Abstract
Rocket salad is an important vegetable for the ready-to-eat sector, normally cultivated under greenhouse conditions, either in soil or soilless systems. In the latter case, as well as in the nurseries, its cultivation is usually carried out by using peat as a growing medium—a non-renewable substrate—for which it is urgent to find a replacement. Similarly to peat, compost may be used as a growing medium; however, depending on its origin, the chemical and physical characteristics may not bet suitable for plants’ cultivation. In this study, we propose the use of agro-industrial compost as a substitute for peat for rocket salad cultivation. Plants grown in compost, alone or in combination with the second cut of rocket salad, gave better results in several biometric parameters, without negatively affecting yield and dry weight percentage. As a mechanistic approach to further understand how compost can affect plants’ stress, the qualitative profile of phytochemicals (glucosinolates and (poly)phenols)—recognized markers of biotic and abiotic plant stress—were monitored and exhibited a decreasing trend in plants grown using compost relative to those cultivated with peat. The analysis of vitamin C provided information on the achievement of an enhanced concentration by the compost, especially in the second cut. It can be inferred from the results obtained that the compost used as a growing medium may be used as a peat-free substrate for rocket crop cultivation.
1. Introduction
Consumers are constantly searching for new vegetable products with a high content of bioactive substances such as vitamins and phytochemicals that may enhance their biological effects [1]. This trend has boosted the sustainable production of vegetables using germplasm of cultivated species [2], but also leveraging the wild species to be cultivated [3] that allows the consumption at different growth stages—namely sprouts, microgreens, and baby leaves (BL)—and at the adult stage [3].
The BL sector, which legal definition is codified by Commission Regulation (EU) N. 752/2014 [4], is commercially established and, in recent years—thanks to soil-less techniques and artificial lighting systems—there has been a chance for consumers to produce the final product (the so-called ‘prosumers’) [5]. Indeed, the need for production to move closer to cities will probably become increasingly important in the future, as about two-thirds of the world’s population will live in urban areas [6].
Nevertheless, when a growing medium is used for vegetable production, either at the nursery level or for the whole crop cycle (e.g., soil-less systems), sustainability cannot be overlooked, particularly when peat is used. Indeed, peat consumption for vegetable production accounts for roughly one-third of European horticultural peat utilization [7], and the trend is expected to increase in the 2020–2050 period [8]. Even if other growing media are used or tested, alone or mixed, the peat remains the major component of substrates in soil-less crop cultivation [7,9], thanks to its excellent physical and chemical characteristics, which entails a slowdown in the search for alternative substrate sources for peat’s replacement. Several growing media based on green waste material (green compost) may be an alternative, but often their use in production—particularly seedlings—is not appropriate due to their high pH and salt concentration [10]. Additionally, the diversity in the properties of the growing media makes it often necessary to design a suitable mixture design to have an appropriate replacer for peat [11].
Coir—another growing media that is also a renewable resource as it derives from agricultural wastes—may have sustainability concerns, as it is a by-product from a tropical crop, a geographical area far from the most important horticultural production zones [12]. Conversely, the compost may be locally obtained starting from local organic wastes, such as household and restaurant waste, and pruning of municipal areas. Particularly for the latter, urban gardening may be an impulse for a more thorough use of compost-based growing media derived from waste biomass [13,14]. Some authors highlighted that such organic material may improve the “3R” requirements (reduce, reuse, and recycle) [9]. Furthermore, the compost may contain useful compounds that may improve the quality of vegetables [15,16,17]. However, the suitability of the different materials for the preparation of composts has to be assessed [18]. More recently, agroindustry compost from organic residues has been proposed as an alternative to peat, because it is less environmentally impactful and has some peculiar characteristics—e.g., suppressiveness against plants’ pathogens and a source for biofertilization and bio-stimulation [19]—which provide an improvement of crop production and quality [20].
Changes in the growing conditions can be associated with plant stress and thus cause deleterious effects on plants’ performance and crops’ viability. In this regard, it is important to monitor parameters that inform on the biotic or abiotic stress induced in plants by crop management. Currently, the key role of phytochemicals (glucosinolates and phenolic compounds) as indicators of plant stress is broadly accepted [21]. Therefore, identifying managing options that help to reduce plants’ stress would be associated with the decrease of the concentration of such molecules that constitutes an advantage from a productive point of view [22]. Our hypothesis was that the cultivation of rocket salad in compost, with a very similar fertigation scheduling to that in peat (comparable substrate moisture), may improve the performance of rocket salad plants due to the chemical characteristics of the compost while also reducing abiotic stress.
Starting from the above premises, the aims of this work were: (i) to test the feasibility of an agro-industrial compost, as a replacement for peat as a growing medium, for the cultivation of the rocket salad [Diplotaxis tenuifolia (L.) DC.] in a soil-less system; and (ii) to test the influence of growing media (peat vs. compost) on the content of phytochemical markers of rocket plant stress.
2. Materials and Methods
2.1. Plant Material and Growing Conditions
The experiment was realized in an unheated greenhouse of the “Estación Experimental Agroalimentaria Tomás Ferro” (37.686376, −0.950268) of the “Universidad Politécnica de Cartagena” (Murcia, Spain).
Seeds of rocket [Diplotaxis tenuifolia (L.) DC.], cv. ‘Apollo’ (Tozer Iberica—Murcia, Spain) were sown on 1 February 2022, in cell plastic trays, using commercial peat 315 (Blond/black 60/40 Turbas y Coco Mar Menor S.L.) as substrate. On 3 March, the seedlings, at the stage of fourth true leaf, were transplanted in metal gutters of pyramidal trunk cross-section, whose dimensions were 1.0/0.15/0.12/0.11 m (length/upper width/lower width/height, respectively), filled with peat (P) or agro-industrial compost (C). The peat was based on a Sphagnum peatmoss F315 mixed with a 60:40—blond: black proportion by volume, supplied by Turbas y Coco Mar Menor S.L. The raw materials for this compost, in dry weight, were tomato and pepper juice waste (41%), leek waste (43%), and vineyard residues (16%). The composting process was carried out at the University Miguel Hernandez (UMH) composting site. The composting process was carried out using open-air piles (15 Tn) with a bio-oxidative phase of 75 days and a maturation phase of 40 days. Once the composting process was finished, the compost was milled and passed through a 2 cm sieve and stored at 4 °C until use.
The characteristics of the growing media are reported in Table 1. The plantlets were transplanted at 0.083 m in the row and 0.25 m between rows, for a final density of 49 plants/m2.

Table 1.
Main characteristics of growing media used.
The daily light integral was measured with a SQ-501 Quantum meter (Apogee Instruments, Inc., North Logan, UT, USA).
During the first cut, light conditions were an average daily light integral (DLI) of 8.96 mol/m2/d; while the minimum, maximum, and average air temperatures were 9.3 °C, 36.1 °C, and 17.9 °C, respectively. During the second cut were a DLI of 15.6 mol/m2/d, while the minimum, maximum, and average air temperatures were 6.1 °C, 36.9 °C, and 18.8 °C, respectively.
Irrigation was applied daily with an automated system using soil moisture sensors (5TM; METER Group, Inc., Pullman, WA, USA). A percentage of the Water Holding Capacity (WHC) was allowed to decrease approximately a 15% of the WHC. When the sensors detected this decreasing (a determined point in the VWC), 3 min of watering events were applied in each growing medium, ensuring to reach the field capacity in both cases and a plus for a 15% of leaching fraction. Previously to the starting the irrigation, the volumetric water content (VWC) of each growing medium was estimated by measuring the voltage of the 5TM output, using a substrate-specific calibration equation according to Valdés et al. [23].
From transplanting to first cut, the total amount of water applied was 2187 L and from first cut to second cut was 2713 L for both growing media.
Fertigation started a week after transplantation by using a nutrient solution (NS) with the following composition (values expressed as mM): 7.2 NO3−, 4.8 NH4+, 2 H2PO4−, 2.5 SO42−, 6 K+, 1.9 Ca2+, and 1.5 Mg2+. The strength of the nutrient solution was ½ for the first week. Afterwards, a full-strength concentration was used for the rest of the crop cycle. Micronutrients and iron were provided as a commercial solution: Nutromix® (2 mg/L for microelements—Biagro, Massalfassar, Valencia, Spain) and Sequestrene® G100 Syngenta (7% soluble iron, 6% chelated iron, 1.5 mg/L—Basel, Switzerland). The values of EC of the NS were 1.47 and 2.37 dS/m, in the first week and for the rest of the crop cycle, respectively; while the pH value was adjusted to 6.1 throughout the crop cycle by adding sulphuric acid.
Two harvests were realized 27 (first cut) and 55 (second cut) days after transplantation (DAT), respectively. A complete randomized block design—with three replicates per substrate—was used, with a single gutter representing the experimental plot.
2.2. Growth Analysis
For every replication, 10 plants were collected by cutting them with sterile scissors to perform the growth analysis.
At both harvest dates, the leaves were weighted to determine yield. Furthermore, they were counted, and their area was determined by using a leaf area meter (LICOR-3100 C; LICOR Biosciences Inc., Lincoln, NE, USA). The specific leaf area (SLA) was determined as the ratio of leaf area per unit of dry leaf biomass.
A sample of the fresh matter was used for dry weight (DW) determination by drying it in an oven at 60 °C until constant weight.
2.3. Phytochemical Analyses
2.3.1. Glucosinolates and (Poly)phenols Extraction
Sample extraction was carried out according to Baenas et al. [24] and Abellán et al. [25] with minor modifications. Freeze-dried samples (100 mg) were extracted with 1.0 mL of methanol/deionized water (70:30, v/v), at 70 °C for 30 min, being vortexed every 5 min in a vortex stirrer. After that, samples were placed in an ice slurry to stop the reaction, for 5 min, and then centrifuged at 10,000 rpm, for 15 min. Supernatants were collected and filtered through 0.22 μm of pore-size PVDF membrane filters (Millipore, Billerica, MA, USA).
2.3.2. HPLC-DAD-ESI–MSn Qualitative and Quantitative Analysis of Glucosinolates and Phenolic Compounds
Qualitative and quantitative analysis of glucosinolates (GSLs) was performed according to Baenas et al. [24]. Briefly, for the identification of GSLs, MS fragmentation patterns (M-H, MS2, and MS3) in HPLC-DAD-ESI-MSn (Agilent Technologies HPLC 1200, Waldbronn, Germany; coupled to an UltraHCT Bruker Ion Trap, Bremen, Germany) were analyzed. For the quantitation of GSLs and phenolic compounds, chromatograms were registered at 227 and 330 nm, respectively. Intact GSLs were identified according to the UV spectra, retention time, and order of elution, together with their characteristic fragmentation patterns in comparison with available data to inform previous experiments and literature. The GSLs were quantified using sinigrin and glucobrassicin (Phytoplan, Germany) as external standards for aliphatic and indole glucosinolates, respectively. The phenolic compounds were quantified using quercetin and chlorogenic acid as external standards for flavonols and phenolic acid derivatives, respectively. Results were expressed as μmol g1 DW.
2.3.3. Vitamin C and Antioxidants Content
Liquid chromatography by the Zapata and Dufour method [26], with minor modifications, was used for measuring vitamin C. Ascorbic acid (AA) and its oxidized form, dehydroascorbic acid (DHA) were quantified. For extraction, three grams of frozen rocket samples were mashed by adding 6 mL of citric acid 0.1 M, 0.05% EDTA, and 4 nM NaF in 5% methanolic. Before filtering the homogenate through a sterile gauze, the mixture was homogenized for 30 seg at high speed (Ultraturrax T25 basic, IKA, Königswinter, Germany) adjusting pH to 2.3–2.4 (6 N HCl). Once obtained the filtrate, it was centrifuged 5 min (13,500 rpm, 4 °C) (Sorvall RC-SB series centrifuge). Then, the samples were passed through a SepPak C18 cartridge (Waters Assoc., Milford, MA, USA) for purification. Samples were filtered once again with a spin filter (0.45 µm). HPLC vials were filled with 750 µL of the filtrate and 250 µL of 1,2-phenylenediamine dihydrochloride (0.83 mg/mL) in methanol/water (5: 95, v/v). Derivatization was attained by allowing the mixture to react (37 min, room temperature). Then, 20 µL were injected on an HPLC (Shimadzu, Kyoto, Japan) equipped with an SPDM-20A photodiode array detector and a Gemini NX C18-110 column (250 mm × 4.6 mm, 5 µm particle size; Phenomenex, Torrance, CA, USA). As mobile phase a 5 mM hexadecyl trimethyl ammonium bromide, 50 mM KH2PO4, and 5% methanol (pH 4.59) solution with an isocratic flow of 1.8 mL/min was used. Chromatograms were recorded for 14 min at 261 nm (AA, Rt = 6.4 min) and 348 nm (DHA, Rt = 3.1 min). Commercial standards (Sigma, St. Louis, MO, USA). of AA and DHA were used for the calibration curves needed for quantification. At least eight data points from 1.25 to 0.1 mM and 1.25 to 0.01 mM for AA and DHA, respectively, were considered. Results were expressed as mg vitamin C/kg FW.
2.4. Statistical Analysis
Data are presented as the mean ± standard deviation (SD). Significant differences between experimental conditions for the different determinations were identified by a one-way analysis of variance (ANOVA) and the Tukey’s multiple-range test using the SPSS 21.0 software package (SPSS Inc., Chicago, IL, USA). Significant differences were set up at p < 0.05.
3. Results and Discussion
3.1. Yield and Growth Parameters
The yield was influenced by the harvest date, as the second cut produced more than twice relative to the first harvest (Table 2). Similar results were reported by other authors [27,28], with the phenological phase may have played a major role in our experiment, as the period for the first and second harvests was almost the same (27 and 28 days for the first and second harvest, respectively). While for the first cut the plants were smaller and needed a certain time to recover from the stress of transplantation, plants before the second cut were already in a phase of intense vegetative growth. Furthermore, in the first part of the cycle which coincided with the first month after transplantation, the weather was unusually cold and rainy, which led to a slowdown in the growth of the crop.

Table 2.
Effects of substrate and harvest date on yield, leaf number, leaf area, water use efficiency (WUE), dry matter percentage (%DW), and specific leaf area (SLA) of rocket crops.
Leaf number and leaf area were both influenced by the interaction of substrate and harvest date (Table 2; Figure 1A,B): while the second harvest did produce a higher number of leaves than the first cut in both substrates, plants from peat showed a greater number of leaves than compost only in the second cut (Figure 1A). Similarly, leaf area was higher in the second harvest, but the differences between substrates were present only in the first cut, as compost resulted in a greater area (48% more than peat—Figure 1B). The higher leaf number and leaf area in the second harvest may be explained by the same pattern of the yield: the plants had already a sharp vegetative growth, and the weather conditions were more favourable in the period between the first and the second harvest, even if such parameters were modulated by the substrate. Several authors reported an influence of the substrate on leaf area and number, sometimes with contrasting results. For example, Nerlich et al. [29] found quite different responses in leaf area and number with lettuce cultivation when using organic or inorganic substrates, with sphagnum giving the best results; while Oberpaur et al. [30], also working with lettuce, did not find any significant differences between different substrates (peat, compost, and vermicompost), whether fertigation was applied or not. Although, other authors [31] have found that compost produces a higher leaf number when it was present at 70% in the substrate [32]. The greater number of leaves in peat—in comparison with compost—in the second harvest (Figure 1A) could be due to a better response of peat to the increased transpiration demand of the crop, as peat is universally known for the optimal hydraulic characteristics for soilless systems. On the other hand, the greater leaf area in compost with respect to peat in the first harvest (Figure 1B) was probably due to a higher concentration of nutrients (especially N) in the compost than in the peat (Table 1). It is well known that nitrogen is essential for leaf growth because when the plants must cope with nitrogen limitation, such a shortage will result in a reduction in leaf area [33].
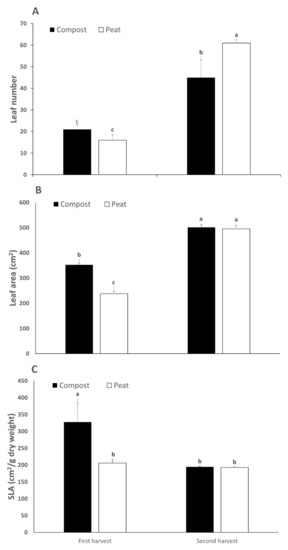
Figure 1.
Effect of harvest date and growing media on leaves number (A), leaf area (B), and specific leaf area (C) of Diplotaxis tenuifolia. Different letters indicate significant differences at p ≤ 0.05 according to Tukey’s test. Vertical bars represent ± standard deviation of mean values (n = 10).
The WUE was significantly higher in the second cut (+76%) compared to the first harvest (Table 2). WUE may be expressed in several ways, but from an agronomic point of view, it represents the unit of production for a unit of water, an important agriculture resource, in particular in semiarid regions [34]. The WUE may be influenced by several factors: its response at the leaf level is related to the gradients of CO2 and H2O and, consequently, to the underlying physiological processes and then, at the canopy level, to the transpiration rate [35]. In our experiment, the total water consumption was not significantly different among the substrate hence the difference was ascribed to a different yield among harvest dates (higher in the second harvest—Table 2), which was due to higher transpiration in the timespan between the first and the second cut.
Finally, SLA was influenced by the substrate, but such an effect was modulated by the harvest date (Figure 1C). Indeed, in the first harvest, the SLA of the compost treatment compost was 49% higher than that of peat (Figure 1C), while in the second cut the substrate did not exert any influence. SLA is an important parameter, as it determines “how much new leaf area to deploy for each unit of biomass produced” [36], thus influencing canopy expansion and growth and, eventually, light interception and light use efficiency [37]. Therefore, the main difference in our experiment was due to the different leaf areas, as the DW percentage was not significant (Table 2). The greater leaf area in the compost treatment, but only in the first harvest, was most probably due to the higher concentration of nitrogen in compost (Table 1), as the leaf area is known to increase with the nitrogen concentration [38], thus leading to a greater leaf area, finally resulting in a higher SLA (Figure 1C) that may result in a greater leaf thickness [39], a positive trait related to the ability of plants to grow in dry environments with high irradiance [40].
3.2. Quantitative Phytochemical Profile of Rocket Salad
3.2.1. Glucosinolates and (Poly)phenolic Profile of Rocket Salad Leaf
A survey of the GSL tentatively annotated in the present work evidence that the relevant GSL such as diglucothiobeinin, gluosativin, glucoerucin, and 4-methoxyglucobrassicin were detected in the negative mode. This operating mode yielded abundant deprotonated ions at MS2 and MS3 fragmentation levels that allowed their proper identification in close comparison with information available in the literature jointly with the annotation of the retention time and parent masses (Table 3).

Table 3.
Identification of glucosinolates present in the rocket salad by HPLC-PDA-ESI-MSn.
The analysis of the parent ions representing GSL showed the presence of [M-H] ions at m/z values of 600, 406, 420, and 477 arbitrary mass units (amu) that corresponded to correspond to diglucothiobeinin, glucosativin, glucoerucin, and 4-methoxyglucobrassicin, correspondingly [41].
When analyzing the mass spectra corresponding to the major negative fragment ions from the GSL identified, along with diagnostic transitions summarized in Table 3, ions with m/z 195, 259, 227, and 97 amu matched to the fragment ions obtained from the glycone side chain, were recorded for almost all GSL identified, as their present a common core structure [42]. The fragment identified at m/z 420 amu corresponded to the loss of SC6H10O5-H2O. The fragments corresponding to the m/z 195 and 97 amu informed on a thioglucose group (C6H11O5S–) and a hydrogen sulfate ion (HSO4−), respectively [42]. Additional typical ions observed were m/z 259 and 227 amu.
According to previous research on the GSL profile of Diplotaxis tenuifolia, the accessions of this species are featured by significant variability concerning the presence/absence or the relative abundance of the different individual GSL, namely glucosativin, glucoerucin, and methoxyglucobrassicin [43]. All GSL were found in plant material of Diplotaxis tenuifolia grown in both compost and peat evidencing no critical influence of the substrate concerning the organosulfur compounds burden in rocket salad.
Regarding (poly)phenols, three flavonols (quercetin-3,3′,4′-triglucosyl, quercetin-3,4′-glucosyl-3′-(6-sinapoyl-glucosyl), and quercetin-3-(2-sinapoyl-glucosyl)-3′-(6-sinapoyl-glucosyl)-4′glucosyl) and two caffeoyl derivatives were identified according to the retention time and parent ions and fragmentation patterns recorded applying a negative mode ESI, in comparison with the information available in the literature (Table 4). All phenolics were present in plants grown in compost and peat.

Table 4.
Identification of phenolic compounds present in the rocket salad by HPLC-PDA-ESI-MSn.
The MSn study of the (poly)phenols found in rocket salad allowed us to identify the presence of quercetin-3,3′,4′-triglucosyl that exhibited a deprotonated molecular ion at m/z 787 amu. This parent mass was complemented with the typical fragmentation pattern attributed to flavonoids esterified with glucose moieties since showed two sequential losses of 162 amu (corresponding to a glucose residue (C6H10O5)) [44,45].
Furthermore, it was recorded a base peak at m/z 993 amu annotated as quercetin-3,4′-glucosyl-3′-(6-sinapoyl-glucosyl), that showed a sequential loss of a glucosyl residue (C6H10O5) from the [M-H] ion to obtain a typical [M-H-C6H10O5] ion at m/z 831 amu, followed by an additional loss of a glucosyl residue to obtain an MS3[M-H] ion at m/z 669 amu [43,44] (Table 4).
The third flavonol eluted at min 32.3 was identified as quercetin-3-(2-sinapoyl-glucosyl)-3′-(6-sinapoyl-glucosyl)-4′glucosyl featured by a deprotonated ion at m/z 1199 amu. The MS2 fragmentation of this flavonol showed the first loss of 162 amu corresponding to a glucose residue to yield two major fragments at m/z 1037 and 831 amu ([M-H- C6H10O5] and the deglycosylated form of quercetin-3,4′-glucosyl-3′-(6-sinapoyl-glucosyl), respectively) [46] (Table 4).
In addition, two caffeoyl-sinapoyl derivatives were identified at min 30.6 and 35.3, featured by the base peak at m/z 993 and 891, respectively. Both of them yielded caffeoyl and sinapoyl residues in the MS3 fragmentation (m/z 179 and 223 amu, correspondingly) [47].
The caffeoyl and sinapoyl derivatives have been broadly reported in cruciferous as well as derivatives of quercetin esterified with glucose moieties, as well as acylated with different hydroxycinnamic acids [22]. More specifically related to the Brassicaceae considered in the present work, Diplotaxis tenuifolia has been shown to contain and accumulate (poly)glycosylated flavonols derived from quercetin to a higher extent than other rocket salad species (e.g., Eruca species and varieties), which instead produce higher amounts of kaempferol derivatives [41,48].
These chemical structures are of special relevance because to date, it has been demonstrated that some glycosides are more bioavailable than other chemical forms of (poly)phenols after dietary intake [49].
3.2.2. Glucosinolate Content
As reported for the yield of Diplotaxis tenuifolia, the phytochemical content was also influenced by the harvest date, as the plant material harvested secondly contained significantly lower concentrations of GSL relative to the first harvest, independently of the substrate considered (Figure 2). In this concern, the GSL identified were found in the following decreasing concentrations: glucoerucin (0.506 mg/g, on average) > 4-methoxyglucobrassicin (0.327 mg/g, on average) > diglucothiobeinin and gluosativin (0.224 mg/g, on average). The relative abundance of the different individual GSL identified in Diplotaxis tenuifolia has been reported as variable, in close connection with the environmental conditions [41], which could be the cause of the differences found in this work relative to previous characterizations of the GLS profile of rocket salad. Although these compounds are not present at high concentrations in the plant material, they can be quite relevant for the sensory attributes and consumer’s liking [50].
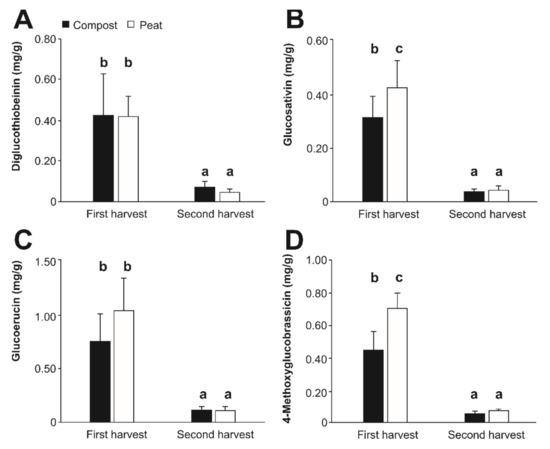
Figure 2.
Content (mg/g) of the glucosinolates diglucothiobeinin (A), glucosativin (B), glucoerucin (C), and 4-methoxyglucobrassicin (D) in Diplotaxis tenuifolia in two separate experiments. Bars represent mean values ± SD (n = 10) for each compound. Bars with different lowercase letters are significantly different at p < 0.05 according to the analysis of variance (ANOVA) and Tukey’s multiple range test.
The amount of the diverse individual GSL allowed us to obtain total average GSL concentrations of up to 2.585 mg/g and 1.945 mg/g for plants grown using peat and compost as substrates, respectively. The concentration of total GSL obtained matched with the lower range reported previously for diverse rocket salad (Eruca sativa L. and Diplotaxis tenuifolia) accessions (0.5–11.6 mg/g) [41,51].
However, the lower concentration recorded in the present work should be interpreted based on the broad variability between rocket salad species and accessions as referred as described by Bell et al. [41], as well as by the composition of the substrate, the focus of the present research. Indeed, in the last years, it has been proposed a high degree of genetic and environmental variability in terms of biosynthesis and accumulation of individual GSL in rocket salad, similar to that affecting other cruciferous species [49,52,53,54].
This fact constitutes an important challenge concerning further rocket-breeding programs focused on enhancing GSL/GHP profiles and sensory characteristics. This is of special relevance because, to date, variability in the GSL accumulation of rocket salad has not been completely documented, although different genetic features condition the capacity of the plant to respond to the changing climatic conditions, the light intensity has been pointed out as the ones with the broadest regulatory capacity concerning phytochemical burden [50].
Concerning the modulatory effect of the substrate (compost and peat) on the content of individual GSL, this was only significant in the plant material corresponding to the first harvest (Figure 2) for two major GSL described in Diplotaxis tenuifolia, glucosativin (the most characteristic GSL in rocket salad [55], despite the wide variation between species and accessions already reported in the literature) [43] and 4-methoxyglucobrassicin [55]. In both cases, the rocket salad plants’ growth when using peat as a substrate (0.421 and 0.707 mg/g, respectively) surpassed the concentration obtained in those grown with compost by 24.9% and 35.5%, on average, correspondingly.
Given the recognized value of GSL as an indicator of plant stress [56], these differences could be due to environmental stresses in plants grown using peat as a substrate relative to those grown using compost. This would mean that using compost reduces the physiological stress of rocket salad plants. The growing substrate cannot be considered the only source of variability and thereby, the responsibility for variations in the burden of secondary metabolites as a result of the stress response should be analyzed in the light of additional environmental factors included in the experimental design (e.g., fertirrigation). As a result, this allows the basic differences between how rocket salad responds to the modification of the substrate as a central element for the appropriate plant development.
3.2.3. (Poly)phenolic Content
As referred to for GSL, the content of phenolics in Brassicaceae plants is modulated by both genetic and environmental factors, existing differences between varieties and tissues. Furthermore, as for GSL, these constitute secondary metabolites of response to biotic and abiotic stress [21]. In the present work, the quantitative analysis of (poly)phenols of Diplotaxis tenuifolia evidenced significantly higher values for individual phenolics in the plant material obtained in the first harvest, relative to the second one, regardless of the specific (poly)phenol or substrate considered (Figure 3). In this regard, the phenolic compounds identified in this work were found in the following decreasing concentrations: quercetin-3,4′-diglucosyl-3′-(6-sinapoyl-glucosyl) (1.940 mg/g, on average) > quercetin-3-(2-sinapoyl-glucosyl)-3′-(6-sinapoyl-glucosyl)-4′-glucosyl (1.259 mg/g, on average) > quercetin-3,3′,4′-triglucosyl (0.461 mg/g, on average) > caffeoyl-sinapoyl derivative II (0.149 mg/g, on average) > caffeoyl-sinapoyl derivative I (0.060 mg/g, on average) (Figure 3).
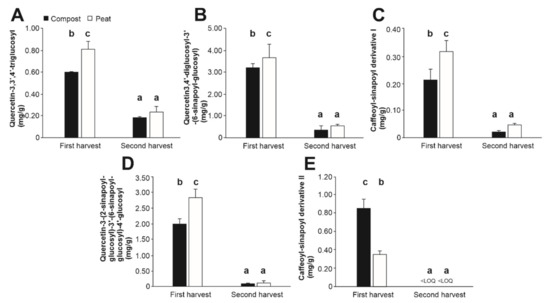
Figure 3.
Content (mg/g) of the phenolic compounds quercetin-3,3′,4′-triglucosyl (A), quercetin-3,4′-diglucosyl-3′-(6-sinapoyl-glucosyl) (B), caffeoyl-sinapoyl derivative I (C), quercetin-3-(2-sinapoyl-glucosyl)-3′-(6-sinapoyl-glucosyl)-4′-glucosyl (D), and caffeoyl-sinapoyl derivative II (E) in Diplotaxis tenuifolia. Bars represent mean values ± SD (n = 10) for each compound. Bars with different lowercase letters are significantly different at p < 0.05 according to the analysis of variance (ANOVA) and Tukey’s multiple range test. <LOQ, lower than the limit of quantification.
These differences have been previously described by Bell et al. [41] that conducted experiments under controlled environmental conditions, thereby speculating that these differences could be due to a large extent to genetic diversity. However, in several studies, the genetic homogeneity of the accessions characterized prompted setting the hypothesis that the environmental conditions, including the substrate and cultivation methods are closely related to the specific phenolic profile [57].
When analyzing the substrates’ effect on the concentration of individual phenolics, unlike the trend recorded for GLS, in the first harvest material all phenolics exhibited differences depending on the growing substrate considered, being obtained significantly higher concentrations in the plant material of rocket salad plants growth in peat than those cultured in compost for four out of the five phenolics present in concentrations higher than the limit of quantification (quercetin-3,3′,4′-triglucosyl, quercetin-3,4′-diglucosyl-3′-(6-sinapoyl-glucosyl) > quercetin-3-(2-sinapoyl-glucosyl)-3′-(6-sinapoyl-glucosyl)-4′-glucosyl, and caffeoyl-sinapoyl derivative I (Figure 3). On average, peat allowed concentrations 1.35-folds higher than compost. Nonetheless, caffeoyl sinapoyl derivative II showed higher concentrations in plants grown in compost (0.085 mg/g) relative to peat (0.035 mg/g) (Figure 3).
Furthermore, the modification of the concentration of phenolic compounds in plants growth in compost in comparison with peat again would indicate a reduction in the plant stress [56], allowing a beneficial behavior of rocket salad plants to the assayed substrate.
3.3. Vitamin C Content
Vitamin C—or ascorbic acid—is one of a major antioxidants in plant cells, and it has a fundamental role in protecting tissues from dangerous and irreversible oxidation that may occur from reactive oxygen species (ROS), in both animal and plant cells [58], and protecting humans against various diseases [59]. Vitamin C exists in two redox states, the active form ascorbic acid [58] and its oxidized form, dehydroascorbic acid [60].
The concentration of vitamin C in our experiment was influenced by the substrate and harvest date jointly (Figure 4). Such results agree with previous findings, in which vitamin C concentration was increased by compost either alone or mixed with soil [61] in strawberries, as well as in pepper when compost was used as organic fertilizer [62]. Furthermore, the total vitamin C and its reduced (ascorbic) and oxidated forms (dehydroascorbic) were higher in the second harvest when compost was the substrate than in the first (Figure 4). In the first cut, the substrate did not produce any difference, except dehydroascorbic acid, where compost again showed a higher value than peat (Figure 4), which is consistent with the above-reported results. Furthermore, apart from compost influence, the higher content of vitamin C and its forms in the second harvest is also related to higher light, as it is well known that exposure to light increases vitamin C content [63].
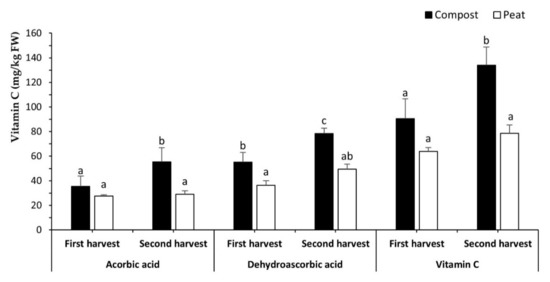
Figure 4.
Content (mg/kg of FW) of the ascorbic acid, dehydroascorbic acid, and vitamin C of Diplotaxis tenuifolia. Bars represent mean values ± SD (n = 10) for each compound. Bars with different lowercase letters are significantly different at p < 0.05 according to the analysis of variance (ANOVA) and Tukey’s multiple range test.
4. Conclusions
Peat is one of the most used growing media in plant nurseries and soilless cultivation. However, it is not renewable, thus alternative substrates should be considered. Our results demonstrate that the agro-industrial compost used in our study may be a potential alternative to peat, since it improved almost all the biometric parameters, as well as the content of vitamin C of the rocket salad leaves—particularly in the second cut—without any negative effect on the production. Indeed, the reduction in the phytochemical burden recorded indicate a reduction in the plant stress that fit well with the enhancement of the productive parameters. According to these results, the use of the compost as growing media besides provides a positive management alternative in terms of production and plant stress, would cope with the circular economy, whose aims are to reduce wastes, thereby creating further value from the use of agro-industrial residues.
Author Contributions
Conceptualization, C.E.-G., J.A.F., and J.O.; Methodology, D.A.M., R.D.-P., A.C.-P., F.A., J.A.P. and P.A.G.; Software, A.S. and V.M.G.-C.; Validation, C.E.-G., J.A.F., J.O., P.A.G., A.S., F.A. and V.M.G.-C.; Formal analysis, D.A.M., R.D.-P., A.C.-P., C.E.-G., J.A.F., J.O, P.A.G., A.S., F.A. and V.M.G.-C.; Investigation, A.S., V.M.G.-C., F.A. and J.O.; Data curation, C.E.-G., J.A.F., J.O, P.A.G., A.S., F.A. and V.M.G.-C.; Writing—original draft preparation, A.S., J.A.F., V.M.G.-C. and A.C.-P.; Writing—review and editing, A.S., C.E.-G., J.A.F., J.O., P.A.G., F.A., V.M.G.-C., D.A.M., J.A.P. and R.D.-P.; Visualization, C.E.-G., J.A.F. and J.O.; Supervision, C.E.-G., J.A.F. and J.O.; Project administration, C.E.-G., J.A.F. and J.O.; Funding acquisition, C.E.-G., J.A.F. and J.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Grant PID2020-114410RB-I00 funded by MCIN/AEI/10.13039/501100011033, and Grant AGROALNEX funded by Comunidad Autónoma de la Región de Murcia through Fundación Séneca—Agencia de Ciencia y Tecnología de la Región de Murcia and European Union NextGenerationEU. Angelo Signore was also funded by a grant of the Re-qualification of the Spanish University System, Maria Zambrano modality (Grant UP2021-033 funded by Ministerio de Universidades and by the “European Union NextGenerationEU/PRTR”). Víctor Gallegos was also funded by a grant of the Re-qualification of the Spanish University System, Margarita Salas modality, by the University of Almería (Grant UP2021-004 funded by Ministerio de Universidades and by the “European Union NextGenerationEU/PRTR”).
Data Availability Statement
Not applicable.
Acknowledgments
Thanks to Noelia Durán López and Almudena Giménez Martínez for technical assistance at the laboratory.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ispizua, E.; Calatayud, Á.; Marsal, J.I.; Cannata, C.; Basile, F.; Abdelkhalik, A.; Soler, S.; Valcárcel, J.V.; Martínez-Cuenca, M.R. The Nutritional Quality Potential of Microgreens, Baby Leaves, and Adult Lettuce: An Underexploited Nutraceutical Source. Foods 2022, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Signore, A.; Renna, M.; Santamaria, P. Agrobiodiversity of Vegetable Crops: Aspect, Needs, and Future Perspectives. Annu. Plant Rev. Online 2019, 2, 41–64. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 752/2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32014R0752&from=EN (accessed on 15 July 2022).
- Verbeek, M.; Hardeweg, B. From consumer to prosumer: Are small-scale home indoor farms economically viable? Eur. J. Hortic. Sci. 2022, 87, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Department of Economic and Social Affairs. World Urbanization Prospects: The 2018 Revision; Department of Economic and Social Affairs: New York, NY, USA, 2018.
- Tietjen, S.; Graubner, I.; Sradnick, A. Reducing peat in substrate mixture formulations for press pots using the Taguchi method. Sci. Hortic. 2022, 295, 110838. [Google Scholar] [CrossRef]
- Blok, C.; Eveleens, B.; van Winkel, A. Growing media for food and quality of life in the period 2020–2050. Acta Hortic. 2021, 1305, 341–355. [Google Scholar] [CrossRef]
- Atzori, G.; Pane, C.; Zaccardelli, M.; Cacini, S.; Massa, D. The role of peat-free organic substrates in the sustainable management of soilless cultivations. Agronomy 2021, 11, 1236. [Google Scholar] [CrossRef]
- Kern, J.; Tammeorg, P.; Shanskiy, M.; Sakrabani, R.; Knicker, H.; Kammann, C.; Tuhkanen, E.-M.; Smidt, G.; Prasad, M.; Tiilikkala, K.; et al. Synergistic use of peat and charred material in growing media—An option to reduce the pressure on peatlands? J. Environ. Eng. Landsc. Manag. 2017, 25, 160–174. [Google Scholar] [CrossRef]
- Ceglie, F.G.; Bustamante, M.A.; Ben Amara, M.; Tittarelli, F. The Challenge of Peat Substitution in Organic Seedling Production: Optimization of Growing Media Formulation through Mixture Design and Response Surface Analysis. PLoS ONE 2015, 10, e0128600. [Google Scholar] [CrossRef]
- Parada, F.; Ercilla-Montserrat, M.; Arcas-Pilz, V.; Lopez-Capel, E.; Carazo, N.; Montero, J.I.; Gabarrell, X.; Villalba, G.; Rieradevall, J.; Muñoz, P. Comparison of organic substrates in urban rooftop agriculture, towards improving crop production resilience to temporary drought in Mediterranean cities. J. Sci. Food Agric. 2021, 101, 5888–5897. [Google Scholar] [CrossRef]
- Zawadzińska, A.; Salachna, P.; Nowak, J.S.; Kowalczyk, W.; Piechocki, R.; Łopusiewicz, Ł.; Pietrak, A. Compost based on pulp and paper mill sludge, fruit-vegetable waste, mushroom spent substrate and rye straw improves yield and nutritional value of tomato. Agronomy 2022, 12, 13. [Google Scholar] [CrossRef]
- Ober Allen, J.; Alaimo, K.; Elam, D.; Perry, E. Growing Vegetables and Values: Benefits of Neighborhood-Based Community Gardens for Youth Development and Nutrition. J. Hunger Environ. Nutr. 2008, 3, 418–439. [Google Scholar] [CrossRef]
- Daneshvar, H.; Babalar, M.; Díaz-Pérez, J.C.; Nambeesan, S.; Delshad, M.; Tabrizi, L. Evaluation of organic and mineral fertilizers on plant growth, minerals, and postharvest quality of celery (Apium graveolens L.). J. Plant Nutr. 2022, 1–18. [Google Scholar] [CrossRef]
- Pant, A.; Radovich, T.J.K.; Hue, N.V.; Arancon, N.Q. Effects of Vermicompost Tea (Aqueous Extract) on Pak Choi Yield, Quality, and on Soil Biological Properties. Compost. Sci. Util. 2011, 19, 279–292. [Google Scholar] [CrossRef]
- Neugart, S.; Wiesner-Reinhold, M.; Frede, K.; Jander, E.; Homann, T.; Rawel, H.M.; Schreiner, M.; Baldermann, S. Effect of Solid Biological Waste Compost on the Metabolite Profile of Brassica rapa ssp. chinensis. Front. Plant Sci. 2018, 9, 305. [Google Scholar] [CrossRef]
- Pascual, J.A.; Ceglie, F.; Tuzel, Y.; Koller, M.; Koren, A.; Hitchings, R.; Tittarelli, F. Organic substrate for transplant production in organic nurseries. A review. Agron. Sustain. Dev. 2018, 38, 35. [Google Scholar] [CrossRef]
- Giménez, A.; Fernández, J.A.; Pascual, J.A.; Ros, M.; Saez-Tovar, J.; Martinez-Sabater, E.; Gruda, N.S.; Egea-Gilabert, C. Promising composts as growing media for the production of baby leaf lettuce in a floating system. Agronomy 2020, 10, 1540. [Google Scholar] [CrossRef]
- Giménez, A.; Fernández, J.A.; Pascual, J.A.; Ros, M.; López-Serrano, M.; Egea-Gilabert, C. An agroindustrial compost as alternative to peat for production of baby leaf red lettuce in a floating system. Sci. Hortic. 2019, 246, 907–915. [Google Scholar] [CrossRef]
- Dominguez-Perles, R.; Martinez-Ballesta, M.C.; Riquelme, F.; Carvajal, M.; Garcia-Viguera, C.; Moreno, D.A. Novel varieties of broccoli for optimal bioactive components under saline stress. J. Sci. Food Agric. 2011, 91, 1638–1647. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (Myricetin, Quercetin, Kaempferol, Luteolin, and Apigenin) Content of Edible Tropical Plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Valdés, R.; Miralles, J.; Ochoa, J.; Sánchez-Blanco, M.J.; Bañón Arias, S. Saline reclaimed wastewater can be used to produce potted weeping fig (Ficus benjamina L.) with minimal effects on plant quality. Spanish J. Agric. Res. 2012, 10, 1167. [Google Scholar] [CrossRef]
- Baenas, N.; Villaño, D.; García-Viguera, C.; Moreno, D.A. Optimizing elicitation and seed priming to enrich broccoli and radish sprouts in glucosinolates. Food Chem. 2016, 204, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Abellán, Á.; Domínguez-Perles, R.; García-Viguera, C.; Moreno, D.A. Evidence on the Bioaccessibility of Glucosinolates and Breakdown Products of Cruciferous Sprouts by Simulated In Vitro Gastrointestinal Digestion. Int. J. Mol. Sci. 2021, 22, 11046. [Google Scholar] [CrossRef] [PubMed]
- Zapata, S.; Dufour, J.-P. Ascorbic, Dehydroascorbic and Isoascorbic Acid Simultaneous Determinations by Reverse Phase Ion Interaction HPLC. J. Food Sci. 1992, 57, 506–511. [Google Scholar] [CrossRef]
- Thapa, U.; Nandi, S.; Rai, R.; Upadhyay, A. Effect of nitrogen levels and harvest timing on growth, yield and quality of lettuce under floating hydroponic system. J. Plant Nutr. 2022, 45, 2563–2577. [Google Scholar] [CrossRef]
- Adamczewska-Sowińska, K.; Sowiński, J.; Jama-Rodzeńska, A. The Effect of Sowing Date and Harvest Time on Leafy Greens of Quinoa (Chenopodium quinoa Willd.) Yield and Selected Nutritional Parameters. Agriculture 2021, 11, 405. [Google Scholar] [CrossRef]
- Nerlich, A.; Dannehl, D. Soilless Cultivation: Dynamically Changing Chemical Properties and Physical Conditions of Organic Substrates Influence the Plant Phenotype of Lettuce. Front. Plant Sci. 2021, 11, 601455. [Google Scholar] [CrossRef]
- Oberpaur, C.; Fernández, C.; Délano, G.; Arévalo, M.E. Inclusion of various controlled release fertilizers in moss substrates(Sphagnum magellanicum). Cienc. E Investig. Agrar. 2012, 39, 435–443. [Google Scholar] [CrossRef]
- Isaka, T.; Clark, S.; Meyer, J. Compost Functions as Effective Replacement for Peat-Based Potting Media in Organic Greenhouse Transplant Production. J 2021, 4, 394–403. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.; Tian, Y.; Gong, X. Composted green waste as a substitute for peat in growth media: Effects on growth and nutrition of Calathea insignis. PLoS ONE 2013, 8, e78121. [Google Scholar] [CrossRef]
- Vos, J.; Van Der Putten, P.E.L.; Birch, C.J. Effect of nitrogen supply on leaf appearance, leaf growth, leaf nitrogen economy and photosynthetic capacity in maize (Zea mays L.). F. Crop. Res. 2005, 93, 64–73. [Google Scholar] [CrossRef]
- Bhattacharya, A. Water-Use Efficiency Under Changing Climatic Conditions. In Changing Climate and Resource Use Efficiency in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 111–180. [Google Scholar]
- Hatfield, J.L.; Dold, C. Water-Use Efficiency: Advances and Challenges in a Changing Climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Kimball, B.A.; Kobayashi, K.; Bindi, M. Responses of Agricultural Crops to Free-Air CO2 Enrichment. Adv. Agron. 2002, 77, 293–368. [Google Scholar]
- Kumar, U.; Singh, P.; Boote, K.J. Effect of Climate Change Factors on Processes of Crop Growth and Development and Yield of Groundnut (Arachis hypogaea L.). Adv. Agron. 2012, 116, 41–69. [Google Scholar]
- Muhammad Arif, S.I. Effect of Nitrogen Levels and Plant Population on Yield and Yield Components of Maize. Adv. Crop Sci. Technol. 2015, 3, 2. [Google Scholar] [CrossRef]
- Vile, D.; Garnier, É.; Shipley, B.; Laurent, G.; Navas, M.-L.; Roumet, C.; Lavorel, S.; Díaz, S.; Hodgson, J.G.; Lloret, F.; et al. Specific Leaf Area and Dry Matter Content Estimate Thickness in Laminar Leaves. Ann. Bot. 2005, 96, 1129–1136. [Google Scholar] [CrossRef]
- Gong, H.; Gao, J. Soil and climatic drivers of plant SLA (specific leaf area). Glob. Ecol. Conserv. 2019, 20, e00696. [Google Scholar] [CrossRef]
- Bell, L.; Oruna-Concha, M.J.; Wagstaff, C. Identification and quantification of glucosinolate and flavonol compounds in rocket salad (Eruca sativa, Eruca vesicaria and Diplotaxis tenuifolia) by LC–MS: Highlighting the potential for improving nutritional value of rocket crops. Food Chem. 2015, 172, 852–861. [Google Scholar] [CrossRef]
- Cataldi, T.R.I.; Rubino, A.; Lelario, F.; Bufo, S.A. Naturally occurring glucosinolates in plant extracts of rocket salad (Eruca sativa L.) identified by liquid chromatography coupled with negative ion electrospray ionization and quadrupole ion-trap mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 2374–2388. [Google Scholar] [CrossRef]
- D’Antuono, L.F.; Elementi, S.; Neri, R. Glucosinolates in Diplotaxis and Eruca leaves: Diversity, taxonomic relations and applied aspects. Phytochemistry 2008, 69, 187–199. [Google Scholar] [CrossRef]
- Ferreres, F.; Llorach, R.; Gil-Izquierdo, A. Characterization of the interglycosidic linkage in di-, tri-, tetra- and pentaglycosylated flavonoids and differentiation of positional isomers by liquid chromatography/electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2004, 39, 312–321. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Llorach, R.; Gil, M.I.; Ferreres, F. Identification of New Flavonoid Glycosides and Flavonoid Profiles To Characterize Rocket Leafy Salads (Eruca vesicaria and Diplotaxis tenuifolia). J. Agric. Food Chem. 2007, 55, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- dos Santos PR, D.; de Lima Moreira, D.; Guimarães, E.F.; Kaplan, M.A.C. Essential oil analysis of 10 Piperaceae species from the Brazilian Atlantic forest. Phytochemistry 2001, 58, 547–551. [Google Scholar] [CrossRef]
- Ma, C.; Whitaker, B.D.; Kennelly, E.J. New 5-O-Caffeoylquinic Acid Derivatives in Fruit of the Wild Eggplant Relative Solanum viarum. J. Agric. Food Chem. 2010, 58, 11036–11042. [Google Scholar] [CrossRef]
- Durazzo, A.; Azzini, E.; Lazzè, M.; Raguzzini, A.; Pizzala, R.; Maiani, G. Italian Wild Rocket [Diplotaxis Tenuifolia (L.) DC.]: Influence of Agricultural Practices on Antioxidant Molecules and on Cytotoxicity and Antiproliferative Effects. Agriculture 2013, 3, 285–298. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.; Wagstaff, C. Rocket science: A review of phytochemical & health-related research in Eruca & Diplotaxis species. Food Chem. X 2019, 1, 100002. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.N.; Carvalho, R.; Mellon, F.A.; Eagles, J.; Rosa, E.A.S. Identification and Quantification of Glucosinolates in Sprouts Derived from Seeds of Wild Eruca sativa L. (Salad Rocket) and Diplotaxis tenuifolia L. (Wild Rocket) from Diverse Geographical Locations. J. Agric. Food Chem. 2007, 55, 67–74. [Google Scholar] [CrossRef]
- Kim, H.W.; Ko, H.C.; Baek, H.J.; Cho, S.M.; Jang, H.H.; Lee, Y.M.; Kim, J.B. Identification and quantification of glucosinolates in Korean leaf mustard germplasm (Brassica juncea var. integrifolia) by liquid chromatography–electrospray ionization/tandem mass spectrometry. Eur. Food Res. Technol. 2016, 242, 1479–1484. [Google Scholar] [CrossRef]
- Lee, M.-K.; Chun, J.-H.; Byeon, D.H.; Chung, S.-O.; Park, S.U.; Park, S.; Arasu, M.V.; Al-Dhabi, N.A.; Lim, Y.-P.; Kim, S.-J. Variation of glucosinolates in 62 varieties of Chinese cabbage (Brassica rapa L. ssp. pekinensis) and their antioxidant activity. LWT—Food Sci. Technol. 2014, 58, 93–101. [Google Scholar] [CrossRef]
- Park, S.; Arasu, M.V.; Lee, M.-K.; Chun, J.-H.; Seo, J.M.; Al-Dhabi, N.A.; Kim, S.-J. Analysis and metabolite profiling of glucosinolates, anthocyanins and free amino acids in inbred lines of green and red cabbage (Brassica oleracea L.). LWT—Food Sci. Technol. 2014, 58, 203–213. [Google Scholar] [CrossRef]
- Avato, P.; Argentieri, M.P. Brassicaceae: A rich source of health improving phytochemicals. Phytochem. Rev. 2015, 14, 1019–1033. [Google Scholar] [CrossRef]
- Variyar, P.S.; Banerjee, A.; Akkarakaran, J.J.; Suprasanna, P. Role of Glucosinolates in Plant Stress Tolerance. In Emerging Technologies and Management of Crop Stress Tolerance; Elsevier: Amsterdam, The Netherlands, 2014; pp. 271–291. [Google Scholar]
- Taranto, F.; Francese, G.; Di Dato, F.; D’Alessandro, A.; Greco, B.; Onofaro Sanajà, V.; Pentangelo, A.; Mennella, G.; Tripodi, P. Leaf Metabolic, Genetic, and Morphophysiological Profiles of Cultivated and Wild Rocket Salad (Eruca and Diplotaxis Spp.). J. Agric. Food Chem. 2016, 64, 5824–5836. [Google Scholar] [CrossRef] [PubMed]
- Locato, V.; Cimini, S.; Gara, L. De Strategies to increase vitamin C in plants: From plant defense perspective to food biofortification. Front. Plant Sci. 2013, 4, 152. [Google Scholar] [CrossRef]
- Ruxton, C.H.S.; Gardner, E.J.; Walker, D. Can pure fruit and vegetable juices protect against cancer and cardiovascular disease too? A review of the evidence. Int. J. Food Sci. Nutra. 2006, 57, 249–272. [Google Scholar] [CrossRef]
- Cisternas, P.; Silva-Alvarez, C.; Martínez, F.; Fernandez, E.; Ferrada, L.; Oyarce, K.; Salazar, K.; Bolaños, J.P.; Nualart, F. The oxidized form of vitamin C, dehydroascorbic acid, regulates neuronal energy metabolism. J. Neurochem. 2014, 129, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Lin, H.-S. Compost as a Soil Supplement Increases the Level of Antioxidant Compounds and Oxygen Radical Absorbance Capacity in Strawberries. J. Agric. Food Chem. 2003, 51, 6844–6850. [Google Scholar] [CrossRef] [PubMed]
- Tortosa, G.; González-Gordo, S.; Ruiz, C.; Bedmar, E.; Palma, J. “Alperujo” Compost Improves the Ascorbate (Vitamin C) Content in Pepper (Capsicum annuum L.) Fruits and Influences Their Oxidative Metabolism. Agronomy 2018, 8, 82. [Google Scholar] [CrossRef]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; de Pinto, M.C. Vitamin C in Plants: From Functions to Biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).