Biofertilizers Improve the Leaf Quality of Hydroponically Grown Baby Spinach (Spinacia oleracea L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Plant Nutrition of Spinach
2.3. Biofetilizer Applications
2.4. Spinach Leaf Antioxidant Measurements
2.5. Mineral Elements and Nitrate Analysis
2.6. Statistical Analysis
3. Results and Discussion
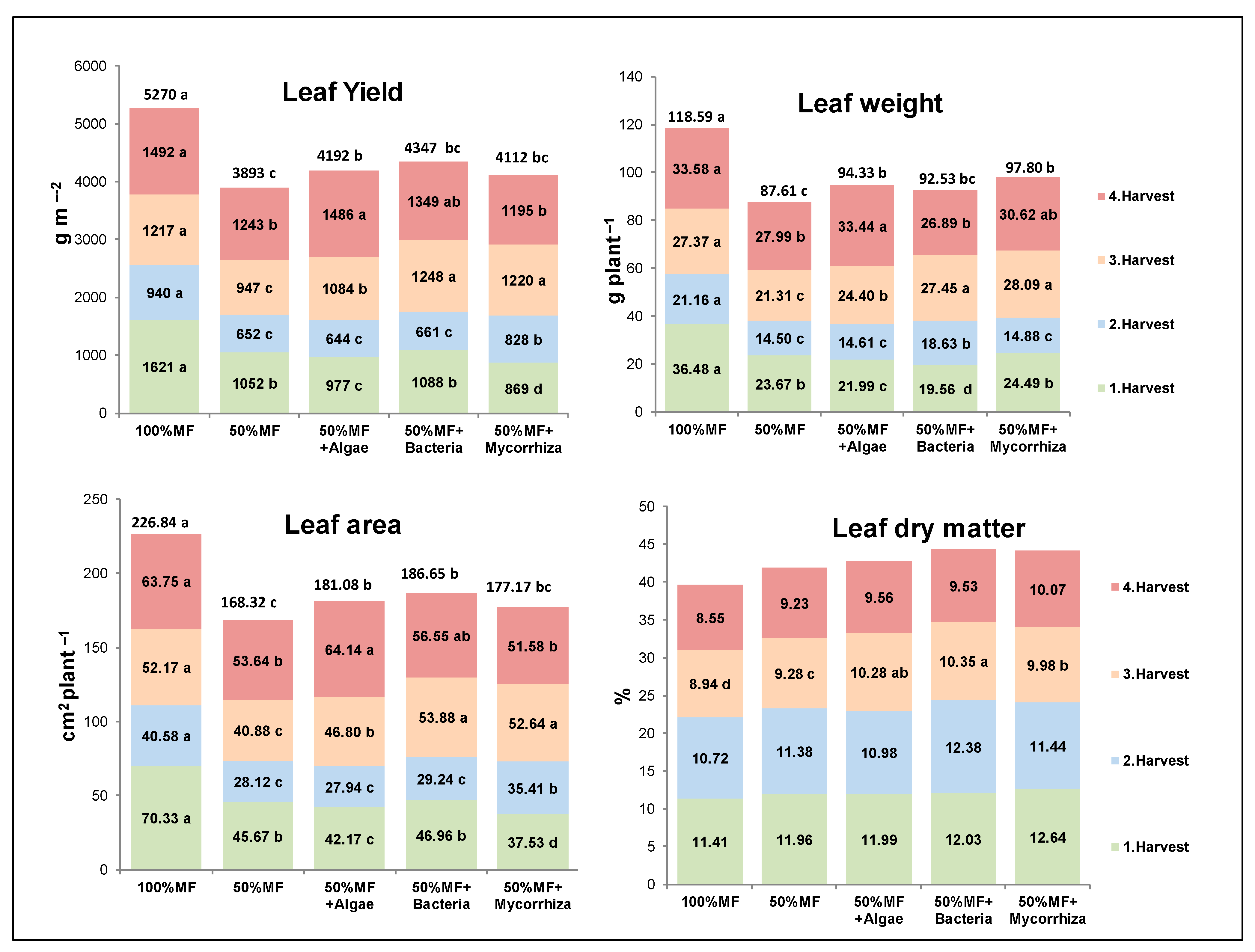
3.1. Agronomic Traits
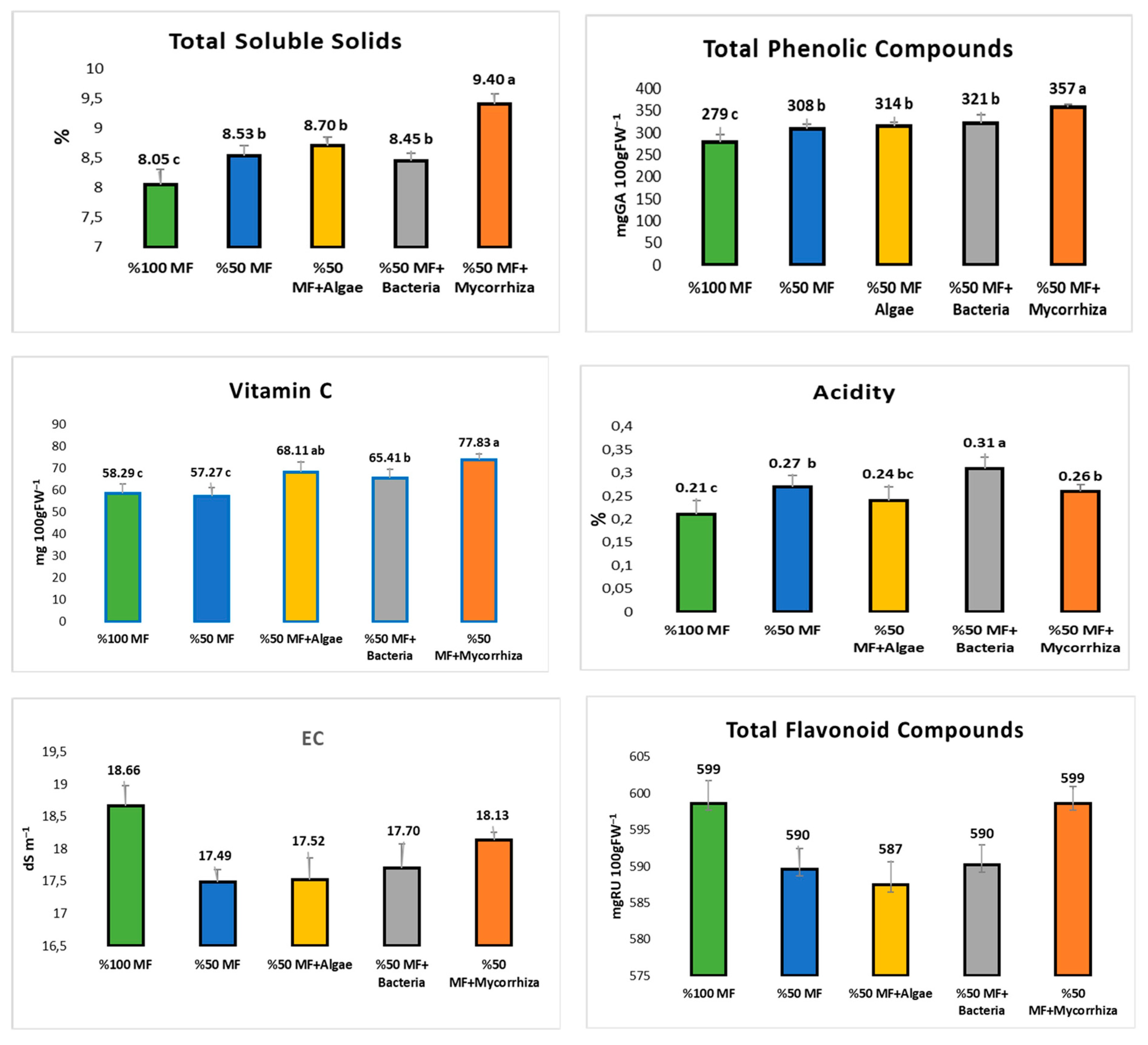
3.2. Baby Spinach Leaf Antioxidant Properties
3.3. Nitrate and Mineral Content of the Baby Spinach Leaf
3.4. Color Properties of Spinach Leaves
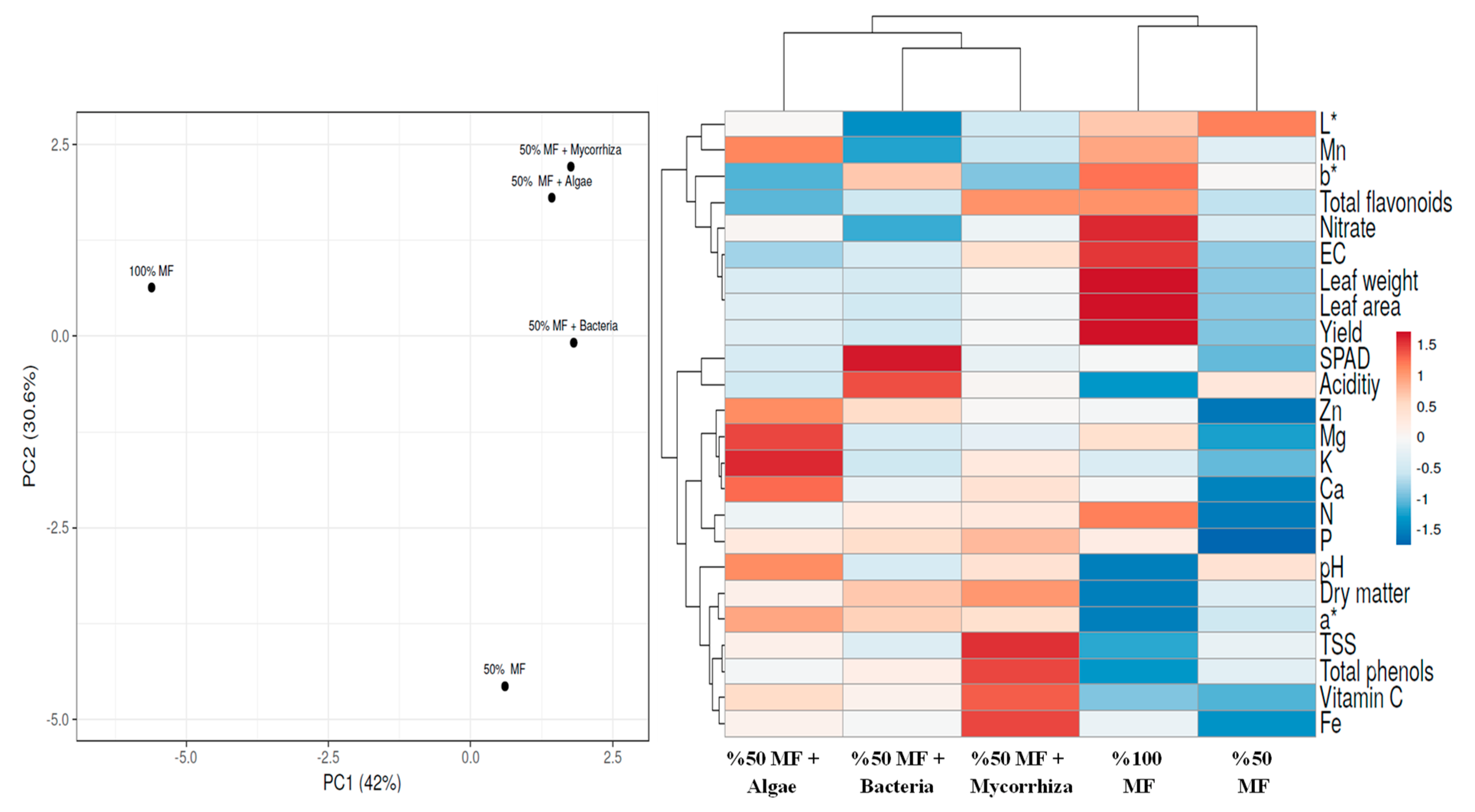
3.5. Heat Map and Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO 2021 Statistics. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 30 December 2022).
- Koh, E.; Charoenprasert, S.; Mitchell, A.E. Effect of organic and conventional cropping systems on ascorbic acid, vitamin c, flavonoids, nitrate and oxalate in 27 varieties of spinach (Spinacia oleracea L.). J. Agric. Food Chem. 2012, 60, 3144–3150. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Tahzeeb-ul-Hassan, M.; Abid, M.; Fahad, S.; Brtnicky, M.; Dokulilova, T.; Danish, S. Potential role of compost mixed biochar with rhizobacteria in mitigating lead toxicity in spinach. Sci. Rep. 2020, 10, 12159. [Google Scholar] [CrossRef]
- Giménez, A.; Fernández, J.A.; Pascual, J.A.; Ros, M.; Saez-Tovar, J.; Martinez-Sabater, E.; Gruda, N.S.; Egea-Gilabert, C. Promising Composts as Growing Media for the Production of Baby Leaf Lettuce in a Floating System. Agronomy 2020, 10, 1540. [Google Scholar] [CrossRef]
- Reddy, C.A.; Saravanan, R.S. Polymicrobial multi-functional approach for enhancement of crop productivity. Adv. Appl. Microbiol. 2013, 82, 53–113. [Google Scholar]
- Baum, C.; El-Tohamy, W.; Gruda, N. Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: A review. Sci. Hortic. 2015, 187, 131–141. [Google Scholar] [CrossRef]
- Dasgan, H.Y.; Yilmaz, M.; Dere, S.; İkiz, B.; Gruda, N.S. Bio-fertilizers reduced the need for the mineral fertilizers in soilless grown capia pepper. Horticulturae 2023, 9, 188. [Google Scholar] [CrossRef]
- Balliu, A.; Zheng, Y.; Sallaku, G.; Fernández, J.A.; Gruda, N.S.; Tuzel, Y. Environmental and Cultivation Factors Affect the Morphology, Architecture and Performance of Root Systems in Soilless Grown Plants. Horticulturae 2021, 7, 243. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Env. 2022, 4, 4–18. [Google Scholar] [CrossRef]
- Nautiyal, C.S.; Bhadauria, S.; Kumar, P.; Lal, H.; Mondal, R.; Verma, D. Stress-induced phosphate solubilization in bacteria isolated from alkaline soils. FEMS Microbiol. Lett. 2000, 182, 291–296. [Google Scholar] [CrossRef]
- Yolcu, H.; Gunes, A.; Gullap, M.K.; Cakmakci, R. Effects of plant growth-promoting rhizobacteria on some morphologic characteristics, yield and quality contents of Hungarian vetch. Turk. J. Field Crop. 2012, 17, 208–214. [Google Scholar]
- Ortas, I.; Ergun, B.; Ortakci, D.; Ercan, S.; Kose, O. Production of mycorrhiza spores and their use in agriculture. Turk. J. Agric. 1999, 23, 959–968. [Google Scholar]
- Win, T.T.; Barone, G.D.; Secundo, F.; Fu, P. Algal biofertilizers and plant growth stimulants for sustainable agriculture. Ind. Biotechnol. 2018, 14, 203–211. [Google Scholar] [CrossRef]
- Aydoner Coban, G.; Dasgan, H.Y.; Akhoundnejad, Y.; Ak Cimen, B. Use of microalgae (Chlorella vulgaris) to save mineral nutrients in soilless grown tomato. Acta Hortic. 2020, 1273, 161–168. [Google Scholar] [CrossRef]
- Ergun, O.; Dasgan, H.Y.; and Isik, O. Effects of microalgae Chlorella vulgaris on hydroponically grown lettuce. Acta Hortic. 2020, 1273, 169–176. [Google Scholar] [CrossRef]
- Dasgan, H.Y.; Aydoner, G.; Akyol, M. Use of some microorganisms as bio-fertilizers in soilless grown squash for saving chemical nutrients. Acta Hortic. 2012, 927, 155–162. [Google Scholar] [CrossRef]
- Gruda, N.; Savvas, D.; Youssuf, R.; Colla, G. Impacts of genetic material and current technologies on product quality of selected greenhouse vegetables–A review. Eur. J. Horti. Sci. 2018, 83, 319–328. [Google Scholar] [CrossRef]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Gruda, N. Impact of environmental factors on product quality of greenhouse vegetables for fresh consumption. Crit. Rev. Plant. Sci. 2005, 24, 227–247. [Google Scholar] [CrossRef]
- Gruda, N. Do soilless culture systems have an influence on product quality of vegetables? J. Appl. Bot Food Qual. 2009, 82, 141–147. [Google Scholar]
- Avio, L.; Sbrana, C.; Giovannetti, M.; Frassinetti, S. Arbuscular mycorrhizal fungi affect total phenolics content and antioxidant activity in leaves of oak leaf lettuce varieties. Sci. Hortic. 2017, 224, 265–271. [Google Scholar] [CrossRef]
- Dasgan, H.Y.; Aldiyab, A.; Elgudayem, F.; İkiz, B.; Gruda, N.S. Effect of biofertilizers on leaf yield, nitrate amount, mineral content and antioxidants of basil (Ocimum basilicum L.) in a floating culture. Sci. Rep. 2022, 12, 20917. [Google Scholar] [CrossRef]
- Spanos, G.A.; Wrolstad, R.E. Influence of Processing and Storage on the Phenolic Composition of Thompson Seedless Grape Juice. J. Agric Food Chem. 1990, 38, 1565–1571. [Google Scholar] [CrossRef]
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, J.; Luyck, M.; Cazin, M.; Cazin, J.C.; Bailleul, F.; Trotin, F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 2000, 72, 35–40. [Google Scholar] [CrossRef]
- Jones, J.B. Laboratory Guide for Conducting Soil Tests Plant Analysis, 1st ed.; Taylor and Francis eBooks; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Cataldo, D.A.; Haroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant. Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Cocetta, G.; Quattrini, E.; Schiavi, M.; Martinetti, L.; Spinardi, A.; Ferrante, A. Nitrate and sucrose content in fresh-cut leaves of spinach plants grown in floating system. Agric. Med. 2007, 137, 79–85. [Google Scholar]
- Duyar, H.; Kilic, C.C. A research on production of rocket and parsley in floating system. J. Agric. Sci. 2016, 8, 54–60. [Google Scholar] [CrossRef]
- Öztekin, G.B.; Uludag, T.; Tüzel, Y. Growing spinach (Spinacia oleracea L.) in a floating system with different concentrations of nutrient solution. Appl. Ecol. Env. Res. 2018, 16, 3333–3350. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Alves-Pereira, I.; Ferreira, R.; Gruda, N.S. Coir, an alternative to peat—Effects on plant growth, phytochemical accumulation, and antioxidant power of spinach. Horticulturae 2021, 7, 127. [Google Scholar] [CrossRef]
- Lara, O.A.; Amorós, A.; Tapia, M.L.; Escalona, V.H. Effect of a photoselective filter on the yield and postharvest quality of “Viroflay” baby spinach (Spinacia oleracea L.) leaves cultivated in a hydroponic system. Sci. Hort. 2021, 277, 109804. [Google Scholar] [CrossRef]
- Levine, C.P.; Mattson, N.S. Potassium-Deficient Nutrient Solution affects the yield, morphology, and tissue mineral elements for hydroponic baby leaf spinach (Spinacia oleracea L.). Horticulturae 2021, 7, 213. [Google Scholar] [CrossRef]
- Kovács, A.B.; Kremper, I.; Kincses, I.; Leviczky, A. Influences of different organic fertilizers on nutrients of humic sandy soil and on the growth of Spinach (Spinacia oleracea L.). Acta Agrar. Debr. 2016, 70, 23–28. [Google Scholar] [CrossRef]
- Saleem, M.; Arshad, M.; Hussain, S.; Bhatti, A.S. Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J. Ind. Microbiol. Biotechnol. 2007, 34, 635–648. [Google Scholar] [CrossRef]
- El-Tohamy, W.A.; El-Abagy, H.M.; El-Greadly, N.H.; Gruda, N. Hormonal changes, growth and yield of tomato plants in response to chemical and bio-fertilization application in sandy soils. J. App. Bot Food Qual. 2009, 82, 179–182. [Google Scholar]
- Azizoglu, U.; Yilmaz, N.; Simsek, O.; Ibal, J.C.; Tagele, S.B.; Shin, J.H. The fate of plant growth-promoting rhizobacteria in soilless agriculture: Future perspectives. 3 Biotech. 2021, 11, 382. [Google Scholar] [CrossRef]
- Jabborova, D.; Annapurna, K.; Paul, S.; Kumar, S.; Saad, H.A.; Desouky, S.; Ibrahim, M.F.M.; Elkelish, A. beneficial features of biochar and arbuscular mycorrhiza for ımproving spinach plant growth, root morphological traits, physiological properties, and soil enzymatic activities. J. Fungi. 2021, 7, 571. [Google Scholar] [CrossRef]
- Howard, L.R.; Pandjaitan, N.; Morelock, T.; Gil, M.I. Antioxidant capacity and phenolic content of spinach as affected by genetics and growing season. J. Agric. Food Chem. 2002, 50, 5891–5896. [Google Scholar] [CrossRef]
- Bangash, J.A.; Arif, M.; Khan, F.; Khan, F.; Amin-Ur-Rahman, H.I. Proximate composition, minerals and vitamins content of selected vegetables grown in Peshawar. J. Chem. Soc. Pak. 2011, 33, 118–122. [Google Scholar]
- Bergquist, S.Å.; Gertsson, U.E.; Nordmark, L.Y.; Olsson, M.E. Ascorbic acid, carotenoids, and visual quality of baby spinach as affected by shade netting and postharvest storage. J. Agric. Food Chem. 2007, 55, 8444–8451. [Google Scholar] [CrossRef] [PubMed]
- Fusco, G.M.; Nicastro, R.; Rouphael, Y.; Carillo, P. The effects of the microbial biostimulants approved by EU regulation 2019/1009 on yield and quality of vegetable crops. Foods 2022, 11, 2656. [Google Scholar] [CrossRef] [PubMed]
- Saraylou, M.; Nadian, H.A.; Enayatizamir, N.; Rangzan, N.; Senn, S. Changes in nitrate concentration, nitrate reductase activity, and accumulation of nutrients in spinach (Spinacia Oleracea L.) in response to Streptomyces sp. inoculation. Preprints 2022, 2022110036. [Google Scholar] [CrossRef]
- European Commission 2011 Regulation no. 1258/2011 of 02 Dec. 2011 Amending Regulation no. 1881/2006 as Regards Maximum Levels for Nitrates in Foodstuffs. OJ. L. 2011 320:15–17. 10 Dec. 2016. Available online: https://www.fsai.ie/uploadedFiles/Reg1258_2011.pdf (accessed on 10 December 2022).
- Bostanci, K.B. Investigation of the Effects of Stagnant Water Culture Technique and Soil Cultivation on the Yield and Quality of Spinach Grown in the Open and under Cover. Master’s Thesis, Akdeniz University, Antalya, Turkey, 2018. [Google Scholar]
- Kacar, B.; Katkat, V. Plant Nutrition; Uludag University Strengthening Foundation Vipas Publications: Bursa, Turkey, 1998; Volume 127. [Google Scholar]
- Plassard, C.; Becquer, A.; Garcia, K. Phosphorus transport in mycorrhiza: How far are we? Trends Plant Sci. 2019, 24, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Kaynar, S.; Hicsonmez, U.; Ozdemir, A.; Ozdemir, C. Evaluation of soil, root and leaf element contents of spinach plant (Spinacia oleracea L.) grown in Manisa. Igdir. Univ. J. Inst. Sci.Tech. 2018, 8, 131–140. [Google Scholar] [CrossRef]
- İbrikci, H.; Gulut, K.Y.; Guzel, N. Plant Analysis Techniques in Fertilization; Textbooks; Cukurova University Faculty of Agriculture Publications: Adana, Turkey, 1994. [Google Scholar]
- Campbell, C.R. Reference Sufficiency Ranges for Vegetable Crops Spinach, Greenhouse. South. Coop. Ser. Bull. 2000, 394, 77. Available online: https://www.clemson.edu/public/regulatory/ag-srvc-lab/plant-tissue/guidelines.html (accessed on 12 December 2022).
- Moncada, A.; Miceli, A.; Vetrano, F. Use of plant growth-promoting rhizobacteria (PGPR) and organic fertilization for soilless cultivation of basil. Sci. Hortic. 2021, 275, 109733. [Google Scholar] [CrossRef]




| STOCK A | STOCK B |
|---|---|
| Calcium nitrate [Ca(NO3)2•4H2O] | Potassium sulfate (K2SO4) |
| Potassium nitrate (KNO3) | Mono potassium phosphate (KH2PO4) |
| Ammonium nitrate (NH4NO3) | magnesium sulfate (MgSO4•7H2O) |
| Fe–EDDHA | Micronutrients |
| Zinc sulfate (ZnSO4•5H2O) | |
| Boric acid (H3BO3) | |
| Manganese sulfate (MnSO4•4H2O) | |
| Copper sulfate (CuSO4•5H2O) | |
| Ammonium molybdate, [(NH4)6Mo7O24•4H2O] |
| Element | mg L−1 | Element | mg L−1 |
|---|---|---|---|
| N | 227.00 | Zn | 0.19 |
| P | 45.00 | B | 0.41 |
| K | 315.00 | Cu | 0.23 |
| Ca | 205.00 | Mo | 0.18 |
| Mg | 75.00 | Mn | 0.78 |
| Fe | 4.62 |
| Treatments | Explanation |
|---|---|
| 100% Mineral fertilizers (MF) | Without supplementation of any biofertilizer and full of the mineral fertilizers requirements (Control 1) |
| 50% Mineral fertilizers (MF) | Without supplementation of any biofertilizer and half of the mineral fertilizers requirements (Control 2) |
| 50% Mineral fertilizers + Microalgae | Supplemented microalgae to half of the mineral fertilizers requirements |
| 50% Mineral fertilizers + PGPR | Supplemented PGPR to half of the mineral fertilizers requirements |
| 50% Mineral fertilizers + AMF | Supplemented AMF to half of the mineral fertilizers requirements |
| Treatments | L | a | b | SPAD-Chlorophyll |
|---|---|---|---|---|
| 100% MF | 35.38 ab | −9.0 | 21.4 | 66.33 |
| 50% MF | 37.84 a | −8.3 | 19.6 | 62.15 |
| 50% MF + Algae | 31.58 a–c | −7.4 | 18.0 | 64.64 |
| 50% MF + Bacteria | 24.15 c | −7.6 | 20.6 | 73.72 |
| 50% MF + Mycorrhiza | 28.93 bc | −7.7 | 18.2 | 65.56 |
| LSD0.05 | 8.34 | NS | NS | NS. |
| P | 0.03 | 0.08 | 0.71 | 0.1582 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasgan, H.Y.; Kacmaz, S.; Arpaci, B.B.; İkiz, B.; Gruda, N.S. Biofertilizers Improve the Leaf Quality of Hydroponically Grown Baby Spinach (Spinacia oleracea L.). Agronomy 2023, 13, 575. https://doi.org/10.3390/agronomy13020575
Dasgan HY, Kacmaz S, Arpaci BB, İkiz B, Gruda NS. Biofertilizers Improve the Leaf Quality of Hydroponically Grown Baby Spinach (Spinacia oleracea L.). Agronomy. 2023; 13(2):575. https://doi.org/10.3390/agronomy13020575
Chicago/Turabian StyleDasgan, Hayriye Yildiz, Sevda Kacmaz, Bekir Bülent Arpaci, Boran İkiz, and Nazim S. Gruda. 2023. "Biofertilizers Improve the Leaf Quality of Hydroponically Grown Baby Spinach (Spinacia oleracea L.)" Agronomy 13, no. 2: 575. https://doi.org/10.3390/agronomy13020575
APA StyleDasgan, H. Y., Kacmaz, S., Arpaci, B. B., İkiz, B., & Gruda, N. S. (2023). Biofertilizers Improve the Leaf Quality of Hydroponically Grown Baby Spinach (Spinacia oleracea L.). Agronomy, 13(2), 575. https://doi.org/10.3390/agronomy13020575









