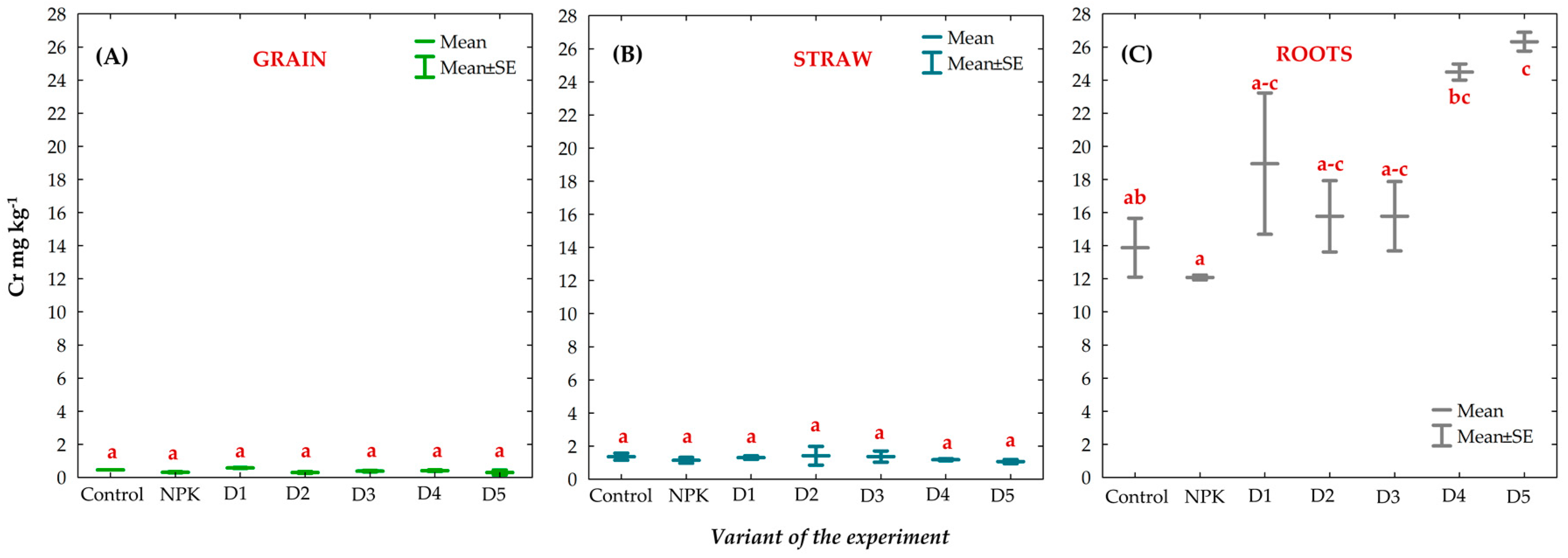
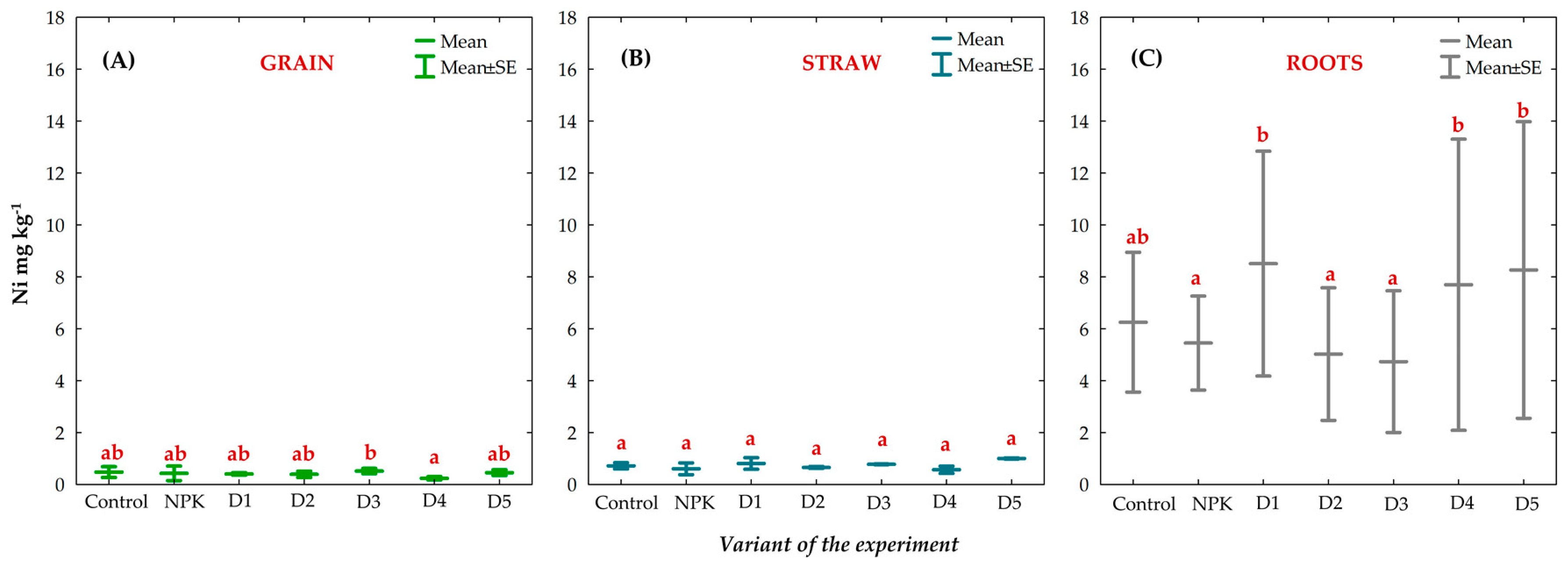
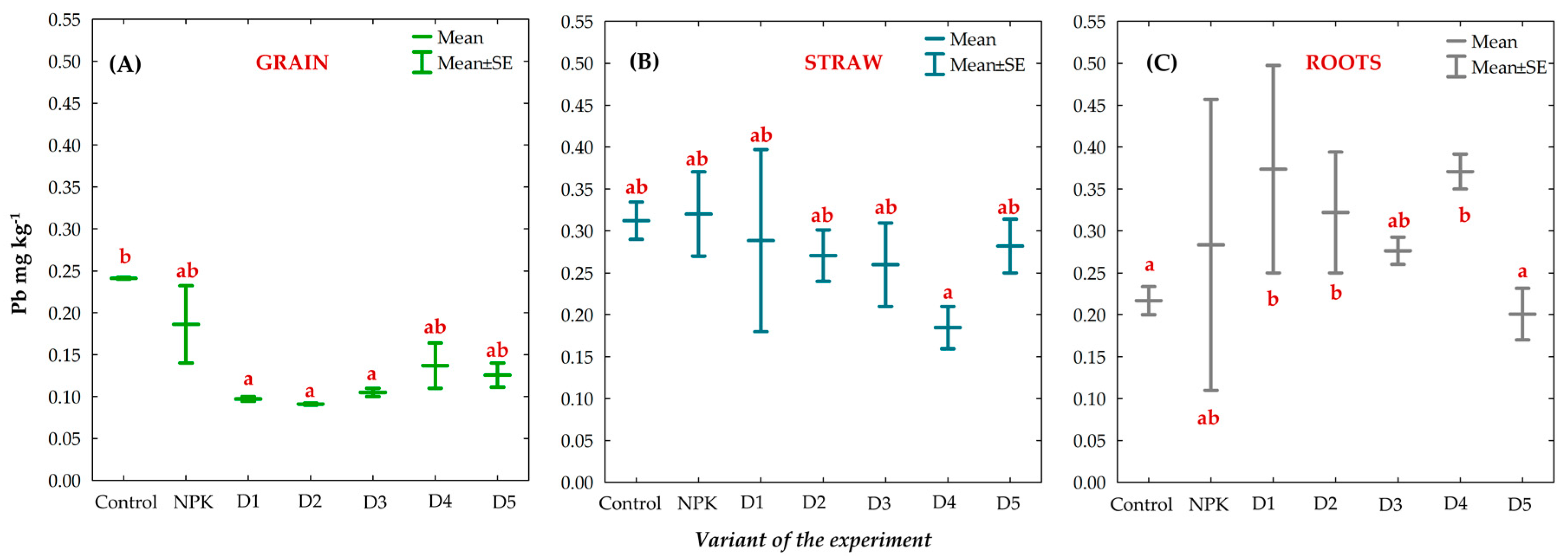
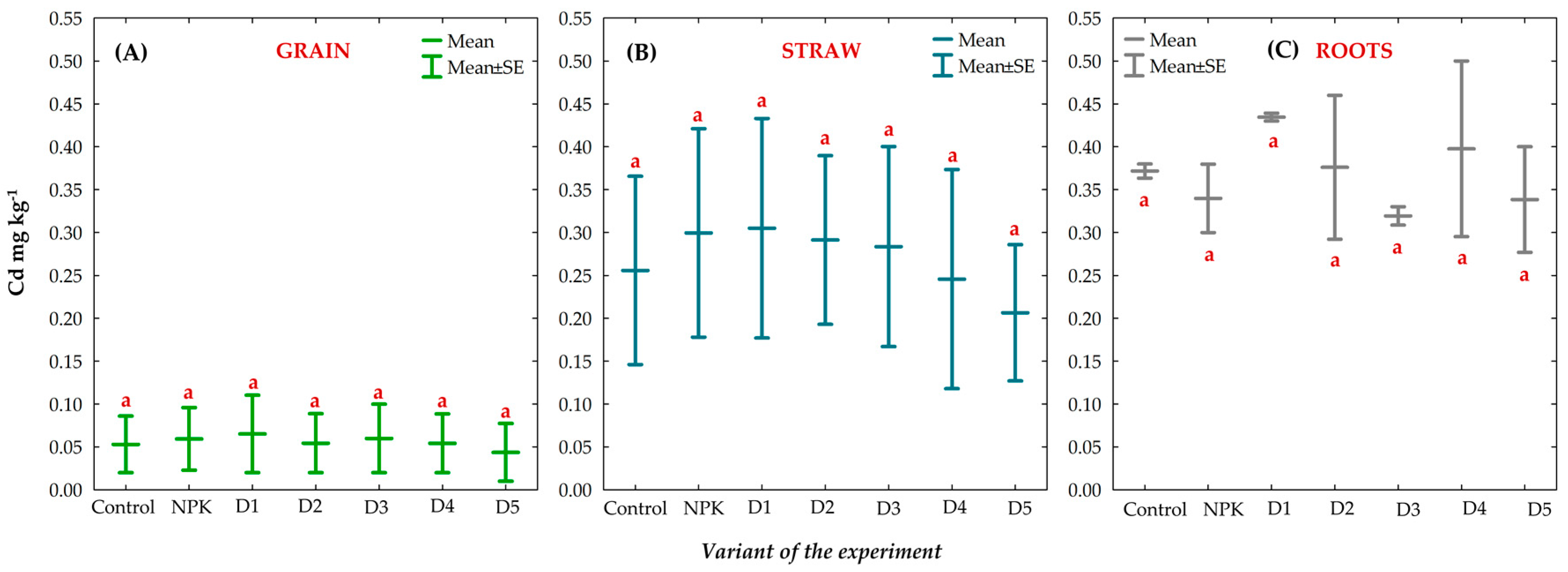
3.8. Effect of BAs on the Concentration of Trace Elements (TEs) in Winter Oilseed Rape Plants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yana, S.; Nizar, M.; Irhamni; Mulyati, D. Biomass waste as a renewable energy in developing bio-based economies in Indonesia: A review. Renew. Sustain. Energy Rev. 2022, 160, 112268. [Google Scholar] [CrossRef]
- Cruz, N.C.; Silva, F.C.; Tarelho, L.A.C.; Rodrigues, S.M. Critical review of key variables affecting potential recycling applications of ash produced at large-scale biomass combustion plants. Resour. Conserv. Recycl. 2019, 150, 104427. [Google Scholar] [CrossRef]
- Zhai, J.; Burke, I.T.; Stewart, D.I. Beneficial management of biomass combustion ashes. Renew. Sustain. Energy Rev. 2021, 151, 111555. [Google Scholar] [CrossRef]
- Silva, F.C.; Cruz, N.C.; Tarelho, L.A.C.; Rodrigues, S.M. Use of biomass ash-based materials as soil fertilizers: Critical review of the existing regulatory framework. J. Clean. Prod. 2019, 214, 112–124. [Google Scholar] [CrossRef]
- Munawar, M.A.; Khoja, A.H.; Hassan, M.; Liaquat, R.; Naqvi, S.R.; Mehran, M.T.; Abdullah, A.; Saleem, F. Biomass ash characterization, fusion analysis and its application in catalytic decomposition of methane. Fuel 2021, 285, 119107. [Google Scholar] [CrossRef]
- Thomas, B.; Yang, J.; Mo, K.; Abdalla, J.; Hawileh, R.; Ariyachandra, E. Biomass ashes from agricultural wastes as supplementary cementitious materials or aggregate replacement in cement/geopolymer concrete: A comprehensive review. J. Build. Eng. 2021, 40, 102332. [Google Scholar] [CrossRef]
- Waste Framework Directive (2008/98/EC) of the European Parliament and of the Council of 19 November 2008 on waste and repealing certain Directives. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32008L0098:EN:NOT (accessed on 1 December 2022).
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical Characteristics of Biomass Ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Kramar, V. Possible utilization directions of ash from biomass combustion. Biomass ash as fertilizer in agriculture. UABIO Bioenergy Assoc. Ukr. 2020, 27, 33–36. [Google Scholar]
- Szpunar-Krok, E.; Szostek, M.; Pawlak, R.; Gorzelany, J.; Migut, D. Effect of Fertilisation with Ash from Biomass Combustion on the Mechanical Properties of Potato Tubers (Solanum tuberosum L.) Grown in Two Types of Soil. Agronomy 2022, 12, 379. [Google Scholar] [CrossRef]
- Szostek, M.; Szpunar-Krok, E.; Jańczak-Pieniążek, M.; Ilek, A. Short-Term Effect of Fly Ash from Biomass Combustion on Spring Rape Plants Growth, Nutrient, and Trace Elements Accumulation, and Soil Properties. Int. J. Environ. Res. Public Health 2023, 20, 455. [Google Scholar] [CrossRef]
- Pasquali, M.; Zanoletti, A.; Benassi, L.; Federici, S.; Depero, L.E.; Bontempi, E. Stabilized biomass ash as a sustainable substitute for commercial P-fertilizers. Land Degrad. Dev. 2018, 29, 2199–2207. [Google Scholar] [CrossRef]
- Poluszyńska, J. Possibilities of applications of fly ash from the biomass combustion in the sludge management. Prace Inst. Ceram. Mater. Bud. Pract. Inst. Ceram. I Mater. Bud. 2013, 6, 49–59. (In Polish) [Google Scholar]
- Trivedi, N.S.; Mandavgane, S.A.; Mehetre, S.; Kulkarni, B.D. Characterization and valorization of biomass ashes. Environ. Sci. Pollut. Res. 2016, 23, 20243–20256. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.J.R.; Matias, J.C.O.; Catalão, J.P.S. Biomass combustion systems: A review on the physical and chemical properties of the ashes. Renew. Sustain. Energy Rev. 2016, 53, 235–242. [Google Scholar] [CrossRef]
- Stankowski, S.; Sobolewska, M.; Jaroszewska, A.; Gibczyńska, M. Influence of biomass ash, lime and gypsum fertilization on macro-and microelement contents in the soil and grains of spring wheat. Soil Sci. Annu. 2018, 69, 177–183. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Gibczyńska, M.; Stankowski, S.; Hury, G.; Kuglarz, K. Effects of limestone, ash from biomass and compost use on chemical properties of soil. Soil Sci. Annu. 2014, 65, 59–64. [Google Scholar] [CrossRef]
- Ure, A.M.; Quevauviller, P.; Muntau, H.; Griepink, B. Speciation of heavy metals in soils and sediments. an account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. Int. J. Environ. Anal. Chem. 1993, 51, 135–151. [Google Scholar] [CrossRef]
- Gleyzes, C.; Tellier, S.; Astruc, M. Fractionation studies of trace elements in contaminated soils and sediments: A review of sequential extraction procedure. Trends Anal. Chem. 2002, 21, 451–467. [Google Scholar] [CrossRef]
- Fernández, E.; Jimènez, R.; Lallena, A.M.; Aguilar, J. Evaluation of the BCR sequential extraction procedure applied for two unpolluted Spanish soil. Environ. Pollut. 2004, 13, 355–364. [Google Scholar] [CrossRef]
- Pan, H.; Enerhadt, T. Fly ash as a soil amendment. Bioresources 2011, 6, 3987–4004. [Google Scholar]
- Karbowska, B.; Zembrzuski, W. Fractionation and Mobility of Thallium in Volcanic Ashes after Eruption of Eyjafjallajökull (2010) in Iceland. Bull. Environ. Contam. Toxicol. 2016, 97, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Gwebu, S.; Tavengw, N.T.; Klink, M.J.; Mtunzi, F.M.; Modise, S.J.; Pakade, V.E. Quantification of Cd, Cu, Pb and Zn from sewage sludge by modified-BCR and ultrasound assisted-modified BCR sequential extraction methods. Afr. J. Pure Appl. Chem. 2017, 11, 9–18. [Google Scholar]
- WRB. World Reference Base for Soil Resources 2014; Update, World Soil Resources Reports, 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Bank Danych o Lasach. Available online: www.bdl.lasy.gov.pl/portal/mapy (accessed on 23 December 2021).
- Zhang, J.; Zhang, J.; Tian, Y.; Li, N.; Kong, L.; Sun, L.; Yu, M.; Zuo, W. Changes of physicochemical properties of sewage sludge during ozonation treatment: Correlation to sludge dewaterability. Chem. Eng. J. 2016, 301, 238–248. [Google Scholar] [CrossRef]
- Tytła, M. Assessment of heavy metal pollution and potential ecological risk in sewage sludge from municipal wastewater treatment plant located in the most industrialized region in Poland–Case study. J. Environ. Res. Public Health 2019, 16, 2430. [Google Scholar] [CrossRef]
- Latosińska, J.; Kowalik, R.; Gawdzik, J. Risk Assessment of Soil Contamination with Trace elements from Municipal Sewage Sludge. Appl. Sci. 2021, 11, 548. [Google Scholar] [CrossRef]
- Adriano, D.C.; Wenzel, W.W.; Vangronsveld, J.; Bolan, N.S. Role of assisted natural remediation in environmental cleanup. Geoderma 2004, 122, 121–142. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, NM, USA; London, UK; New York, NY, USA; Washington, DC, USA, 2001. [Google Scholar]
- Alvarez, A.; Catalano, S.A.; Amoroso, M.J. Heavy metal resistant strains are widespread along Streptomyces phylogeny. Mol. Phylogenet. Evol. 2013, 66, 1083–1088. [Google Scholar] [CrossRef]
- Barančíková, G.; Makovníková, J. The influence of humic acid quality on the sorption and mobility of heavy metals. Plant Soil Environ. 2003, 49, 565–571. [Google Scholar] [CrossRef]
- Pourret, O.; Davranche, M.; Gruau, G.; Dia, A. Rare earth elements complexation with humic acid. Chem. Geol. 2007, 243, 128–141. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Tsadilas, C.D.; Rinklebe, J. A review of the distribution coefficients of trace elements in soils: Influence of sorption system, element characteristics, and soil colloidal properties. Adv. Colloid Interface Sci. 2013, 201–202, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Ernst, W.H.O. Evolution of metal tolerance in higher plants. For. Snow Landsc. Res. 2006, 80, 251–274. [Google Scholar]
- Koniuszy-Nycz, J. Evaluation of leaching and bioavailability of heavy metals from industrial biomass ashes. Uniw. Zielonogórski Zesz. Nauk. Inżynieria Środowiska 2018, 167, 18–33. (In Polish) [Google Scholar] [CrossRef]
- Jukić, M.; Ćurković, L.; Šabarić, J.; Kerolli-Mustafa, M. Fractionation of Heavy Metals in Fly Ash from Wood Biomass Using the BCR Sequential Extraction Procedure. Bull. Environ. Contam. Toxicol. 2017, 99, 524–529. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef]
- Bonanno, G. Trace element accumulation and distribution in the organs of Phragmites australis (common reed) and biomonitoring applications. Ecotoxicol. Environ. Saf. 2011, 74, 1057–1064. [Google Scholar] [CrossRef]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Factors affecting phytoextraction: A review. Pedosphere 2016, 26, 148–166. [Google Scholar] [CrossRef]
- Kushwaha, A.; Hans, N.; Kumar, S.; Rani, R. A critical review on speciation, mobilization and toxicity of lead in soilmicrobe-plant system and bioremediation strategies. Ecotoxicol. Environ. Saf. 2018, 147, 1035–1045. [Google Scholar] [CrossRef]
- Gerhardt, K.E.; Gerwing, P.D.; Greenberg, B.M. Opinion: Taking phytoremediation from proven technology to accepted practice. Plant Sci. 2017, 256, 170–185. [Google Scholar] [CrossRef]
- Gucwa-Przepióra, E.; Nadgórska-Socha, A.; Fojcik, B.; Chmura, D. Enzymatic activities and arbuscular mycorrhizal colonization of Plantago lanceolata and Plantago major in a soil root zone under heavy metal stress. Environ. Sci. Pollut. Res. 2016, 23, 4742–4755. [Google Scholar] [CrossRef] [PubMed]
- Wierzbowska, J.; Sienkiewicz, S.; Żarczyński, P.; Krzebietke, S. Environmental Application of Ash from Incinerated Biomass. Agronomy 2020, 10, 482. [Google Scholar] [CrossRef]
- Murtaza, G.; Usman, Y.; Niazi, N.K.; Usman, M.; Hussain, T. Bioaccumulation of Potentially Toxic Elements in Cereal and Legume Crops: A Review. Clean Soil Air Water 2017, 45, 1700548. [Google Scholar] [CrossRef]
- Shahkolaie, S.S.; Baranimotlagh, M.; Dordipour, E.; Khormali, F. Cadmium and lead immobilization in a calcareous contaminated soil using the cost-effective amendments. Arab. J. Geosci. 2019, 12, 733. [Google Scholar] [CrossRef]
- Jakubus, M.; Graczyk, M. The Effect of Compost and Fly Ash Treatment of Contaminated Soil on the Immobilisation and Bioavailability of Lead. Agronomy 2021, 11, 1188. [Google Scholar] [CrossRef]
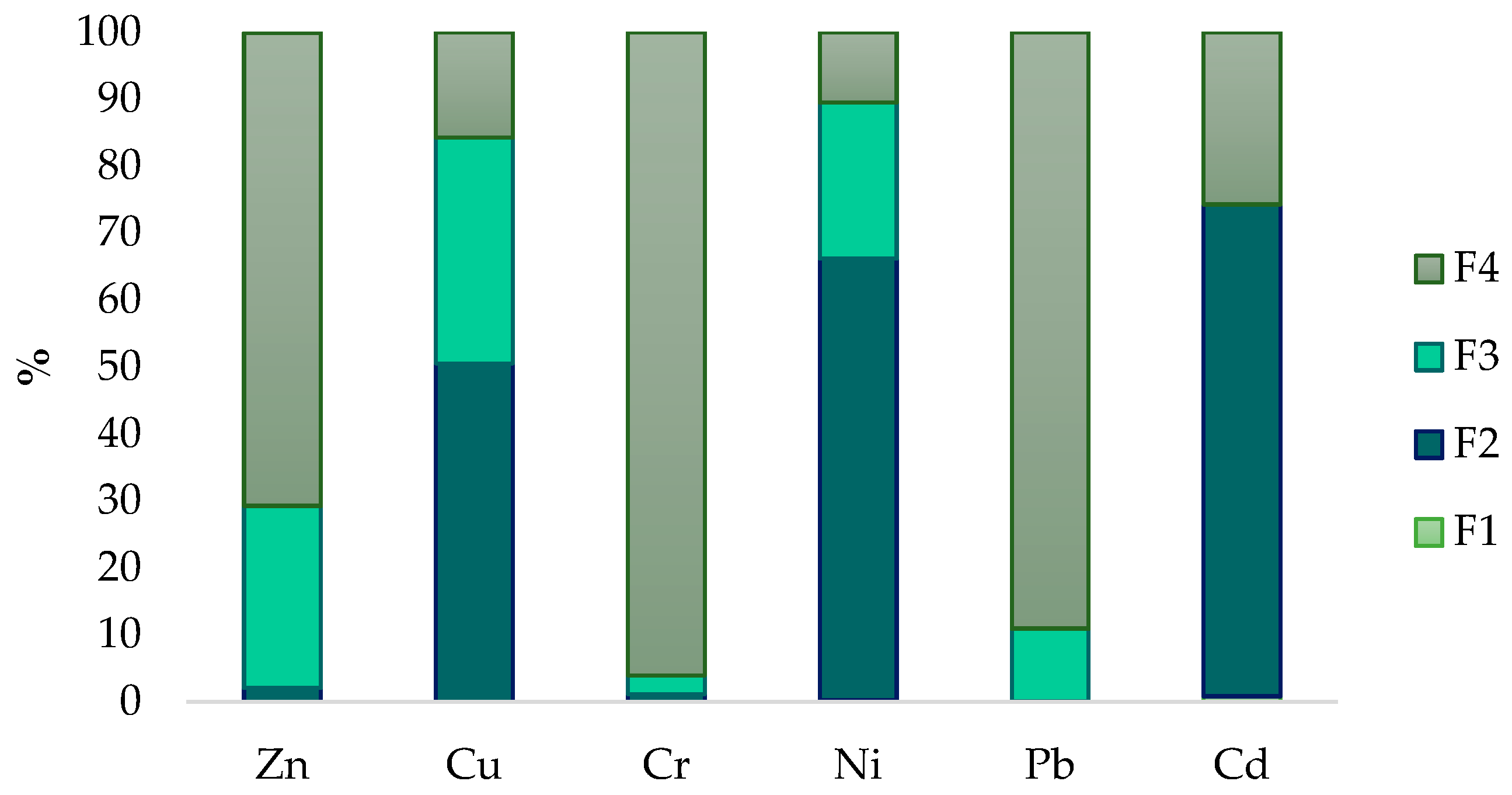

| Parameter | F1 | F2 | F3 | F4 | Total Form | RAC |
|---|---|---|---|---|---|---|
| mg kg−1 | % | |||||
| Zn | 0.05 ± 0.001 | 9.67 ± 2.34 | 127.8 ± 9.12 | 332 ± 13.72 | 470 ± 2.19 | <1 |
| Cu | 0.01 ± 0.00 | 157.04 ± 18.29 | 104.77 ± 3.04 | 48.98 ± 22.74 | 310.8 ± 1.41 | <1 |
| Cr | <0.001 | 0.88 ± 0.14 | 2.17 ± 0.09 | 74.65 ± 1.07 | 77.7 ± 1.30 | <1 |
| Ni | 0.08 ± 0.17 | 18.95 ± 0.52 | 6.69 ± 0.63 | 3.03 ± 0.79 | 28.74 ± 0.54 | <1 |
| Pb | <0.001 | 0.026 ± 0.03 | 4.09 ± 0.68 | 33.51 ± 0.14 | 37.63 ± 0.81 | <1 |
| Cd | 0.03 ± 0.01 | 2.71 ± 0.09 | <0.001 | 0.95 ± 0.14 | 3.69 ± 0.06 | <1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szostek, M.; Szpunar-Krok, E.; Ilek, A. Chemical Speciation of Trace Elements in Soil Fertilized with Biomass Combustion Ash and Their Accumulation in Winter Oilseed Rape Plants. Agronomy 2023, 13, 942. https://doi.org/10.3390/agronomy13030942
Szostek M, Szpunar-Krok E, Ilek A. Chemical Speciation of Trace Elements in Soil Fertilized with Biomass Combustion Ash and Their Accumulation in Winter Oilseed Rape Plants. Agronomy. 2023; 13(3):942. https://doi.org/10.3390/agronomy13030942
Chicago/Turabian StyleSzostek, Małgorzata, Ewa Szpunar-Krok, and Anna Ilek. 2023. "Chemical Speciation of Trace Elements in Soil Fertilized with Biomass Combustion Ash and Their Accumulation in Winter Oilseed Rape Plants" Agronomy 13, no. 3: 942. https://doi.org/10.3390/agronomy13030942
APA StyleSzostek, M., Szpunar-Krok, E., & Ilek, A. (2023). Chemical Speciation of Trace Elements in Soil Fertilized with Biomass Combustion Ash and Their Accumulation in Winter Oilseed Rape Plants. Agronomy, 13(3), 942. https://doi.org/10.3390/agronomy13030942








