Seed-Primed and Foliar Oxozinc Nanofiber Application Increased Wheat Production and Zn Biofortification in Calcareous-Alkaline Soil
Abstract
1. Introduction
2. Materials and Methods
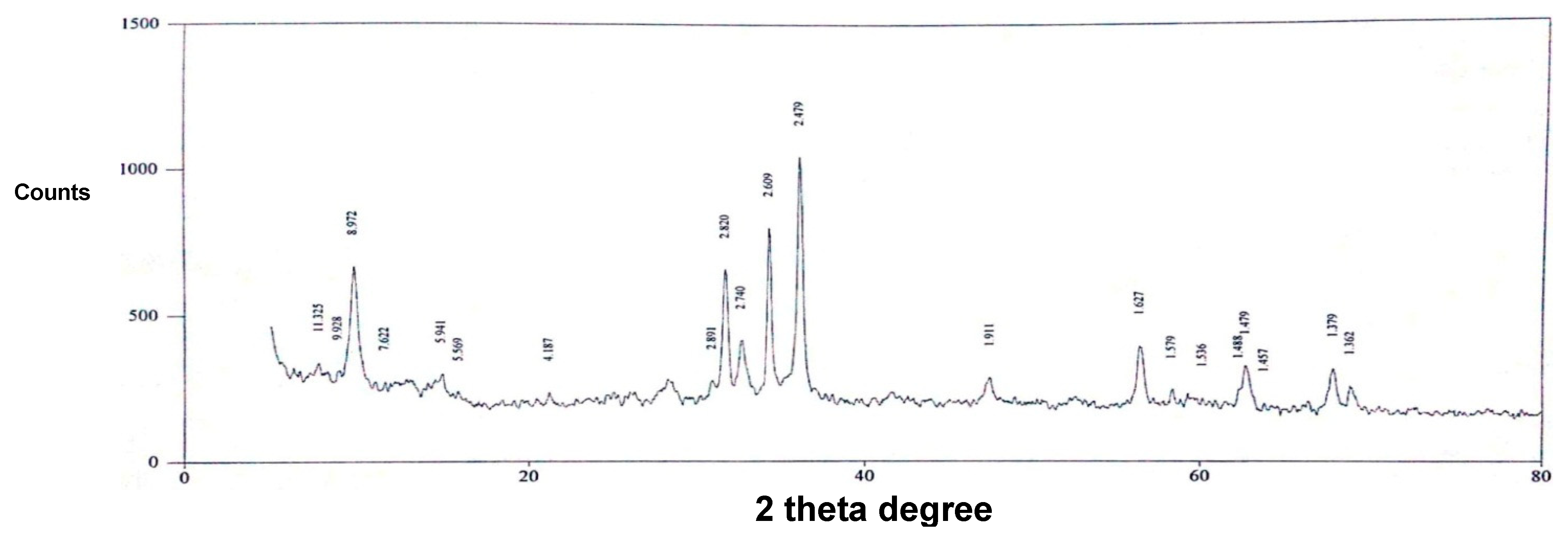
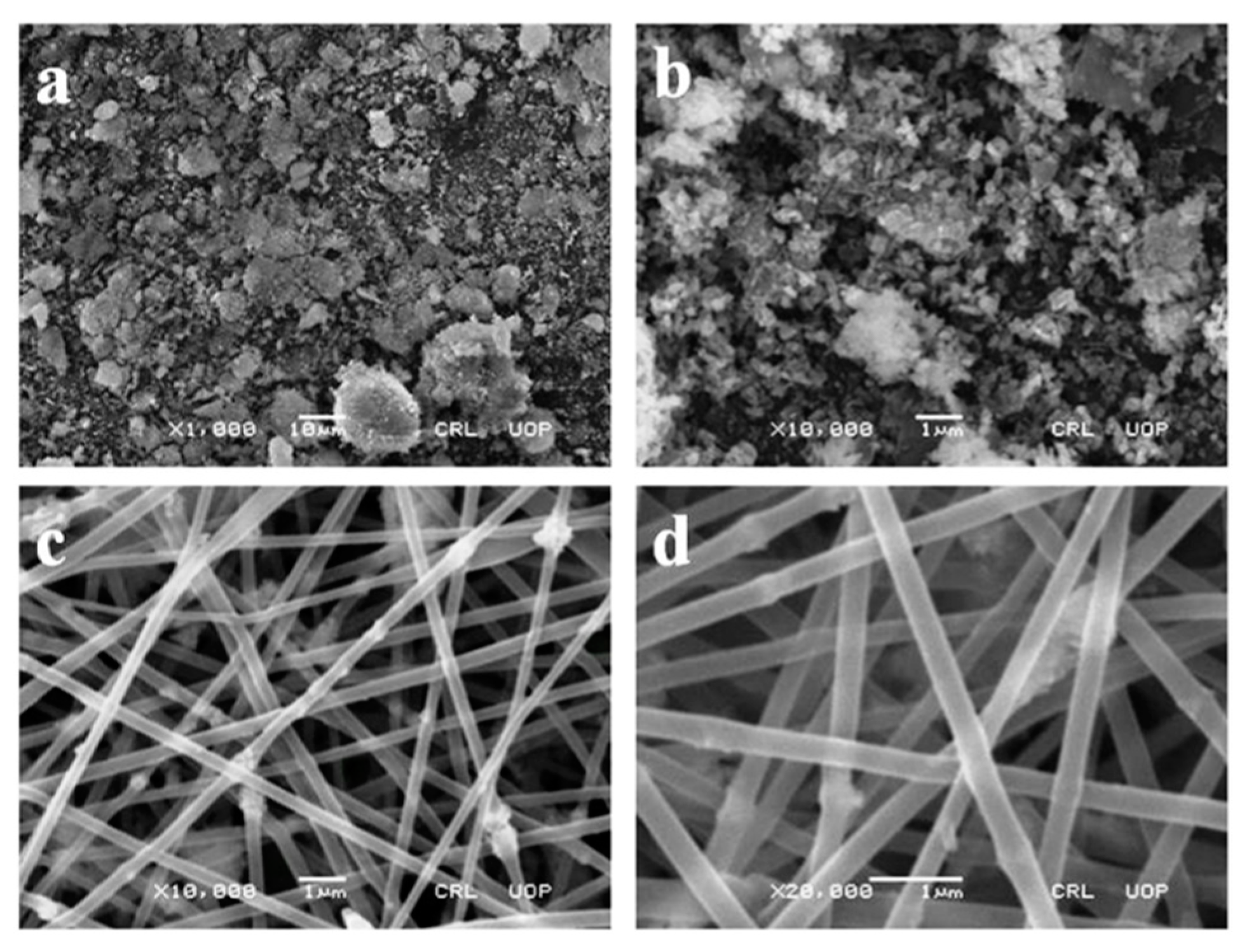
2.1. Preparation and Characterization of ZnO Nanofiber
2.2. Application of ZnO Nanofibers to Wheat Crop in Pots
2.3. Data Collection
2.4. Plant Sample Analysis
2.5. Statistical Analysis
3. Results
3.1. Growth Parameters
3.2. Yield Parameters
3.3. Zn Concentration in Plant Tissues
3.4. Zn Uptake
4. Discussion
4.1. Effect of Zn on Wheat Growth and Yield Parameters
4.2. Comparative Effects on Zn Uptake and Use Efficiency
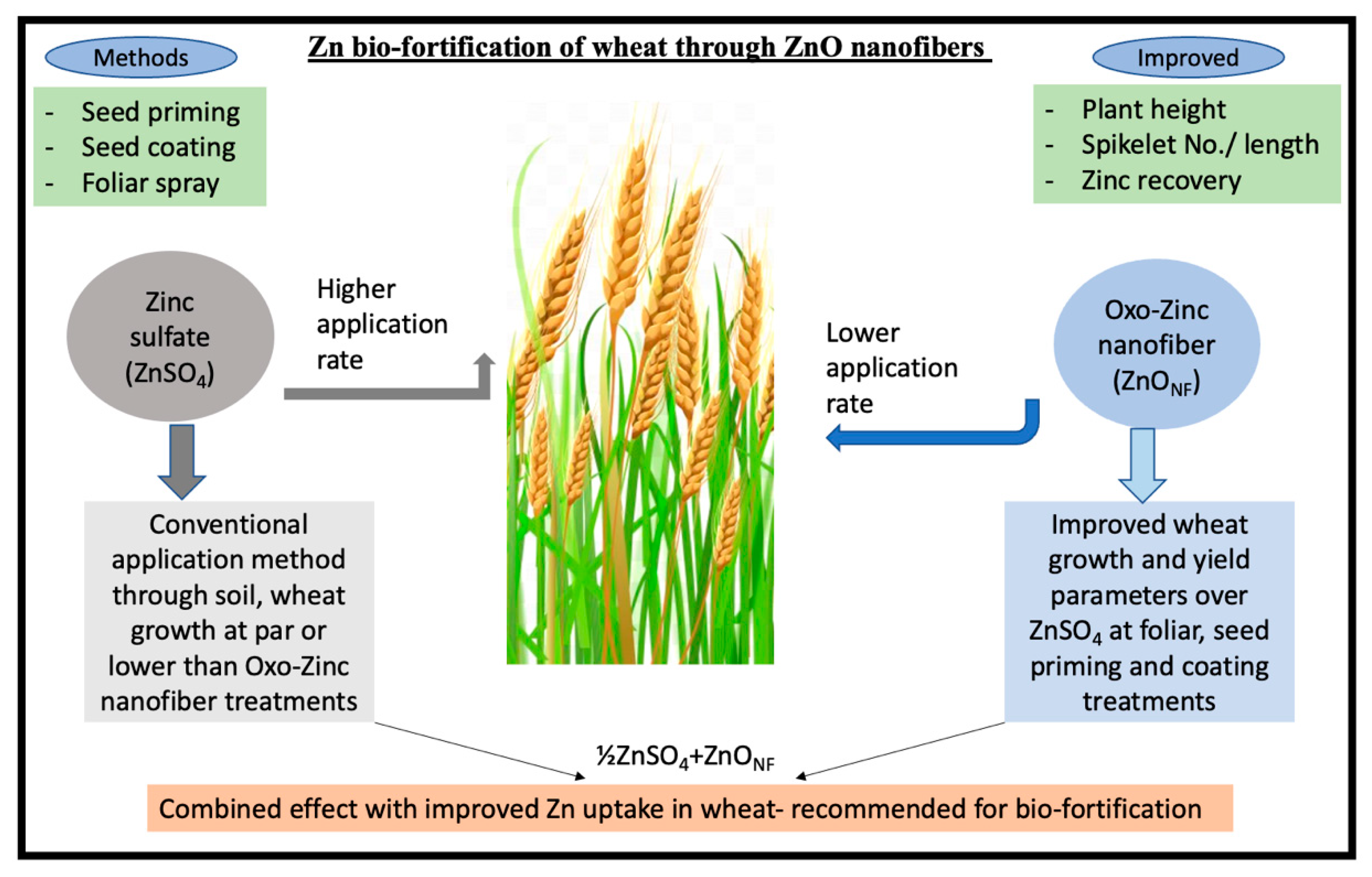
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatt, R.; Hossain, A.; Sharma, P. Zinc biofortification as an innovative technology to alleviate the zinc deficiency in human health: A review. Open Agric. 2020, 5, 176–186. [Google Scholar] [CrossRef]
- Gondal, A.H.; Zafar, A.; Zainab, D.; Toor, M.D.; Sohail, S.; Ameen, S.; Ijaz, A.B.; Imran, B.C.; Hussain, I.; Haider, S.; et al. A Detailed Review Study of Zinc Involvement in Animal, Plant and Human Nutrition. Ind. J. Pure Appl. Biosci. 2021, 9, 262–271. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Schulte, E.E. Soil and AppLIed Zinc (A2528). Understanding Plant Nutrients. 2004. Available online: file:///C:/Users/MDPI/Downloads/a2528.pdf (accessed on 20 December 2022).
- Recena, R.; García-López, A.M.; Delgado, A. Zinc Uptake by Plants as Affected by Fertilization with Zn Sulfate, Phosphorus Availability, and Soil Properties. Agronomy 2021, 11, 390. [Google Scholar] [CrossRef]
- Gregory, P.J.; Wahbi, J.; Adu-Gyamfi, M.; Heiling, R.; Gruber, R. Approaches to reduce zinc and iron deficits in food systems. Glob. Food Secur. 2017, 15, 1–10. [Google Scholar] [CrossRef]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Znic and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Elemike, E.E.; Uzoh, I.M.; Onwudiwe, D.C.; Babalola, O.O. The role of nanotechnology in the fortification of plant nutrients and improvement of crop production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M.; Ullah, A.; Nadeem, F.; Im, S.Y.; Park, S.K.; Lee, D.-J. Agronomic Biofortification of Zinc in Pakistan: Status, Benefits, and Constraints. Front. Sustain. Food Syst. 2020, 4, 591722. [Google Scholar] [CrossRef]
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide research trends on wheat and barley: A bibliometric comparative analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef]
- United Nations. World Population Projected to Reach 9.8 Billion in 2050; Department of Economic and Social Affairs: New York City, NY, USA, 2019; Volume 1, pp. 6–11. Available online: https://www.un.org/en/desa/world-population-projected-reach-98-billion-2050-and-112-billion-2100 (accessed on 29 December 2022).
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050 The 2012 Revision Proof Copy; ESA Working Paper; FAO: Rome, Italy, 2012. [Google Scholar]
- Kareem, S.A.; Mohamed, H.; Qayyum, M.; Abdel-Hadi, A.M.; Rehman, R.A. Interactive effect of salinity and silver nanoparticles on photosynthetic and biochemical parameters of wheat. Arch. Agron. Soil Sci. 2017, 63, 1736–1747. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mater. 2017, 322, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.A.; Zayed, B.A.; Bassiouni, S.M.A.; Mahdi, A.H.A.; Majrashi, A.; Ali, E.F.; Seleiman, M.F. Influence of Nano Silicon and Nano Selenium on Root Characters, Growth, Ion Selectivity, Yield, and Yield Components of Rice (Oryza sativa L.) under Salinity Conditions. Plants 2021, 10, 1657. [Google Scholar] [CrossRef] [PubMed]
- Taha, R.S.; Seleiman, M.F.; Shami, A.; Alhammad, B.A.; Mahdi, A.H.A. Integrated Application of Selenium and Silicon Enhances Growth and Anatomical Structure, Antioxidant Defense System and Yield of Wheat Grown in Salt-Stressed Soil. Plants 2021, 10, 1040. [Google Scholar] [CrossRef]
- Al-Selwey, W.A.; Alsadon, A.A.; Ibrahim, A.A.; Labis, J.P.; Seleiman, M.F. Effects of Zinc Oxide and Silicon Dioxide Nanoparticles on Physiological, Yield, and Water Use Efficiency Traits of Potato Grown under Water Deficit. Plants 2023, 12, 218. [Google Scholar] [CrossRef]
- Moghaddasi, S.; Fotovat, A.; Khoshgoftarmanesh, A.H.; Karimzadeh, F.; Khazaei, H.R. Bioavailability of coated and uncoated ZnO nanoparticles to cucumber in soil with or without organic matter. Ecotoxicol. Environ. Saf. 2017, 144, 543–551. [Google Scholar] [CrossRef]
- Iavicoli, I.; Leso, V.; Beezhold, D.H.; Shvedova, A.A. Nanotechnology in agriculture: Opportunities, toxicological implications, and occupational risks. Toxicol. Appl. Pharmacol. 2017, 329, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Sabir, S.; Arshad, M.; Chaudhari, S.K. Zinc oxide nanoparticles for revolutionizing agriculture: Synthesis and applications. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Prasad, T.N.V.K.V.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Sreeprasad, T.S.; Sajanlal, P.R.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Batsmanova, L.M.; Gonchar, L.M.; Yu, N.; Taran, A.A.O. Using a Colloidal Solution of Metal Nanoparticles as Micronutrient Fertiliser for Cereals. Ph.D. Thesis, Sumy State University, Sumy, Ukraine, 2013; pp. 2–3. [Google Scholar]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Li, J.H.; Liu, X.R.; Zhang, Y.; Tian, F.F.; Zhao, G.Y. Toxicity of nano zinc oxide to mitochondria. Toxicol. Res. 2012, 1, 137–144. [Google Scholar] [CrossRef]
- Joseph, H.M.; Poornima, N. Synthesis and characterization of ZnO nanoparticles. Mater. Today Proc. 2019, 9, 7–12. [Google Scholar] [CrossRef]
- Ristić, M.; Musić, S.; Ivanda, M.; Popović, S. Sol–gel synthesis and characterization of nanocrystalline ZnO powders. J. Alloys Compd. 2005, 397, L1–L4. [Google Scholar] [CrossRef]
- Jia, Y.T.; Gong, J.; Gu, X.H.; Kim, H.Y.; Dong, J. Fabrication and characterization of poly (vinyl alcohol)/chitosan blend nanofibers produced by electrospinning method. Carbohydr. Polym. 2007, 67, 403–409. [Google Scholar] [CrossRef]
- Rhoades, J.D. Soluble salts. In Methods of Soil Analysis (Part 2) Chemical and Microbiological Properties, 2nd ed.; ASA-SSSA, 667S; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 167–179. [Google Scholar]
- Mclean, E.O. Soil pH and Lime Requirement. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 199–224. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Hanlon, E.A.; Schaffer, B. Communications in Soil Science and Plant Analysis Ammonium bicarbonate—DTPA extraction of elements from waste—Amended calcareous soil. Commun. Soil Sci. Plant Anal. 2008, 27, 2321–2335. [Google Scholar] [CrossRef]
- Jones, J.I., Jr. Plants. In Official Methods of Analysis of the Association of Official Analytical Chemists, 14th ed.; Williams, S., Ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1984; pp. 38–64. [Google Scholar]
- Atil, H.; Unver, Y.U. Multiple comparisons. Online J. Biol. Sci. 2001, 1, 723–727. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Haslett, B.S.; Reid, R.J. Uptake and distribution of zinc applied to leaves or roots. Ann. Bot. 2001, 87, 379–386. [Google Scholar] [CrossRef]
- Rico, C.M.; Lee, S.C.; Rubenecia, R.; Mukherjee, A.; Hong, J. Cerium oxide nanoparticles impact yield and modify nutritional parameters in wheat (Triticum aestivum L.). J. Agric. Food Chem. 2014, 62, 9669–9675. [Google Scholar] [CrossRef]
- Doolette, C.L.; Read, T.L.; Howell, N.R.; Cresswell, T.; Lombi, E. Zinc from foliar-applied nanoparticle fertiliser is translocated to wheat grain: A 65Zn radiolabelled translocation study comparing conventional and novel foliar fertilisers. Sci. Total Environ. 2020, 749, 142369. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.P.; Joshi, A.; Rajput, V.D.; Singh, M. Nanofertilizer Possibilities for Healthy Soil, Water, and Food in Future: An Overview. Front. Plant Sci. 2022, 13, 865048. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, T.; Kundu, S.; Rao, A.S. Zinc Delivery to Plants through Seed Coating with Nano Zinc Oxide Particles. J. Plant Nutr. 2015, 39, 136–146. [Google Scholar] [CrossRef]
- Adrees, M.; Khan, Z.S.; Hafeez, M.; Rizwan, M.; Hussain, K. Foliar exposure of zinc oxide nanoparticles improved the growth of wheat (Triticum aestivum L.) and decreased cadmium concentration in grains under simultaneous Cd and water deficient stress. Ecotoxicol. Environ. Saf. 2020, 208, 111627. [Google Scholar] [CrossRef] [PubMed]
- Tarafdar, J.C.; Raliya, R.; Mahawar, H.; Rathore, I. Development of Zinc Nanofertilizer to Enhance Crop Production in Pearl Millet (Pennisetum americanum). Agric. Res. 2014, 3, 257–262. [Google Scholar] [CrossRef]
- Liu, D.Y.; Zhang, W.; Liu, Y.M.; Chen, X.P.; Zou, C.Q. Soil Application of Zinc Fertilizer Increases Maize Yield by Enhancing the Kernel Number and Kernel Weight of Inferior Grains. Front. Plant Sci. 2020, 11, 188. [Google Scholar] [CrossRef]
- Elshayb, O.M.; Farroh, K.Y.; Amin, H.E.; Atta, A.M. Green Synthesis of Zinc Oxide Nanoparticles: Fortification for Rice Grain Yield and Nutrients Uptake Enhancement. Molecules 2021, 26, 584. [Google Scholar] [CrossRef]
- Pandey, A.C.; Sanjay, S.S.; Yadav, R.S. Application of ZnO nanoparticles in influencing the growth rate of Cicer arietinum. J. Exp. Nanosci. 2010, 5, 488–497. [Google Scholar] [CrossRef]
- Munir, T.; Rizwan, M.; Kashif, M.; Shahzad, A.; Ali, S. Effect of zinc oxide nanoparticles on the growth and Zn uptake in wheat (Triticum aestivum L.) by seed priming method. Dig. J. Nanomater. Biostruct. 2018, 13, 315–323. [Google Scholar]
- Saleem, I.; Javid, S.; Ehsan, S.; Niaz, A.; Bibi, F. Improvement of Wheat Grain Zinc and Zinc Daily Intake by Biofortification with Zinc. Int. J. Plant Soil Sci. 2015, 8, 1–6. [Google Scholar] [CrossRef]
- Hart, J.J.; Norvell, W.A.; Welch, R.M.; Sullivan, L.A.; Kochian, L.V. Characterization of zinc uptake, binding, and translocation in intact seedlings of bread and durum wheat cultivars. Plant Physiol. 1998, 118, 219–226. [Google Scholar] [CrossRef]
- Tsonev, T.; Lidon, F.J.C. Zinc in plants—An overview. Emir. J. Food Agric. 2012, 24, 322–333. [Google Scholar]
- Muthukumararaja, T.; Sriramachandrasekharan, M.V. Critical limit of zinc for rice soils of Veeranam command area, Tamilnadu, India. J. Agric. Biol. Sci. 2012, 7, 23–34. [Google Scholar]
- Kulhare, P.; Tagore, G.; Sharma, G. Effect of foliar spray and sources of zinc on yield, zinc content and uptake by rice grown in a vertisol of central India. Int. J. Chem. Stud. 2017, 5, 35–38. [Google Scholar]

| Parameters | Unit | Value |
|---|---|---|
| Texture | - | Silty loam |
| pH (1:5 H2O) | - | 7.7 |
| EC (1:5) | (dS m−1) | 0.467 |
| OM | (%) | 1.13 |
| Lime (CaCO3) | (%) | 9.4 |
| Total soil N | (%) | 0.22 |
| AB-DTPA Ext. Zn | mg kg−1 | 0.81 |
| AB-DTPA Ext. P | mg kg−1 | 5.77 |
| AB-DTPA Ext. K | mg kg−1 | 102.6 |
| Treatments | Plant Height | Spike Length | Spikelets Spike−1 | Grain Spike−1 |
|---|---|---|---|---|
| Control | 78 b | 10.5 | 17.7 c | 39.3 d |
| ZnSO4(f) | 86 ab | 11.3 | 18.7 bc | 47.0 b |
| ZnSO4(s) | 92 a | 11.2 | 19.3 abc | 51.0 ab |
| ZnSO4(p) | 88 ab | 11.7 | 19.3 abc | 44.3 c |
| ZnONF(f) | 82 ab | 11.0 | 19.0 abc | 43.7 c |
| ZnONF(c) | 92 a | 12.0 | 20.7 a | 52.3 a |
| ZnONF(p) | 94 a | 11.8 | 20.7 a | 52.0 a |
| ½ ZnSO4(s) + ZnONF(f) | 85 ab | 11.3 | 20.7 a | 49.0 b |
| ½ ZnSO4(s) + ZnONF(c) | 92 a | 12.1 | 20.0 ab | 42.0 c |
| ½ ZnSO4(s) + ZnONF(p) | 92 a | 11.2 | 18.7 bc | 50.3 ab |
| LSD (p ≤ 0.05) | 12 | ns | 1.8 | 2.6 |
| Treatments | Zn Concentration (µg g−1) | |||
|---|---|---|---|---|
| Grain | Leaf | Root | Stem | |
| Control | 20.8 d | 17.8 d | 21.5 f | 11.1 d |
| ZnSO4(f) | 29.0 ab | 21.2 abc | 53.5 a | 17.5 b |
| ZnSO4(s) | 25.6 bc | 20.8 abc | 26.3 ef | 15.0 c |
| ZnSO4(p) | 26.0 bc | 18.8 cd | 36.0 bc | 16.6 bc |
| ZnONF(f) | 29.3 ab | 18.9 cd | 34.1 cd | 11.7 d |
| ZnONF(c) | 26.1 bc | 20.0 bcd | 37.4 bc | 16.6 bc |
| ZnONF(p) | 25.7 bc | 19.1 cd | 29.3 de | 20.4 a |
| ½ ZnSO4(s) + ZnONF(f) | 32.9 a | 18.9 cd | 38.4 bc | 12.9 d |
| ½ ZnSO4(s) + ZnONF(c) | 25.7 bc | 22.4 ab | 28.0 e | 16.5 bc |
| ½ ZnSO4(s) + ZnONF(p) | 31.1 a | 23.3 a | 40.8 b | 20.2 a |
| LSD (p ≤ 0.05) | 3.9 | 2.7 | 5.4 | 2.1 |
| Treatments | Zn Uptake (µg pot−1) | ZUE (%) | ||||
|---|---|---|---|---|---|---|
| Grain | Leaf | Root | Stem | Total Uptake | ||
| Control | 148 d | 84 b | 75 d | 119 d | 427 d | |
| ZnSO4(f) | 242 bcd | 127 ab | 188 a | 220 bc | 776 ab | 3 c |
| ZnSO4(s) | 233 bcd | 114 ab | 76 d | 211 c | 633 bcd | 2 c |
| ZnSO4(p) | 211 cd | 105 ab | 103 cd | 224 bc | 642 bcd | 2 c |
| ZnONF(f) | 215 cd | 84 b | 91 cd | 126 d | 516 cd | 7 c |
| ZnONF(c) | 232 bcd | 123 ab | 132 abcd | 235 bc | 722 abc | 23 b |
| ZnONF(p) | 283 abc | 111 ab | 113 bcd | 340 a | 847 ab | 34 a |
| ½ ZnSO4(s) + ZnONF(f) | 380 a | 129 ab | 139 abc | 190 cd | 838 ab | 5 c |
| ½ ZnSO4(s) + ZnONF(c) | 252 bcd | 145 a | 87 cd | 247 bc | 731 abc | 4 c |
| ½ ZnSO4(s) + ZnONF(p) | 334 ab | 139 a | 162 ab | 291 ab | 926 a | 6 c |
| LSD (p ≤ 0.05) | 106 | 52 | 58 | 75 | 259 | 8.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, W.; Zou, Z.; Awais, M.; Munsif, F.; Khan, A.; Nepal, J.; Ahmad, M.; Akbar, S.; Ahmad, I.; Khan, M.S.; et al. Seed-Primed and Foliar Oxozinc Nanofiber Application Increased Wheat Production and Zn Biofortification in Calcareous-Alkaline Soil. Agronomy 2023, 13, 400. https://doi.org/10.3390/agronomy13020400
Ahmad W, Zou Z, Awais M, Munsif F, Khan A, Nepal J, Ahmad M, Akbar S, Ahmad I, Khan MS, et al. Seed-Primed and Foliar Oxozinc Nanofiber Application Increased Wheat Production and Zn Biofortification in Calcareous-Alkaline Soil. Agronomy. 2023; 13(2):400. https://doi.org/10.3390/agronomy13020400
Chicago/Turabian StyleAhmad, Wiqar, Zhiyou Zou, Muhammad Awais, Fazal Munsif, Aziz Khan, Jaya Nepal, Masood Ahmad, Sultan Akbar, Ijaz Ahmad, Muhammad Shahid Khan, and et al. 2023. "Seed-Primed and Foliar Oxozinc Nanofiber Application Increased Wheat Production and Zn Biofortification in Calcareous-Alkaline Soil" Agronomy 13, no. 2: 400. https://doi.org/10.3390/agronomy13020400
APA StyleAhmad, W., Zou, Z., Awais, M., Munsif, F., Khan, A., Nepal, J., Ahmad, M., Akbar, S., Ahmad, I., Khan, M. S., Qamar, Z., & Khan, H. (2023). Seed-Primed and Foliar Oxozinc Nanofiber Application Increased Wheat Production and Zn Biofortification in Calcareous-Alkaline Soil. Agronomy, 13(2), 400. https://doi.org/10.3390/agronomy13020400







