Different Non-Target Site Mechanisms Endow Different Glyphosate Susceptibility in Avena Species from Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection
2.2. Biometric Study and Taxonomic Identify
2.3. Dose-Response Assays
2.4. Spray Retention Assays
2.5. Shikimate Accumulation Assay
2.6. Glyphosate Absorption and Mobilization Assays
2.7. Glyphosate Metabolism
2.8. Activity of Target Enzyme of Glyphosate
2.9. Statistical Study
3. Results
3.1. Biometric Study and Taxonomic Identify
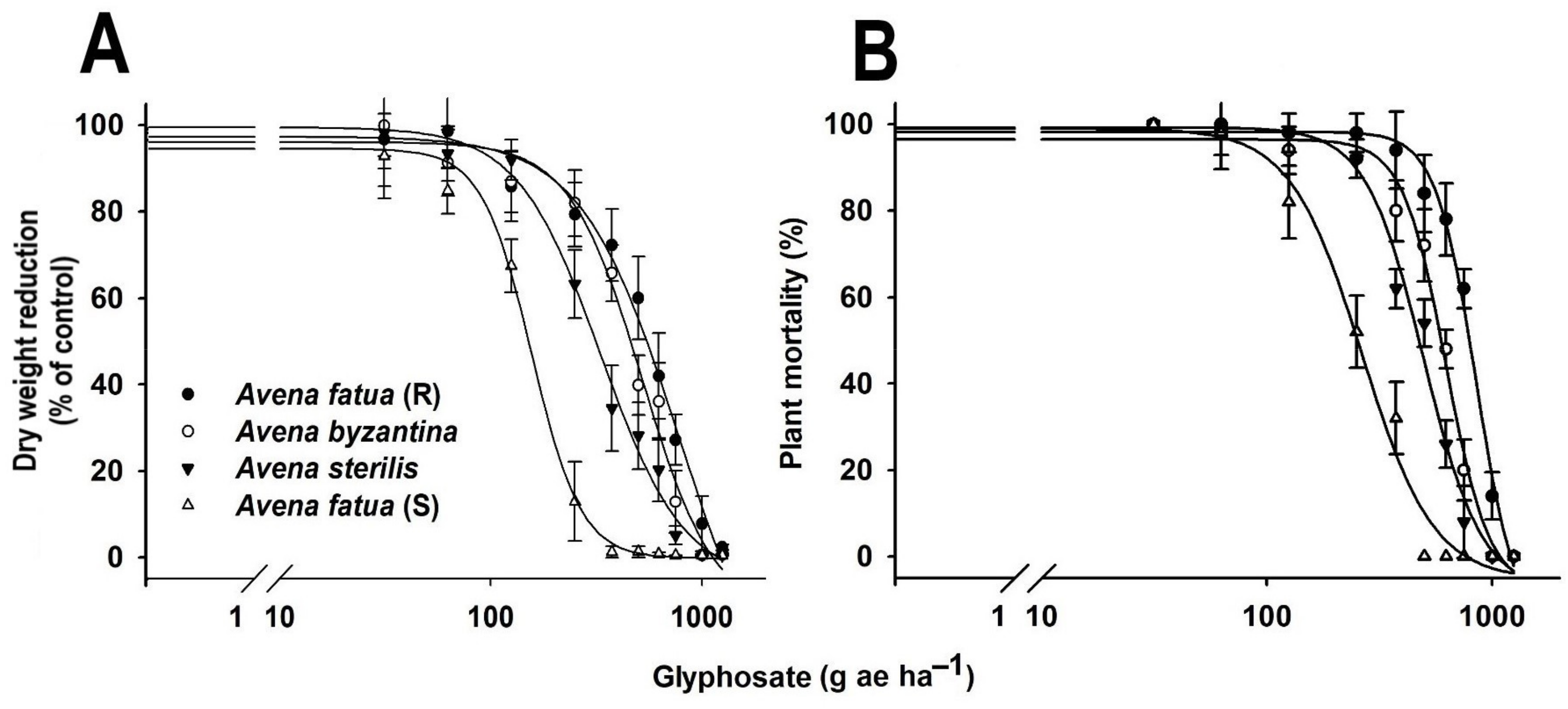
3.2. Dose-Response Assay
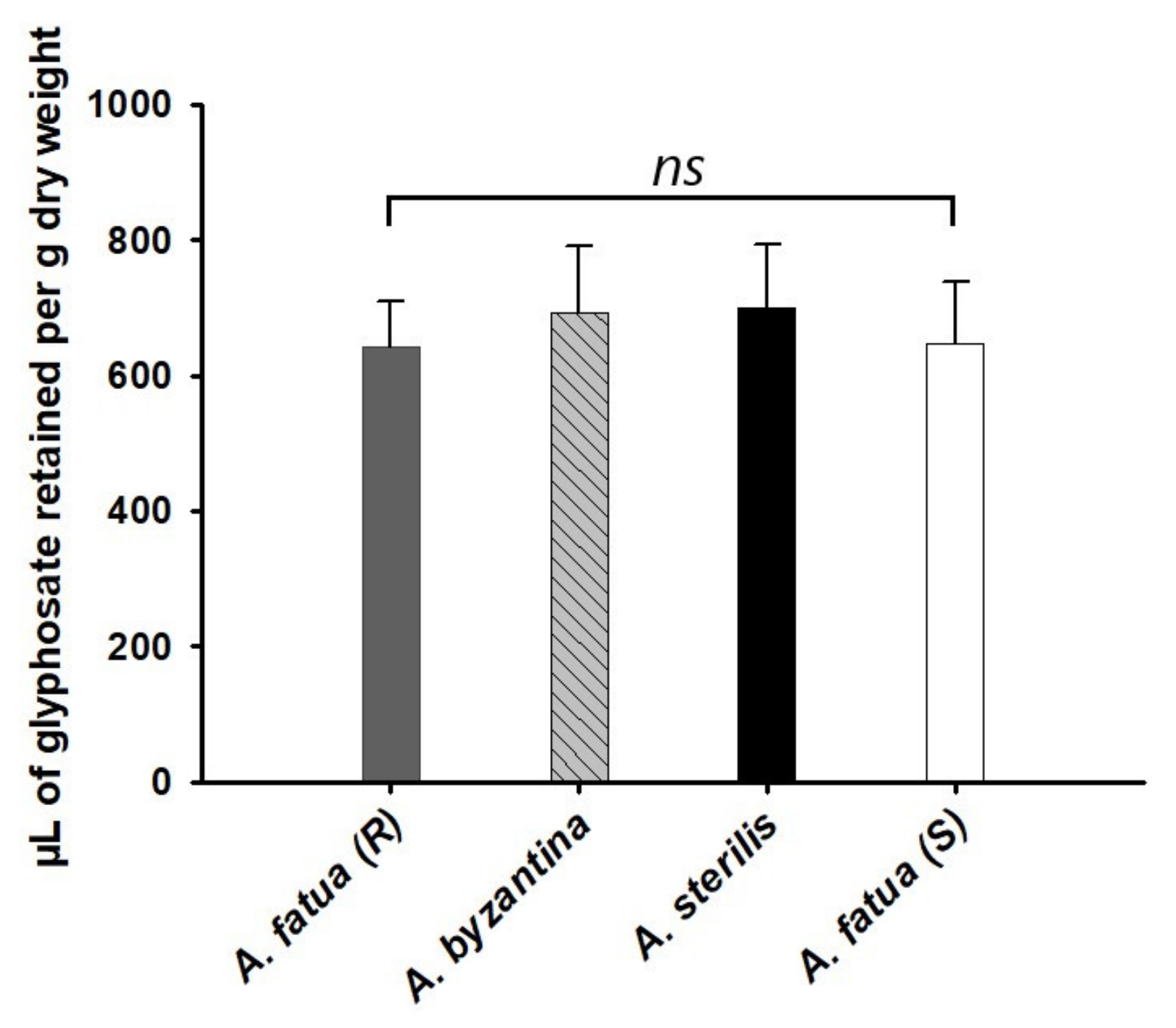
3.3. Herbicide Retention
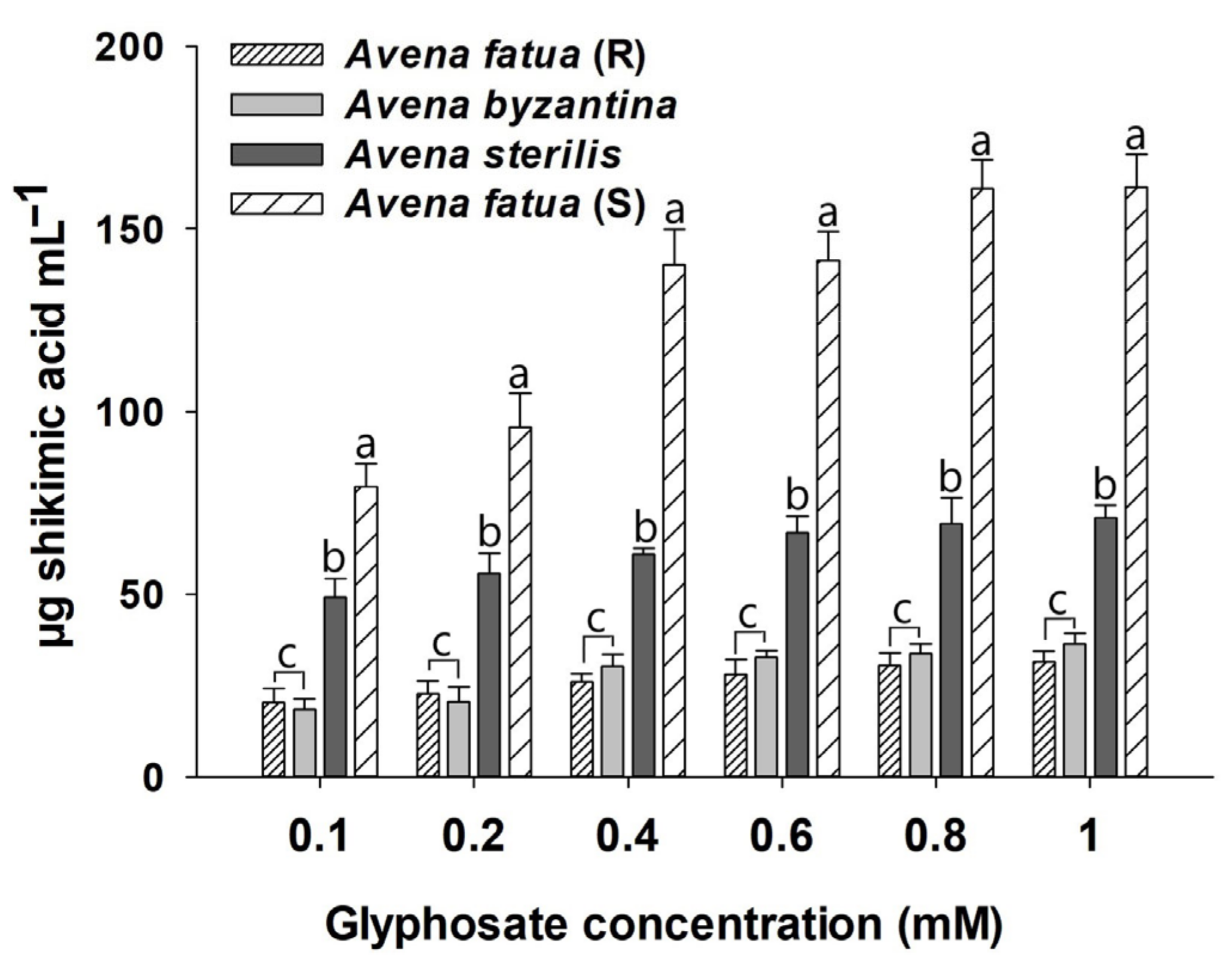
3.4. Shikimic Acid Accumulation
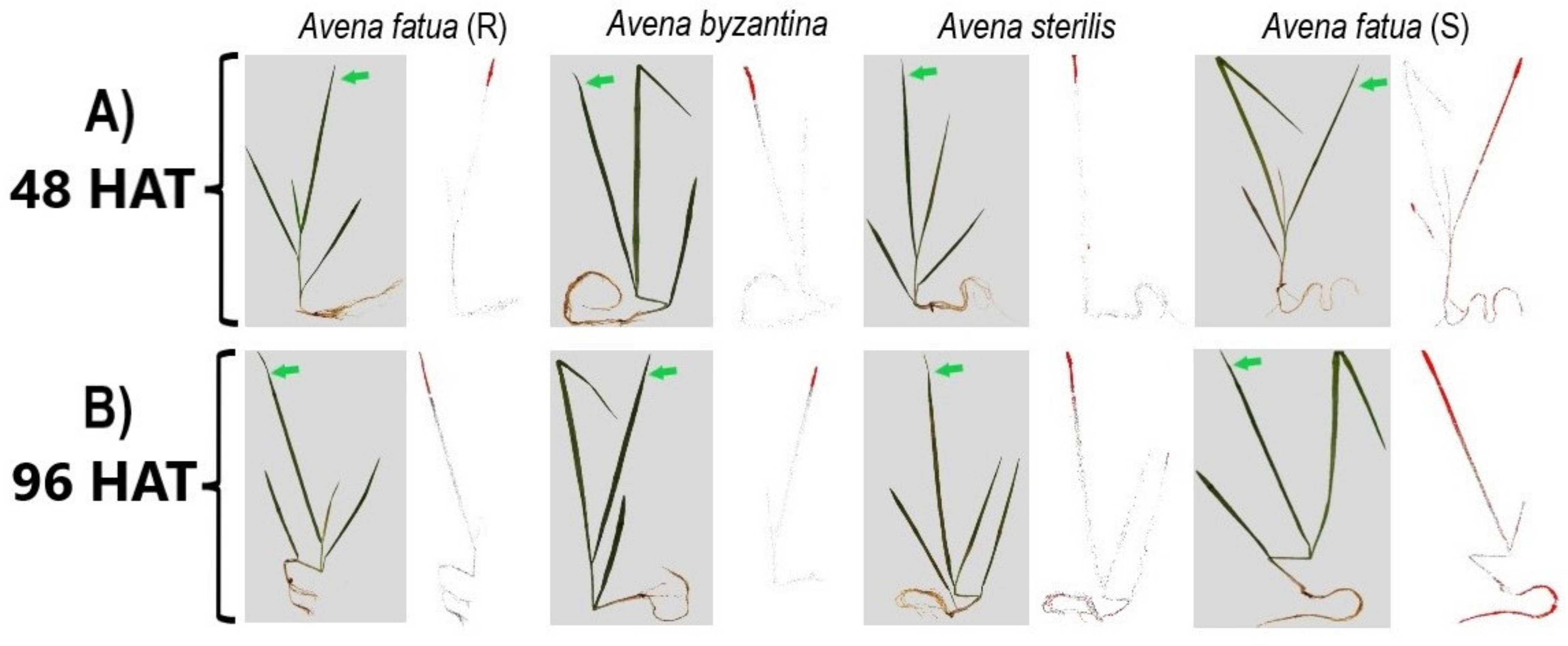
3.5. 14C-Glyphosate Absorption, Mobilization and Visualization
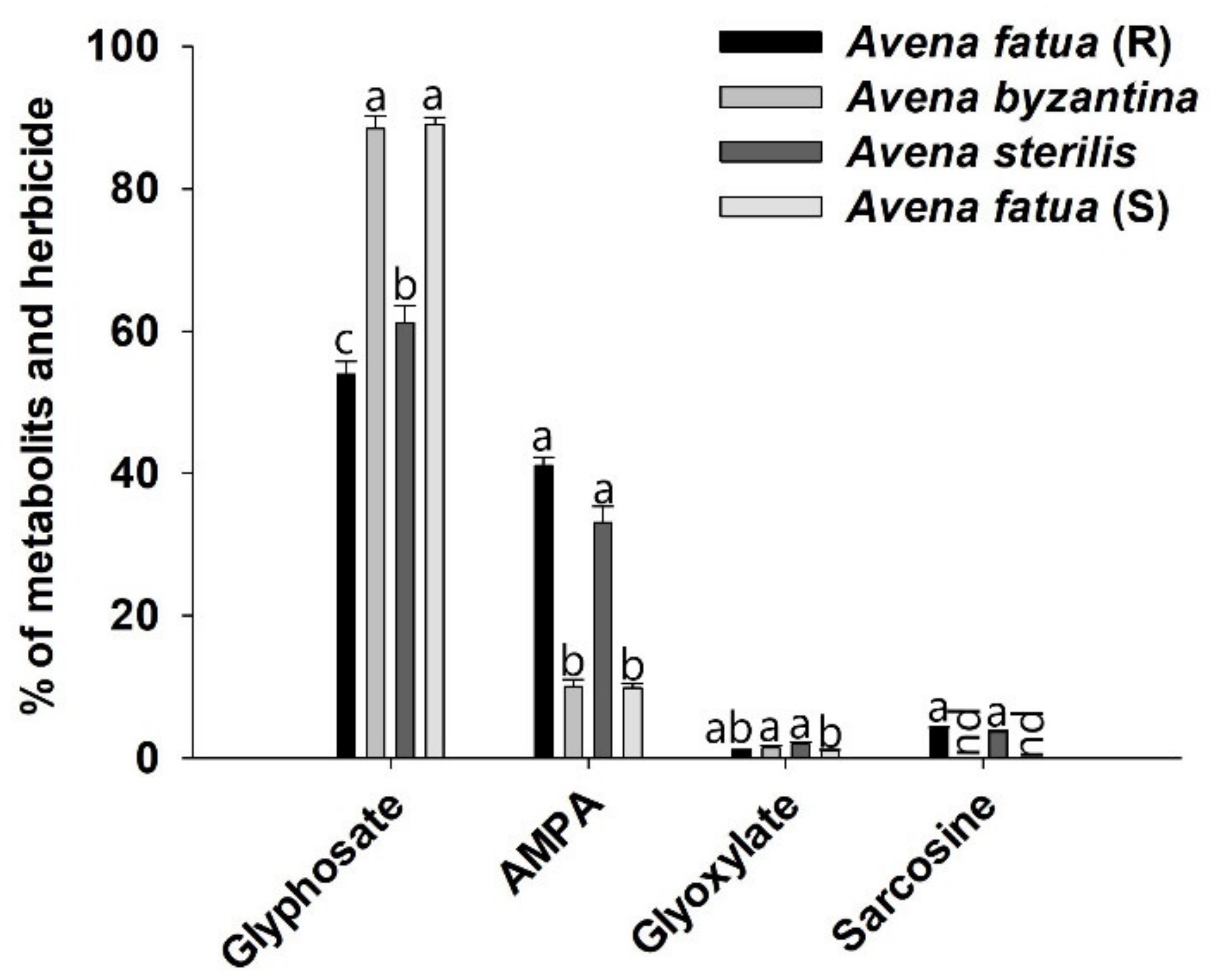
3.6. Metabolism Study
3.7. Activity of Glyphosate Target Enzyme
4. Discussion
4.1. Glyphosate-Resistance Confirmation
4.2. Unraveling Resistance Mechanisms to Glyphosate
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Barroso, J.; Navarrete, L.; Sanchez Del Arco, M.J.; Fernandez-Quintanilla, C.; Lutman, P.J.W.; Perry, N.H.; Hull, R.I. Dispersal of Avena fatua and Avena sterilis patches by natural dissemination, soil tillage and combine harvesters. Weed Res. 2006, 46, 118–128. [Google Scholar] [CrossRef]
- Chauhan, B.S. The world’s first glyphosate-resistant case of Avena fatua L. and Avena sterilis ssp. ludoviciana (Durieu) Gillet & Magne and alternative herbicide options for their control. PLoS ONE 2022, 17, e0262494. [Google Scholar] [CrossRef]
- Gómez de Barreda, D.; Pardo, G.; Osca, J.M.; Catala-Forner, M.; Consola, S.; Garnica, I.; López-Martínez, N.; Palmerín, J.A.; Osuna, M.D. An overview of rice cultivation in Spain and the management of herbicide-resistant weeds. Agronomy 2021, 11, 1095. [Google Scholar] [CrossRef]
- Alshallash, K.S. Germination of weed species (Avena fatua, Bromus catharticus, Chenopodium album and Phalaris minor) with implications for their dispersal and control. Ann. Agric. Sci. 2018, 63, 91–97. [Google Scholar] [CrossRef]
- Sousa-Ortega, C.; Royo-Esnal, A.; Urbano, J.M. Predicting seedling emergence of three canarygrass (Phalaris) species under semi-arid conditions using parametric and non-parametric models. Agronomy 2021, 11, 893. [Google Scholar] [CrossRef]
- Sammons, R.D.; Gaines, T.A. Glyphosate resistance: State of knowledge. Pest Manag. Sci. 2014, 70, 1367–1377. [Google Scholar] [CrossRef]
- Fernández-Moreno, P.T.; Alcantara-de la Cruz, R.; Cruz-Hipólito, H.E.; Rojano-Delgado, A.M.; Travlos, I.; De Prado, R. Non-target site tolerance mechanisms describe tolerance to glyphosate in Avena sterilis. Front. Plant Sci. 2016, 7, 1220. [Google Scholar] [CrossRef]
- Cirujeda, A.; Aibar, J.; Zaragoza, C. Remarkable changes of weed species in Spanish cereal fields from 1976 to 2007. Agron. Sustain. Dev. 2011, 31, 675–688. [Google Scholar] [CrossRef]
- Juárez-Escario, A.; Solé-Senan, X.O.; Recasens, J.; Taberner, A.; Conesa, J.A. Long-termi compositional and functional changes in alien and native weed communities in annual and perennial irrifated crops. Ann. App. Biol. 2018, 173, 42–54. [Google Scholar] [CrossRef]
- De Prado, R.; Palma-Bautista, C.; Vázquez-García, J.G.; Alcántara-de la Cruz, R. Influence of Herbicide Environmental Behavior on Weed Management. In Interactions of Biochar and Herbicides in the Environment; Mendes, K., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 53–77. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; Ward, S.M.; Shaw, D.R.; Llewellyn, R.S.; Nichols, R.L.; Webster, T.M.; Bradley, K.W.; Frisvold, G.; Powles, S.B.; Burgos, N.R.; et al. Reducing the risks of herbicide resistance: Best management practices and recommendations. Weed Sci. 2012, 60, 31–62. [Google Scholar] [CrossRef]
- Ghanizadeh, H.; Harrington, K.C. Non-target site mechanisms of resistance to herbicides. Crit. Rev. Plant Sci. 2017, 36, 24–34. [Google Scholar] [CrossRef]
- Belz, R.G.; Carbonari, C.A.; Duke, S.O. The potential influence of hormesis on evolution of resistance to herbicides. Curr. Opin. Environ. Sci. Health 2022, 27, 100360. [Google Scholar] [CrossRef]
- Jugulam, M.; Shyam, C. Non-target-site resistance to herbicides: Recent developments. Plants 2019, 8, 417. [Google Scholar] [CrossRef]
- Yanniccari, M.; Vázquez-García, J.G.; Gómez-Lobato, M.E.; Rojano-Delgado, A.M.; Alves, P.L.D.C.A.; De Prado, R. First case of glyphosate resistance in Bromus catharticus Vahl.: Examination of endowing resistance mechanisms. Front. Plant Sci. 2021, 12, 617945. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-García, J.G.; Rojano-Delgado, A.M.; Alcántara-de la Cruz, R.; Torra, J.; Dellaferrera, I.; Portugal, J.; De Prado, R. Distribution of glyphosate-resistance in Echinochloa crus-galli across agriculture areas in the Iberian Peninsula. Front. Plant Sci. 2021, 12, 617040. [Google Scholar] [CrossRef]
- Rojano-Delgado, A.M.; Ruiz-Jiménez, J.; de Castro, M.D.L.; De Prado, R. Determination of glyphosate and its metabolites in plant material by reversed-polarity CE with indirect absorptiometric detection. Electrophoresis 2010, 31, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Owens, D.K.; Corniani, N.; Lima-Silva, F.M.; Watson, S.B.; Howell, J.; Shaner, D. Biochemical markers and enzyme assays for herbicide mode of action and resistance studies. Weed Sci. 2015, 63, 23–63. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Benedetti, L.; Rangani, G.; Ebeling Viana, V.; Carvalho-Moore, P.; Rabaioli Camargo, E.; Avila, L.A.; Roma-Burgos, N. Recurrent selection by herbicide sublethal Dose and drought stress results in rapid reduction of herbicide sensitivity in junglerice. Agronomy 2020, 10, 1619. [Google Scholar] [CrossRef]
- Bracamonte, E.; Silveira, H.M.; Alcántara-de la Cruz, R.; Domínguez-Valenzuela, J.A.; Cruz-Hipolito, H.E.; De Prado, R. From tolerance to resistance: Mechanisms governing the differential response to glyphosate in Chloris barbata. Pest Manag. Sci. 2018, 74, 1118–1124. [Google Scholar] [CrossRef]
- Ding, W.; Reddy, K.N.; Zablotowicz, R.M.; Bellaloui, N.; Arnold Bruns, H. Physiological responses of glyphosate-resistant and glyphosate-sensitive soybean to aminomethylphosphonic acid, a metabolite of glyphosate. Chemosphere 2011, 83, 593–598. [Google Scholar] [CrossRef]
- Vemanna, R.S.; Vennapusa, A.R.; Easwaran, M.; Chandrashekar, B.K.; Rao, H.; Ghanti, K.; Sudhakar, C.; Mysore, K.S.; Makarla, U. Aldo-keto reductase enzymes detoxify glyphosate and improve herbicide resistance in plants. Plant Biotechnol. J. 2017, 15, 794–804. [Google Scholar] [CrossRef]
- Shaner, D.L. The impact of glyphosate-tolerant crops on the use of other herbicides and on resistance management. Pest Manag. Sci. 2000, 56, 320–326. [Google Scholar] [CrossRef]
- Pan, L.; Yu, Q.; Wang, J.; Han, H.; Mao, L.; Nyporko, A.; Maguza, A.; Fan, L.; Bai, L.; Powles, S. An ABCC-type transporter endowing glyphosate resistance in plants. Proc. Nat. Acad. Sci. USA 2021, 118, e2100136118. [Google Scholar] [CrossRef] [PubMed]
- Michitte, P.; De Prado, R.; Espinoza, N.; Pedro Ruiz-Santaella, J.; Gauvrit, C. Mechanisms of resistance to glyphosate in a ryegrass (Lolium multiflorum) biotype from Chile. Weed Sci. 2007, 55, 435–440. [Google Scholar] [CrossRef]
- Nandula, V.K.; Reddy, K.N.; Poston, D.H.; Rimando, A.M.; Duke, S.O. Glyphosate tolerance mechanism in Italian ryegrass (Lolium multiflorum) from Mississippi. Weed Sci. 2008, 56, 344–349. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Benitez, J.J.; Dominguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and raman spectroscopic features of plant cuticles: A review. Front. Plant Sci. 2014, 5, 305. [Google Scholar] [CrossRef]
- Gaines, T.A.; Duke, S.O.; Morran, S.; Rigon, C.A.G.; Tranel, P.J.; Küpper, A.; Dayan, F.E. Mechanisms of evolved herbicide resistance. J. Biol. Chem. 2020, 295, 10307–10330. [Google Scholar] [CrossRef]
- Vila-Aiub, M.M.; Balbi, M.C.; Distéfano, A.J.; Fernández, L.; Hopp, E.; Yu, Q.; Powles, S.B. Glyphosate resistance in perennial Sorghum halepense (Johnsongrass), endowed by reduced glyphosate translocation and leaf uptake. Pest Manag. Sci. 2012, 68, 430–436. [Google Scholar] [CrossRef]
- Ribeiro, D.N.; Nandula, V.K.; Dayan, F.E.; Rimando, A.M.; Duke, S.; Reddy, K.N.; Shaw, D.R. Possible Glyphosate Tolerance Mechanism in Pitted Morningglory (Ipomoea lacunosa L.). J. Agric. Food Chem. 2015, 63, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.N.; Rimando, A.M.; Duke, S.O. Aminomethylphosphonic Acid, a metabolite of glyphosate, causes injury in glyphosate-treated, glyphosate-resistant soybean. J. Agric. Food Chem. 2004, 52, 5139–5143. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O. Enhanced Metabolic Degradation: The last evolved glyphosate resistance mechanism of weeds? Plant Physiol. 2019, 181, 1401–1403. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Yu, Q.; Han, H.; Mao, L.; Nyporko, A.; Fan, L.; Bai, L.; Powles, S. Aldo-keto reductase metabolizes glyphosate and confers glyphosate resistance in Echinochloa colona. Plant Physiol. 2019, 181, 1519–1534. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Reverte, R.; García, A.; Watson, S.B.; Abdallah, I.; Sabaté, S.; Hernández, M.J.; Dayan, F.E.; Fischer, A.J. Concerted action of target-site mutations and high EPSPS activity in glyphosate-resistant junglerice (Echinochloa colona) from California. Pest Manag. Sci. 2015, 71, 996–1007. [Google Scholar] [CrossRef]
- Palma-Bautista, C.; Torra, J.; Garcia, M.J.; Bracamonte, E.; Rojano-Delgado, A.M.; Alcántara-de la Cruz, R.; De Prado, R. Reduced absorption and impaired translocation endows glyphosate resistance in Amaranthus palmeri harvested in glyphosate-resistant soybean from Argentina. J. Agric. Food Chem. 2019, 67, 1052–1060. [Google Scholar] [CrossRef]
- Gaines, T.A.; Zhang, W.; Wang, D.; Bukun, B.; Chisholm, S.T.; Shaner, D.L.; Nissen, S.J.; Patzoldt, W.L.; Tranel, P.J.; Culpepper, A.S.; et al. Gene amplification confers glyphosate resistance in Amaranthus palmeri. Proc. Natl. Acad. Sci. USA 2010, 107, 1029–1034. [Google Scholar] [CrossRef]



| Avena fatua (R) (n = 7) | Avena byzantina (n = 7) | Avena sterilis (n = 7) | Avena fatua (S) (n = 8) | |
|---|---|---|---|---|
| Spikelet | ||||
| Lower glume length | 2.5 ± 0.12 | 2.4 ± 0.08 | 3.1 ± 0.16 | 2.5 ± 0.16 |
| Upper glume length | 2.6 ± 0.08 | 2.7 ± 0.13 | 3.1 ± 0.08 | 2.6 ± 0.18 |
| Number of fertile florets | 2 | 2 | 2 | 2–3 |
| Disarticulation of the rhachilla at maturity | between florets | no | below the basal floret only | between florets |
| Lemma of the basal floret | ||||
| Length | 1.6 ± 0.06 | 1.6 ± 0.07 | 2.5 ± 0.11 | 1.8 ± 0.14 |
| Hairiness | lower half | only at floret callus | lower half | lower half |
| Apical awns | absent | absent | absent | absent |
| Dorsal awn, length of the basal twisting portion (column) | 1.4 ± 0.12 | without a column | 2.0 ± 0.14 | 1.5 ± 0.14 |
| Dorsal awn, total length | 3.6 ± 0.33 | 2.5 ± 0.14 | 5.7 ± 0.41 | 3.9 ± 0.20 |
| Accession | b | d | GR50 | 95% CI | Ri a | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| A. fatua (R) | 3.1 | 93.1 | 560.5 | 525.5 | 595.6 | 4.2 |
| A. byzantina | 3.5 | 93.3 | 467.5 | 439.7 | 495.2 | 3.4 |
| A. sterilis | 2.5 | 97.4 | 314.2 | 288.3 | 340.0 | 2.4 |
| A. fatua (S) | 2.9 | 100.5 | 130.5 | 117.1 | 143.8 | - |
| LD50 | ||||||
| A. fatua (R) | 6.5 | 97.2 | 788.8 | 764.9 | 812.8 | 3.5 |
| A. byzantina | 5.4 | 95.7 | 604.0 | 582.8 | 625.3 | 2.7 |
| A. sterilis | 3.9 | 99.0 | 458.2 | 437.5 | 479.0 | 2 |
| A. fatua (S) | 3.1 | 98.0 | 222.1 | 201.5 | 242.74 | - |
| Accession | b | d | I50 | 95% CI | Ri a | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| A. fatua (R) | 0.9 | 96.7 | 43.7 | 34.0 | 53.4 | 1.19 |
| A. byzantina | 0.6 | 99.9 | 37.7 | 26.3 | 49.0 | 1.03 |
| A. sterilis | 0.8 | 100.2 | 30.7 | 26.8 | 44.1 | 0.84 |
| A. fatua (S) | 0.8 | 94.5 | 36.5 | 23.7 | 37.7 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-García, J.G.; Torra, J.; Palma-Bautista, C.; Bastida, F.; Alcántara-de la Cruz, R.; Portugal, J.; Jorrin-Novo, J.V.; De Prado, R. Different Non-Target Site Mechanisms Endow Different Glyphosate Susceptibility in Avena Species from Spain. Agronomy 2023, 13, 763. https://doi.org/10.3390/agronomy13030763
Vázquez-García JG, Torra J, Palma-Bautista C, Bastida F, Alcántara-de la Cruz R, Portugal J, Jorrin-Novo JV, De Prado R. Different Non-Target Site Mechanisms Endow Different Glyphosate Susceptibility in Avena Species from Spain. Agronomy. 2023; 13(3):763. https://doi.org/10.3390/agronomy13030763
Chicago/Turabian StyleVázquez-García, José G., Joel Torra, Candelario Palma-Bautista, Fernando Bastida, Ricardo Alcántara-de la Cruz, João Portugal, Jesús V. Jorrin-Novo, and Rafael De Prado. 2023. "Different Non-Target Site Mechanisms Endow Different Glyphosate Susceptibility in Avena Species from Spain" Agronomy 13, no. 3: 763. https://doi.org/10.3390/agronomy13030763
APA StyleVázquez-García, J. G., Torra, J., Palma-Bautista, C., Bastida, F., Alcántara-de la Cruz, R., Portugal, J., Jorrin-Novo, J. V., & De Prado, R. (2023). Different Non-Target Site Mechanisms Endow Different Glyphosate Susceptibility in Avena Species from Spain. Agronomy, 13(3), 763. https://doi.org/10.3390/agronomy13030763









