Abstract
Rapid global modernization, urbanization, and industrialization have accelerated the release of heavy metals, causing soil pollution. These highly noxious environmental pollutants induce oxidative stress in plants via stimulation of the production of reactive oxygen species (ROS). Thioredoxin (Trxs) is a highly conserved disulfide reductase that plays a crucial role in intracellular redox homeostasis in both eukaryotes and prokaryotes. Herein, the presence of heavy metals highly upregulated LmTrxh2 transcription in Lobularia maritima seedlings and its overexpression-conferred tolerance to Cd, Cu, Mn, and Zn in Saccharomyces cerevisiae. In addition, LmTrxh2-overexpressing tobacco plants had higher seedling survival rates than non-transgenic plants (NT), with enhanced root length and biomass production and reduced ROS accumulation, following Cd and Cu stress. These plants also accumulated more Cd, Cu, and Mn than the NT plants. Moreover, LmTrxh2 overexpression stimulated the transcription of genes encoding metallothioneins (Met-1, Met-2, Met-3, and Met-4), a copper transport protein, a Snakin/GASA protein (Snakin-2), and ROS-scavenging enzymes (SOD, APX1, and CAT), which might contribute to heavy metal tolerance in tobacco plants. These results suggest that LmTrxh2 overexpression helps to improve heavy metal tolerance by stimulating antioxidant capacities and the expression of several stress-responsive genes in plants.
1. Introduction
The environmental pollution of heavy metals is becoming an increasingly serious problem worldwide, owing to its adverse effects on organisms and humans. Globally, heavy metals have been found in elevated concentrations in soil. Generally, some elements, such as Cu, Fe, Mn, Mo, and Zn, are essential to plants, whereas other elements, such as Ag, As, Cd, Hg, Pb, and Sb, have no role in plant growth and development. No matter their importance, their accumulation hinders plant development [1] and may displace other elements, thereby affecting the plant’s molecular and physiological mechanisms. In addition, plants with high concentrations of heavy metals show signs of genotoxic effects, explained by an increase in the ratio of DNA damage and chromosome aberrations [2,3]. As an essential element for plants, Cu is involved in carbon assimilation and ATP synthesis, and in vegetative growth, development, and proliferation [4]. However, high levels of Cu can be fatal to plants. It can cause significant damage to membranes and macromolecules and can be harmful to the DNA and diverse metabolic processes [4]. The effects of Cu toxicity include stunted root growth, plant growth inhibition, chlorosis, and ion leakage. Moreover, Cu toxicity can adversely affect germination, the root number, and seedling length [4]. Similarly, Cd toxicity is usually associated with stunted growth, and Cd stress limits photosynthesis, lowers chlorophyll content, changes plant water status, and interferes with mineral absorption [5]. Additionally, Cd prevents the absorption of Ca, P, K, Mg, and water, and inhibits the activity of nitrate reductase, which decreases nitrate translocation and absorption. Similar to Cu, Zn is an essential element for plant growth. It is involved in many plant metabolic processes as a cofactor for many enzymatic reactions, pollen formation, and resistance against many pathogens. Nonetheless, excessive Zn accumulation results in chlorosis in young leaves, stunted growth, changes in metabolic processes, and oxidative damage [6]. Furthermore, similar to Cu and Mn, Zn poisoning promotes the accumulation of other heavy metals in the roots and shoots of plants, and causes phosphorus deficiencies [6].
The increased formation of ROS is one of the earliest biochemical responses to heavy metal stress [7]. High ROS accumulation can result in lipid peroxidation of the cell membrane, which consequently leads to cell death [7,8]. An imbalance between ROS production and removal by the antioxidant system induces oxidative stress in cells. Both eukaryotes and prokaryotes have antioxidant systems that can protect against the damages caused by oxidative stress via the synthesis of enzymatic and non-enzymatic antioxidants [9].
Metals/metalloids affect the molecular and physiological mechanisms of plants by interacting with functional proteins, such as thiol, imidazole, and carboxyl groups, and altering their functionality and shape through misfolding and aggregation [10]. The thiol–disulfide redox reaction is a widely distributed post-translational modification that is critical for the regulation of plant cell metabolism [11,12]. Thioredoxins (Trxs) are relatively small, ubiquitous proteins with two redox-active cysteine residues that are separated by a pair of amino acids (WC[G/P]PC motif) in their WCG/PPC active site [13]. Additionally, Trxs assists in protein folding and activating/deactivating target proteins by catalyzing redox reactions [13]. These proteins are present in all species, from prokaryotes to multicellular eukaryotes. In plants, six types of Trx (h, o, f, m, x, y) have been reported. They differ in their amino acid sequences and subcellular locations [14]. These proteins play crucial roles in regulating hormone synthesis, enzyme activation, translation, metabolism, and the response to abiotic stress [15]. Recent advancements in molecular biology have assisted in discovering several functions of these Trx genes in plants in response to oxidative stress [16]. The expression of these genes is induced in response to ROS. For instance, the expression level of Arabidopsis Trxh5 genes was enhanced under conditions of oxidative stress [17]. Additionally, there are Trx regulated enzymes, such as catalase (CAT) and superoxide dismutase (SOD), and germin-like proteins [18]. Furthermore, in vivo studies have reported that Trx can protect plants from oxidative damage by acting as an antioxidant. Based on these results, we can postulate that plant Trx may play an important role in protecting plants against oxidative damage.
Previously, Ben Saad et al. [19] isolated and characterized the first Trxh2 gene from Lobularia maritima (Brassicaceae), LmTrxh2, which was induced by abiotic stress in transgenic tobacco plants. Its overexpression resulted in resistance to salt and osmotic stress. In the present study, we investigated the role of LmTrxh2 in developing a response and tolerance to heavy metals in L. maritima. Analysis of the expression profile of the LmTrxh2 gene in L. maritima showed that LmTrxh2 was rapidly regulated by heavy metals, such as Cd, Cu, Mn, and Zn. Furthermore, analysis in both yeast and transgenic tobacco plants revealed that LmTrxh2 enhanced the tolerance of these eukaryotic systems to heavy metals. Moreover, tobacco transgenic lines that harbor the LmTrxh2 gene exhibited enhanced resistance against phytotoxic concentrations of Cd and Cu via the stimulation of ROS-scavenging and metal-binding mechanisms. LmTrxh2-mediated metal tolerance may involve other detoxification mechanisms, possibly through its putative role in regulating gene expression.
2. Materials and Methods
2.1. Plant Growth and Metal Stress Treatments
The seeds of L. maritima were harvested from salt marshes near Chebba, Mahdia in Tunisia. They were surface sterilized according to the method previously described by Ben Saad et al. [20]. They were then germinated in a nutrient solution as described elsewhere [21] and kept under high humidity conditions at 25 °C with a 16 h light/8 h dark cycle. Later, the seeds were left to grow for 4 weeks. Finally, in order to induce metal stress, the following solutions were added to the hydroponic nutrition medium: 100 μM CdCl2, 1 mM MnCl2, 500 μM ZnSO4, or 50 μM CuSO4. The concentrations of heavy metals used in the present study were chosen based on Ben Saad et al. [20]. The plant tissues (leaf and root) were collected at indicated time points (6 h, 12 h, 24 h, 48 h, and 72 h), frozen in liquid nitrogen, and stored at −80 °C for RNA extraction.
Three homozygous LmTrxh2-transformed lines with different gene levels (Tr1, Tr2, and Tr3), constitutively expressing the LmTrxh2 coding sequence under the control of the CaMV35S promoter [19], were evaluated together with NT plants. The seeds from the two groups were surface sterilized [19] and germinated on solid MS medium supplemented with or without 100 μM CdCl2, or 50 μM CuSO4, and were then left in the growth chamber under controlled conditions (22 °C and a 18/6 h light/dark photoperiod). The germination rates were measured twenty days later. In order to study the effects of metal stress on the plant’s growth, 10-day-old seedlings were transferred to MS agar medium, where 100 μM CdCl2, or 50 μM CuSO4 was added, and incubated under the same conditions described above. The seedlings were left to grow for two weeks. At the end of this period, the plants were photographed, and root length and dry weight were measured. The tissues were also collected for further analyses.
2.2. Stress-Response Expression of LmTrxh2 and Cd-Tolerance Genes
In order to analyze LmTrxh2 expression under different stress treatments in L. maritima, a quantitative real-time PCR (RT-qPCR) assay was conducted, with qLmTrxh2-F and qLmTrxh2-R as forward and reverse primers, respectively (Table S1). Then, the expression of nine Cd-tolerance genes (Table S1) was investigated using homozygous transgenic lines and NT tobacco plants collected from both the control and 100 μM CdCl2 groups after 24 h.
Isolation of the total RNA was effectuated using either L. maritima or tobacco plants with the TRIzol reagent (Invitrogen, Waltham, MA, USA). The synthesis of first-strand cDNA and RT-qPCR was carried out as previously described [22]. In order to check the primer specificity, a melting curve analysis is usually carried out using the UBQ10 gene (UBQ10-F and UBQ10-R) and the L25 ribosomal protein (L25-F and L25-R) as internal references for L. maritima and tobacco plants, respectively (Table S1). The relative expression levels were calculated according to the formula 2−ΔΔCT. Three biological replicates were analyzed.
2.3. Test of the Resistance of a Transgenic Yeast Harboring the LmTrxh2 Gene to Heavy Metals
The full-length cDNA of the LmTrxh2 gene was cloned into the pYES2 vector (Invitrogen, Karlsruhe, Germany) using EcoRI/XbaI restriction enzymes, downstream of the galactose-inducible promoter GAL1 (Figure S1), and was used to transform the W303 S. cerevisiae strain (MATa ade2 ura3 leu2 his3 trp1). The empty pYES2 vector was considered the control and was mobilized into W303 cells. The standard PEG lithium acetate method was used to mobilize the four recombinant plasmids and the empty pYES2 vector (EV) into the W303 strain. The entire protocol was performed as described before [23].
2.4. NBT and DBA Staining Superoxide Anions (O2−) and Hydrogen Peroxide (H2O2)
The presence of O2− and H2O2 was determined using NBT and DAB histochemical staining as described previously [24]. Briefly, a group of treated seedlings were stained with DAB or NBT solutions. Pictures of the stained leaves were then taken using an Olympus W120 digital still camera. The experiment was repeated three times for each line.
2.5. Determination of Oxidative Stress in LmTrxh2 Transgenic Tobacco
The determination of malondialdehyde (MDA) and (H2O2) content in the treated transgenic tobacco seedlings (Tr1, Tr2, and Tr3) and the NT plants were used to analyze oxidative stress. The production of MDA and H2O2 contents in transgenic tobacco and NT leaves were determined by the method described before [25]. Analyses were repeated three times for each line.
2.6. Statistical Analyses
The presented data were each the result of at least three repetitions of three biological replicates. The tests used for the statistical analyses were the one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test to compare the means of the NT and transgenic plants that were tested for each treatment. Statistical analyses were carried out using the SPSS 19 statistical package software (SPSS Ltd., Woking, UK). The difference was considered significant when p < 0.05. Differences between the means were marked using different lowercase letters on the figures.
3. Results
3.1. Expression Profile of the LmTrxh2 Gene Following Heavy Metal Exposure in L. maritima
The expression patterns of the LmTrxh2 gene, in response to metal stress (Cd, Cu, Zn, and Mn), in the leaves and roots tissues at various time points were investigated using RT-qPCR. No significant differences in LmTrxh2 expression were found under control conditions (Figure 1). The induction of LmTrxh2 expression was perceived in both the leaves and root tissues after heavy metal exposure (Figure 1). The LmTrxh2 gene was moderately upregulated (1- to 2-fold upregulation) by the different above-mentioned heavy metal treatments after 6 h of exposure. At 24 h, LmTrxh2 gene expression was highly upregulated by ZnSO4 (4- to 6-fold upregulation) and was moderately upregulated by CdCl2 (2- to 3-fold upregulation) and CuSO4 and MnCl2 (3- to 4-fold upregulation) in the leaves and roots tissues, respectively. However, LmTrxh2 gene expression showed the highest response to Cd, Mn, and Zn treatments in the roots and leaves after 48 h of treatment (Figure 1). After 72 h of exposure to Cu, a substantial increase in LmTrxh2 gene expression was recorded in the leaves, but not in the roots. Hence, these data show that LmTrxh2 gene expression was induced by the heavy metals used in the present study. This observation emphasizes the role of this gene in the adaptation of L. maritima to its climate.
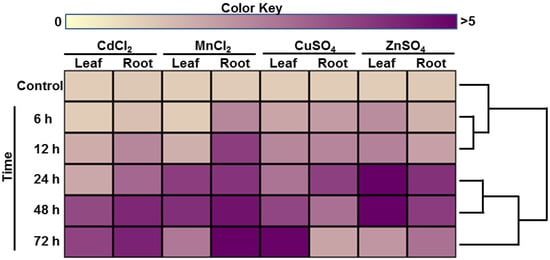
Figure 1.
Heatmap of the LmTrxh2 gene expression profile in L. maritima subjected to different treatments. The dendrogram was constructed by hierarchical clustering of the expression values of the LmTrxh2 gene using the Euclidian distance metric. The data represent the means of three independent experiments.
3.2. LmTrxh2 Gene Confers Heavy Metal Tolerance to Yeast Cells
The upregulation of LmTrxh2 gene expression in response to heavy metal stresses led to the investigation of its role in protecting cells against environmental stress. Since transgenic yeast is widely used as a simple and rapid eukaryotic system that allows several stress-related genes to be investigated under driven conditions, transgenic yeast harboring the pYES2-LmTrxh2 vector was used in this study, where LmTrxh2 gene expression was controlled by the galactose-inducible promoter.
Under controlled growth conditions, no significant differences in growth were found between the pYES2-LmTrxh2 construct and the empty vector pYES2 (EV) (Figure 2). Under Cd, Cu, Zn, and Mn treatments, the yeast cells overexpressing the LmTrxh2 gene exhibited enhanced growth compared to the control (Figure 2). Furthermore, under Cd, Cu, and Mn stress, the yeast cells expressing the LmTrxh2 gene exhibited better growth than the cells transformed with the EV. On the contrary, under Zn treatment, the yeast cells overexpressing the LmTrxh2 gene showed moderate growth enhancement compared with the EV (Figure 2).

Figure 2.
Expression of the LmTrxh2 gene confers the heavy metals stress tolerance of LmTrxh2-transformed yeast.
3.3. Transgenic Tobacco Plants Harboring the LmTrxh2 Gene Exhibit Tolerance to Heavy Metal Stress
We utilized three previously established homozygous LmTrxh2 tobacco lines (Tr1, Tr2, and Tr3) constitutively expressing the LmTrxh2 gene [19]. Their tolerances in response to heavy metals (Cd and Cu) were investigated. The seeds of the Tr1, Tr2, Tr3, and NT lines were germinated in vitro on MS media (control without metals) or media containing Cd (100 μM CdCl2) or Cu (50 μM CuSO4).
As illustrated in Figure 3A, the three transgenic lines were phenotypically characterized by growth that was more vigorous with greener leaves, and the transgenic lines had significantly higher germination rates compared to the NT plants under Cd or Cu treatments after 2 weeks of growth (Figure 3B). As shown in Figure 4A, the transgenic lines showed better growth parameters compared to the NT plants. Dry weight (Figure 4B) and root length (Figure 4C) were significantly higher in transgenic lines (Tr1, Tr2, and Tr3) than in the NT plants. It is also worth mentioning that the root system of the LmTrxh2 overexpression lines was very well developed and significantly longer in the presence of CdCl2 (~2 cm for NT and ~8 cm for the LmTrxh2 transgenic lines) and CuSO4 (~2 cm for NT and ~7 cm for the LmTrxh2 transgenic lines). Indeed, its biomass was 3-fold and 2.5-fold higher compared to the roots of the NT plants when grown in the presence of Cd and Cu, respectively (Figure 4B,C).
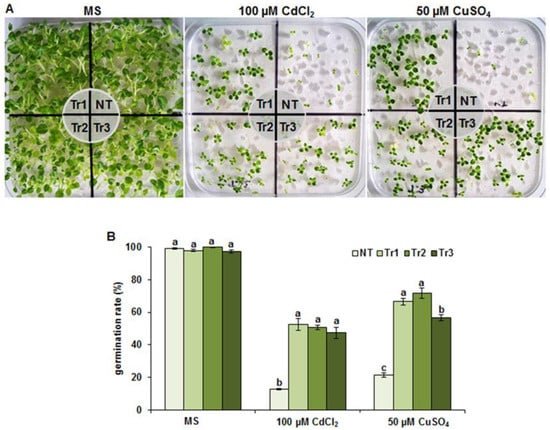
Figure 3.
Effect of Cd (100 µmM) and Cu (50 µmM) stress on seed germination and plant phenotype in the transgenic tobacco lines (Tr1, Tr2, and Tr3) overexpressing LmTrxh2. (A) Twenty days after seed germination; (B) the seed germination rates of the transgenic and NT plants. The means denoted by the same letter did not differ significantly at p < 0.05.

Figure 4.
Response of LmTrxh2 tobacco lines and NT plants to heavy metal (Cd and Cu) stress conditions. (A) The phenotype of LmTrxh2 transgenic lines, and the dry weight (B) and root length (C) of the NT and transgenic tobacco lines grown under control and stress conditions for 15 days. The means denoted by the same letter did not differ significantly at p < 0.05.
3.4. Transgenic Plants Overexpressing LmTrxh2 Showed Higher Cd and Cu Accumulation
The accumulation levels of Cd and Cu in the LmTrxh2 transgenic lines were evaluated in order to determine whether the increase in tolerance to heavy metals in the LmTrxh2 tobacco seedling lines was caused by the phenomenon of metal exclusion. When left to grow for 15 days with Cd and Cu added to the medium, the LmTrxh2-overexpressing lines had a higher accumulation of metal ions compared to the NT plants (Figure 5A,B). These findings demonstrate that the increased tolerance to Cd and Cu exhibited by the LmTrxh2 transgenic seedlings was due to their survival capacity rather than their metal exclusion capacity.
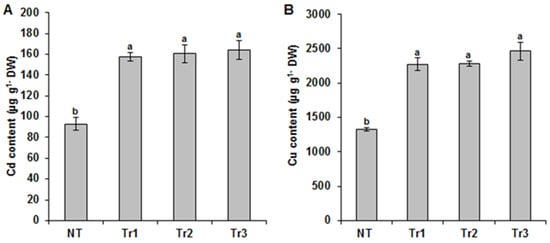
Figure 5.
Cd (A) and Cu (B) accumulation in LmTrxh2-overexpressing tobacco lines and NT plants. Plants were cultivated for 15 days on MS medium supplemented with or without Cd or Cu. The means denoted by the same letter were not significantly different at p < 0.05.
3.5. LmTrxh2 Transgenic Tobacco Lines Accumulated Less MDA and H2O2
One major drawback to metal stress exposure was the generation of ROS and the stimulation of oxidative stress, which consequently results in damage to the different cell components. Therefore, evaluation of the accumulation of H2O2 and the content of lipid peroxidation in LmTrxh2 transgenic seedlings grown under normal growth or subjected to metal stress (Cd or Cu) was performed. In order to detect the production of O2− and H2O2, the NBT and DAB staining methods were used.
As shown in Figure 6A, under normal conditions, no significant differences were found in the accumulations of O2− and H2O2 in the leaves of LmTrxh2 transgenic tobacco compared to those of the NT plants. However, under Cd or Cu treatment, the NBT and DAB coloration intensifies in all the lines. The LmTrxh2-overexpressing lines that were exposed to Cd or Cu led to less accumulation of O2− and H2O2 compared to the NT plants (Figure 6A). Additionally, we measured the production of MDA and H2O2 after exposure of the NT and transgenic tobacco lines to different heavy metals. According to Figure 6B,C, exposing the NT and transgenic lines to Cd or Cu led to an increase in MDA and H2O2 accumulation for all the tested lines. Furthermore, the increment was notably higher for the NT plants compared to the LmTrxh2 transgenic lines (Tr1, Tr2, and Tr3). Hence, from these data, it can be concluded that the LmTrxh2-overexpressing lines respond better to the oxidative damage caused by heavy metal exposure compared to the NT plants.

Figure 6.
Biochemical analysis of the NT and transgenic lines overexpressing the LmTrxh2 gene under different heavy metal stress conditions. (A) The revelation of O2− localization by NBT and H2O2 localization by DAB staining. MDA (B) and H2O2 (C) contents in the NT and transgenic lines that were exposed to MS liquid medium supplemented with or without 100 µmM Cd or 50 µmM Cu. The means denoted by the same letter did not differ significantly at p < 0.05.
3.6. Expression Analysis of Cd-Tolerance Genes in Overexpressed LmTrxh2 Tobacco under Heavy Metal Stress
In order to further investigate the potential regulatory role of LmTrxh2 in heavy metal tolerance, several tobacco genes involved in Cd detoxification were examined. The genes encoding metallothioneins (Met-1, Met-2, Met-3, and Met-4), a copper transport protein (Cu transport), a Snakin/GASA protein (Snakin-2), and ROS-scavenging genes (SOD, CAT, and APX1) were analyzed using RT-qPCR. As shown in Figure 7, all the examined stress-related genes showed significantly higher transcription levels in transgenic LmTrxh2 tobacco subjected to metal stress after Cd exposure for 24 h. These findings demonstrate that the Cd-tolerance-related genes may be positively regulated by the LmTrxh2 gene.
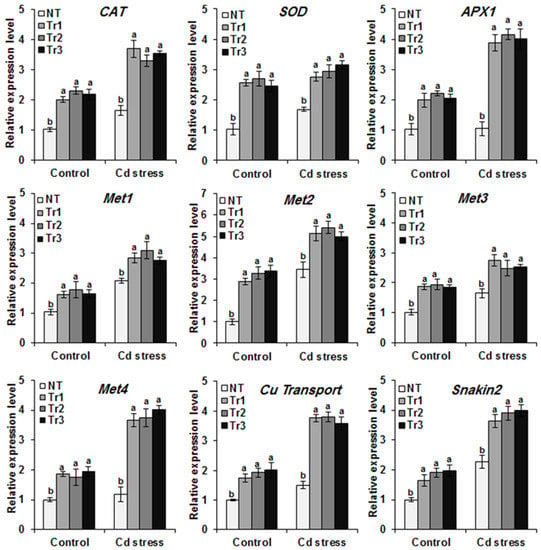
Figure 7.
Expression of the pattern of Cd-tolerance/detoxification genes in NT and transgenic plants (Tr1, Tr2, and Tr3) under MS (control), Cd, or Cu stress conditions. The means denoted by the same letter did not differ significantly at p < 0.05.
4. Discussion
The contamination of soil by heavy metals is a challenging issue globally. Industrial activities, wastewater irrigation, insecticides, and fertilizers release high concentrations of heavy metals into the environment [26]. Several kinds of research have emphasized the importance of small quantities of heavy metals (Fe, Mn, Zn, Cu, Mo, and Co) that benefit plant growth and development. In contrast, the presence of heavy metals in excessive quantities in soil and water can cause the inactivation of several biomolecules, decrease protein levels, and compete with other essential metal ions. This can become toxic and can cause major health problems in humans [1]. Heavy metals such as Cd and trace elements such as Cu are phytotoxic partly because they interfere with the antioxidant defense system and create oxidative stress, which indirectly produces oxygen-free radicals [26]. Tolerance to heavy metals is a complex process that is controlled by several genes [27]. Therefore, the improvement of heavy metal tolerance using engineering approaches requires the investigation of genes that are directly involved in the signaling and regulatory processes of protein- and metabolite-mediated heavy metal tolerance [28,29]. Several organisms have developed a defense antioxidant system in which Trx is a crucial component for protecting against oxidative damage [30].
This study demonstrated that the LmTrxh2 gene in L. maritima is induced by different heavy metals (Cd, Cu, Zn, and Mn), and when it is overexpressed in yeast and transgenic tobacco plants, an enhanced tolerance to Cd and Cu is observed. These findings demonstrate that LmTrxh2 is a potential regulator of Cd and Cu stress tolerance. Ben Saad et al. [19] suggested that overexpression of the LmTrxh2 gene in tobacco conferred tolerance to salt and osmotic stress. This suggests that this gene enhances plants’ responses to abiotic stress, which is similar to reports regarding the Trxhs from other plants [31,32,33]. Previous reports have shown the involvement of Trxhs in heavy metal tolerance in non-halophyte plants. However, studies on the role of the L. maritima Trxh family members in heavy metal tolerance are limited. In this study, we provided evidence that the expression of LmTrxh2 is upregulated by different heavy metals in the leaves and roots of L. maritima. Chibani et al. [13] reported that the expression of thioredoxins (Trx) m and h in Chlamydomonas reinhardtii were highly regulated by heavy metals (Cd and Hg) in the cells. Several investigations have reported that Trxl is abundant, stable, and widely expressed in the cells of different tissues [34,35,36]. Meng et al. [16] detected PeTrxl mRNA from P. esculenta in all the plant tissues examined, which emphasizes the role of this gene in the detoxification of P. esculenta in the early stages of Cd stress. However, little information is available on the reactivity of the other Trxs in plants upon exposure to metal ions. The overexpression of TRX-h confers increased tolerance to Al in barley roots [37]. In has been suggested that TRX proteins act by reducing free Cu ions by regulating the binding capacity of the reduced form of TRX-Cu [18].
In order to better understand the involvement of the LmTrxh2 gene in heavy metal tolerance, we overexpressed it in yeast. The yeast system is routinely used for the functional characterization of plant genes and for the validation of the different genes implicated in tolerance to abiotic stress in plants. The LmTrxh2 gene was previously identified in L. maritima by Ben Saad et al. [19], and via the current study, we found that it conferred high tolerance to heavy metal stress in transgenic tobacco plants. Additionally, these findings are similar to those obtained when using the original W303 system, showing that LmTrxh2 provided resistance for yeast in solid media to Cu, Cd, Mn, and Zn. Forty-eight candidate genes from S. alfredii that are involved in Cd tolerance were investigated [38]. The results showed that all the identified genes encoded the thioredoxin protein from different families.
In A. thaliana, the binding of metals by the cysteines present in the protein sequence was proposed as the mechanism by which heavy metal ATPases (HMAs) confer resistance to Zn and Cd [39]. The overexpression of HMAs in A. thaliana and tobacco showed that these enzymes play a major role in Cd hyper-accumulation and hyper-tolerance due to sequestration in the vacuole and to Zn-distribution, based on their identity [39]. In a recent in silico prediction study of the LmTrxh2 structure conducted by Ben Saad et al. [19], it was reported that the catalytic Trx motif, which encompasses two catalytic cysteine residues (59 and 62), could potentially be implicated in metal binding. Therefore, the presented results suggest that LmTrxh2 contributes to metal tolerance by enhanced metal sequestration (Figure 8).

Figure 8.
A hypothetical model for LmTrxh2-mediated heavy metal stress tolerance.
In contrast to the localization of HMAs in several cell compartments (plasma and vacuolar membranes) [39], the results from our lab have shown previously that the expression of LmTrxh2 was exclusive to the plasma membrane [19], which makes it unlikely that this gene is directly involved in metal sequestration. Therefore, the LmTrxh2 gene could have conferred tolerance to heavy metals due to its high capacity to cope with the internal toxic effects of metals. In addition, the expression of the thioredoxin gene and several genes encoding Cys-rich proteins were previously reported to be enhanced by aluminum stress [40,41], which may suggest that thioredoxins could have a role as an antioxidant. For instance, the overexpression of the Phalaris coerulescens thioredoxin gene (PTrx) was shown to have a protective effect against oxidative damage in barley roots through an increase in the antioxidant activity generated by AlCl3 during germination [37]. The common result of exposure to heavy metals in plants is the production of ROS, which are known to cause damage [27]. O2−, H2O2, and MDA are the major indicators of oxidative stress. The overexpression of LmTrxh2 decreased the levels of O2−, H2O2, and MDA in the transgenic lines compared to NT. This suggests that the LmTrxh2-overexpressing lines better prevent the oxidative damage caused by heavy metal exposure. Similarly, in the present study, the overexpression of LmTrxh2 in tobacco seedlings induced the expression of several genes that were previously reported to be a part of the response of tobacco to Cd [42]. Four of these genes (Met-1, Met-2, Met-3, and Met-4) that encode MTs, a family of Cys-rich proteins, had a high ability to bind mono- or bivalent metal ions, especially Cu, Zn, and Cd, using the thiol group of the cysteine residues. This could have given these genes the advantage of being implicated in the detoxification of toxic metals and the protection against oxidative damage. Two other genes (Snakin-2 and Cu-transport) were reported to be implicated in the maintenance of redox homeostasis through their involvement in resistance to Cd stress [42,43]. They were found to be expressed at a higher level in all the LmTrxh2 lines compared with NT plants, with further activation after Cd treatment. In fact, due to the phytochelatin activity, Cu is partly sequestered, which causes Cu deficiency, the inactivation of several proteins involved in Cu transport, and maintains Cu homeostasis [44]. Finally, the mRNA transcripts of genes encoding antioxidant enzymes (APX1, CAT, and SOD) were expressed at higher levels in all the LmTrxh2-overexpressing lines. This suggests that the tolerance to metal stress shown in LmTrxh2-transgenic plants is related to the attenuation of ROS accumulation and the maintenance of redox homeostasis via improved antioxidant enzymes. Due to their impact in controlling ROS levels, the CAT- and SOD-encoding genes were overexpressed in transgenic plants and conferred stronger tolerance against heavy metals [45]. It has been shown that high levels of antioxidant enzymes increase tolerance to heavy metal stress conditions [46]. Based on these findings, LmTrxh2 expression could enhance the capacity of the antioxidant enzymes to protect the plant cells against oxidative damage by scavenging ROS, which in turn helps the plants tolerate heavy metals stress. LmTrxh2-mediated metal tolerance further implicates metal-detoxification-specific mechanisms through their potential role in regulating the expression of the genes that encode MTs.
5. Conclusions
In conclusion, new insights into the importance of LmTrxh2, a thioredoxin protein-encoding gene, from L. maritima for heavy metal (Cd and Cu) stress tolerance have been provided here. The neutralization of ROS is found to be one of the most effective plant mechanisms to defend themselves against heavy metals and to avoid cell damage. Future research is necessary in order to understand the physiological functions of LmTrxh2 regulation by heavy metals. We have provided a new understanding of how LmTrxh2 improves heavy metal tolerance in plants, which could be used for the phytoremediation of heavy-metal-contaminated soils.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agronomy13020399/s1. Table S1: Sequences of primers used in RT-qPCR analysis. Figure S1: Schematic diagram of pYES2::LmTrxh2 construct.
Author Contributions
Conceptualization, R.B.S.; methodology, R.B.S. and A.B.H.; validation, R.B.S., A.M. and S.Ć.Z.; formal analysis, R.B.S. and M.T.B.; resources, S.Ć.Z.; data curation, R.B.S., M.T.B. and A.B.H.; writing—original draft preparation, R.B.S.; writing—review and editing, R.B.S., N.B., W.B.R. and S.Ć.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the large research Groups projects to Narjes Baazaoui (Project under grant number (RGP. 2/73/44)). This research was partially funded by the Tunisian Ministry of Higher Education and Scientific Research (Program contract 2019–2022). This work was funded by the project MZE-RO0423 by the Ministry of Agriculture, Czech Republic, and by the project “Plants as a tool for sustainable global development” (registration number: CZ.02.1.01/0.0/0.0/16_019/0000827) within the program Research, Development, and Education (OP RDE)
Data Availability Statement
All data is contained within the article or Supplementary Material.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the large research Groups projects to Narjes Baazaoui (Project under grant number (RGP. 2/73/44)).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bharti, R.; Sharma, R. Effect of heavy metals: An overview. Mater. Today Proc. 2022, 51, 880–885. [Google Scholar]
- Pizzaia, D.; Nogueira, M.L.; Mondin, M.; Carvalho, M.E.A.; Piotto, F.A.; Rosario, M.F.; Azevedo, R.A. Cadmium toxicity and its relationship with disturbances in the cytoskeleton, cell cycle and chromosome stability. Ecotoxicology 2019, 28, 1046–1055. [Google Scholar] [CrossRef]
- Jeena, A.S.; Pandey, D. Metal induced genotoxicity and oxidative stress in plants, assessment methods, and role of various factors in genotoxicity regulation. In Induced Genotoxicity and Oxidative Stress in Plants; Springer: Berlin/Heidelberg, Germany, 2021; pp. 133–149. [Google Scholar]
- Mir, A.R.; Pichtel, J.; Hayat, S. Copper: Uptake, toxicity and tolerance in plants and management of Cu-contaminated soil. Biometals 2021, 34, 737–759. [Google Scholar] [CrossRef]
- Đukić-Ćosić, D.; Baralić, K.; Javorac, D.; Djordjevic, A.B.; Bulat, Z. An overview of molecular mechanisms in cadmium toxicity. Curr. Opin. Toxicol. 2020, 19, 56–62. [Google Scholar] [CrossRef]
- Kaur, H.; Garg, N. Zinc toxicity in plants: A review. Planta 2021, 253, 129. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Qureshi, N.; Mubarik, M.; Bukhari, S. Heavy metals (chromium, copper, and cadmium) induced oxidative stress in Labeo rohita (Hamilton, 1822) during acute and chronic toxicity experiment. Int. J. Biosci. 2015, 6, 64–72. [Google Scholar]
- Zhang, H.; Cai, C.; Shi, C.; Cao, H.; Han, Z.; Jia, X. Cadmium-induced oxidative stress and apoptosis in the testes of frog Rana limnocharis. Aquat. Toxicol. 2012, 122–123, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Tamás, M.J.; Sharma, S.K.; Ibstedt, S.; Jacobson, T.; Christen, P. Heavy metals and metalloids as a cause for protein misfolding and aggregation. Biomolecules 2014, 4, 252–267. [Google Scholar] [CrossRef]
- Mock, H.P.; Dietz, K.J. Redox proteomics for the assessment of redox-related posttranslational regulation in plants. Biochim. Biophys. Acta 2016, 1864, 967–973. [Google Scholar] [CrossRef]
- Mata-Perez, C.; Spoel, S.H. Thioredoxin-mediated redox signalling in plant immunity. Plant Sci. Int. J. Exp. Plant Biol. 2019, 279, 27–33. [Google Scholar] [CrossRef]
- Chibani, K.; Pucker, B.; Dietz, K.J.; Cavanagh, A. Genome-wide analysis and transcriptional regulation of the typical and atypical thioredoxins in Arabidopsis thaliana. FEBS Lett. 2021, 595, 2715–2730. [Google Scholar] [CrossRef] [PubMed]
- Geigenberger, P.; Thormahlen, I.; Daloso, D.M.; Fernie, A.R. The unprecedented versatility of the plant thioredoxin system. Trends Plant Sci. 2017, 22, 249–262. [Google Scholar] [CrossRef]
- Elasad, M.; Wei, H.; Wang, H.; Su, J.; Ondati, E.; Yu, S. Genome- wide analysis and characterization of the TRX gene family in upland cotton. Trop. Plant Biol. 2018, 11, 119–130. [Google Scholar] [CrossRef]
- Meng, J.; Gao, X.; Luo, S.; Lin, C.; Du, C.; Hou, C.; Wang, J.; Jin, S.; Tang, D.; Zhang, C.; et al. Cloning, functional characterization and response to cadmium stress of the thioredoxin-like protein 1 gene from Phascolosoma esculenta. Int. J. Mol. Sci. 2021, 23, 332. [Google Scholar] [CrossRef] [PubMed]
- Laloi, C.; Mestres-Ortega, D.; Marco, Y.; Meyer, Y.; Reichheld, J.P. The Arabidopsis cytosolic thioredoxin h5 gene induction by oxidative stress and its W-box-mediated response to pathogen elicitor. Plant Physiol. 2004, 134, 1006–1016. [Google Scholar] [CrossRef]
- Jedelská, T.; Luhová, L.; Petřivalský, M. Thioredoxins: Emerging players in the regulation of protein S-nitrosation in plants. Plants 2020, 9, 1426. [Google Scholar] [CrossRef] [PubMed]
- Ben Saad, R.; Ben Romdhane, W.; Bouteraa, M.T.; Jrad, O.; Ben Hsouna, A. Lobularia maritima thioredoxin-h2 gene mitigates salt and osmotic stress damage in tobacco by modeling plant antioxidant system. Plant Growth Regul. 2022, 97, 101–115. [Google Scholar] [CrossRef]
- Ben Saad, R.; Ben Hsouna, A.; Saibi, W.; Ben Hamed, K.; Brini, F.; Ghneim-Herrera, T. A stress-associated protein, LmSAP, from the halophyte Lobularia maritima provides tolerance to heavy metals in tobacco through increased ROS scavenging and metal detoxification processes. J. Plant Physiol. 2018, 231, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Zouari, N.; Ben Saad, R.; Legavre, T.; Azaza, J.; Sabau, X.; Jaoua, M.; Masmoudi, K.; Hassairi, A. Identification and sequencing of ESTs from the halophyte grass Aeluropus littoralis. Gene 2007, 404, 61–69. [Google Scholar] [CrossRef]
- Ben Romdhane, W.; Ben-Saad, R.; Meynard, D.; Verdeil, J.L.; Azaza, J.; Zouari, N.; Fki, L.; Guiderdoni, E.; Al-Doss, A.; Hassairi, A. Ectopic expression of Aeluropus littoralis plasma membrane protein gene AlTMP1 confers abiotic stress tolerance in transgenic tobacco by improving water status and cation homeostasis. Int. J. Mol. Sci. 2017, 18, 692. [Google Scholar] [CrossRef]
- Soni, R.; Carmichael, J.P.; Murray, J.A. Parameters affecting lithium acetate-mediated transformation of Saccharomyces cere-visiae and development of a rapid and simplified procedure. Curr. Genet. 1993, 24, 455–459. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Ghneim-Herrera, T.; Ben Romdhane, W.; Dabbous, A.; Ben Saad, R.; Brini, F.; Abdelly, C.; Ben Hamed, K. Early effects of salt stress on the physiological and oxidative status of the halophyte Lobularia maritima. Funct. Plant Biol. 2020, 47, 912–924. [Google Scholar] [CrossRef]
- Bouteraa, M.T.; Mishra, A.; Ben Romdhane, W.; Ben Hsouna, A.; Siddique, K.H.M.; Ben Saad, R. Bio-stimulating effect of natural polysaccharides from Lobularia maritima on durum wheat seedlings: Improved plant growth, salt stress tolerance by modulating biochemical responses and ion homeostasis. Plants 2022, 11, 1991. [Google Scholar] [CrossRef]
- Cuypers, A.; Keunen, E.; Bohler, S.; Jozefczak, M.; Opdenakker, K.; Gielen, H.; Vercampt, H.; Bielen, A.; Schellingen, K.; Vangronsveld, J.; et al. Cadmium and copper stress induce a cellular oxidative challenge leading to damage versus signalling. In Metal Toxicity in Plants: Perception, Signaling and Remediation; Gupta, D.K., Sandalio, L.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 65–90. [Google Scholar]
- Ghori, N.-H.; Ghori, T.; Hayat, M.; Imadi, S.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Michalak, M.; Kukula-Koch, W.; Ben Saad, R.; ben Romdhane, W.; Zeljković, S.Ć.; Mnif, W. Evaluation of halophyte biopotential as an unused natural resource: The case of Lobularia maritima. Biomolecules 2022, 12, 1583. [Google Scholar] [CrossRef] [PubMed]
- Jamla, M.; Khare, T.; Joshi, S.; Patil, S.; Penna, S.; Kumar, V. Omics approaches for understanding heavy metal responses and tolerance in plants. Curr. Plant Biol. 2021, 27, 100213. [Google Scholar] [CrossRef]
- Rohwer, J.M.; Viljoen, C.; Christensen, C.D.; Mashamaite, L.N.; Pillay, C.S. Identifying the conditions necessary for the thioredoxin ultrasensitive response. Perspect. Sci. 2016, 9, 53–59. [Google Scholar] [CrossRef]
- Duan, X.; Wang, Z.; Zhang, Y.; Li, H.; Yang, M.; Yin, H.; Cui, J.; Chai, H.; Gao, Y.; Hu, G.; et al. Overexpression of a thioredoxin-protein-encoding gene, MsTRX, from Medicago sativa enhances salt tolerance to transgenic tobacco. Agronomy 2022, 12, 1467. [Google Scholar] [CrossRef]
- Lv, H.; Zhu, C.; Wei, W.; Lv, X.; Yu, Q.; Deng, X.; Ci, X. Enhanced Keap1-Nrf2/Trx-1 axis by daphnetin protects against oxidative stress-driven hepatotoxicity via inhibiting ASK1/JNK and Txnip/NLRP3 inflammasome activation. Phytomedicine 2020, 71, 153241. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Zhang, H.; Wang, Y.; Li, T.; Che, Y.; Wang, J.; Guo, D.; Sun, G.; Li, X. Thioredoxin-like protein CDSP32 alleviates Cd-induced photosynthetic inhibition in tobacco leaves by regulating cyclic electron flow and excess energy dissipation. Plant Physiol. Biochem. 2021, 167, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, J.; Xie, X.; Wu, C. Cloning and functional characterization of thioredoxin genes from large yellow croaker Larimichthys crocea. Fish Shellfish Immunol. 2018, 77, 385–391. [Google Scholar] [CrossRef]
- Cheng, S.; Li, C.; Wang, Y.; Yang, L.; Chang, Y. Characterization and expression analysis of a thioredoxin-like protein gene in the sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2016, 58, 165–173. [Google Scholar] [CrossRef]
- Kugapreethan, R.; Umasuthan, N.; Wan, Q.; Thulasitha, W.S.; Kim, C.; Lee, J. Comparative analysis of two thioredoxin-like genes in black rockfish Sebastes schlegelii and their possible involvement in redox homeostasis and innate immune responses. Dev. Comp. Immunol. 2017, 67, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Niu, H.B.; Yin, J.; Shao, H.B.; Niu, J.S.; Ren, J.P.; Li, Y.C.; Wang, X. Transgenic barley with overexpressed PTrx increases aluminum resistance in roots during germination. J. Zhejiang Univ. Sci. B 2010, 11, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; He, X.; Feng, T.; Zhuo, R.; Qiu, W.; Han, X.; Qiao, G.; Zhang, D. cDNA library for mining functional genes in Sedum alfredii hance related to cadmium tolerance and characterization of the roles of a novel SaCTP2 gene in enhancing cadmium hyperaccumulation. Environ. Sci. Technol. 2019, 53, 10926–10940. [Google Scholar] [CrossRef]
- Takahashi, R.; Bashir, K.; Ishimaru, Y.; Nishizawa, N.K.; Nakanishi, H. The role of heavy-metal ATPases, HMAs, in zinc and cadmium transport in rice. Plant Signal. Behav. 2012, 7, 1605–1607. [Google Scholar] [CrossRef]
- Goodwin, S.B.; Sutter, T.R. Microarray analysis of Arabidopsis genome response to aluminum stress. Biol. Plant. 2009, 53, 85–99. [Google Scholar] [CrossRef]
- Maron, L.G.; Kirst, M.; Mao, C.; Milner, M.J.; Menossi, M.; Kochian, L.V. Transcriptional profiling of aluminum toxicity and tolerance responses in maize roots. New Phytol. 2008, 179, 116–128. [Google Scholar] [CrossRef]
- Zhang, M.; Mo, H.; Sun, W.; Guo, Y.; Li, J. Systematic isolation and characterization of cadmium tolerant genes in tobacco: A cDNA library Construction and screening approach. PLoS ONE 2016, 11, e0161147. [Google Scholar] [CrossRef]
- Nahirnak, V.; Almasia, N.I.; Hopp, H.E.; Vazquez-Rovere, C. Snakin/GASA proteins: Involvement in hormone crosstalk and redox homeostasis. Plant Signal. Behav. 2012, 7, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Gielen, H.; Vangronsveld, J.; Cuypers, A. Cd-induced Cu deficiency responses in Arabidopsis thaliana: Are phytochelatins involved? Plant Cell Environ. 2017, 40, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2015, 6, 1143. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Kumar, D.; Soni, V. Copper and mercury induced oxidative stresses and antioxidant responses of Spirodela polyrhiza (L.) Schleid. Biochem. Biophys. Rep. 2020, 23, 100781. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).