Effect of Inoculants and Sealing Delay on the Fermentation Quality of Early Harvested Wheat Forage
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Silage Preparation and Conservation
2.3. Proximate Composition, Fermentative Analysis and Physical Characteristics of Pre- and Post-Ensiled Wheat
2.4. Microbiological Analysis
2.5. Statistical Analysis
3. Results
3.1. Effects of the DM at Harvest, Inoculation and Sealing Delay on the Final Silage Quality (T60)
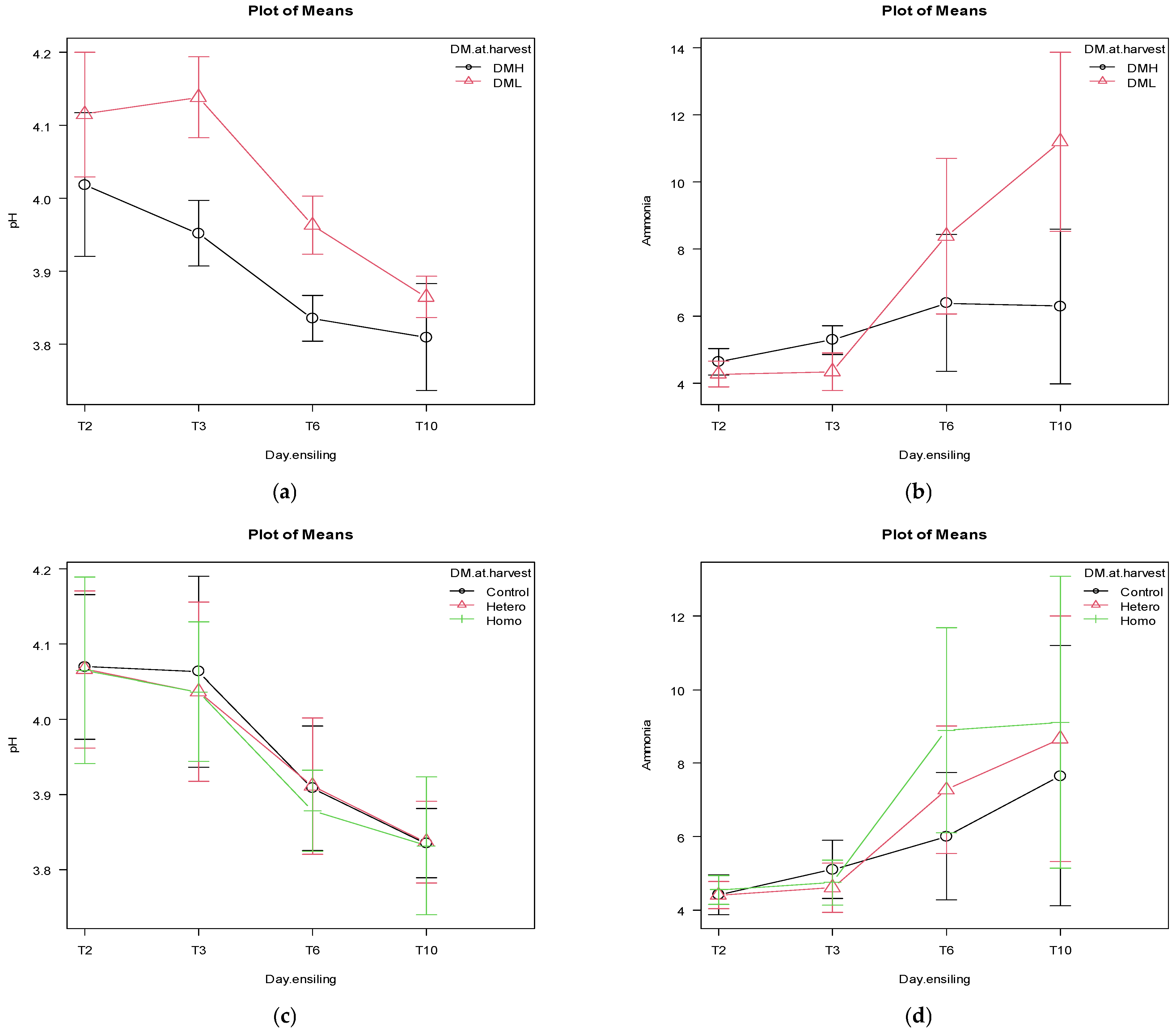
3.2. Effects of the DM at Harvest, Inoculation and Sealing Delay on the First Ten d for pH and Ammonia
3.3. Silage Quality on T67 and T74
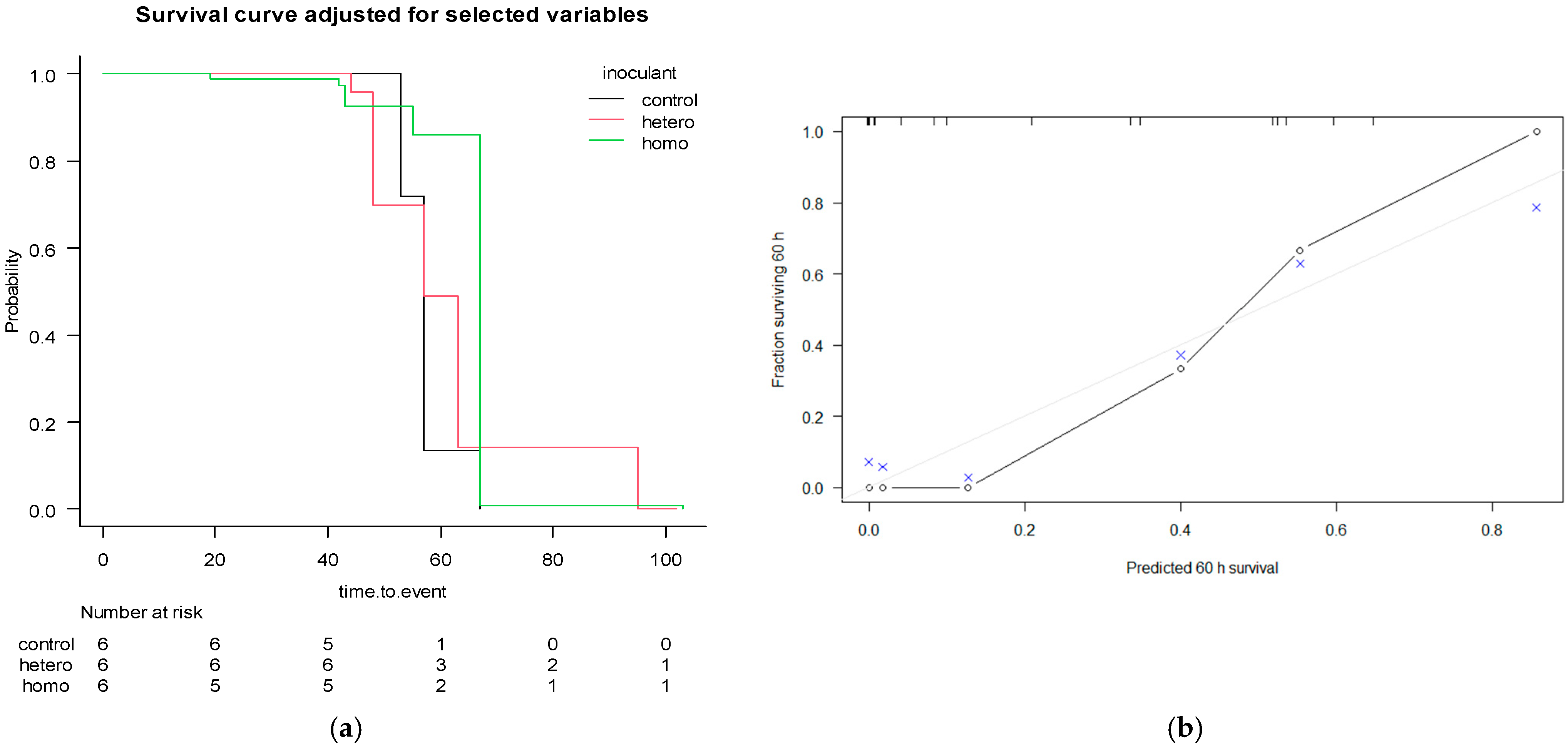
3.4. Risk Analysis for Aerobic Stability
3.5. Microbiological Quality of Silage at the Final Fermentation Time (T60)
4. Discussion
4.1. Effects of the DM at Harvest, Inoculation and Sealing Delay on the Final Silage Quality (T60)
4.2. Effects of the DM at Harvest, Inoculation and Sealing Delay on the First Ten Days for pH and Ammonia
4.3. Silage Quality on T67 and T74
4.4. Risk Analysis for Aerobic Stability
4.5. Microbiological Quality of Silage at the Final Fermentation Time (T60)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harper, M.T.; Oh, J.; Giallongo, F.; Roth, G.W.; Hristov, A.N. Inclusion of Wheat and Triticale Silage in the Diet of Lactating Dairy Cows. J. Dairy Sci. 2017, 100, 6151–6163. [Google Scholar] [CrossRef]
- Migliorati, L.; Boselli, L.; Pirlo, G.; Moschini, M.; Masoero, F. Corn Silage Replacement with Barley Silage in Dairy Cows’ Diet Does Not Change Milk Quality, Cheese Quality and Yield. J. Sci. Food Agric. 2017, 97, 3396–3401. [Google Scholar] [CrossRef] [PubMed]
- Carey, P.L.; Cameron, K.C.; Di, H.J.; Edwards, G.R.; Chapman, D.F. Sowing a Winter Catch Crop Can Reduce Nitrate Leaching Losses from Winter-Applied Urine under Simulated Forage Grazing: A Lysimeter Study. Soil Use Manag. 2016, 32, 329–337. [Google Scholar] [CrossRef]
- Istituto Nazionale Di Statistica. Available online: http://dati.istat.it/Index.aspx?DataSetCode=DCSP_LATTE (accessed on 9 May 2022).
- Giorgi, F.; Lionello, P. Climate Change Projections for the Mediterranean Region. Glob. Planet. Change 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Dipartimento Regionale per la Sicurezza del Territorio. Rapporto Sulla Risorsa Idrica in Veneto; Dipartimento Regionale per la Sicurezza del Territorio: Padova, Italy, 2022. [Google Scholar]
- Garofalo, P.; Ventrella, D.; Kersebaum, K.C.; Gobin, A.; Trnka, M.; Giglio, L.; Dubrovský, M.; Castellini, M. Water Footprint of Winter Wheat under Climate Change: Trends and Uncertainties Associated to the Ensemble of Crop Models. Sci. Total Environ. 2019, 658, 1186–1208. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Ayuso, M.; Quemada, M.; Vanclooster, M.; Ruiz-Ramos, M.; Rodriguez, A.; Gabriel, J.L. Assessing Cover Crop Management under Actual and Climate Change Conditions. Sci. Total Environ. 2018, 621, 1330–1341. [Google Scholar] [CrossRef]
- Ronga, D.; Prà, A.D.; Immovilli, A.; Ruozzi, F.; Davolio, R.; Pacchioli, M.T. Effects of Harvest Time on the Yield and Quality of Winter Wheat Hay Produced in Northern Italy. Agronomy 2020, 10, 917. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage Review: Interpretation of Chemical, Microbial, and Organoleptic Components of Silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Woolford, M.K.K. The Detrimental Effects of Air on Silage. J. Appl. Bacteriol. 1990, 68, 101–116. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Bolsen, K.K.; Lin, C.J. History of Silage. In Silage Science and Technology; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; American Society of Agronomy: Madison, WI, USA, 2003; pp. 1–30. [Google Scholar] [CrossRef]
- Vanbelle, M.; Bertin, G. L’ensilage, Aspects Biologiques Nouveaux; Sanofi Santé Animale: Paris, France, 1985. [Google Scholar]
- Gallo, A.; Giuberti, G.; Bruschi, S.; Fortunati, P.; Masoero, F. Use of Principal Factor Analysis to Generate a Corn Silage Fermentative Quality Index to Rank Well- or Poorly Preserved Forages. J. Sci. Food Agric. 2016, 96, 1686–1696. [Google Scholar] [CrossRef]
- Kaiser, E.; Weiß, K.; Nussbaum, H.; Kalzendorf, C.; Pahlow, G.; Schenkel, H.; Schwarz, F.J.; Spiekers, H.; Staudacher, W.; Thaysen, J. Grobfutterbewertung Teil B—DLG-Schlüssel Zur Beurteilung Der Gärqualität von Grünfuttersilagen Auf Basis Der Chemischen Untersuchung. Available online: https://www.lksh.de/fileadmin/PDFs/Landwirtschaft/Futter-_und_Substratkonservierung/FuKo_DLG_Grobfutterbewertung_B.pdf (accessed on 5 September 2022).
- Xiccato, G.; Cinetto, M.; Carazzolo, A.; Cossu, M.E. The Effect of Silo Type and Dry Matter Content on the Maize Silage Fermentation Process and Ensiling Loss. Anim. Feed Sci. Technol. 1994, 49, 311–323. [Google Scholar] [CrossRef]
- Xiccato, G.; Trocino, A.; Carazzolo, A. Ensiling and Nutritive Value of Kenaf (Hibiscus Cannabinus). Anim. Feed Sci. Technol. 1998, 71, 229–240. [Google Scholar] [CrossRef]
- Segato, S.; Marchesini, G.; Serva, L.; Contiero, B.; Magrin, L.; Andrighetto, I. Assessment of Fermentative Quality of Ensiled High-Moisture Maize Grains by a Multivariate Modelling Approach. Agronomy 2022, 12, 429. [Google Scholar] [CrossRef]
- Andrighetto, I.; Serva, L.; Gazziero, M.; Tenti, S.; Mirisola, M.; Garbin, E.; Contiero, B.; Grandis, D.; Marchesini, G. Proposal and Validation of New Indexes to Evaluate Maize Silage Fermentative Quality in Lab-Scale Ensiling Conditions through the Use of a Receiver Operating Characteristic Analysis. Anim. Feed Sci. Technol. 2018, 242, 31–40. [Google Scholar] [CrossRef]
- Serva, L.; Marchesini, G.; Chinello, M.; Contiero, B.; Tenti, S.; Mirisola, M.; Grandis, D.; Andrighetto, I. Use of Near-Infrared Spectroscopy and Multivariate Approach for Estimating Silage Fermentation Quality from Freshly Harvested Maize. Ital. J. Anim. Sci. 2021, 20, 859–871. [Google Scholar] [CrossRef]
- Archibald, J.G.; Kuzmeski, J.W.; Russell, S. Grass Silage Quality as Affected by Crop Composition and by Additives. J. Dairy Sci. 1960, 43, 1648–1653. [Google Scholar] [CrossRef]
- Ranjit, N.K.; Kung, L. The Effect of Lactobacillus buchneri, Lactobacillus plantarum, or a Chemical Preservative on the Fermentation and Aerobic Stability of Corn Silage. J. Dairy Sci. 2000, 83, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Brüning, D.; Gerlach, K.; Weiß, K.; Südekum, K.H. Effect of Compaction, Delayed Sealing and Aerobic Exposure on Maize Silage Quality and on Formation of Volatile Organic Compounds. Grass Forage Sci. 2018, 73, 53–66. [Google Scholar] [CrossRef]
- Jia, T.; Wang, B.; Yu, Z.; Wu, Z. The Effects of Stage of Maturity and Lactic Acid Bacteria Inoculants on the Ensiling Characteristics, Aerobic Stability and in Vitro Digestibility of Whole-Crop Oat Silages. Grassl. Sci. 2021, 67, 55–62. [Google Scholar] [CrossRef]
- Addah, W.; Baah, J.; Groenewegen, P.; Okine, E.K.; McAllister, T.A. Comparison of the Fermentation Characteristics, Aerobic Stability and Nutritive Value of Barley and Corn Silages Ensiled with or without a Mixed Bacterial Inoculant. Can. J. Anim. Sci. 2011, 91, 133–146. [Google Scholar] [CrossRef]
- Muck, R.E. A Lactic Acid Bacterial Strain to Improve Aerobic Stability of Silages; US Dairy Forage Research Center: Madison, WI, USA, 1996; pp. 42–43. [Google Scholar]
- Daniel, J.L.P.P.; Queiroz, O.C.M.M.; Arriola, K.G.; Daetz, R.; Basso, F.; Romero, J.J.; Adesogan, A.T. Effects of Homolactic Bacterial Inoculant on the Performance of Lactating Dairy Cows. J. Dairy Sci. 2018, 101, 5145–5152. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Weinberg, Z.G.; Ogunade, I.M.; Cervantes, A.A.P.P.; Arriola, K.G.; Jiang, Y.; Kim, D.; Li, X.; Gonçalves, M.C.M.M.; Vyas, D.; et al. Meta-Analysis of Effects of Inoculation with Homofermentative and Facultative Heterofermentative Lactic Acid Bacteria on Silage Fermentation, Aerobic Stability, and the Performance of Dairy Cows. J. Dairy Sci. 2017, 100, 4587–4603. [Google Scholar] [CrossRef]
- Tabacco, E.; Piano, S.; Cavallarin, L.; Bernardes, T.F.; Borreani, G. Clostridia Spore Formation during Aerobic Deterioration of Maize and Sorghum Silages as Influenced by Lactobacillus buchneri and Lactobacillus plantarum Inoculants. J. Appl. Microbiol. 2009, 107, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Serva, L.; Magrin, L.; Marchesini, G.; Andrighetto, I. Short Communication: Prognostic Values of a Multiparametric Risk Score in Maize Silage Undergoing Different Ensiling Conditions. Agronomy 2022, 12, 774. [Google Scholar] [CrossRef]
- Serva, L.; Andrighetto, I.; Marchesini, G.; Contiero, B.; Grandis, D.; Magrin, L. Prognostic Capacity Assessment of a Multiparameter Risk Score for Aerobic Stability of Maize Silage Undergoing Heterofermentative Inoculation (Lactobacillus buchneri) in Variable Ensiling Conditions. Anim. Feed Sci. Technol. 2021, 281, 115116. [Google Scholar] [CrossRef]
- Berzaghi, P.; Serva, L.; Piombino, M.; Mirisola, M.; Benozzo, F. Prediction Performances of Portable near Infrared Instruments for at Farm Forage Analysis. Ital. J. Anim. Sci. 2005, 4, 145–147. [Google Scholar] [CrossRef]
- Marchesini, G.; Serva, L.; Garbin, E.; Mirisola, M.; Andrighetto, I. Near-Infrared Calibration Transfer for Undried Whole Maize Plant between Laboratory and on-Site Spectrometers. Ital. J. Anim. Sci. 2017, 17, 66–72. [Google Scholar] [CrossRef]
- Evangelista, C.; Basiricò, L.; Bernabucci, U. An Overview on the Use of near Infrared Spectroscopy (Nirs) on Farms for the Management of Dairy Cows. Agriculture 2021, 11, 296. [Google Scholar] [CrossRef]
- Harris, P.A.; Nelson, S.; Carslake, H.B.; Argo, C.M.G.; Wolf, R.; Fabri, F.B.; Brolsma, K.M.; van Oostrum, M.J.; Ellis, A.D. Comparison of NIRS and Wet Chemistry Methods for the Nutritional Analysis of Haylages for Horses. J. Equine Vet. Sci. 2018, 71, 13–20. [Google Scholar] [CrossRef]
- Marchesini, G.; Serva, L.; Chinello, M.; Gazziero, M.; Tenti, S.; Mirisola, M.; Garbin, E.; Contiero, B.; Grandis, D.; Andrighetto, I. Effect of Maturity Stage at Harvest on the Ensilability of Maize Hybrids in the Early and Late FAO Classes, Grown in Areas Differing in Yield Potential. Grass Forage Sci. 2019, 74, 415–426. [Google Scholar] [CrossRef]
- Abrams, S.M.; Shenk, J.S.; Harpster, H.W. Potential of Near Infrared Reflectance Spectroscopy for Analysis of Silage Composition. J. Dairy Sci. 1988, 71, 1955–1959. [Google Scholar] [CrossRef]
- Park, R.S.; Agnew, R.E.; Gordon, F.J.; Steen, R.W.J.J. The Use of near Infrared Reflectance Spectroscopy (NIRS) on Undried Samples of Grass Silage to Predict Chemical Composition and Digestibility Parameters. Anim. Feed Sci. Technol. 1998, 72, 155–167. [Google Scholar] [CrossRef]
- Reeves, J.B.; Blosser, T.H.; Colenbrander, V.F. Near Infrared Reflectance Spectroscopy for Analyzing Undried Silage. J. Dairy Sci. 1989, 72, 79–88. [Google Scholar] [CrossRef]
- Sørensen, L.K. Prediction of Fermentation Parameters in Grass and Corn Silage by near Infrared Spectroscopy. J. Dairy Sci. 2004, 87, 3826–3835. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Lee, S.H.; Choi, K.C.; Lim, Y.C.; Kim, J.H.; Lee, K.W.; Choi, G.J. Prediction of the Chemical Composition and Fermentation Parameters of Winter Rye Silages by Near Infrared Spectroscopy. J. Korean Soc. Grassl. Forage Sci. 2014, 34, 209–213. [Google Scholar] [CrossRef]
- Liu, X.; Han, L.J. Evaluation of Near-Infrared Reflectance Spectroscopy (NIRS) for Predicting Chemical Composition of Straw Silage. J. Anim. Feed Sci. 2006, 15, 329–336. [Google Scholar] [CrossRef][Green Version]
- Schuchmann, G.H. DLG Test Report 7020. Available online: https://pruefberichte.dlg.org/filestorage/7020_e.pdf (accessed on 19 July 2022).
- Tabacco, E.; Righi, F.; Quarantelli, A.; Borreani, G. Dry Matter and Nutritional Losses during Aerobic Deterioration of Corn and Sorghum Silages as Influenced by Different Lactic Acid Bacteria Inocula. J. Dairy Sci. 2011, 94, 1409–1419. [Google Scholar] [CrossRef]
- Segato, S.; Marchesini, G.; Serva, L.; Magrin, L.; Contiero, B.; Andrighetto, I.; Serva, L. A Machine Learning-Based Assessment of Maize Silage Dry Matter Losses by Net-Bags Buried in Farm Bunker Silos. Agriculture 2022, 12, 785. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 2nd Revision, 17th ed.; Association of Official Analytical Chemist: Gaithersburg, MD, USA, 2003. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemist: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Ferreira, G.; Mertens, D.R. Measuring Detergent Fibre and Insoluble Protein in Corn Silage Using Crucibles or Filter Bags. Anim. Feed Sci. Technol. 2007, 133, 335–340. [Google Scholar] [CrossRef]
- Schlau, N.; Mertens, D.R.; Taysom, K.; Taysom, D. Technical Note: Effects of Filter Bags on Neutral Detergent Fiber Recovery and Fiber Digestion in Vitro. J. Dairy Sci. 2021, 104, 1846–1854. [Google Scholar] [CrossRef]
- Vogel, K.P.; Pedersen, J.F.; Masterson, S.D.; Toy, J.J. Evaluation of a Filter Bag System for NDF, ADF, and IVDMD Forage Analysis. Crop Sci. 1999, 39, 276–279. [Google Scholar] [CrossRef]
- Ankom Determining Acid Detergent Lignin in Beakers. Available online: http//www.ankom.com/media/documents/Method_8_Lignin_in_beakers_3_13_13.pdf (accessed on 9 March 2014).
- Martillotti, F.; Puppo, S. Liquid Chromatographic Determination of Organic Acids in Silages and Rumen Fluids. Ann. dell’Istituto Sper. Zootec. 1985, 18, 1–10. [Google Scholar]
- Urea/Ammonia Assay Procedure. UREA/AMMONIA (Rapid). Available online: https://secure.megazyme.com/files/Booklet/K-URAMR_DATA.pdf (accessed on 5 July 2022).
- Martillotti, F.; Anotngiovanni, M.; Rizzi, L.; Santi, E.; Bittante, G. Metodi Di Analisi per La Valutazione Degli Alimenti D’impiego Zootecnico; CNR IPRA; Quaderni Metodologici: Milano, Italy, 1987. [Google Scholar]
- Richard, T.L.; Veeken, A.H.M.; de Wilde, V.; Hamelers, H.V.M. Air-Filled Porosity and Permeability Relationships during Solid-State Fermentation. Biotechnol. Prog. 2004, 20, 1372–1381. [Google Scholar] [CrossRef]
- Grambsch, P.M.; Therneau, T.M. Proportional Hazards Tests and Diagnostics Based on Weighted Residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Abeysekera, W.W.M.M.; Sooriyarachchi, M.R. Use of Schoenfeld’s Global Test to Test Proportional Hazards Assumption in the Cox Proportional Hazards Model: An Application to Clinical Study. J. Natl. Sci. Found. Sri Lanka 2009, 37, 41–51. [Google Scholar] [CrossRef]
- Downie, T. Using the R Commander: A Point-and-Click Interface for R. J. Stat. Softw. 2016, 75, 1–4. [Google Scholar] [CrossRef]
- Fox, J. The R Commander: A Basic-Statistics Graphical User Interface to R. J. Stat. Softw. 2005, 14, 1–42. [Google Scholar] [CrossRef]
- Pesarin, F.; Salmaso, L. Permutation Tests for Complex Data: Theory, Applications and Software. Available online: http://www.wiley.com/legacy/wileychi/pesarin/material.html (accessed on 30 November 2022).
- Fasolato, L.; Carraro, L.; Facco, P.; Cardazzo, B.; Balzan, S.; Taticchi, A.; Andreani, N.A.; Montemurro, F.; Martino, M.E.; Di Lecce, G.; et al. Agricultural By-Products with Bioactive Effects: A Multivariate Approach to Evaluate Microbial and Physicochemical Changes in a Fresh Pork Sausage Enriched with Phenolic Compounds from Olive Vegetation Water. Int. J. Food Microbiol. 2016, 228, 34–43. [Google Scholar] [CrossRef]
- Chandler, J.A.; Jewell, W.J.; Gossett, J.M.; Van Soest, P.J.; Robertson, J.B. Predicting Methane Fermentation Biodegradability. Biotechnol. Bioeng. Symp. 1980, 10, 93–107. [Google Scholar]
- Raffrenato, E.; Ross, D.A.; Van Amburgh, M.E. Development of an in Vitro Method to Determine Rumen Undigested ANDFom for Use in Feed Evaluation. J. Dairy Sci. 2018, 101, 9888–9900. [Google Scholar] [CrossRef]
- Raffrenato, E.; Fievisohn, R.; Cotanch, K.W.; Grant, R.J.; Chase, L.E.; Van Amburgh, M.E. Effect of Lignin Linkages with Other Plant Cell Wall Components on in Vitro and in Vivo Neutral Detergent Fiber Digestibility and Rate of Digestion of Grass Forages. J. Dairy Sci. 2017, 100, 8119–8131. [Google Scholar] [CrossRef]
- Oba, M.; Allen, M.S. Evaluation of the Importance of the Digestibility of Neutral Detergent Fiber from Forage: Effects on Dry Matter Intake and Milk Yield of Dairy Cows. J. Dairy Sci. 1999, 82, 589–596. [Google Scholar] [CrossRef]
- Allen, M.S. Effects of Diet on Short-Term Regulation of Feed Intake by Lactating Dairy Cattle. J. Dairy Sci. 2000, 83, 1598–1624. [Google Scholar] [CrossRef]
- Xie, Z.L.; Zhang, T.F.; Chen, X.Z.; Li, G.D.; Zhang, J.G. Effects of Maturity Stages on the Nutritive Composition and Silage Quality of Whole Crop Wheat. Asian-Australas. J. Anim. Sci. 2012, 25, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Randby, T.; Nadeau, E.; Karlsson, L.; Johansen, A. Effect of Maturity Stage at Harvest and Kernel Processing of Whole Crop Wheat Silage on Digestibility by Dairy Cows. Anim. Feed Sci. Technol. 2019, 253, 141–152. [Google Scholar] [CrossRef]
- Walsh, K.; O’Kiely, P.; Moloney, A.P.; Boland, T.M. Intake, Performance and Carcass Characteristics of Beef Cattle Offered Diets Based on Whole-Crop Wheat or Forage Maize Relative to Grass Silage or Ad Libitum Concentrates. Livest. Sci. 2008, 116, 223–236. [Google Scholar] [CrossRef]
- Oude Elferink, S.J.W.H.; Krooneman, E.J.; Gottschal, J.C.; Spoelstra, S.F.; Faber, F.; Driehuis, F. Anaerobic Conversion of Lactic Acid to Acetic Acid and 1,2-Propanediol by Lactobacillus buchneri. Appl. Environ. Microbiol. 2001, 67, 125–132. [Google Scholar] [CrossRef]
- Kleinschmit, D.H.; Kung, L. A Meta-Analysis of the Effects of Lactobacillus buchneri on the Fermentation and Aerobic Stability of Corn and Grass and Small-Grain Silages. J. Dairy Sci. 2006, 89, 4005–4013. [Google Scholar] [CrossRef] [PubMed]
- Keshri, J.; Chen, Y.; Pinto, R.; Kroupitski, Y.; Weinberg, Z.G.; Sela, S. Microbiome Dynamics during Ensiling of Corn with and without Lactobacillus plantarum Inoculant. Appl. Microbiol. Biotechnol. 2018, 102, 4025–4037. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Chen, Y. Effects of Storage Period on the Composition of Whole Crop Wheat and Corn Silages. Anim. Feed Sci. Technol. 2013, 185, 196–200. [Google Scholar] [CrossRef]
- Chen, Y.; Weinberg, Z.G. The Effect of Relocation of Whole-Crop Wheat and Corn Silages on Their Quality. J. Dairy Sci. 2014, 97, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Pieper, R.; Hackl, W.; Korn, U.; Zeyner, A.; Souffrant, W.B.; Pieper, B. Effect of Ensiling Triticale, Barley and Wheat Grains at Different Moisture Content and Addition of Lactobacillus plantarum (DSMZ 8866 and 8862) on Fermentation Characteristics and Nutrient Digestibility in Pigs. Anim. Feed Sci. Technol. 2011, 164, 96–105. [Google Scholar] [CrossRef]
- Keshri, J.; Chen, Y.; Pinto, R.; Kroupitski, Y.; Weinberg, Z.G.; Saldinger, S.S. Bacterial Dynamics of Wheat Silage. Front. Microbiol. 2019, 10, 1532. [Google Scholar] [CrossRef] [PubMed]
- Agarussi, M.C.N.; Pereira, O.G.; da Silva, L.D.; da Silva, V.P.; de Paula, R.A.; Fonesca e Silva, F.; Ribeiro, K.G. Effect of Various Strains of Lactobacillus buchneri on the Fermentation Quality and Aerobic Stability of Corn Silage. Agriculture 2022, 12, 95. [Google Scholar] [CrossRef]
- Baah, J.; Addah, W.; Okine, E.K.; McAllister, T.A. Effects of Homolactic Bacterial Inoculant Alone or Combined with an Anionic Surfactant on Fermentation, Aerobic Stability and in Situ Ruminal Degradability of Barley Silage. Asian-Australas. J. Anim. Sci. 2011, 24, 369–378. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E. Quantifying the Extent of Aerobic Deterioration in Corn Bunker and Pile Silages at a Farm Level. In Proceedings of the 15th International Silage Conference, Madison, WI, USA, 27–29 July 2009; US Dairy Forage Research Centre, USDA-ARS: Madison, WI, USA, 2009; pp. 321–322. [Google Scholar]
- Schmidt, R.J.; Kung, L. The Effects of Lactobacillus buchneri with or without a Homolactic Bacterium on the Fermentation and Aerobic Stability of Corn Silages Made at Different Locations. J. Dairy Sci. 2010, 93, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Huisden, C.M.; Adesogan, A.T.; Kim, S.C.; Ososanya, T. Effect of Applying Molasses or Inoculants Containing Homofermentative or Heterofermentative Bacteria at Two Rates on the Fermentation and Aerobic Stability of Corn Silage. J. Dairy Sci. 2009, 92, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Mari, L.J.; Schmidt, R.J.; Nussio, L.G.; Halladas, C.M.; Kung, L. Short Communication: An Evaluation of the Effectiveness of Lactobacillus buchneri 40788 to Alter Fermentation and Improve the Aerobic Stability of Corn Silage in Farm Silos. J. Dairy Sci. 2009, 92, 1174–1176. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage Review: Factors Affecting Dry Matter and Quality Losses in Silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef]
- Borreani, G.; Bernardes, T.F.; Tabacco, E. Aerobic Deterioration Influences the Fermentative, Microbiological and Nutritional Quality of Maize and Sorghum Silages on Farm in High Quality Milk and Cheese Production Chains. Rev. Bras. Zootec. 2008, 37, 68–77. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E. The Relationship of Silage Temperature with the Microbiological Status of the Face of Corn Silage Bunkers. J. Dairy Sci. 2010, 93, 2620–2629. [Google Scholar] [CrossRef]
- Santos, M.C.; Golt, C.; Joerger, R.D.; Mechor, G.D.; Mourão, G.B.; Kung, L. Identification of the Major Yeasts Isolated from High Moisture Corn and Corn Silages in the United States Using Genetic and Biochemical Methods. J. Dairy Sci. 2017, 100, 1151–1160. [Google Scholar] [CrossRef]
- Spadaro, D.; Bustos-Lopez, M.P.; Gullino, M.L.; Piano, S.; Tabacco, E.; Borreani, G. Evolution of Fungal Populations in Corn Silage Conserved under Polyethylene or Biodegradable Films. J. Appl. Microbiol. 2015, 119, 510–520. [Google Scholar] [CrossRef]
- Jonsson, A. Enumeration and Confirmation of Clostridium Tyrobutyricum in Silages Using Neutral Red, D-Cycloserine, and Lactate Dehydrogenase Activity. J. Dairy Sci. 1990, 73, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.M.; Davies, D.R. The Aerobic Stability of Silage: Key Findings and Recent Developments. Grass Forage Sci. 2013, 68, 1–19. [Google Scholar] [CrossRef]
- Gharechahi, J.; Kharazian, Z.A.; Sarikhan, S.; Jouzani, G.S.; Aghdasi, M.; Hosseini Salekdeh, G. The Dynamics of the Bacterial Communities Developed in Maize Silage. Microb. Biotechnol. 2017, 10, 1663–1676. [Google Scholar] [CrossRef] [PubMed]
- Benjamim da Silva, É.; Liu, X.; Mellinger, C.; Gressley, T.F.; Stypinski, J.D.; Moyer, N.A.; Kung, L. Effect of Dry Matter Content on the Microbial Community and on the Effectiveness of a Microbial Inoculant to Improve the Aerobic Stability of Corn Silage. J. Dairy Sci. 2022, 105, 5024–5043. [Google Scholar] [CrossRef]
- Arriola, K.G.; Oliveira, A.S.; Jiang, Y.; Kim, D.; Silva, H.M.; Kim, S.C.; Amaro, F.X.; Ogunade, I.M.; Sultana, H.; Pech Cervantes, A.A.; et al. Meta-Analysis of Effects of Inoculation with Lactobacillus buchneri, with or without Other Bacteria, on Silage Fermentation, Aerobic Stability, and Performance of Dairy Cows. J. Dairy Sci. 2021, 104, 7653–7670. [Google Scholar] [CrossRef]
- Weiß, K.; Kroschewski, B.; Auerbach, H.U. The Influence of Delayed Sealing and Repeated Air Ingress during the Storage of Maize Silage on Fermentation Patterns, Yeast Development and Aerobic Stability. Fermentation 2022, 8, 48. [Google Scholar] [CrossRef]
- De Melo, N.N.; Carvalho-Estrada, P.d.A.; Tavares, Q.G.; de Moura Pereira, L.; Delai Vigne, G.L.; Camargo Rezende, D.M.L.; Schmidt, P. The Effects of Short-Time Delayed Sealing on Fermentation, Aerobic Stability and Chemical Composition on Maize Silages. Agronomy 2023, 13, 223. [Google Scholar] [CrossRef]
- Kroschewski, B.; Auerbach, H.; Weiss, K. Statistics and experimental design in silage research: Some comments on design and analysis of comparative silage experiments. In Proceedings of the XVIII International Silage Conference, Bonn, Germany, 24–26 July 2018; Gerlach, K., Südekum, K.-H., Eds.; University of Bonn: Bonn, Germany, 2018; pp. 554–560. [Google Scholar]
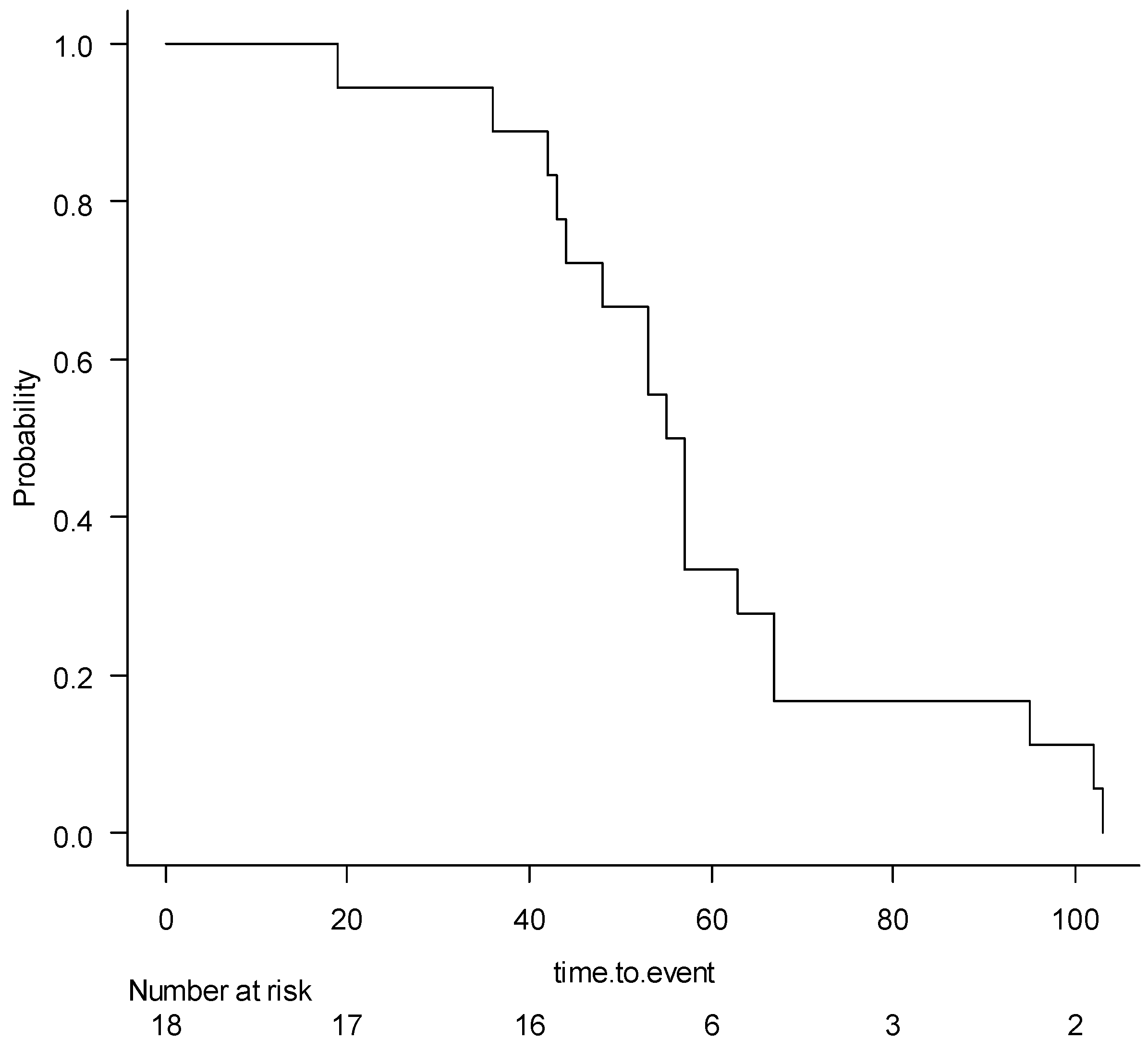
| DML | DMH | SEM | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Sd | Median | IQR | Mean | Sd | Median | IQR | |||
| Dry matter (DM, g/kg) | 312 | 6.93 | 311 | 7.35 | 348 | 9.75 | 345 | 14.6 | 1.75 | <0.001 |
| Ash (g/kg of the DM) | 90.7 | 3.78 | 90.55 | 3.90 | 78.3 | 2.60 | 78.3 | 3.15 | 0.76 | <0.001 |
| Crude protein (g/kg of the DM) | 100 | 3.42 | 100 | 4.35 | 73.2 | 3.47 | 73.9 | 3.33 | 0.72 | <0.001 |
| Ether extract (g/kg of the DM) | 22.3 | 1.32 | 22.4 | 0.79 | 21.7 | 0.88 | 21.9 | 1.18 | 0.24 | 0.094 |
| aNDF 1 (g/kg of the DM) | 515 | 21.3 | 513 | 28.6 | 559 | 10.3 | 561 | 13.0 | 2.50 | <0.001 |
| ADF 2 (g/kg of the DM) | 289 | 13.2 | 292 | 18.3 | 339 | 7.89 | 337 | 11.2 | 1.54 | <0.001 |
| Lignin (sa) (g/kg of the DM) | 42.9 | 6.78 | 43.4 | 8.73 | 41.8 | 5.59 | 41.3 | 7.25 | 1.36 | 0.566 |
| Starch (g/kg of the DM) | 158 | 11.47 | 158 | 11.95 | 143 | 13.6 | 147 | 19.0 | 2.40 | <0.001 |
| WSC 3 (g/kg of the DM) | 55.4 | 13.17 | 54.6 | 23.7 | 39.8 | 11.2 | 37.6 | 16.9 | 2.17 | <0.001 |
| Density (kg/m3) | 148 | 8.80 | 147 | 7.10 | 155 | 6.40 | 156 | 4.45 | 1.56 | 0.006 |
| Porosity (decimals) | 0.61 | 0.02 | 0.61 | 0.03 | 0.65 | 0.02 | 0.65 | 0.03 | 0.01 | <0.001 |
| DM at Harvest (g/kg of the DM) | ||||
|---|---|---|---|---|
| DML 1 | DMH 2 | SEM 3 | p | |
| Dry matter (DM, g/kg) | 281 | 313 | 1.85 | <0.0001 |
| pH | 3.94 | 3.89 | 0.02 | 0.062 |
| Ammonia (% of total N) | 7.18 | 8.00 | 0.13 | <0.0001 |
| Lactic acid (g/kg of the DM) | 61.5 | 46.2 | 1.16 | <0.0001 |
| Acetic acid (g/kg of the DM) | 23.5 | 18.1 | 0.48 | <0.0001 |
| Propionic acid (g/kg of the DM) | 4.84 | 5.06 | 0.07 | 0.055 |
| Butyric acid (g/kg of the DM) | 2.75 | 2.44 | 0.03 | <0.0001 |
| Ethanol (g/kg of the DM) | 5.62 | 1.46 | 0.27 | <0.0001 |
| FQI 4 | 55.4 | 51.2 | 1.59 | 0.076 |
| FZs 5 | 65.8 | 61.7 | 1.68 | 0.098 |
| DM loss (%) | 10.7 | 11.7 | 0.77 | 0.378 |
| Inoculant Type | |||||
|---|---|---|---|---|---|
| Control | Heterofermentative | Homofermentative | SEM 1 | p | |
| Dry matter (DM, g/kg) | 298 | 297 | 295 | 2.27 | 0.734 |
| pH | 3.92 | 3.92 | 3.90 | 0.02 | 0.722 |
| Ammonia (% of total N) | 7.36 | 7.90 | 7.44 | 0.16 | 0.056 |
| Lactic acid (g/kg of the DM) | 52.6 | 52.8 | 56.2 | 1.43 | 0.159 |
| Acetic acid (g/kg of the DM) | 19.9 b | 20.4 b | 22.0 a | 0.59 | 0.048 |
| Propionic acid (g/kg of the DM) | 4.93 a | 4.76 b | 5.15 a | 0.09 | 0.017 |
| Butyric acid (g/kg of the DM) | 2.62 | 2.53 | 2.63 | 0.04 | 0.160 |
| Ethanol (g/kg of the DM) | 3.63 | 3.90 | 3.08 | 0.33 | 0.227 |
| FQI 2 | 53.5 | 50.1 | 56.4 | 1.94 | 0.099 |
| FZs 3 | 62.7 | 63.3 | 65.3 | 2.06 | 0.654 |
| DM loss (%) | 11.0 | 11.2 | 11.5 | 0.87 | 0.893 |
| Sealing Delay (h) | |||||
|---|---|---|---|---|---|
| 0 h | 6 h | 20 h | SEM 1 | p | |
| Dry matter (DM, g/kg) | 298 | 296 | 297 | 2.27 | 0.823 |
| pH | 3.91 | 3.91 | 3.92 | 0.02 | 0.955 |
| Ammonia (% of total N) | 7.46 | 7.50 | 7.75 | 0.16 | 0.410 |
| Lactic acid (g/kg of the DM) | 55.0 | 53.1 | 53.5 | 1.43 | 0.638 |
| Acetic acid (g/kg of the DM) | 20.0 | 21.3 | 20.9 | 0.59 | 0.299 |
| Propionic acid (g/kg of the DM) | 4.95 | 4.99 | 4.91 | 0.09 | 0.809 |
| Butyric acid (g/kg of the DM) | 2.63 | 2.56 | 2.59 | 0.04 | 0.390 |
| Ethanol (g/kg of the DM) | 3.61 | 3.23 | 3.78 | 0.33 | 0.486 |
| FQI 2 | 54.8 | 53.1 | 52.1 | 1.94 | 0.598 |
| FZs 3 | 65.3 | 61.8 | 64.1 | 2.06 | 0.471 |
| DM loss (%) | 12.7 a | 9.46 b | 11.6 ab | 0.87 | 0.048 |
| DM at Harvest × Inoculant | DM at Harvest × Sealing Delay | Inoculant ×Sealing Delay | |
|---|---|---|---|
| p | p | p | |
| Dry matter (DM, g/kg) | 0.340 | 0.449 | 0.673 |
| pH | 0.739 | 0.046 | 0.330 |
| Ammonia (% of total N) | 0.291 | 0.192 | 0.232 |
| Lactic acid (g/kg of the DM) | 0.722 | 0.084 | 0.397 |
| Acetic acid (g/kg of the DM) | 0.158 | 0.260 | 0.285 |
| Propionic acid (g/kg of the DM) | 0.531 | 0.656 | 1.000 |
| Butyric acid (g/kg of the DM) | 0.938 | 0.813 | 0.380 |
| Ethanol (g/kg of the DM) | 0.142 | 0.391 | 0.652 |
| FQI 1 | 0.856 | 0.009 | 0.675 |
| FZs 2 | 0.743 | 0.190 | 0.896 |
| DM loss (%) | 0.845 | 0.048 | 0.614 |
| Variable | All Samples | Univariate HR (95% C.I.) |
|---|---|---|
| Pre-ensiled traits | ||
| Dry matter (DM, g/kg) | 329 ± 21.7 | 1.02 (0.995–1.05) |
| Ash (g/kg of the DM) | 83.8 ± 7.59 | 0.929 (0.865–0.998) |
| Crude protein (g/kg of the DM) | 75.9 ± 21.6 | 0.979 (0.943–1.01) |
| Ether extract (g/kg of the DM) | 20.3 ± 5.6 | 0.650 (0.357–1.18) |
| aNDF 1 (g/kg of the DM) | 509 ± 139.8 | 1.01 (0.996–1.03) |
| ADF 2 (g/kg of the DM) | 303 ± 82 | 1.01 (0.995–1.03) |
| Lignin (sa) (g/kg of the DM) | 38.8 ± 10.7 | 1.03 (0.950–1.12) |
| Starch (g/kg of the DM) | 138 ± 38 | 0.99 (0.960–1.03) |
| WSC 3 (g/kg of the DM) | 41.3 ± 16.2 | 0.994 (0.963–1.03) |
| Density (kg/m3) | 150 ± 8.40 | 0.980 (0.926–1.04) |
| Porosity (decimals) | 0.63 ± 0.03 | 1.08 × 1013 (418–2.8 × 1023) |
| Post-ensiled traits | ||
| Dry matter (DM, g/kg) | 28.4 ± 7.83 | 1.15 (0.887–1.49) |
| pH | 3.61 ± 1.02 | 4.48 (0.0003–750) |
| Ammonia (% of total N) | 7.19 ± 2.01 | 1.21 (0.504–2.89) |
| Lactic acid (g/kg of the DM) | 4.94 ± 1.42 | 0.601 (0.313–1.15) |
| Acetic acid (g/kg of the DM) | 1.87 ± 0.54 | 0.214 (0.046–1.00) |
| Propionic acid (g/kg of the DM) | 0.46 ± 0.13 | 0.630 (1.4 × 10−8–3.8 × 107) |
| Butyric acid (g/kg of the DM) | 0.23 ± 0.06 | 2.4 × 10−5 (3.1 × 10−13–1837) |
| Ethanol (g/kg of the DM) | 0.24 ± 0.18 | 0.153 (0.016–1.45) |
| Factors | ||
| Dry matter at harvest (g/kg) | ||
| DML | 9 (50%) | |
| DMH | 9 (50%) | 2.08 (0.753–5.76) |
| Use of inoculants | ||
| Control | 6 (33.3%) | |
| Heterofermentative | 6 (33.3%) | 0.648 (0.200–2.10) |
| Homofermentative | 6 (33.3%) | 0.758 (0.220–2.61) |
| Delay in sealing (hour) | ||
| 0 h | 6 (33.3%) | |
| 6 h | 6 (33.3%) | 0.259 (0.067–1.004) |
| 20 h | 6 (33.3%) | 0.535 (0.161–1.776) |
| Coefficient | HR | |
|---|---|---|
| Crude protein (g/kg of the DM) | −0.19 | 0.82—(0.69–0.98) |
| aNDF (g/kg of the DM) | 0.72 | 2.06—(1.29–3.283) |
| ADF (g/kg of the DM) | −0.95 | 0.39—(0.2–3.28) |
| Lignin (sa) (g/kg of the DM) | 0.48 | 1.62—(1.14–2.31) |
| Starch (g/kg of the DM) | −0.22 | 0.8—(0.67–0.96) |
| WSC 1 (g/kg of the DM) | 0.28 | 1.33—(1.1–1.6) |
| Density (kg/m3) | 0.39 | 1.47—(1.06–2.05) |
| Porosity (decimals) | 179.80 | 1.27 × 1078—(8.15 × 1018–1.97 × 10137) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serva, L.; Currò, S.; Andrighetto, I.; Marchesini, G.; Magrin, L. Effect of Inoculants and Sealing Delay on the Fermentation Quality of Early Harvested Wheat Forage. Agronomy 2023, 13, 508. https://doi.org/10.3390/agronomy13020508
Serva L, Currò S, Andrighetto I, Marchesini G, Magrin L. Effect of Inoculants and Sealing Delay on the Fermentation Quality of Early Harvested Wheat Forage. Agronomy. 2023; 13(2):508. https://doi.org/10.3390/agronomy13020508
Chicago/Turabian StyleServa, Lorenzo, Sarah Currò, Igino Andrighetto, Giorgio Marchesini, and Luisa Magrin. 2023. "Effect of Inoculants and Sealing Delay on the Fermentation Quality of Early Harvested Wheat Forage" Agronomy 13, no. 2: 508. https://doi.org/10.3390/agronomy13020508
APA StyleServa, L., Currò, S., Andrighetto, I., Marchesini, G., & Magrin, L. (2023). Effect of Inoculants and Sealing Delay on the Fermentation Quality of Early Harvested Wheat Forage. Agronomy, 13(2), 508. https://doi.org/10.3390/agronomy13020508







