Abstract
Slugs are cryptic terrestrial pests that target a wide range of crops and are especially damaging to seedlings. Management of these invertebrates mostly relies on synthetic chemistry. These molecules can be efficient against slugs and snails but can be toxic to other organisms (e.g., dogs) and harmful to the environment (e.g., leaching into surface and groundwater). The usage of pathogenic nematodes has been effective in several crops and European countries. A survey was conducted to investigate the presence of natural populations of malacopathogenic nematodes in soybean in the mid-Atlantic region. Slugs were sampled in nine fields across Delaware at various distances from the field edges (0 m, 5 m, 10 m, 15 m). Soil cover and soil type were also recorded. Invertebrates were brought back to the laboratory. Slugs were monitored for four weeks, and mortality was classified into one of three categories: (1) death with the presence of nematodes; (2) death with the presence of fungi; (3) death without the presence of nematodes or fungi. Nematodes associated with slugs were identified based on 18S rRNA sequencing. The distance from the field edge did not impact the number of trapped slugs and the incidence of slug death associated with the presence of nematodes. Overall, nematodes were collected from ca. 20% of the slug cadavers, and most have previously been associated with slugs (ca. 35% of deaths associated with fungi and ca. 45% not associated with nematodes or fungi). The number of captured slugs and slug death associated with the presence of nematodes were positively correlated with ground cover. Soil type impacted both the number of captured slugs and the presence of pathogenic nematodes. This survey provides a first insight into the natural populations of mollusk-associated nematodes in the mid-Atlantic region. This knowledge may contribute to implementing cultural practices favoring these natural enemies of slug pests.
1. Introduction
Slugs and snails are voracious mollusk pests that have the potential to damage virtually all crops, ranging from specialty to field crops. Because they are particularly hard on young and emerging plants, slugs are especially problematic during crop establishment in spring or fall. With the growing popularity of conservation agriculture, mollusk outbreaks have become a serious concern for growers in no-till systems, which provide stable, residue-rich habitats [1,2,3]. Slug herbivory can damage up to 37% of the total biomass of annual crucifer species [4]. Among mollusks, slugs tend to be more damaging than snails because of their greater resistance to mechanical disturbance (e.g., planting, tillage) and lower need for calcium to build shells [5]. Due to their generalist diets, slugs are among the most important pests in areas that get frequent rain, such as Northern and Western Europe, and are an increasing challenge in some parts of the United States, such as the Mid-Atlantic region and the Pacific Northwest, that tend to receive ample precipitation [1]. Aside from mechanical control (i.e., tillage) [6], currently, the most widely used slug management practice is the application of granular baits containing active ingredients of metaldehyde or iron phosphate. In addition to the potential leaching of these chemicals into groundwater and streams [7], metaldehyde can be toxic to mammals such as cannids [8] or rodents [9]. Furthermore, these baits are expensive (US$ 16–20/hectare) yet are not always effective, partly because they are somewhat water-soluble. Fortunately, these active ingredients do not show toxicity to natural enemies of slugs, such as predatory insects [10], meaning they can be used in integrated pest management programs.
Given the limited management options, slug control would undoubtedly benefit from alternative management approaches. Currently, the main cultural approach to controlling slugs is tillage, which can facilitate soil erosion and the transport of nutrients and pesticides into nearby waterways. In the Mid-Atlantic region of the U.S., about 70% of agricultural lands are not tilled to prevent soil erosion and run-off into streams and rivers leading into the Chesapeake Bay, the world’s largest estuary [11]. Unfortunately, no-till farming in this region favors the growth of slug populations by providing a stable, relatively undisturbed environment.
One alternative approach to slug management that needs to be better explored is promoting or augmenting populations of natural enemies that can kill slugs, especially ground beetles [12] and malacopathogenic nematodes (MPN) [13,14]. MPNs have received some research attention [15,16,17,18,19,20], but more research is necessary for them to become both viable and safe candidates for helping to control pest slug populations. These particular nematodes are spread across various taxonomic families (i.e., Alloionematidae, Cosmocercidae, Mermithidae, and Rhabditidae) [21]. While several pathogenic nematode species have been considered in sustainable insect pest management for several years [22], there are only two MPN species registered as commercial products for slug and snail control, (Phasmarhabitis hermaphorita Shneider (Nematoda: Rhabditidae) and P. californica De Ley et al.) [13,23,24,25].
Phasmarhabditis hermaphrodita is a facultative parasite of terrestrial mollusks (Wilson 1993). Similar to entomopathogenic nematodes, this mollusk-killing species evolved a symbiosis with bacteria that partially serve as food sources. After penetrating its mollusk host, P. hermaphrotita and its symbiont kill the host within 4 to 21 days [13,25,26]. This symbiosis between P. hermaphrodita and bacteria is not as strict as with their entomopathogenic counterparts; rather, it occurs with complex and variable natural assemblages of bacterial species [17,27], a phenomenon that may also occur with other MPN species but remains to be elucidated. After depleting resources from the cadaver, MPNs leave their host and forage for new hosts. As facultative parasites, MPNs can also survive on alternative resources, such as decomposing organic matter or invertebrate feces [13,26,28]. Because of their potential for pest management, efforts to isolate different Phasmarhabitis species have been pursued in Europe and elsewhere. Over various field campaigns, the following species have been isolated and described: P. hermaphrodita [29], P. papillosa Schneider [30] and P. neopapillosa Mengert in Osche [31], P. bonaquaense Nermut’, Půža & Mráček [32], P. apuliae Nermut’, Půža & Mráček [33], P. bohemica Nermut’, Půža & Mráček [34], P. tawfiki Azzam [35], P. huizhouensis Huang, Ye, Ren & Zhao [36], P. californica [37], P. meridionalis Ivanova & Spiridonov [38], P. safricana Ross, Pieterse, Malan & Ivanova, [39], and P. thesamica Gorgadze et al. [40]. Other genera have been largely overlooked.
The present survey contributes to the current global effort to identify distributions of MPNs in gastropod hosts on agricultural soils. Focusing on soybeans, we sampled various farms across Delaware, USA. To get better insights into the impact of the environment on slugs and associated MPNs, we hypothesized that the distance from the edge of the field, providing shelter and resources, negatively impacts the slug density as well as the incidence of MPN infections. In addition, we hypothesized a positive impact of ground cover (weed and crop debris) on both slug populations and MPN incidence.
2. Materials and Methods
2.1. Field Location
We worked in nine soybean fields in Delaware, USA, three sites per County (New Castle, Kent, and Sussex). A brief description of each sampling site is available in Table 1.

Table 1.
Description of the field conditions.
2.2. Slug Sampling
In each selected field, we established a transect perpendicular to the field edge, with a trap positioned every 5 m up to 15 m into the field (i.e., 0 m, 5 m, 10 m, and 15 m from the field edge). Slug traps were squares of roofing shingle (1 × 1 m, White Fiberglass Mineral Roll, Home Depot, Inc., Atlanta, GA, USA). This particular shingle was selected for its white/grey color, which reflects some sunlight and helps prevent overheating of the traps. We visited the transects weekly and checked each shingle trap and residues underneath for the number of slugs present for a max. of 1 h. We collected the first 100 slugs that we encountered and brought them back to the laboratory for further processing. Slugs were identified based on Chichester and Getz [41].
2.3. Ground Cover
To evaluate the ground cover along the transects, we captured digital images of the shingle traps from a height of 1.5 m perpendicular to the center of each trap. We analyzed the images with CANOPEO [42] and recorded the portion of ground covered by debris and vegetation.
2.4. Soil Type
Based on the GPS coordinates, soil types of each site were extracted from the website Web Soil Survey managed by the USDA-NRCS [43]. Soil types encountered in the current study are briefly described in Table 1.
2.5. Slug Parasitic Nematodes
We individually placed the slugs taken from the field in Petri dishes (10 cm diameter) with moist filter paper and provided small slices of organic carrots as diet; water and food were provided as needed. We weekly checked each slug for one month (i.e., four weeks) or until death. Dead slugs were placed in White traps [44] and monitored every other day for nematode emergence. Emerging nematodes were prepared for molecular identification [18]. Briefly, we placed aliquots of max. 15 nematodes into 1 mL of 70% ethanol in a 1.5 mL sample tube (ThermoFisher Scientific, Waltham, MA, USA). Tubes were stored at −80 °C until processing with molecular identification. We classified dead slugs into three categories; (1) death with the presence of nematodes; (2) death with the presence of fungi; (3) death without nematodes and fungi. We discarded slugs still alive four weeks after capture.
2.6. Molecular Identification of Slug Parasitic Nematodes
After removing the storage ethanol, each sample was washed twice with sterile water, and then the nematodes were transferred to a proteinase-K-based lysis buffer for DNA extraction [45]. We performed PCR amplification of the 18S ribosomal DNA region as previously described using the primer set 18A (5′-AAAGATTAAGCCATGCATG-3′) and 26R (5′-CATTCTTGGCAAATGCTTTCG-3′) [46,47]. The resulting ∼800 bp amplicons were purified using a modified SPRI method [48] and direct end sequenced using primer 18A. All 18S rDNA sequences were compared against GenBank’s non-redundant (nr) database using blast [49]. Nematode BLAST matches with a percent identity of 98–100 were used for species identification. One representative sequence for each nematode species identified in this study was submitted to GenBank (see Table 2 for accession numbers).

Table 2.
Nematodes associated with dead slugs trapped in soybean fields.
2.7. Statistical Analysis
We tested statistical differences in the number of slugs collected between sampling dates with an RM-ANOVA. As there were no differences between sampling dates, we applied GLMs to fields, distance from the field edge, soil type, and the number of dead slugs associated with the presence of nematodes with a QuasiPoisson distribution. Further differences between factors were tested with Tukey HSD post-hoc tests.
We tested for interactions between the number of slugs and ground cover as well as between the number of slug deaths with the presence of nematodes and ground cover with Spearman correlations.
All tests were conducted in R Statistical Software (v4.2.1; [50]).
3. Results
3.1. Slug Sampling
From 36 traps deployed across three counties, we collected 1808 slugs, representing only three species. The majority of individuals were Deroceras laeve (98.3%), whereas grey garden slug (Deroceras reticulatum; 1.6%) and leopard slug (Limax maximus; 0.1%) were rare by comparison. Without a formal population estimate, the data suggests seasonality in slug population densities, with most (38.4%) captured on 19 April 2018 and fewer collected on other dates (19.9% on 12 April 2018, 19.9% on 26 April 2018, 21.8% on 3 May 2018; F3-140 = 2.426, p = 0.06).
The distance from the field edge did not influence the number of collected slugs (F3-140 = 0.108, p = 0.956). However, the number of slugs collected per trap (4) and sampling date (4) varied among fields (Figure 1, F8-135 = 20.034, p < 0.001) and soil types (Figure 2, F6-137 = 10.693, p < 0.001).
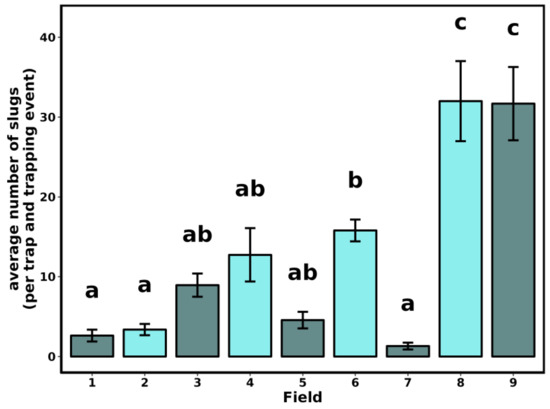
Figure 1.
Average number of slugs per trap (4 traps of 1 m2) and per trapping event (4) in each survey field. Details on the field conditions are provided in Table 1. Bars indicate SEM. Letters indicate statistical differences among fields.
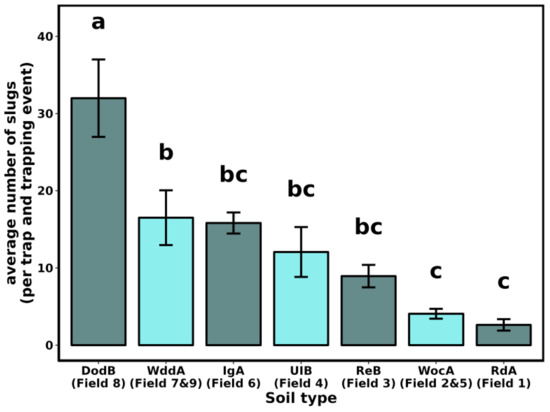
Figure 2.
Average number of slugs collected per trap (4 traps of 1 m2) and per trapping event (4) by soil types. Soil types were identified on Web Soil Survey [43], based on field location, as Downer sandy loam (DodB), Ingleside sandy loam (IgA), Reybold-Queponco complex (RdA), Reybold silt loam (ReB), Unicorn loam (UIB), Woodstown sandy loam (WddA), Woodbridge loam (WocA). Short descriptions of the soil types are provided in Table 1. Bars indicate SEM. Letters indicate statistical differences between soil types.
3.2. Ground Cover
Ground cover (before planting) was variable among fields and transects. We found a strong positive relationship between the % of ground cover and the number of slugs under shingles (rs(19) = 0.74, p < 0.001). Similarly, the total number of nematodes associated with dead slugs was positively correlated with ground cover (Figure 3, rs(19) = 0.70, p < 0.001)
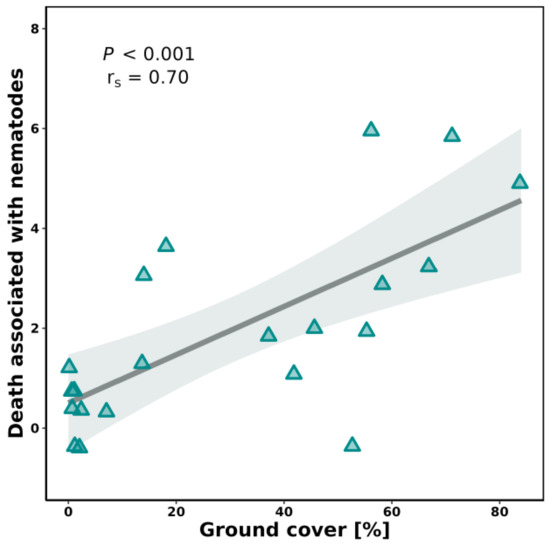
Figure 3.
Spearman correlation between ground cover and the number of dead slugs with the presence of killed by nematodes.
3.3. Slug Parasitic Nematodes
Of the slugs we collected, 616 died within four weeks after collection, and 122 deaths were associated with the presence of nematodes (Table 2). The mortality of slugs associated with the presence of nematodes varied among fields (F8-135 = 3.181, p < 0.01) but was not influenced by the distance from the field edge (F3-140 = 2.172, p = 0.094) nor the trapping events (F3-140 = 1.356, p = 0.07. Soil type also affected slug mortality associated with nematodes (Figure 4, F6-137 = 2.505, p = 0.025).
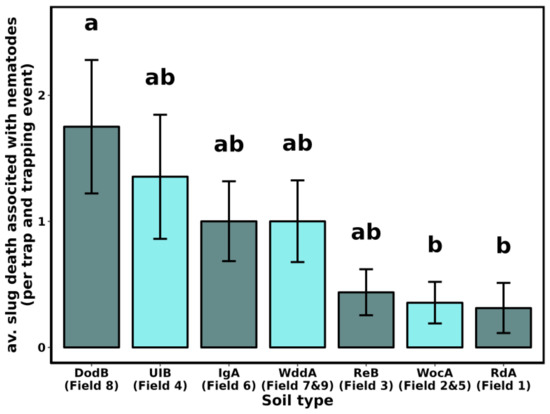
Figure 4.
Average number of slugs death associated with the presence of nematodes per trap (4 traps of 1 m2) and per trapping event (4) by soil types. Soil types were identified on Web Soil Survey [43], based on field location, as Downer sandy loam (DodB), Ingleside sandy loam (IgA), Reybold-Queponco complex (RdA), Reybold silt loam (ReB), Unicorn loam (UIB), Woodstown sandy loam (WddA), Woodbridge loam (WocA). Short descriptions of the soil types are provided in Table 1. Bars indicate SEM. Letters indicate statistical differences between soil types.
Overall, ca. 19% of the slug death was associated with the presence of nematodes. Fungi were associated with 35%, and 46% of the dead slugs were not associated with the presence of nematodes or fungi. These mortality rates varied across the field, but these percentages were quite constant across the state, even though Field 7 had >50% of death associated with the presence of nematodes (Figure 5B).
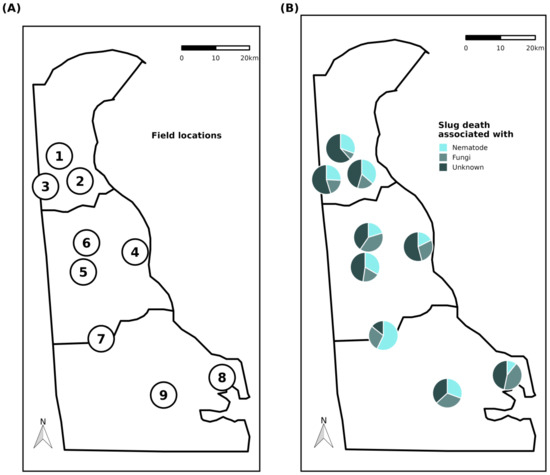
Figure 5.
(A) Geographical locations of the surveyed fields across Delaware. Details on the field conditions are provided in Table 1. (B) Slug death associated with the presence of nematodes, fungi, or unknown causes at the different surveyed sites. Pie charts illustrate the proportion of slug death associated with the different factors measured among all individuals dead within 4 weeks after capture (4 traps per field and 4 sampling dates).
4. Discussion
We conducted this study to isolate MPN from slugs in Delaware, with the hope of finding Phasmarhabditis, which may be able to facilitate sustainable control of pest slugs in North America. Failing to achieve this ambitious aim, the present study still offers interesting insights into the ecology of slugs, slug-associated nematodes, and other potential pathogens.
The three slug species that we captured are invasive species. Exotic populations of D. laeve were probably established in North America in the 1700s but this Holarctic species also has native populations in the U.S. [61]. Deroceras reticulatum was introduced into the USA in the 1800s [62]. L. maximus was introduced into the USA in the late 1800s [62], yet this, to our knowledge, is its first record in Delaware [63]. For slugs to be abundant, an area must receive adequate moisture, which helps them avoid desiccation [64]. However, on a local level, crop residue and soil coverage by weeds can influence slug abundance. Increased soil coverage likely retains soil moisture, favoring slug development and survival [64]. Even though we found no differences in the number of slugs collected from the edge of the fields and along transects, there were differences among fields with the highest number of slugs found in southern Delaware. This result is counterintuitive because soil conditions tend to be dryer in southern Delaware than in the north; perhaps this difference can partly be explained by the greater atmospheric humidity in southern Delaware.
In several instances, slug mortality was associated with the presence of fungal hyphae and spores on the surface of cadavers. Attempts to isolate these fungi were unsuccessful, but further investigations on pathogenic fungi against slugs could be a worthwhile area for future research. Literature on this topic is extremely scarce, yet fungi seem to have the potential to produce metabolites having repellent or even molluscicide effects [65]. It is also possible that the fungi we observed were secondary colonizers and not responsible for slug death.
Our study also highlighted aspects of the ecology of nematodes associated with dead slugs. Associations between nematodes and mollusks likely vary along a continuum of intimacy. Certain vertebrate nematode species use mollusks as transitional hosts (paratenic hosts) in which little or no development takes place [66]. Other nematode species complete part of their life cycle in the mollusk [67]. Finally, mollusks can be definitive hosts of nematodes, with some species only developing partially in the host (non-pathogenic) while finishing their life cycle as free-living organisms. Other nematode species that use mollusks as definitive hosts are pathogenic and kill their hosts at the completion of their life cycle. From the nematode species that we isolated, Cosmocercoides dukae belongs to the class of nematodes using slugs as definitive hosts [51,52,53,54,55]. Other nematode species isolated from soybean fields, such as Rhabditoides inermiformis, also closely interact with slug species [60], yet their lifestyle may or may not be parasitic. We also found several individuals from the genus Panagrolaimus, associated with mollusks in a phoretic interaction [57,68]. Nematodes from the genus Panagrolaimus are bacterivorous and can be found in various niches, from the soil in Antarctica to arid soil in Iran [57,58]. Some species use mollusks as final hosts [67], yet the nematodes we isolated have not been described to have such an association. We isolated nematodes from the genus Rhabditis, which has been associated with slugs and mollusks [60], but it is unclear whether they were parasites even if there is evidence of parasitism of mollusks within the genus [30,60].
Overall, regardless of the type of association that nematode species have with mollusks, soil type influenced nematodes associated with slugs. The highest densities of nematodes were found in soils that better retain water and provided suitable conditions for nematodes and their gastropod hosts. Related to moisture (and, to some extent, the soil’s capacity to retain moisture), the instances when a nematode was associated with a slug increased with soil cover, suggesting that, in addition to favoring slugs, ground cover would also favor the presence of slug-associated nematodes.
Even though we failed to isolate Phasmarhabditis from Delaware, this genus has previously been collected in North America. Phasmarhabditis hermaphrodita was found in a number of pest slug species in California and Oregon [69,70], and these US strains were subsequently shown to be lethal to a number of invasive gastropod species in lab assays [14,71,72], suggesting that they may have a role to play in biological control of mollusks [73]. Furthermore, P. papillosa [37] and P. californica were also collected from multiple invasive gastropod species in both California and Oregon [37,74]. More recently, P. californica was found in Canada for the first time [60], and this, combined with its known lethality to pest slugs [71], has led to suggestions that it may have the potential to be used as a biological control agent in different areas of North America, as congener P. hermaphrodita was used in Europe. It should be noted that our survey focused on soybeans. More comprehensive surveys should include other crops and habitats, including horticultural sites and/or natural habitats. In addition, the methods used to collect slugs could be widened (e.g., use of soil cores) to increase the probability that mollusks infected by Phasmarhabitis sp. are sampled. Indeed, it has been suggested that these nematodes manipulate the behavior of their infected host, as infected slugs tend to be found underneath the soil surface, which prevents the parasite-infected host from being predated or scavenged [75]. The trapping method used herein sampled slugs from the soil surface only.
To the best of our knowledge, our MPN survey was the first on the east coast of North America. While the slug-associated nematode species we found are not obvious candidates for biological control of pest slugs, our data demonstrate that a diversity of nematodes can be found along with slugs in crop fields. Even though we did not find Phasmarhabditis, this first effort has highlighted some pitfalls that could be avoided in future surveys and demonstrated the importance of soil and ground cover on MPNs and their hosts.
Author Contributions
Conceptualization, B.K., W.J.C., J.F.T. and I.H.; methodology, B.K., W.J.C., J.F.T., R.J.M.D. and I.H.; formal analysis, D.K.H. and I.H.; investigation, B.K., W.J.C. and I.H.; resources, I.H.; data curation, D.K.H. and I.H.; writing—original draft preparation, I.H.; writing—review and editing, B.K., W.J.C., J.F.T., D.K.H., R.J.M.D. and D.R.D.; visualization, I.H.; supervision, I.H.; project administration, I.H.; funding acquisition, B.K., W.J.C., J.F.T. and I.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Mid-Atlantic Soybean Association. Funds were granted to I.H.
Data Availability Statement
Data are available on demand from the corresponding author.
Acknowledgments
The authors are grateful to the administrative staff of the Department of Entomology and Wildlife Ecology for their support in conducting this project. We also warmly thank the students and interns who have taken care of the numerous slugs in the laboratory. We are grateful to the reviewer who helped improve the clarity and quality of the present contribution.
Conflicts of Interest
R.J.M.D declares he is a co-inventor on a patent application entitled Mollusk-killing Biopesticide (U.S. application Serial No. 62/236,674). B.K., W.J.C., J.F.T., D.K.H., D.R.D. and I.H. declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Douglas, M.R.; Tooker, J.F. Slug (Mollusca: Agriolimacidae, Arionidae) Ecology and Management in No-Till Field Crops, With an Emphasis on the mid-Atlantic Region. J. Integr. Pest Manag. 2012, 3, C1–C9. [Google Scholar] [CrossRef]
- Le Gall, M.; Tooker, J.F. Developing ecologically based pest management programs for terrestrial molluscs in field and forage crops. J. Pest Sci. 2017, 90, 825–838. [Google Scholar] [CrossRef]
- Tulli, M.C.; Carmona, D.M.; López, A.N.; Manetti, P.L.; Vincini, A.M.; Cendoya, G. Predation on the slug Deroceras reticulatum (Pulmonata: Stylommatophora) by Scarites anthracinus (Coleoptera: Carabidae). Ecol. Austral 2009, 19, 55–61. [Google Scholar]
- Rees, M.; Brown, V.K. Interactions between Invertebrate Herbivores and Plant Competition. J. Ecol. 1992, 80, 353. [Google Scholar] [CrossRef]
- Beeby, A.; Richmond, L. Differential growth rates and calcium-allocation strategies in the garden snail Cantareus aspersus. J. Molluscan Stud. 2007, 73, 105–112. [Google Scholar] [CrossRef]
- Busscher, W. Soil Tillage in Agroecosystems. Vadose Zone J. 2005, 4, 442. [Google Scholar] [CrossRef]
- Kay, P.; Grayson, R.; Mciwem, P.K. Using water industry data to assess the metaldehyde pollution problem. Water Environ. J. 2013, 28, 410–417. [Google Scholar] [CrossRef]
- Dodler, L.K. Metaldehyde toxicosis. Vererinary Med. 2003, 98, 213–215. [Google Scholar]
- Bailey, S. Molluscicidal baits for control of terrestrial gastropods. In Molluscs as Crop Pests; Barker, G.M., Ed.; CABI Publishing: Wallingford, UK, 2002; pp. 33–54. [Google Scholar]
- Langan, A.M.; Taylor, A.; Wheater, C.P. Effects of metaldehyde and methiocarb on feeding preferences and survival of a slug predator (Pterostichus melanarius (F.): Carabidae, Pterostichini). J. Appl. Èntomol. 2004, 128, 51–55. [Google Scholar] [CrossRef]
- Dean, S. Tillage Practices with Updated Alfalfa Seedings and Final Acreages; USDA-NASS: Washington, DC, USA, 2014; p. 2. [Google Scholar]
- Lövei, G.L.; Sunderland, K.D. Ecology and Behavior of Ground Beetles (Coleoptera: Carabidae). Annu. Rev. Èntomol. 1996, 41, 231–256. [Google Scholar] [CrossRef]
- Wilson, M.; Rae, R. Phasmarhabditis hermaphrodita as a control agent for slugs. In Nematode Pathogenesis of Insects and Other Pests; Springer: Berlin/Heidelberg, Germany, 2015; pp. 509–521. [Google Scholar]
- Mc Donnell, R.; De Ley, T.I.; Paine, T.D. Susceptibility of neonate Lissachatina fulica (Achatinidae: Mollusca) to a US strain of the nematode Phasmarhabditis hermaphrodita (Rhabditidae: Nematoda). Biocontrol Sci. Technol. 2018, 28, 1091–1095. [Google Scholar] [CrossRef]
- Grewal, P.; Grewal, S.; Taylor, R.; Hammond, R. Application of Molluscicidal Nematodes to Slug Shelters: A Novel Approach to Economic Biological Control of Slugs. Biol. Control 2001, 22, 72–80. [Google Scholar] [CrossRef]
- Jaworska, M. Laboratory Infection of Slugs (Gastropoda: Pulmonata) with Entomopathogenic Nematodes (Rhabditida: Nematoda). J. Invertebr. Pathol. 1993, 61, 223–224. [Google Scholar] [CrossRef]
- Rae, R.G.; Tourna, M.; Wilson, M.J. The slug parasitic nematode Phasmarhabditis hermaphrodita associates with complex and variable bacterial assemblages that do not affect its virulence. J. Invertebr. Pathol. 2010, 104, 222–226. [Google Scholar] [CrossRef]
- Ross, J.; Ivanova, E.; Sirgel, W.; Malan, A.; Wilson, M. Diversity and distribution of nematodes associated with terrestrial slugs in the Western Cape Province of South Africa. J. Helminthol. 2011, 86, 215–221. [Google Scholar] [CrossRef]
- Schley, D.; Bees, M.A. The role of time delays in a non-autonomous host–parasitoid model of slug biocontrol with nematodes. Ecol. Model. 2006, 193, 543–559. [Google Scholar] [CrossRef]
- Wynne, R.; Morris, A.; Rae, R. Behavioural avoidance by slugs and snails of the parasitic nematode Phasmarhabditis hermaphrodita. Biocontrol Sci. Technol. 2016, 26, 1129–1138. [Google Scholar] [CrossRef]
- Puža, V.; Mráček, Z.; Nermut, J. Novelties in Pest Control by Entomopathogenic and Mollusc-Parasitic Nematodes. Intech. Open 2016, 71–102. [Google Scholar] [CrossRef]
- Koppenhöfer, A.M.; Shapiro-Ilan, D.I.; Hiltpold, I. Entomopathogenic Nematodes in Sustainable Food Production. Front. Sustain. Food Syst. 2020, 4, 125. [Google Scholar] [CrossRef]
- Speiser, B.; Zaller, J.; Neudecker, A. Size-specific susceptibility of the pest slugs Deroceras reticulatum and Arion lusitanicus to the nematode biocontrol agent Phasmarhabditis hermaphrodita. Biocontrol 2001, 46, 311–320. [Google Scholar] [CrossRef]
- Glen, D.; Wilson, M.; Brain, P.; Stroud, G. Feeding Activity and Survival of Slugs, Deroceras reticulatum, Exposed to the Rhabditid Nematode, Phasmarhabditis hermaphrodita: A Model of Dose Response. Biol. Control 2000, 17, 73–81. [Google Scholar] [CrossRef]
- Wilson, M.J.; Glen, D.M.; George, S.K. The rhabditid nematode Phasmarhabditis hermaphrodita as a potential biological control agent for slugs. Biocontrol Sci. Technol. 1993, 3, 503–511. [Google Scholar] [CrossRef]
- Tan, L.; Grewal, P.S. Infection Behavior of the Rhabditid Nematode Phasmarhabditis hermaphrodita to the Grey Garden Slug Deroceras reticulatum. J. Parasitol. 2001, 87, 1349. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; Glen, D.; Pearce, J.; Rodgers, P. Monoxenic culture of the slug parasite Phasmarhabditis hermaphrodita (Nematoda: Rhabditidae) with different bacteria in liquid and solid phase. Fundam. Appl. Nematol. 1995, 18, 159–166. [Google Scholar]
- MacMillan, K.; Haukeland, S.; Rae, R.; Young, I.; Crawford, J.; Hapca, S.; Wilson, M. Dispersal patterns and behaviour of the nematode Phasmarhabditis hermaphrodita in mineral soils and organic media. Soil Biol. Biochem. 2009, 41, 1483–1490. [Google Scholar] [CrossRef]
- Schneider, A. Ueber eine Nematodenlarve und gewisse Verschiedenheiten in den Geschlechtsorganen der Nematoden. Z. Fur Wiss. Zool. 1859, 10, 176–178. [Google Scholar]
- Mengert, H. Nematoden und schnecken. Z. Für Morphol. Und Okol. Der Tiere 1953, 41, 311–349. [Google Scholar] [CrossRef]
- Schneider, A. Monographie Der Nematoden; Reimer, G., Ed.; Walter De Gruyter Incorporated: Berlin, Germany, 1866. [Google Scholar]
- Nermuť, J.; Půža, V.; Mekete, T.; Mráček, Z. Phasmarhabditis bonaquaense n. sp. (Nematoda: Rhabditidae), a new slug-parasitic nematode from the Czech Republic. Zootaxa 2016, 4179, 530–546. [Google Scholar] [CrossRef]
- Nermuť, J.; Půža, V.; Mráček, Z. Phasmarhabditis apuliae n. sp. (Nematoda: Rhabditidae), a new rhabditid nematode from milacid slugs. Nematology 2016, 18, 1095–1112. [Google Scholar] [CrossRef]
- Nermuť, J.; Půža, V.; Mekete, T.; Mráček, Z. Phasmarhabditis bohemica n. sp. (Nematoda: Rhabditidae), a slug-parasitic nematode from the Czech Republic. Nematology 2017, 19, 93–107. [Google Scholar] [CrossRef]
- Azzam, K.M. Description of the nematode Phasmarhabditis tawfiki n. sp. isolated from Egyptian terrestrial snails and slugs. J. Egypt. Ger. Soc. Zool. 2003, 42, 79–88. [Google Scholar]
- Huang, R.-E.; Ye, W.; Ren, X.; Zhao, Z. Morphological and Molecular Characterization of Phasmarhabditis huizhouensis sp. nov. (Nematoda: Rhabditidae), a New Rhabditid Nematode from South China. PLoS ONE 2015, 10, e0144386. [Google Scholar] [CrossRef]
- De Ley, I.T.; Holovachov, O.; Mc Donnell, R.J.; Bert, W.; Paine, T.D.; De Ley, P. Description of Phasmarhabditis californica n. sp. and first report of P. papillosa (Nematoda: Rhabditidae) from invasive slugs in the USA. Nematology 2016, 18, 175–193. [Google Scholar] [CrossRef]
- Ivanova, E.S.; Spiridonov, S.E. Phasmarhabditis meridionalis sp. n.(Nematoda: Rhabditidae) from a land snail Quantula striata (Gastropoda: Dyakiidae) from southern Vietnam. Russ. J. Nematol. 2017, 25, 129–140. [Google Scholar]
- Ross, J.L.; Pieterse, A.; Malan, A.P.; Ivanova, E. Phasmarhabditis safricana n. sp. (Nematoda: Rhabditidae), a parasite of the slug Deroceras reticulatum from South Africa. Zootaxa 2018, 4420, 391–404. [Google Scholar] [CrossRef]
- Gorgadze, O.; Troccoli, A.; Fanelli, E.; Tarasco, E.; De Luca, F. Phasmarhabditis thesamica n. sp. (Nematoda: Rhabditidae), a new slug nematode from southern slope of Caucasus, Georgia. Nematology 2022, 24, 617–629. [Google Scholar] [CrossRef]
- Chichester, L.F.; Getz, L.L. The terrestrial slugs of northeastern North America. Sterkiana 1973, 51, 11–42. [Google Scholar]
- Patrignani, A.; Ochsner, T.E. Canopeo: A Powerful New Tool for Measuring Fractional Green Canopy Cover. Agron. J. 2015, 107, 2312–2320. [Google Scholar] [CrossRef]
- USDA NRCS. Web Soil Survey. Available online: https://websoilsurvey.sc.egov.usda.gov/App/HomePage.htm (accessed on 12 March 2022).
- White, G.F.; Rose, M.C.; Styr, B.; Báez-Mendoza, R.; Mastrobattista, E.P.; Ni, J.; Huang, C. A Method for Obtaining Infective Nematode Larvae from Cultures. Science 1927, 66, 302–303. [Google Scholar] [CrossRef]
- Williams, B.D.; Schrank, B.; Huynh, C.; Shownkeen, R.; Waterston, R.H. A genetic mapping system in Caenorhabditis elegans based on polymorphic sequence-tagged sites. Genetics 1992, 131, 609–624. [Google Scholar] [CrossRef]
- Blaxter, M.L.; De Ley, P.; Garey, J.R.; Liu, L.X.; Scheldeman, P.; Vierstraete, A.; Vanfleteren, J.R.; Mackey, L.Y.; Dorris, M.; Frisse, L.M.; et al. A molecular evolutionary framework for the phylum Nematoda. Nature 1998, 392, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Denver, D.R.; Morris, K.; Thomas, W.K. Phylogenetics in Caenorhabditis elegans: An Analysis of Divergence and Outcrossing. Mol. Biol. Evol. 2003, 20, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Elkin, C.J.; Richardson, P.M.; Fourcade, H.M.; Hammon, N.M.; Pollard, M.J.; Predki, P.F.; Glavina, T.; Hawkins, T.L. High-Throughput Plasmid Purification for Capillary Sequencing. Genome Res. 2001, 11, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- NCBI Resource Coordinators. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018, 46, D8–D13. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.R-project.org/ (accessed on 24 October 2022).
- Ogren, R.E. A Contribution to the Life Cycle of Cosmocercoides in Snails (Nematoda: Cosmocercidae). Trans. Am. Microsc. Soc. 1953, 72, 87. [Google Scholar] [CrossRef]
- Ogren, R.E. The nematode Cosmocercoides dukae as a parasite of the slug. In Proceedings of the Pennsylvania Academy of Science 26 March 1959, State College, PA, USA; Volume 33, pp. 236–241.
- Anderson, R.C. On the development and transmission of Cosmocercoides dukae of terrestrial molluscs in ontario. Can. J. Zool. 1960, 38, 801–825. [Google Scholar] [CrossRef]
- Morand, S. Cycle évolutif de Angiostoma aspersae Morand, 1986 parasite de la cavité palléale de Helix aspersa Müller. Ann. Parasitol. Hum. Comparée 1989, 64, 340–346. [Google Scholar] [CrossRef]
- Vanderburgh, D.J.; Anderson, R.C. The relationship between nematodes of the genus Cosmocercoides Wilkie, 1930 (Nematoda: Cosmocercoidea) in toads (Bufo americanus) and slugs (Deroceras laeve). Can. J. Zool. 1987, 65, 1650–1661. [Google Scholar] [CrossRef]
- Dillman, A.R.; Chaston, J.M.; Adams, B.J.; Ciche, T.A.; Goodrich-Blair, H.; Stock, S.P.; Sternberg, P.W. An Entomopathogenic Nematode by Any Other Name. PLoS Pathog. 2012, 8, e1002527. [Google Scholar] [CrossRef]
- Ross, J.L.; Ivanova, E.S.; Sirgel, W.F.; Malan, A.P.; Wilson, M.J. Diversity and distribution of nematodes associated with terrestrial slugs in the Western Cape Province of South Africa. Journal of Helminthology 2012, 86, 215–221. [Google Scholar] [CrossRef]
- Mehdizadeh, S.; Shokoohi, E.; Abolafia, J. Morphological and molecular characterisation of Panagrolaimus Fuchs, 1930 (Nematoda, Rhabditida, Panagrolaimidae) species from Iran. Russ. J. Nematol. 2013, 21, 99–115. [Google Scholar]
- Félix, M.-A.; Ailion, M.; Hsu, J.-C.; Richaud, A.; Wang, J. Pristionchus nematodes occur frequently in diverse rotting vegetal substrates and are not exclusively necromenic, while Panagrellus redivivoides is found specifically in rotting fruits. PLoS ONE 2018, 13, e0200851. [Google Scholar] [CrossRef]
- Brophy, T.; Mc Donnell, R.; Howe, D.; Denver, D.; Ross, J.; Luong, L. Nematodes associated with terrestrial slugs in the Edmonton region of Alberta, Canada. J. Helminthol. 2020, 94, e200. [Google Scholar] [CrossRef]
- Mc Donnell, R.J.; Paine, T.D.; Gormally, M.J. ) Slugs: A Guide to the Invasive and Native Fauna of California. Available online: https://anrcatalog.ucanr.edu/pdf/8336.pdf (accessed on 22 February 2023).
- South, A. Terrestrial Slugs: Biology, Ecology and Control; Chapman and Hall Ltd.: London, UK, 1992; p. 428. [Google Scholar]
- CABI. Invasive Species Compedium. Available online: https://www.cabi.org/isc/datasheet/30825#tohistoryOfIntroductionAndSpread (accessed on 2 September 2022).
- Becker, J.E.; Mirochnitchenko, N.A.; Ingram, H.; Everett, A.; McCluney, K.E. Water-seeking behavior among terrestrial arthropods and mollusks in a cool mesic region: Spatial and temporal patterns. PLoS ONE 2021, 16, e0260070. [Google Scholar] [CrossRef]
- Khoja, S.; Eltayef, K.M.; Baxter, I.; Bull, J.C.; Loveridge, E.J.; Butt, T. Fungal volatile organic compounds show promise as potent molluscicides. Pest Manag. Sci. 2019, 75, 3392–3404. [Google Scholar] [CrossRef]
- Anderson, R.C. Nematode Parasites of Vertebrates: Their Development and Transmission; CABI Publishing: Wallingford, UK, 2000. [Google Scholar]
- Grewal, P.; Grewal, S.; Tan, L.; Adams, B. Parasitism of molluscs by nematodes: Types of associations and evolutionary trends. J. Nematol. 2003, 35, 146. [Google Scholar] [CrossRef]
- Sudhaus, W. Dispersion of nematodes (Rhabditida) in the guts of slugs and snails. Soil Org. 2018, 90, 101–114. [Google Scholar] [CrossRef]
- De Ley, I.T.; McDonnell, R.D.; Lopez, S.; Paine, T.D.; De Ley, P. Phasmarhabditis hermaphrodita (Nematoda: Rhabditidae), a potential biocontrol agent isolated for the first time from invasive slugs in North America. Nematology 2014, 16, 1129–1138. [Google Scholar] [CrossRef]
- Donnell, R.M.; Lutz, M.S.; Howe, D.K.; Denver, D.R. First Report of the Gastropod-Killing Nematode, Phasmarhabditis hermaphrodita, in Oregon, U.S.A. J. Nematol. 2018, 50, 77–78. [Google Scholar] [CrossRef]
- Donnell, R.M.; Colton, A.J.; Howe, D.K.; Denver, D.R. Lethality of four species of Phasmarhabditis (Nematoda: Rhabditidae) to the invasive slug, Deroceras reticulatum (Gastropoda: Agriolimacidae) in laboratory infectivity trials. Biol. Control 2020, 150, 104349. [Google Scholar] [CrossRef]
- Mc Donnell, R.; Colton, A.; Howe, D.; Denver, D. Susceptibility of Testacella haliotidea (Testacellidae: Mollusca) to a U.S. strain of Phasmarhabditis hermaphrodita (Rhabditidae: Nematoda). Biocontrol Sci. Technol. 2022, 32, 262–266. [Google Scholar] [CrossRef]
- Rae, R.; Verdun, C.; Grewal, P.S.; Robertson, J.F.; Wilson, M.J. Biological control of terrestrial molluscs using Phasmarhabditis hermaphrodita—Progress and prospects. Pest Manag. Sci. 2007, 63, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Howe, D.K.; Ha, A.D.; Colton, A.; De Ley, I.T.; Rae, R.G.; Ross, J.; Wilson, M.; Nermut, J.; Zhao, Z.; Mc Donnell, R.J.; et al. Phylogenetic evidence for the invasion of a commercialized European Phasmarhabditis hermaphrodita lineage into North America and New Zealand. PLoS ONE 2020, 15, e0237249. [Google Scholar] [CrossRef] [PubMed]
- Pechova, H.; Foltan, P. The parasitic nematode Phasmarhabditis hermaphrodita defends its slug host from being predated or scavenged by manipulating host spatial behaviour. Behav. Process. 2008, 78, 416–420. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).