Precision Phenotyping of Agro-Physiological Responses and Water Use of Sorghum under Different Drought Scenarios
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Methods
2.2.1. Experimental Conditions
2.2.2. Experiment Design and Management
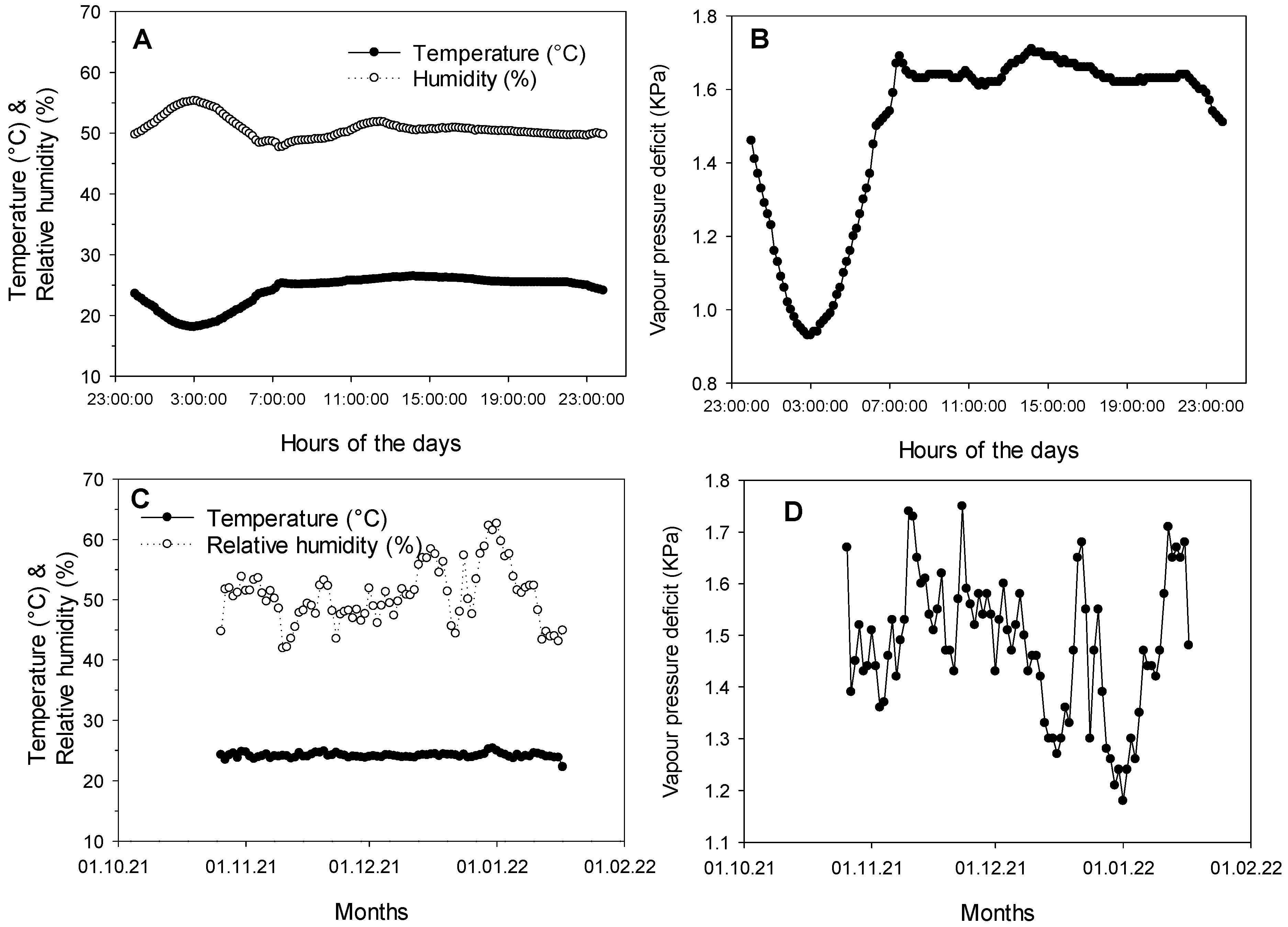
2.2.3. Weather Conditions
2.3. Data Collection
2.3.1. Plant Phenology and Morphology
2.3.2. Biomass Production
2.3.3. Grain Production
2.3.4. Water Use Efficiency
2.3.5. Gas Exchange, Chlorophyll Fluorescence, and Chlorophyll Content
2.3.6. Drought Recovery Index
2.3.7. Leaf Anatomical Structures Analysis
2.4. Data Analysis
3. Results
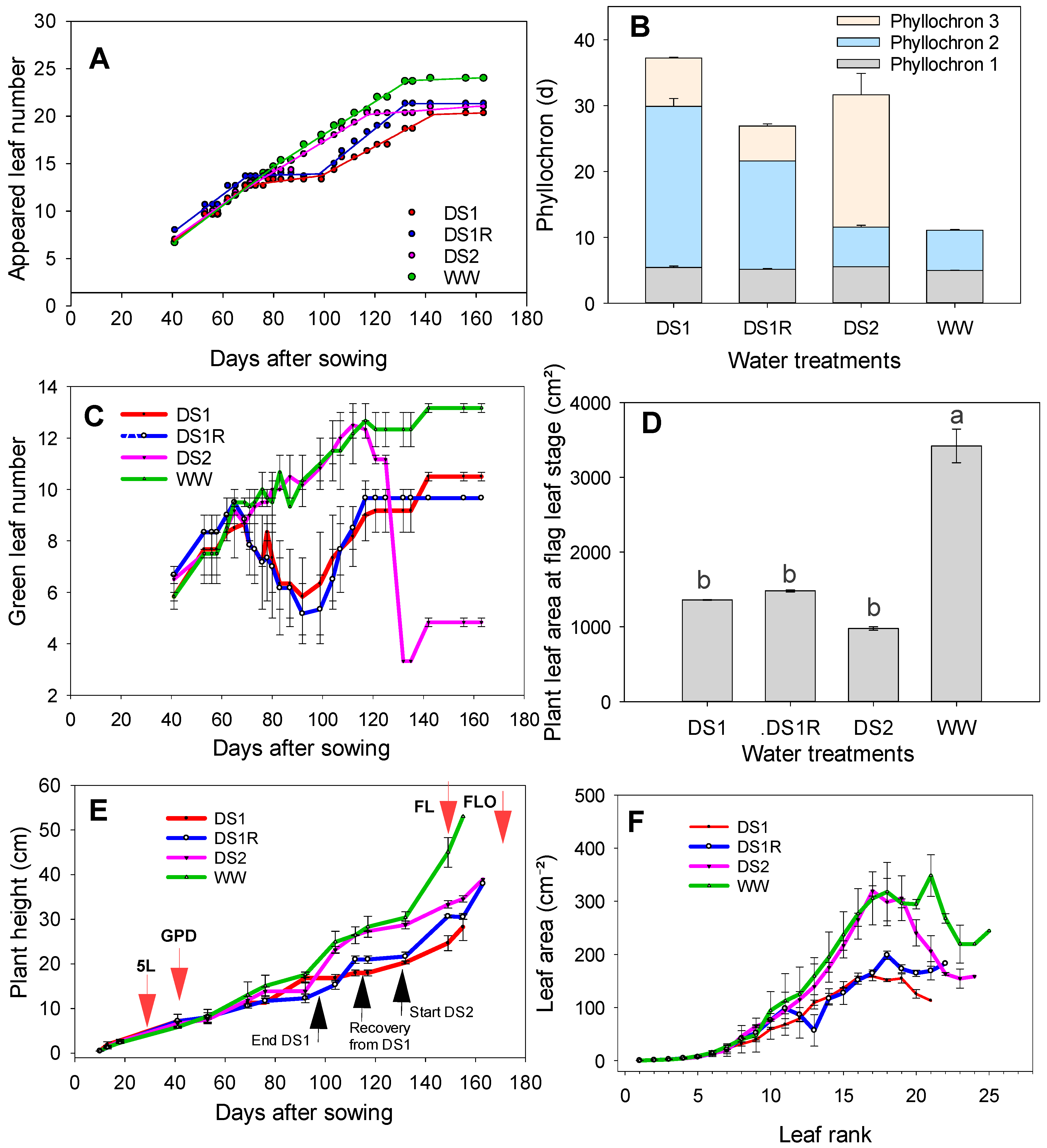
3.1. Effect of Different Drought Scenarios on Plant Phenology
3.2. Effect of Different Drought Scenarios on Plant Growth and Development
3.3. Effect of Different Drought Scenarios on Sorghum Agro-Morphological Parameters Measured at Physiological Maturity
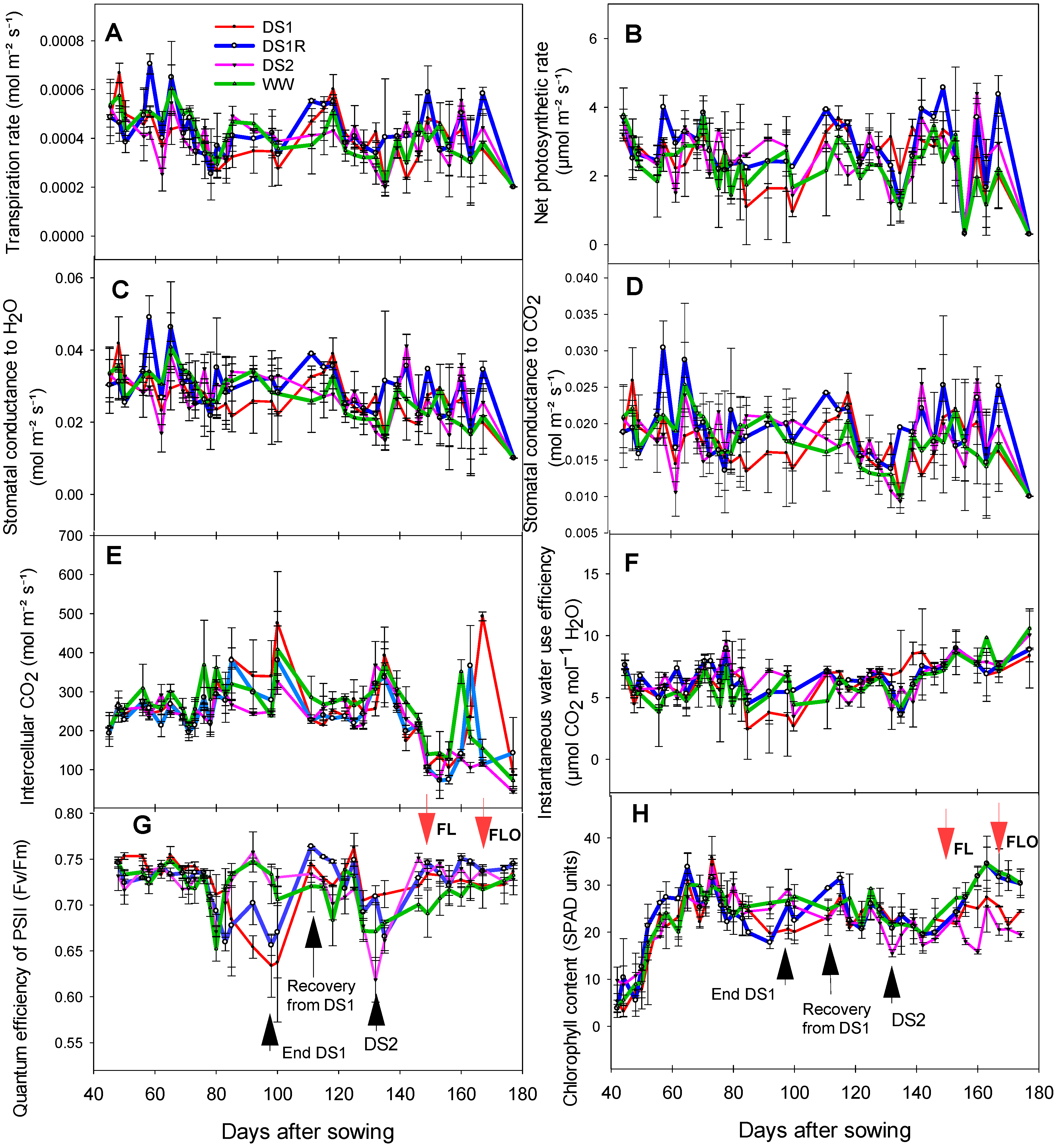
3.4. Effect of Early and Late Vegetative Drought Stresses on Sorghum Physiology
Gas Exchange, Chlorophyll Content, and Fluorescence
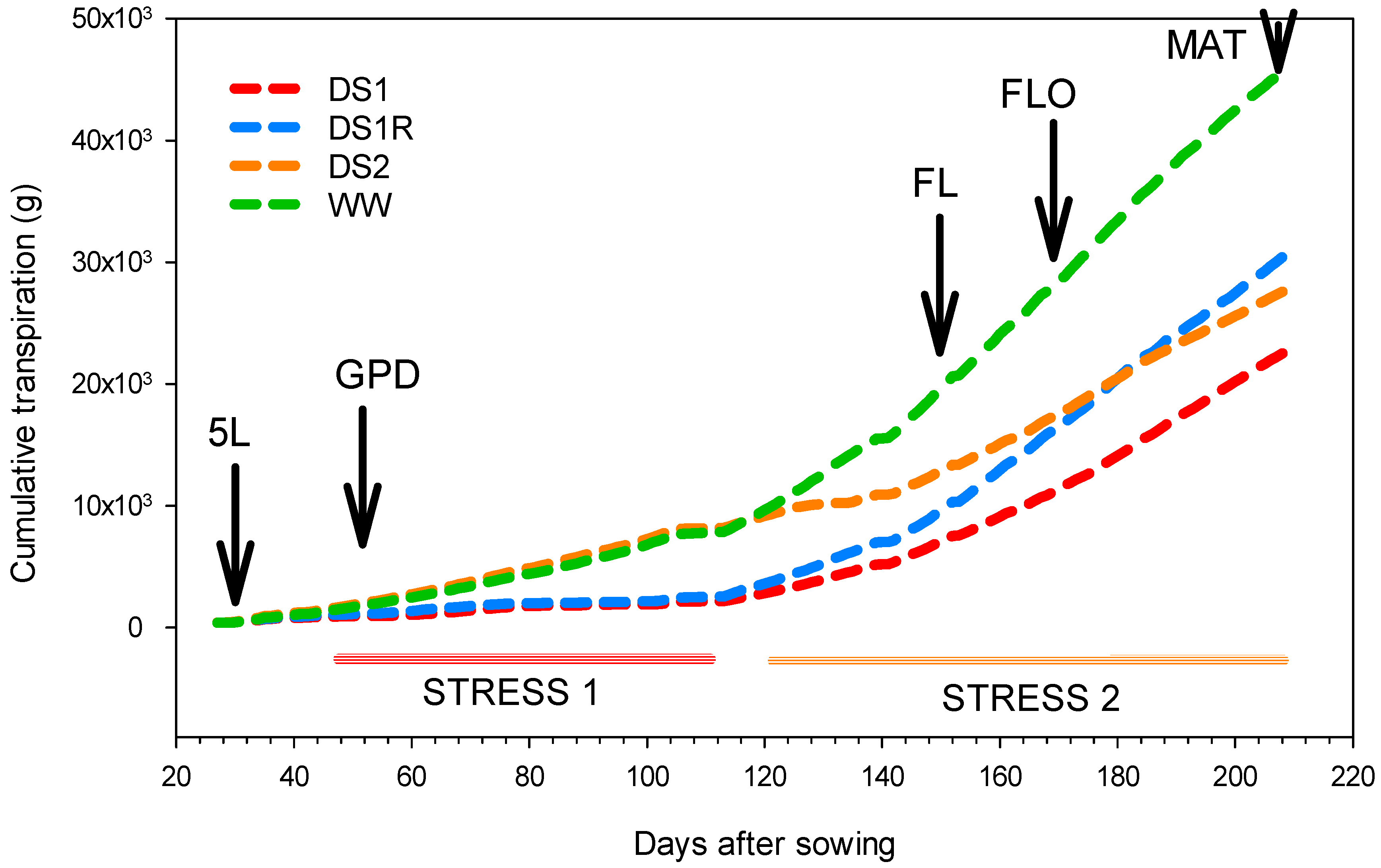
3.5. Drought Recovery Index and Water Use Efficiency
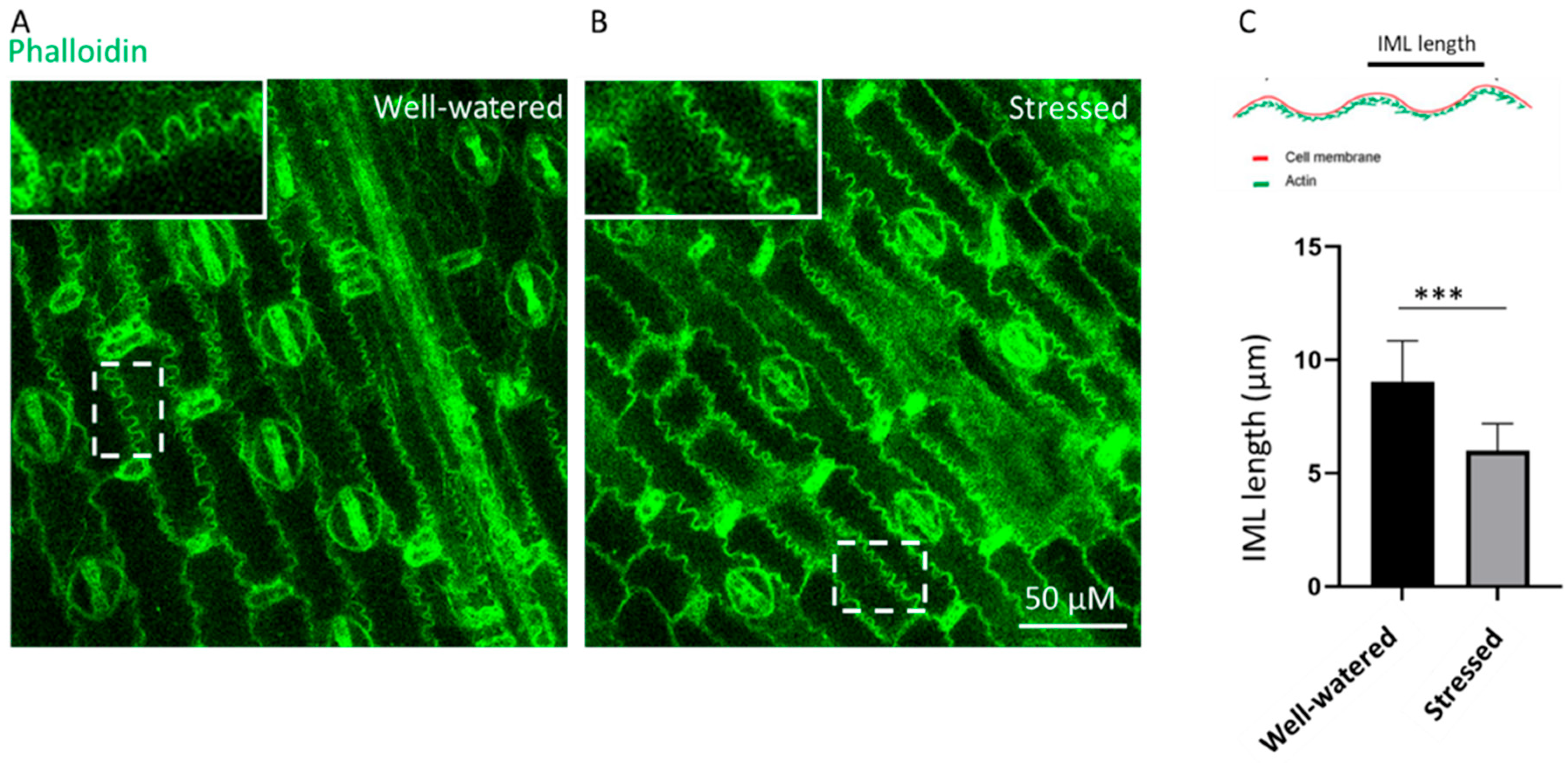
3.6. Early Drought Effect on Sorghum Leaf Anatomical Structures
4. Discussion
4.1. Drought Stress Effect on Plant Phenology, Growth and Development, and Production
4.2. Drought Stress Effect on Plant Physiology
4.3. Sorghum Water Use Efficiency and Ability to Recover from Early Drought Stress
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belton, P.S.; Taylor, J.R.N. Sorghum and Millets: Protein Sources for Africa. Trends Food Sci. Technol. 2004, 15, 94–98. [Google Scholar] [CrossRef]
- Windpassinger, S.; Friedt, W.; Frauen, M.; Snowdon, R.; Wittkop, B. Designing Adapted Sorghum Silage Types with an Enhanced Energy Density for Biogas Generation in Temperate Europe. Biomass Bioenergy 2015, 81, 496–504. [Google Scholar] [CrossRef]
- Mace, E.S.; Tai, S.; Gilding, E.K.; Li, Y.; Prentis, P.J.; Bian, L.; Campbell, B.C.; Hu, W.; Innes, D.J.; Han, X.; et al. Whole-Genome Sequencing Reveals Untapped Genetic Potential in Africa’s Indigenous Cereal Crop Sorghum. Nat. Commun. 2013, 4, 2320. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.L.; Pot, D.; Latrille, E.; Trouche, G.; Bonnal, L.; Bastianelli, D.; Carrère, H. Sorghum Biomethane Potential Varies with the Genotype and the Cultivation Site. Waste Biomass Valorization 2017, 10, 783–788. [Google Scholar] [CrossRef]
- Vo, L.T.T.; Girones, J.; Beloli, C.; Chupin, L.; di Giuseppe, E.; Vidal, A.C.; Soutiras, A.; Pot, D.; Bastianelli, D.; Bonnal, L.; et al. Processing and Properties of Sorghum Stem Fragment-Polyethylene Composites. Ind. Crops Prod. 2017, 107, 386–398. [Google Scholar] [CrossRef]
- Bobojonov, I.; Lamers, J.P.A.; Djanibekov, N.; Ibragimov, N.; Begdullaeva, T.; Ergashev, A.-K.; Kienzler, K.; Eshchanov, R.; Rakhimov, A.; Ruzimov, J.; et al. Crop Diversification in Support of Sustainable Agriculture in Khorezm. In Cotton, Water, Salts and Soums: Economic and Ecological Restructuring in Khorezm, Uzbekistan; Martius, C., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 219–233. [Google Scholar]
- Prom, L.K.; Perumal, R.; Cissé, N.; Little, C.R. Evaluation of Selected Sorghum Lines and Hybrids for Resistance to Grain Mold and Long Smut Fungi in Senegal, West Africa. Plant Health Prog. 2014, 15, 28–31. [Google Scholar] [CrossRef]
- Perrier, L.; Rouan, L.; Jaffuel, S.; Clément-Vidal, A.; Roques, S.; Soutiras, A.; Baptiste, C.; Bastianelli, D.; Fabre, D.; Dubois, C.; et al. Plasticity of Sorghum Stem Biomass Accumulation in Response to Water Deficit: A Multiscale Analysis from Internode Tissue to Plant Level. Front. Plant Sci. 2017, 8, 1516. [Google Scholar] [CrossRef]
- Amelework, B.; Shimelis, H.; Tongoona, P.; Laing, M. Physiological Mechanisms of Drought Tolerance in Sorghum, Genetic Basis and Breeding Methods: A Review. Afr. J. Agric. Res. 2015, 10, 3029–3040. [Google Scholar] [CrossRef]
- Krupa, K.N.; Dalawai, N.; Shashidhar, H.E.; Harinikumar, K.M.; Manojkumar, H.B.; Bharani, S.; Turaidar, V. Mechanisms of Drought Tolerance in Sorghum: A Review. Int. J. Pure Appl. Biosci. 2017, 5, 221–237. [Google Scholar] [CrossRef]
- Gano, B.; Dembele, J.S.B.; Tovignan, T.K.; Sine, B.; Vadez, V.; Diouf, D.; Audebert, A. Adaptation Responses to Early Drought Stress of West Africa Sorghum Varieties. Agronomy 2021, 11, 443. [Google Scholar] [CrossRef]
- Martínez-Goñi, X.S.; Robredo, A.; Pérez-López, U.; Muñoz-Rueda, A.; Mena-Petite, A. Sorghum Bicolor Prioritizes the Recovery of Its Photosynthetic Activity When Re-Watered after Severe Drought Stress, While Manages to Preserve It under Elevated CO2 and Drought. J. Agron. Crop Sci. 2022. [Google Scholar] [CrossRef]
- Jedmowski, C.; Ashoub, A.; Beckhaus, T.; Berberich, T.; Karas, M.; Brüggemann, W. Comparative Analysis of Sorghum Bicolor Proteome in Response to Drought Stress and Following Recovery. Int. J. Proteom. 2014, 2014, 395905. [Google Scholar] [CrossRef]
- Mahamat, H.H.; Belko, N.; Cisse, N.; Sine, B.; Ndoye, I. Amélioration de l’adaptation à La Sécheresse Chez Le Niébé (Vigna unguiculata L. Walpers). J. Appl. Biosci. 2014, 77, 6550–6563. [Google Scholar]
- Zhang, F.; Zhu, K.; Wang, Y.Q.; Zhang, Z.P.; Lu, F.; Yu, H.Q.; Zou, Q. Changes in Photosynthetic and Chlorophyll Fluorescence Characteristics of Sorghum under Drought and Waterlogging Stress. Photosynthetica 2019, 57, 1156–1164. [Google Scholar] [CrossRef]
- Chowdhury, M.K.; Hasan, M.A.; Bahadur, M.M.; Islam, M.R.; Hakim, M.A.; Iqbal, M.A.; Javed, T.; Raza, A.; Shabbir, R.; Sorour, S.; et al. Evaluation of Drought Tolerance of Some Wheat (Triticum aestivum L.) Genotypes through Phenology, Growth, and Physiological Indices. Agronomy 2021, 11, 1792. [Google Scholar] [CrossRef]
- Borrell, A.K.; Mullet, J.E.; George-Jaeggli, B.; van Oosterom, E.J.; Hammer, G.L.; Klein, P.E.; Jordan, D.R. Drought Adaptation of Stay-Green Sorghum Is Associated with Canopy Development, Leaf Anatomy, Root Growth, and Water Uptake. J. Exp. Bot. 2014, 65, 6251–6263. [Google Scholar] [CrossRef]
- Ejeta, G.; Tuinstra, M.R.; Grote, E.M.; Goldsbrough, P.B. Genetic Analysis of Pre-Flowering and Post-Flowering Drought Tolerance in Sorghum. In Molecular Approaches for the Genetic Improvement of Cereals for Stable Production in Water-Limited Environments; Ribaut, J.M., Poland, D., Eds.; CIMMYT: Texcoco, Mexico, 2000; pp. 137–141. [Google Scholar]
- Rosenow, D.T.; Quisenberry, J.E.; Wendt, C.W.; Clark, L.E. Drought Tolerant Sorghum and Cotton Germplasm. Agric. Water Manag. 1983, 12, 207–222. [Google Scholar] [CrossRef]
- Stephens, J.C.; Miller, F.R.; Rosenow, D.T. Conversion of Alien Sorghums to Early Combine Genotypes1. Crop Sci. 1967, 7, 396. [Google Scholar] [CrossRef]
- Stahl, A.; Wittkop, B.; Snowdon, R.J. High-Resolution Digital Phenotyping of Water Uptake and Transpiration Efficiency. Trends Plant Sci. 2020, 25, 429–433. [Google Scholar] [CrossRef]
- Alduchov, O.A.; Eskridge, R.E. Improved Magnus Form Approximation of Saturation Vapor Pressure. J. Appl. Meteorol. Climatol. 1996, 35, 601–609. [Google Scholar] [CrossRef]
- Kim, H.K.; van Oosterom, E.; Dingkuhn, M.; Luquet, D.; Hammer, G. Regulation of Tillering in Sorghum: Environmental Effects. Ann. Bot. 2010, 106, 57–67. [Google Scholar] [CrossRef]
- Tovignan, T.K.; Fonceka, D.; Ndoye, I.; Cisse, N.; Luquet, D. The Sowing Date and Post-Flowering Water Status Affect the Sugar and Grain Production of Photoperiodic, Sweet Sorghum through the Regulation of Sink Size and Leaf Area Dynamics. Field Crops Res. 2016, 192, 67–77. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kobayashi, I.; Funaki, Y.; Fujimoto, S.; Takemoto, T.; Kunoh, H. Dynamic Reorganization of Microfilaments and Microtubules Is Necessary for the Expression of Non-Host Resistance in Barley Coleoptile Cells. Plant J. 1997, 11, 525–537. [Google Scholar] [CrossRef]
- Opalski, K.S.; Schultheiss, H.; Kogel, K.H.; Hückelhoven, R. The Receptor-like MLO Protein and the RAC/ROP Family G-Protein RACB Modulate Actin Reorganization in Barley Attacked by the Biotrophic Powdery Mildew Fungus Blumeria graminis f.Sp. Hordei. Plant J. 2005, 41, 291–303. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Craufurd, P.Q.; Flower, D.J.; Peacock, J.M. Effect of Heat and Drought Stress on Sorghum (Sorghum bicolor). I. Panicle Development and Leaf Appearance. Exp. Agric. 1993, 29, 61–76. [Google Scholar] [CrossRef]
- Rakshit, S.; Swapna, M.; Dalal, M.; Sushma, G.; Ganapathy, K.N.; Dhandapani, A.; Karthikeyan, M.; Talwar, H.S. Post-Flowering Drought Stress Response of Post-Rainy Sorghum Genotypes. Indian J. Plant Physiol. 2015, 21, 8–14. [Google Scholar] [CrossRef]
- Akman, H.; Zhang, C.; Ejeta, G. Physio-Morphological, Biochemical, and Anatomical Traits of Drought-Tolerant and Susceptible Sorghum Cultivars under Pre- and Post-Anthesis Drought. Physiol. Plant 2021, 172, 912–921. [Google Scholar] [CrossRef]
- de Menezes, C.B.; da Silva, K.J.; Teodoro, L.P.R.; dos Santos, C.V.; Julio, B.H.M.; Portugal, A.F.; Batista, P.S.C.; Carvalho, A.J.; Teodoro, P.E. Grain Sorghum Hybrids under Drought Stress and Full-Irrigation Conditions in the Brazilian Semiarid. J. Agron. Crop Sci. 2022, 208, 868–875. [Google Scholar] [CrossRef]
- Fracasso, A.; Magnanini, E.; Marocco, A.; Amaducci, S. Real-Time Determination of Photosynthesis, Transpiration, Water-Use Efficiency and Gene Expression of Two Sorghum Bicolor (Moench) Genotypes Subjected to Dry-Down. Front. Plant Sci. 2017, 8, 932. [Google Scholar] [CrossRef]
- Tsuji, W.; Ali, M.E.K.; Inanaga, S.; Sugimoto, Y. Growth and Gas Exchange of Three Sorghum Cultivars under Drought Stress. Biol. Plant. 2003, 46, 583–587. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.P.; Zeng, Y.; Li, D.M.; Guo, D.J.; Rajput, V.D.; Chen, G.L.; Barakhov, A.; Minkina, T.M.; Li, Y.R. Characteristics of Leaf Stomata and Their Relationship with Photosynthesis in Saccharum Officinarum under Drought and Silicon Application. ACS Omega 2020, 5, 24145–24153. [Google Scholar] [CrossRef]
- Wang, N.; Gao, J.; Zhang, S. Overcompensation or Limitation to Photosynthesis and Root Hydraulic Conductance Altered by Rehydration in Seedlings of Sorghum and Maize. Crop J. 2017, 5, 337–344. [Google Scholar] [CrossRef]
- Endris, S.; Fetene, M.; Amede, T. CO2 Exchange, Dry Matter Accumulation and Growth Response of Sorghum (Sorghum bicolor L. Moench) to Terminal Drought as Affected by Potassium and Blended-NPSBZn Fertilization. J. Agron. Crop Sci. 2021, 207, 450–464. [Google Scholar] [CrossRef]
- Hasan, S.A.; Rabei, S.H.; Nada, R.M.; Abogadallah, G.M. Water Use Efficiency in the Drought-Stressed Sorghum and Maize in Relation to Expression of Aquaporin Genes. Biol. Plant 2017, 61, 127–137. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Shan, L. Responsibility of Non-Stomatal Limitations for the Reduction of Photosynthesis-Response of Photosynthesis and Antioxidant Enzyme Characteristics in Alfalfa (Medicago sativa L.) Seedlings to Water Stress and Rehydration. Front. Agric. China 2007, 1, 255–264. [Google Scholar] [CrossRef]
- Ermakova, M.; Osborn, H.; Groszmann, M.; Bala, S.; Bowerman, A.; McGaughey, S.; Byrt, C.; Alonso-Cantabrana, H.; Tyerman, S.; Furbank, R.T.; et al. Expression of a CO2-Permeable Aquaporin Enhances Mesophyll Conductance in the C4 Species Setaria Viridis. eLife 2021, 10, e70095. [Google Scholar] [CrossRef]
- Olsovska, K.; Kovar, M.; Brestic, M.; Zivcak, M.; Slamka, P.; Shao, H.B. Genotypically Identifying Wheat Mesophyll Conductance Regulation under Progressive Drought Stress. Front. Plant Sci. 2016, 7, 1111. [Google Scholar] [CrossRef]
- Sonawane, B.V.; Cousins, A.B. Mesophyll CO2 Conductance and Leakiness Are Not Responsive to Short- and Long-Term Soil Water Limitations in the C4 Plant Sorghum Bicolor. Plant J. 2020, 103, 1590–1602. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, L.; Eduardo Ñústez, C.L.; Patricia Moreno, L.F. Drought Stress Affects Physiological Parameters but Not Tuber Yield in Three Andean Potato (Solanum tuberosum L.) Cultivars. Agron. Colomb. 2017, 35, 158–170. [Google Scholar] [CrossRef]
- Shahenshah; Isoda, A. Effects of Water Stress on Leaf Temperature and Chlorophyll Fluorescence Parameters in Cotton and Peanut. Plant Prod. Sci. 2015, 13, 269–278. [Google Scholar] [CrossRef]
- Galicia-Juárez, M.; Zavala-García, F.; Sinagawa-García, S.R.; Gutiérrez-Diez, A.; Williams-Alanís, H.; Cisneros-lópez, M.E.; Valle-Gough, R.E.; Flores-Garivay, R.; Santillano-Cázares, J. Identification of Sorghum (Sorghum bicolor (L.) Moench) Genotypes with Potential for Hydric and Heat Stress Tolerance in Northeastern Mexico. Plants 2021, 10, 2265. [Google Scholar] [CrossRef]
- Getnet, Z.; Husen, A.; Fetene, M.; Yemata, G. Growth, Water Status, Physiological, Biochemical and Yield Response of Stay Green Sorghum (Sorghum bicolor (L.) Moench) Varieties-a Field Trial under Drought-Prone Area in Amhara Regional State, Ethiopia. J. Agron. 2015, 14, 188–202. [Google Scholar] [CrossRef]
- Kapanigowda, M.H.; Perumal, R.; Djanaguiraman, M.; Aiken, R.M.; Tesso, T.; Prasad, P.V.; Little, C.R. Genotypic Variation in Sorghum [Sorghum bicolor (L.) Moench] Exotic Germplasm Collections for Drought and Disease Tolerance. Springerplus 2013, 2, 650. [Google Scholar] [CrossRef]
- Augustine, S.M.; Cherian, A.V.; Seiling, K.; di Fiore, S.; Raven, N.; Commandeur, U.; Schillberg, S. Targeted Mutagenesis in Nicotiana Tabacum ADF Gene Using Shockwave-Mediated Ribonucleoprotein Delivery Increases Osmotic Stress Tolerance. Physiol. Plant 2021, 173, 993–1007. [Google Scholar] [CrossRef]
- Augustine, S.M.; Cherian, A.V.; Syamaladevi, D.P.; Subramonian, N. Erianthus Arundinaceus HSP70 (EaHSP70) Acts as a Key Regulator in the Formation of Anisotropic Interdigitation in Sugarcane (Saccharum spp. Hybrid) in Response to Drought Stress. Plant Cell Physiol. 2015, 56, 2368–2380. [Google Scholar] [CrossRef]
- Hussain, T.; Hussain, N.; Tahir, M.; Raina, A.; Ikram, S.; Maqbool, S.; Fraz Ali, M.; Duangpan, S. Impacts of Drought Stress on Water Use Efficiency and Grain Productivity of Rice and Utilization of Genotypic Variability to Combat Climate Change. Agronomy 2022, 12, 2518. [Google Scholar] [CrossRef]
- Bhattarai, B.; Singh, S.; West, C.P.; Ritchie, G.L.; Trostle, C.L. Water Depletion Pattern and Water Use Efficiency of Forage Sorghum, Pearl Millet, and Corn Under Water Limiting Condition. Agric. Water Manag. 2020, 238, 106206. [Google Scholar] [CrossRef]
- Abdel-Motagally, F.M.F. Evaluation of Water Use Efficiency under Different Water Regimes in Grain Sorghum (Sorghum bicolor, L. Moench). World J. Agric. Sci. 2010, 6, 499–505. [Google Scholar]
- Mastrorilli, M.; Katerji, N.; Rana, G. Productivity and Water Use Efficiency of Sweet Sorghum as Affected by Soil Water Deficit Occurring at Different Vegetative Growth Stages. Eur. J. Agron. 1999, 11, 207–215. [Google Scholar] [CrossRef]
- Bell, J.M.; Schwartz, R.; McInnes, K.J.; Howell, T.; Morgan, C.L.S. Deficit Irrigation Effects on Yield and Yield Components of Grain Sorghum. Agric. Water Manag. 2018, 203, 289–296. [Google Scholar] [CrossRef]
- van Oosterom, E.J.; Hammer, G.L. Determination of Grain Number in Sorghum. Field Crops Res. 2008, 108, 259–268. [Google Scholar] [CrossRef]
- Devnarain, N.; Crampton, B.G.; Chikwamba, R.; Becker, J.V.W.; O’Kennedy, M.M. Physiological Responses of Selected African Sorghum Landraces to Progressive Water Stress and Re-Watering. S. Afr. J. Bot. 2016, 103, 61–69. [Google Scholar] [CrossRef]


| Analysis of Soil Texture | |||
|---|---|---|---|
| Type | Size [mm] | Unit | Value |
| Fine sand | 0.063–0.2 | % | 31.50 |
| Middle sand | 0.2–0.63 | % | 25.00 |
| Large silt | 0.02–0.063 | % | 20.60 |
| Clay | <0.002 | % | 8.30 |
| Middle silt | 0.0063–0.02 | % | 7.50 |
| Fine silt | 0.002–0.0063 | % | 3.70 |
| Large sand | 0.63–2 | % | 3.50 |
| Soil nutrient contents | |||
| Element | Symbol | Unit | Value |
| Phosphorus | P2O5 | [mg/100 g] | 14.00 |
| Potassium | K2O | [mg/100 g] | 8.00 |
| Magnesium | Mg | [mg/100 g] | 10.00 |
| Iron | Fe | mg/kg | 76.40 |
| Copper | Cu | mg/kg | 1.44 |
| Zinc | Zn | mg/kg | 1.75 |
| Manganese | Mn | mg/kg | 37.40 |
| Boron | B | mg/kg | 0.17 |
| Molybdenum | Mo | mg/kg | <0.0150 |
| DS1 | DS1 R | DS2 | WW | p-Value | |
|---|---|---|---|---|---|
| Plant morphology | |||||
| Diam (mm) | 6 ± 0.3 b | 7.5 ± 1.3 b | 9.1 ± 0.9 ab | 11.7 ± 1.4 a | 0.0164 * |
| PH (cm) | 82.6 ± 7.6 b | 94.3 ± 1.8 ab | 82.7 ± 0.5 b | 106.7 ± 0 a | 0.027 * |
| IN | 7.7 ± 0.5 a | 6.7 ± 0.5 a | 6.7 ± 1.4 a | 8.2 ± 1.2 a | 0.36 |
| Lped (cm) | 26.0 ± 1.4 a | 29.3 ± 1.4 a | 19.5 ± 0.7 b | 23.3 ± 0.5 ab | 0.0151 * |
| Lpan (cm) | 17 ± 1.9 a | 19.5 ± 0.2 a | 18.2 ± 0.7 a | 21.5 ± 0.7 a | 0.0805 |
| Wpan (g) | 2.6 ± 3.7 a | 3.3 ± 5.1 a | 2.8 ± 2.2 a | 4.1 ± 5.8 a | 0.0805 |
| Biomass | |||||
| SFW (g) | 28.1 ± 6.1 b | 43.7 ± 3.1 b | 39.2 ± 1 b | 71.6 ± 4.6 a | 0.00928 ** |
| LFW (g) | 24.1 ± 0.8 b | 31 ± 2.3 b | 30.6 ± 1.3 b | 49.9 ± 2.5 a | 0.00411 ** |
| SDW (g) | 8.5 ± 2.1 b | 12 ± 0.7 b | 13.7 ± 0.4 b | 23.2 ± 1.6 a | 0.00874 ** |
| LDW (g) | 7.6 ± 0.4 b | 9.3 ± 0.8 b | 14.5 ± 2.2 ab | 18.1 ± 1.6 a | 0.0155 * |
| Grain production | |||||
| PFW (g) | 13 ± 3.3 b | 25 ± 0.8 ab | 15.3 ± 1.8 b | 34.2 ± 4.8 a | 0.0265 * |
| PDW (g) | 9.5 ± 3.1 b | 18.1 ± 0.8 ab | 10.9 ± 1.3 ab | 25.1 ± 4.3 a | 0.0155 * |
| GWP (g) | 8.3 ± 1.9 c | 15.5 ± 0.6 ab | 10.2 ± 1.3 bc | 19.8 ± 0.8 a | 0.0119 * |
| P100 (g) | 1.4 ± 0.1 a | 1.9 ± 0.2 a | 1.2 ± 0.1 a | 1.6 ± 0.2 a | 0.0178 * |
| GNP | 575.6 ± 123.2 b | 833.8 ± 44.1 ab | 867 ± 178.2 ab | 1248.5 ± 54.4 a | 0.0521 |
| Drought Recovery Index (DRI) | |
|---|---|
| Plant height, PH | −0.36 |
| Number of appeared leaf | −0.17 |
| Intercellular CO2 | −0.20 |
| Vapor pressure deficit, VPD | −0.04 |
| Chlorophyll content (SPAD) | −0.02 |
| Leaf temperature | 0.02 |
| Maximum yield of PSII (Fv/Fm) | 0.02 |
| Transpiration rate | 0.10 |
| Stomatal conductance to CO2, Gtc | 0.32 |
| Stomatal conductance to H2O, Gsw | 0.32 |
| Instantaneous water use efficiency, iWUE | 0.38 |
| Photosynthetic rate | 0.52 |
| DS1 | DS1R | DS2 | WW | p-Value | |
|---|---|---|---|---|---|
| Cumulative transpiration (Kg) | 22.8 ± 3.2 a | 30.8 ± 1.5 ab | 27.8 ± 1.9 ab | 46 ± 2.3 b | 0.0486 * |
| Water use efficiency (g Kg−1) | |||||
| SFW | 1.23 ± 0.19 a | 1.42 ± 0.07 a | 1.41 ± 0.03 a | 1.56 ± 0.07 a | 0.413 |
| SDW | 0.42 ± 0.02 ab | 0.39 ± 0.02 b | 0.49 ± 0.01 ab | 0.52 ± 0.01 a | 0.0277 * |
| LFW | 1.06 ± 0.03 a | 1.01 ± 0.05 a | 1.1 ± 0.03 a | 1.09 ± 0.04 a | 0.552 |
| LDW | 0.33 ± 0.01 b | 0.30 ± 0.02 b | 0.55 ± 0.03 a | 0.39 ± 0.03 ab | 0.0163 * |
| PFW | 0.2157 ± 0.1 a | 0.81 ± 0.02 a | 0.55 ± 0.05 a | 0.74 ± 0.07 a | 0.111 |
| PDW | 0.46 ± 0.05 a | 0.59 ± 0.02 a | 0.41 ± 0.02 a | 0.55 ± 0.07 a | 0.227 |
| GWP | 0.39 ± 0.03 a | 0.5 ± 0.01 a | 0.37 ± 0.03 a | 0.43 ± 0.01 a | 0.123 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tovignan, T.K.; Basha, Y.; Windpassinger, S.; Augustine, S.M.; Snowdon, R.; Vukasovic, S. Precision Phenotyping of Agro-Physiological Responses and Water Use of Sorghum under Different Drought Scenarios. Agronomy 2023, 13, 722. https://doi.org/10.3390/agronomy13030722
Tovignan TK, Basha Y, Windpassinger S, Augustine SM, Snowdon R, Vukasovic S. Precision Phenotyping of Agro-Physiological Responses and Water Use of Sorghum under Different Drought Scenarios. Agronomy. 2023; 13(3):722. https://doi.org/10.3390/agronomy13030722
Chicago/Turabian StyleTovignan, Thierry Klanvi, Yasmeen Basha, Steffen Windpassinger, Sruthy Maria Augustine, Rod Snowdon, and Stjepan Vukasovic. 2023. "Precision Phenotyping of Agro-Physiological Responses and Water Use of Sorghum under Different Drought Scenarios" Agronomy 13, no. 3: 722. https://doi.org/10.3390/agronomy13030722
APA StyleTovignan, T. K., Basha, Y., Windpassinger, S., Augustine, S. M., Snowdon, R., & Vukasovic, S. (2023). Precision Phenotyping of Agro-Physiological Responses and Water Use of Sorghum under Different Drought Scenarios. Agronomy, 13(3), 722. https://doi.org/10.3390/agronomy13030722





