Characterization of Lupin Cultivars Based on Phenotypical, Molecular and Metabolomic Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Phenotypic Data
Morphological and Agronomic Traits
2.3. Nutritional Traits
2.4. Antinutritional Traits
2.4.1. Determination of Total Alkaloids
2.4.2. Extraction of Phenolic Compounds
2.4.3. Determination of Total Phenols
2.4.4. Determination of Hydrolyzed Tannins
2.4.5. Determination of Condensed Tannins
2.5. Statistical Analysis
2.6. Molecular Analysis
2.6.1. SSR-HRM Genotyping
2.6.2. Analysis of Molecular Genetic Diversity
2.7. Metabolomic Analysis
Metabolomic Profiling of Lupin Seeds
3. Results
3.1. Phenotypical Analysis: Morphological and Agronomical Traits
3.2. Nutritional Traits and Antinutritional Traits
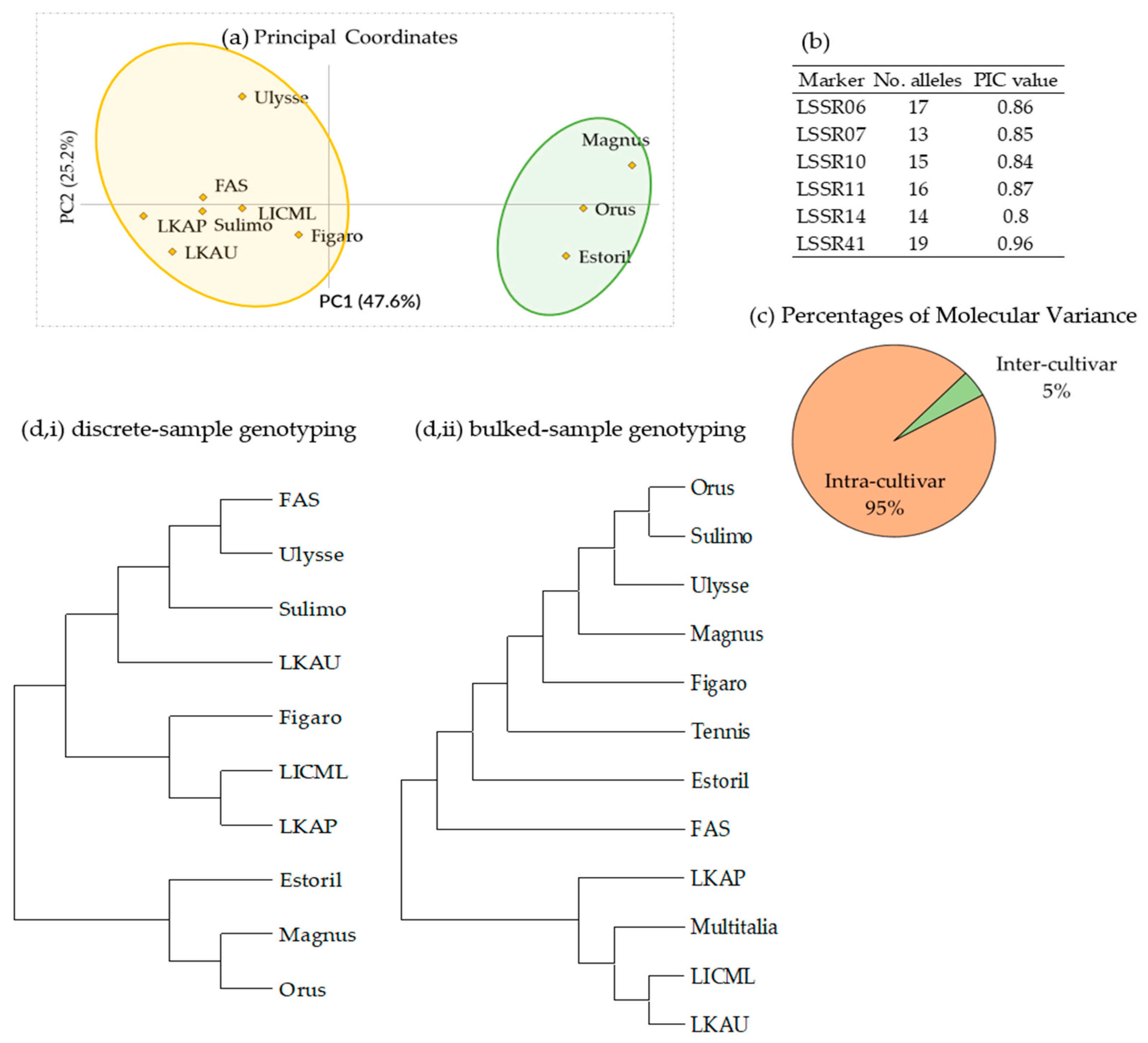
3.3. Molecular Analysis
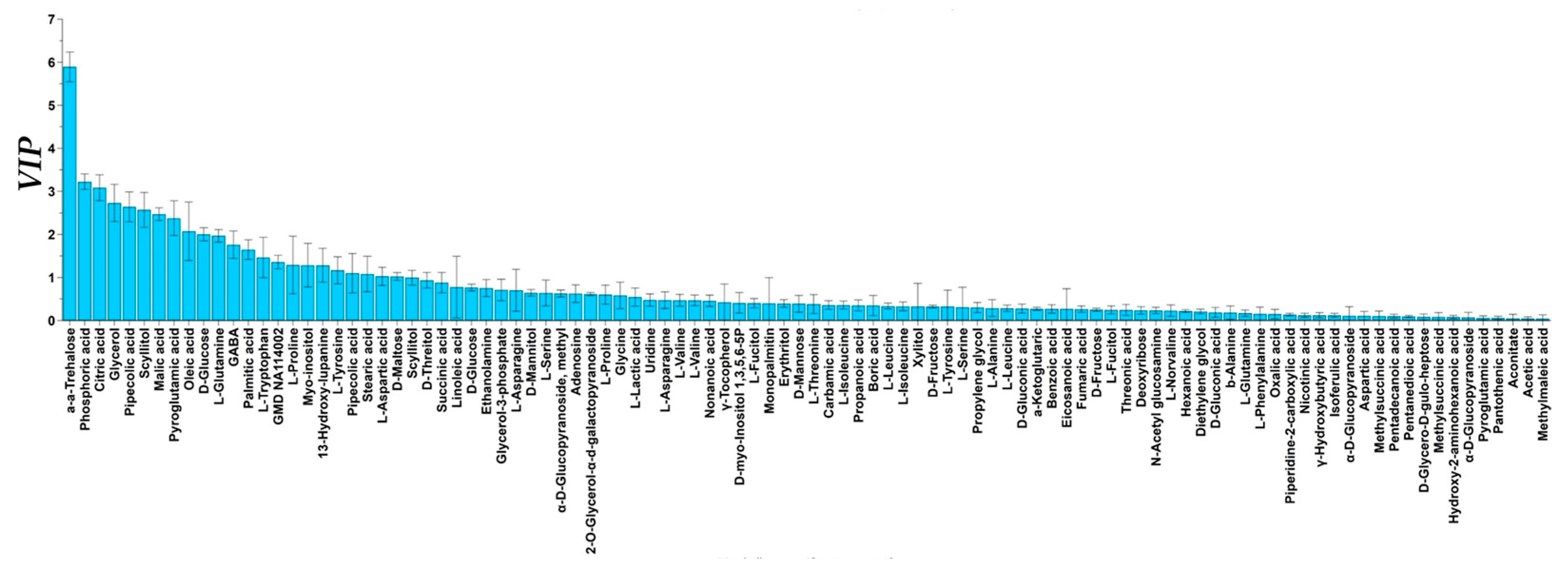
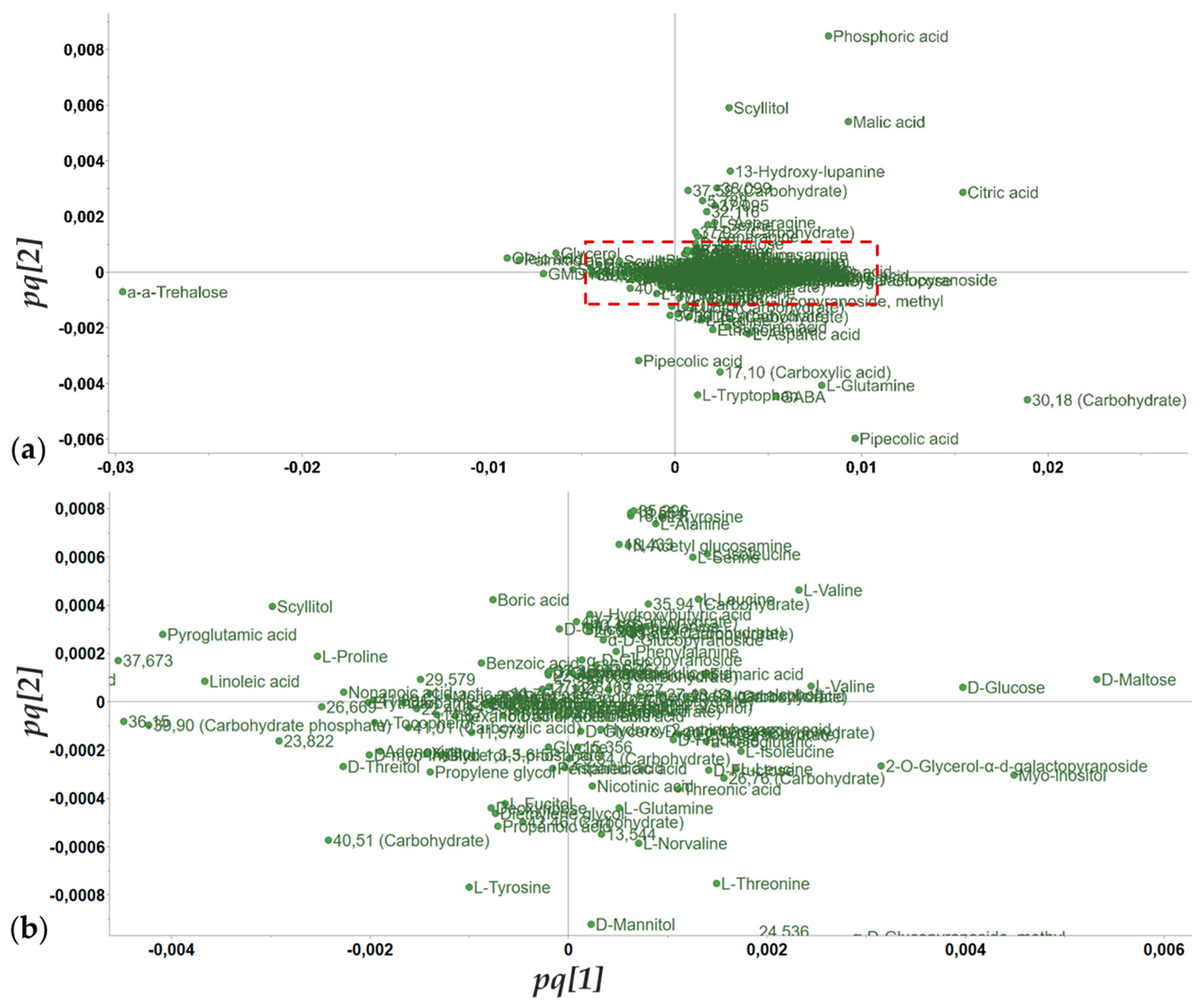
3.4. Metabolomics Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ainouche, A.-K.; Bayer, R.J. Phylogenetic relationships in Lupinus (Fabaceae: Papilionoideae) based on internal transcribed spacer sequences (ITS) of nuclear ribosomal DNA. Am. J. Bot. 1999, 86, 590–607. [Google Scholar] [CrossRef]
- Abraham, E.M.; Ganopoulos, I.; Madesis, P.; Mavromatis, A.; Mylona, P.; Nianiou-Obeidat, I.; Parissi, Z.; Polidoros, A.; Tani, E.; Vlachostergios, D. The Use of Lupin as a Source of Protein in Animal Feeding: Genomic Tools and Breeding Approaches. Int. J. Mol. Sci. 2019, 20, 851. [Google Scholar] [CrossRef]
- Clements, J.C.; Buirchell, B.J.; Yang, H.; Smith, P.M.C.; Sweetingham, M.W.; Smith, C.G. Lupin. Chapter 9. In Genetic Resources, Chromosome Engineering, and Crop Improvement, Grain Legumes; Singh, R.J., Jauhar, P.P., Eds.; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2005. [Google Scholar]
- Kurlovich, B.S.; Kartuzova, L.T. Lupin breeding. Chapter 13. In Lupins (Geography, Classification, Genetic Resources and Breeding); Kurlovich, B.S., Ed.; OY International North Express: St. Petersburg, Russia; Pellosniemi, Finland, 2002; pp. 351–374. [Google Scholar]
- Walker, J.; Hertel, K.; Parker, P.; Edwards, J. Lupin Growth and Development. Industry & Investment NSW (I&I NSW). 2011. Available online: www.industry.nsw.gov.au (accessed on 23 January 2023).
- COM. Report from the Commission to the Council and the European Parliament on the Development of Plant Proteins in the European Union. 757 Final. pp. 1–15. Available online: https://ec.europa.eu/.../report-plant-proteins-com2018-7 (accessed on 23 January 2023).
- Sedláková, K.; Straková, E.; Suchý, P.; Krejcarová, J.; Herzig, I. Lupin as a perspective protein plant for animal and human nutrition—A review. Acta Veter-Brno 2016, 85, 165–175. [Google Scholar] [CrossRef]
- White, C.L.; Staines, V.E.; Staines, M.V.H. A review of the nutritional value of lupins for dairy cows. Aust. J. Agric. Res. 2007, 58, 185–202. [Google Scholar] [CrossRef]
- Tadele, Y. White lupin (Lupinus albus) grain, a potential source of protein for ruminants: A review. Res. J. Agric. Environ. Manag. 2015, 4, 180–188. [Google Scholar]
- Huyghe, C. White lupin (Lupinus albus L.). Field Crops Res. 1997, 53, 147–160. [Google Scholar] [CrossRef]
- Suchý, P.; Straková, E.; Kroupa, L.; Večerek, V. The fatty acid content of oil from seeds of some lupin varieties. In Lupins for Health and Wealth; Palta, J.A., Berger, J.B., Eds.; International Lupin Association Canterbury: Canterbury, New Zealand, 2008; pp. 188–191. [Google Scholar]
- Sawicka-Sienkiewicz, E.J.; Galek, R.; Clements, J.C.; Wilson, J. Difficulties with interspecific hybridization in the genus Lupinus. In Lupins for Health and Wealth, Proceedings of the 12th international lupin conference, 14–18 September 2008, Fremantle, Western Australia; Palta, J.A., Berger, J.D., Eds.; International Lupin Association: Canterbury, New Zealand, 2008; pp. 135–142. [Google Scholar]
- Steenfeldt, S.; González, E.; Knudsen, K.B. Effects of inclusion with blue lupins (Lupinus angustifolius) in broiler diets and enzyme supplementation on production performance, digestibility and dietary AME content. Anim. Feed Sci. Technol. 2003, 110, 185–200. [Google Scholar] [CrossRef]
- Palander, S.; Laurinen, P.; Perttilä, S.; Valaja, J.; Partanen, K. Protein and amino acid digestibility and metabolizable energy value of pea (Pisum sativum), faba bean (Vicia faba) and lupin (Lupinus angustifolius) seeds for turkeys of different age. Anim. Feed Sci. Technol. 2006, 127, 89–100. [Google Scholar] [CrossRef]
- Wolko, B.; Clements, J.C.; Naganowska, B.; Nelson, M.N.; Yang, H.A. Lupinus. In Wild Crop Relatives: Genomic and Breeding Resources; Springer: Berlin/Heidelberg, Germany, 2011; pp. 153–206. [Google Scholar]
- Amir, R.; Han, T.; Ma, F. Bioengineering approaches to improve the nutritional values of seeds by increasing their methionine content. Mol. Breed. 2012, 29, 915–924. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Manunza, P.; Proietti, S. White Lupin Tolerance to Winter Cold, Terminal Drought and Soil Lime: Patterns of Genetic Variation and Their Exploitation in Breeding for Southern Europe; International Lupin Association: Canterbury, New Zealand, 2011; pp. 99–103. [Google Scholar]
- Annicchiarico, P.; Alami, I.T. Enhancing white lupin (Lupinus albus L.) adaptation to calcareous soils through selection of lime-tolerant plant germplasm and Bradyrhizobium strains. Plant Soil 2012, 350, 131–144. [Google Scholar] [CrossRef]
- Atnaf, M.; Wegary, D.; Dagne, K.; Tesfaye, K. Genotype by environment interaction and grain yield stability of Ethiopian white lupin (Lupinus albus L.) landraces. Exp. Agric. 2018, 54, 943–956. [Google Scholar] [CrossRef]
- Nalle, C.L.; Ravindran, V.; Ravindran, G. Nutritional value of white lupins (Lupinus albus) for broilers: Apparent metabolisable energy, apparent ileal amino acid digestibility and production performance. Animal 2012, 6, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Kroc, M.; Tomaszewska, M.; Czepiel, K.; Bitocchi, E.; Oppermann, M.; Neumann, K.; Guasch, L.; Bellucci, E.; Alseekh, S.; Graner, A.; et al. Towards Development, Maintenance, and Standardized Phenotypic Characterization of Single-Seed-Descent Genetic Resources for Lupins. Curr. Protoc. 2021, 1, e191. [Google Scholar] [CrossRef] [PubMed]
- Beyene, C. Genetic variation among white lupin (Lupinus albus L.) landraces from Northwestern and Southern Ethiopia for agronomic traits and nutrient contents of grain. J. Plant Breed. Crop Sci. 2020, 12, 156–169. [Google Scholar]
- Zafeiriou, I.; Polidoros, A.N.; Baira, E.; Kasiotis, K.M.; Machera, K.; Mylona, P.V. Mediterranean White Lupin Landraces as a Valuable Genetic Reserve for Breeding. Plants 2021, 10, 2403. [Google Scholar] [CrossRef] [PubMed]
- Atnaf, M.; Yao, N.; Martina, K.; Dagne, K.; Wegary, D.; Tesfaye, K. Molecular genetic diversity and population structure of Ethiopian white lupin landraces: Implications for breeding and conservation. PLoS ONE 2017, 12, e0188696. [Google Scholar] [CrossRef]
- Bellucci, E.; Aguilar, O.M.; Alseekh, S.; Bett, K.; Brezeanu, C.; Cook, D.; De la Rosa, L.; Delledonne, M.; Dostatny, D.F.; Ferreira, J.J.; et al. The INCREASE project: Intelligent Collections of food-legume genetic resources for European agrofood systems. Plant J. 2021, 108, 646–660. [Google Scholar] [CrossRef]
- Alves, M.; Chicau, P.; Matias, H.; Passarinho, J.; Pinheiro, C.; Ricardo, C.P. Metabolic analysis revealed altered amino acid profiles in Lupinus albus organs as a result of boron deficiency. Physiol. Plant. 2011, 142, 224–232. [Google Scholar] [CrossRef]
- Zhong, L.; Wu, G.; Fang, Z.; Wahlqvist, M.L.; Hodgson, J.M.; Clarke, M.W.; Junaldi, E.; Johnson, S.K. Characterization of polyphenols in Australian sweet lupin (Lupinus angustifolius) seed coat by HPLC-DAD-ESI-MS/MS. Food Res. Int. 2019, 116, 1153–1162. [Google Scholar] [CrossRef]
- Farag, M.A.; Khattab, A.R.; Maamoun, A.; Kropf, M.; Heiss, A.G. UPLC-MS metabolome based classification of Lupinus and Lens seeds: A prospect for phyto-equivalency of its different accessions. Food Res. Int. 2019, 115, 379–392. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2002. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses. USDA Agric. Handb. 1970, 379, 20. [Google Scholar]
- Buzuk, G.N.; Lovkova, M.Y.; Sokolova, S.M.; Tyutekin, Y.V. Evaluation of the correlation between the content of alkaloids and chemical elements in washington lupin (Lupinus polyphyllus lindl.) based on statistical analysis and mathematical simulation. Appl. Biochem. Microbiol. 2002, 38, 286–293. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and anti-oxidants by means of Folin-Ciocalteu reagents. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Makkar, H.P.S.; Blümmel, M.; Borowy, N.K.; Becker, K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J. Sci. Food Agric. 1993, 61, 161–165. [Google Scholar] [CrossRef]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1986, 25, 223–230. [Google Scholar] [CrossRef]
- Nelson, M.N.; Phan, H.T.T.; Ellwood, S.R.; Moolhuijzen, P.M.; Hane, J.; Williams, A.; O‘Lone, C.E.; Fosu-Nyarko, J.; Scobie, M.; Cakir, M.; et al. The first gene-based map of Lupinus angustifolius L.-location of domestication genes and conserved synteny with Medicago truncatula. Theor. Appl. Genet. 2006, 113, 225–238. [Google Scholar] [CrossRef]
- Nei, M.; Tajima, F.; Tateno, Y. Accuracy of estimated phylogenetic trees from molecular data. II. Gene frequency data. J. Mol. Evol. 1983, 19, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.C.; Chin, E.C.L.; Shu, H.; Smith, O.S.; Wall, S.J.; Senior, M.L.; Mitchell, S.E.; Kresovich, S.; Ziegle, J. An evaluation of the utility of SSR loci as molecular markers in maize (Zea mays L.): Comparisons with data from RFLPS and pedigree. Theor. Appl. Genet. 1997, 95, 163–173. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2018. Available online: https://www.R-project.org/ (accessed on 23 January 2023).
- Kostopoulou, S.; Ntatsi, G.; Arapis, G.; Aliferis, K.A. Assessment of the effects of metribuzin, glyphosate, and their mixtures on the metabolism of the model plant Lemna minor L. applying metabolomics. Chemosphere 2020, 239, 124582. [Google Scholar] [CrossRef]
- Papadopoulou, E.-A.; Giaki, K.; Angelis, A.; Skaltsounis, A.-L.; Aliferis, K.A. A Metabolomic Approach to Assess the Toxicity of the Olive Tree Endophyte Bacillus sp. PTA13 Lipopeptides to the Aquatic Macrophyte Lemna minor L. Toxics 2022, 10, 494. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- De Cortes Sánchez, M.; Altares, P.; Pedrosa, M.M.; Burbano, C.; Cuadrado, C.; Goyoaga, C.; Muzquiz, M.; Jiménez-Martínez, C.; Dávila-Ortiz, G. Alkaloid variation during germination in different lupin species. Food Chem. 2005, 90, 347–355. [Google Scholar] [CrossRef]
- Sujak, A.; Kotlarz, A.; Strobel, W. Compositional and nutritional evaluation of several lupin seeds. Food Chem. 2006, 98, 711–719. [Google Scholar] [CrossRef]
- Musco, N.; Cutrignelli, M.I.; Calabrò, S.; Tudisco, R.; Infascelli, F.; Grazioli, R.; Presti, V.L.; Gresta, F.; Chiofalo, B. Comparison of nutritional and antinutritional traits among different species (Lupinus albus L., Lupinus luteus L., Lupinus angustifolius L.) and varieties of lupin seeds. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Nigussie, Z. Contribution of White Lupin for Food Security in North-Western Ethiopia. Asian J. Plant Sci. 2012, 11, 200–205. [Google Scholar] [CrossRef]
- Kroc, M.; Rybiński, W.; Wilczura, P.; Kamel, K.; Kaczmarek, Z.; Barzyk, P.; Święcicki, W. Quantitative and qualitative analysis of alkaloids composition in the seeds of a white lupin (Lupinus albus L.) collection. Genet. Resour. Crop. Evol. 2017, 64, 1853–1860. [Google Scholar] [CrossRef]
- Khan, M.K.; Karnpanit, W.; Nasar-Abbas, S.M.; Huma, Z.-E.; Jayasena, V. Phytochemical composition and bioactivities of lupin: A review. Int. J. Food Sci. Technol. 2015, 50, 2004–2012. [Google Scholar] [CrossRef]
- Min, B.; Barry, T.; Attwood, G.; McNabb, W. The effect of condensed tannins on the nutrition of ruminants fed fresh temperate forages: A review. Anim. Feed Sci. Technol. 2003, 106, 3–19. [Google Scholar] [CrossRef]
- Besharati, M.; Maggiolino, A.; Palangi, V.; Kaya, A.; Jabbar, M.; Eseceli, H.; De Palo, P.; Lorenzo, J.M. Tannin in Ruminant Nutrition: Review. Molecules 2022, 27, 8273. [Google Scholar] [CrossRef]
- Fijen, T.P.; Morra, E.; Kleijn, D. Pollination increases white and narrow-leaved lupin protein yields but not all crop visitors contribute to pollination. Agric. Ecosyst. Environ. 2021, 313, 107386. [Google Scholar] [CrossRef]
- Kleijn, D.; Raemakers, I. A retrospective analysis of pollen host plant use by stable and declining bumble bee species. Ecology 2008, 89, 1811–1823. [Google Scholar] [CrossRef]
- Peeters, M.J.T. Bijen: Bijenstudie in Nederland. Nat. Ned. 2012, 11, 13–19. [Google Scholar]
- Mohammad, N.; Mahesh, S.; Jain, Y.K.; Ansari, S.A. Effect of discrete (individual) and mixed (bulk) genomic DNA on genetic diversity estimates and population structure in Teak (Tectona grandis L. f.). Indian J. Exp. Biol. 2017, 55, 44–48. [Google Scholar] [PubMed]
- Wang, P.; Luo, Q.; Yang, W.; Ahammed, G.J.; Ding, S.; Chen, X.; Wang, J.; Xia, X.; Shi, K. A Novel Role of Pipecolic Acid Biosynthetic Pathway in Drought Tolerance through the Antioxidant System in Tomato. Antioxidants 2021, 10, 1923. [Google Scholar] [CrossRef] [PubMed]
- Sita, K.; Kumar, V. Role of Gamma Amino Butyric Acid (GABA) against abiotic stress tolerance in legumes: A review. Plant Physiol. Rep. 2020, 25, 654–663. [Google Scholar] [CrossRef]
- Ben Massoud, M.; Sakouhi, L.; Chaoui, A. Effect of plant growth regulators, calcium and citric acid on copper toxicity in pea seedlings. J. Plant Nutr. 2018, 42, 1230–1242. [Google Scholar] [CrossRef]
- Altamirano-Hernández, J.; López, M.G.; Acosta-Gallegos, J.A.; Farías-Rodríguez, R.; Peña-Cabriales, J.J. Influence of Soluble Sugars on Seed Quality in Nodulated Common Bean (Phaseolus vulgaris L.): The Case of Trehalose. Crop Sci. 2007, 47, 1193–1205. [Google Scholar] [CrossRef]
- Ali, I.; Shah, S.A.; Ahmed, H.M.; Rehman, K.U.; Ahmad, M. Studies on genetic diversity for seed quality in rapeseed (Brassica napus L.) germplasm of Pakistan through near infrared spectroscopy and principal component analysis. Pak. J. Bot. 2012, 44, 219–222. Available online: https://www.researchgate.net/publication/286516476 (accessed on 23 January 2023).
- Ghosh, S.; Zhang, S.; Azam, M.; Gebregziabher, B.S.; Abdelghany, A.M.; Shaibu, A.S.; Qi, J.; Feng, Y.; Agyenim-Boateng, K.G.; Liu, Y.; et al. Natural Variation of Seed Tocopherol Composition in Diverse World Soybean Accessions from Maturity Group 0 to VI Grown in China. Plants 2022, 11, 206. [Google Scholar] [CrossRef]
- Al-Amrousi, E.F.; Badr, A.N.; Abdel-Razek, A.G.; Gromadzka, K.; Drzewiecka, K.; Hassanein, M.M.M. A Comprehensive Study of Lupin Seed Oils and the Roasting Effect on Their Chemical and Biological Activity. Plants 2022, 11, 2301. [Google Scholar] [CrossRef]
- Rybiński, W.; Święcicki, W.; Bocianowski, J.; Börner, A.; Starzycka-Korbas, E.; Starzycki, M. Variability of fat content and fatty acids profiles in seeds of a Polish white lupin (Lupinus albus L.) collection. Genet. Resour. Crop Evol. 2018, 65, 417–431. [Google Scholar] [CrossRef]
- Wang, L.; Cao, C.; Ma, Q.; Zeng, Q.; Wang, H.; Cheng, Z.; Zhu, G.; Qi, J.; Ma, H.; Nian, H.; et al. RNA-seq analyses of multiple meristems of soybean: Novel and alternative transcripts, evolutionary and functional implications. BMC Plant Biol. 2014, 14, 169. [Google Scholar] [CrossRef] [PubMed]

| Code | Genetic Material | Origin | Genotype/Type of Cultivar | Species |
|---|---|---|---|---|
| L1 | Estoril | Portugal | Commercial cultivar | Lupinus albus |
| L2 | Fas Sweet | Serbia | Commercial cultivar | Lupinus albus |
| L3 | Figaro | France | Commercial cultivar | Lupinus albus |
| L4 | Magnus | France | Commercial cultivar | Lupinus albus |
| L5 | Multitalia | Italy | Commercial cultivar | Lupinus albus |
| L6 | Orus | France | Commercial cultivar | Lupinus albus |
| L7 | Sulimo | France | Commercial cultivar | Lupinus albus |
| L8 | Ulysse | France | Commercial cultivar | Lupinus albus |
| L9 | LKML | Greece | Advanced breeding line | Lupinus albus |
| L10 | LKAP | Greece | Advanced breeding line | Lupinus albus |
| L11 | LKAU | Greece | Advanced breeding line | Lupinus albus |
| A/A | Morphological Traits |
|---|---|
| 1 | Seed size (coDES) |
| 2 | Color of leaf: intensity of green color prior to bud emergence |
| 3 | Shape of leaflets |
| 4 | Type of inflorescence |
| 5 | Date of anthesis and flowering period |
| 6 | Plant height at the beginning of anthesis |
| 7 | Color of flower wings |
| 8 | Color of the flower keel |
| 9 | Type of development |
| 10 | Pod maturation time |
| 11 | Seed decoration |
| Marker | Primers | Ta (°C) |
|---|---|---|
| LSSR06a | F: 5′-GTTGTTTTGGGACAACACCC-3′ | 54 |
| R: 5′-AAAACCCGAACCTGTGTAGC-3′ | ||
| LSSR07 | F; 5′-GGATCAGGTGCTTTCAGTCTTG-3′ | 56 |
| R: 5′-AACCTCATCGAGTGTGAGACTGTAC-3′ | ||
| LSSR10 | F: 5′-CGTCATCCATCAATGTTTGG-3′ | 54 |
| R: 5′-TTAAGGAAACACTGGCCCAT-3′ | ||
| LSSR11 | F: 5′-TCCCTTGCTCTTTTCCTCAC-3′ | 53 |
| R: 5′-CCGTTTAGCACATTGGCAC-3′ | ||
| LSSR14 | F: 5′-GGTGACCCTCACCAGAACAT-3′ | 54 |
| R: 5′-GGTCCTTTGATGATGGTGCT-3′ | ||
| LSSR41 | F: 5′-TCAAGGGTGCTCAGTATGGG-3′ | 56 |
| R: 5′-TCATCCTTTCCCTCCAAAGA-3′ |
| Lupin Cultivar Genotype | Flower Color | Leaf: Intensity of Green Color | Central Leaflet: Width | Type of Inflorescence | Color of the Flower Keel | Color of Flower Wings | Type of Development |
|---|---|---|---|---|---|---|---|
| Estoril | White | medium | medium | raceme | blue-black | white–violet | Ιndeterminate |
| FAS Sweet | White | dark | medium | raceme | blue-black | white | Ιndeterminate |
| Figaro | White | medium | medium | raceme | blue-black | white | Ιndeterminate |
| Magnus | White | dark | medium | raceme | blue-black | white | Ιndeterminate |
| Multitalia | White | medium | medium | raceme | blue-black | white-violet | Indeterminate |
| Orus | White | dark | medium | raceme | blue-black | white | Ιndeterminate |
| Sulimo | White | dark | medium | raceme | blue-black | white | Ιndeterminate |
| Ulysse | White | dark | medium | raceme | blue-black | white–violet | Ιndeterminate |
| LKML | White | medium | board | raceme | blue-black | blue–white | Ιndeterminate |
| LKAP | White | medium | board | raceme | blue-black | violet | Ιndeterminate |
| LKAU | White | light | board | raceme | blue-black | white | Ιndeterminate |
| Code | Lupin Cultivar | Yield (gr) | Chlorophyll Content at Stage of Anthesis | Weight of 100 Pods (gr) | Weight of 100 Seeds (gr) | Size of 10 Random Pods (cm) | Number of Seeds/Pod |
|---|---|---|---|---|---|---|---|
| L1 | Estoril | 163.6 c* | 31.12 b | 172 c | 42.6 a | 8.95 b | 4 |
| L2 | Fas Sweet | 89.9 de | 42.50 a | 185 c | 28.8 c | 8.15 b | 4 |
| L3 | Figaro | 111.4 d | 39.94 ab | 180 c | 35.4 b | 6.7 d | 3 |
| L4 | Magnus | 80.4 de | 34.20 ab | 178 c | 35.3 b | 7.85 c | 4 |
| L5 | Multitalia | 185.6 c | 30.6 b | 180 c | 26.8 c | 7.45 c | 4 |
| L6 | Orus | 66.9 e | 33.80 b | 160 d | 24.2 c | 7.75 c | 4 |
| L7 | Sulimo | 248.5 b | 31.85 b | 204 b | 37.6 b | 7.3 c | 4 |
| L8 | Ulysse | 120.9 cd | 32.40 b | 157 d | 34.6 b | 7.35 c | 3 |
| L9 | LKML | 287.7 b | 18.54 c | 170 cd | 36.9 b | 9.25 a | 5 |
| L10 | LKAP | 356.5 a | 19.22 c | 193 bc | 33.7 b | 8.55 cd | 5 |
| L11 | LKAU | 266.8 b | 18.04 c | 243 a | 34.3 b | 9.25 a | 5 |
| Mean | 179.26 | 30.161 | 184.2 | 34.34 | 8.11 | 4.1 |
| Code | Lupin Cultivar | Total Alkaloids (%) | Total Phenols (mg GAE/100 g) | Total Hydrolyzed Tannins (mg GAE/100 g) | Total Condensed Tannins (mg PCNB/100 g) |
|---|---|---|---|---|---|
| L1 | Estoril | 0.025 ef * | 80.11 efg | 36.82 d | 13.93 g |
| L2 | Fas Sweet | 0.035 de | 93.75 d | 24.09 f | 22.01 b–e |
| L3 | Figaro | 0.009 f | 71.93 g | 35.46 de | 20.91 b–f |
| L4 | Magnus | 0.015 ef | 88.30 de | 27.27 def | 23.45 bcd |
| L5 | Multitalia | 0.23 c | 377.05 a | 298.18 b | 18.40 ef |
| L6 | Orus | 0.020 ef | 77.73 fg | 25.91 ef | 24.75 b |
| L7 | Sulimo | 0.054 d | 60.34 h | 30.91 def | 41.05 a |
| L8 | Ulysse | 0.004 f | 83.86 ef | 35.46 de | 23.60 bc |
| L9 | LKML | 0.312 a | 366.82 b | 309.54 a | 19.27 c–f |
| L10 | LKAP | 0.260 b | 322.16 c | 272.27 c | 17.15 fg |
| L11 | LKAU | 0.233 c | 326.59 c | 280.91 c | 19.12 def |
| Code | Lupin Cultivar | CP (g/kg DM) | NDF (g/kg DM) | ADF (g/kg DM) | ADL (g/kg DM) |
|---|---|---|---|---|---|
| L1 | Estoril | 321.83 ef * | 216.93 c | 140.22 cd | 9.49 ab |
| L2 | Fas Sweet | 387.26 c | 204.22 de | 135.04 cd | 8.86 ab |
| L3 | Figaro | 459.83 b | 208.69 cde | 134.09 cd | 8.70 ab |
| L4 | Magnus | 310.14 f | 256.63 a | 184.99 a | 11.73 a |
| L5 | Multitalia | 364.45 cde | 199.89 e | 133.46 d | 6.18 b |
| L6 | Orus | 339.85 def | 209.17 cde | 143.13 c | 7.69 ab |
| L7 | Sulimo | 383.97 c | 215.65 c | 138.21 cd | 9.00 ab |
| L8 | Ulysse | 334.01 def | 232.70 b | 166.7 b | 8.74 ab |
| L9 | LKML | 527.66 a | 208.87 cde | 140.09 cd | 8.79 ab |
| L10 | LKAP | 308.68 f | 212.74 cd | 141.78 cd | 9.49 ab |
| L11 | LKAU | 373.23 cd | 206.67 cde | 141.29 cd | 9.15 ab |
| Lupin Cultivar | Na | Ne | I | h | uh | P% | No. Alleles | No. Private Alleles |
|---|---|---|---|---|---|---|---|---|
| Estoril | 0.6 ± 0.1 | 1.14 ± 0.03 | 0.14 ± 0.02 | 0.09 ± 0.02 | 0.10 ± 0.02 | 29.8% | 28 | 4 |
| FAS | 0.6 ± 0.1 | 1.15 ± 0.03 | 0.14 ± 0.02 | 0.09 ± 0.02 | 0.11 ± 0.02 | 29.8% | 28 | 3 |
| Figaro | 0.7 ± 0.1 | 1.14 ± 0.03 | 0.15 ± 0.02 | 0.10 ± 0.02 | 0.11 ± 0.02 | 35.1% | 33 | 3 |
| Magnus | 0.5 ± 0.1 | 1.13 ± 0.03 | 0.13 ± 0.02 | 0.08 ± 0.02 | 0.09 ± 0.02 | 25.5% | 24 | 3 |
| Orus | 0.6 ± 0.1 | 1.15 ± 0.03 | 0.15 ± 0.02 | 0.09 ± 0.02 | 0.11 ± 0.02 | 25.5% | 28 | 2 |
| Sulimo | 0.6 ± 0.1 | 1.15 ± 0.03 | 0.15 ± 0.02 | 0.09 ± 0.02 | 0.11 ± 0.02 | 31.1% | 30 | 5 |
| Ulysse | 0.5 ± 0.1 | 1.13 ± 0.03 | 0.12 ± 0.02 | 0.08 ± 0.02 | 0.09 ± 0.02 | 25.5% | 24 | 2 |
| LKML | 0.6 ± 0.1 | 1.14 ± 0.03 | 0.14 ± 0.02 | 0.09 ± 0.01 | 0.11 ± 0.02 | 30.9% | 29 | 3 |
| LKAP | 0.6 ± 0.1 | 1.14 ± 0.03 | 0.14 ± 0.02 | 0.09 ± 0.02 | 0.11 ± 0.02 | 29.8% | 28 | 5 |
| LKAU | 0.6 ± 0.1 | 1.13 ± 0.03 | 0.14 ± 0.02 | 0.09 ± 0.02 | 0.11 ± 0.02 | 29.8% | 28 | 5 |
| Mean | 0.6 ± 0.03 | 1.14 ± 0.01 | 0.14 ± 0.01 | 0.09 ± 0.01 | 0.1 ± 0.01 | 29.8% | 28 | 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavromatis, A.; Nianiou-Obeidat, I.; Polidoros, A.; Parissi, Z.; Tani, E.; Irakli, M.; Aliferis, K.A.; Zafeiriou, I.; Mylona, P.V.; Sarri, E.; et al. Characterization of Lupin Cultivars Based on Phenotypical, Molecular and Metabolomic Analyses. Agronomy 2023, 13, 370. https://doi.org/10.3390/agronomy13020370
Mavromatis A, Nianiou-Obeidat I, Polidoros A, Parissi Z, Tani E, Irakli M, Aliferis KA, Zafeiriou I, Mylona PV, Sarri E, et al. Characterization of Lupin Cultivars Based on Phenotypical, Molecular and Metabolomic Analyses. Agronomy. 2023; 13(2):370. https://doi.org/10.3390/agronomy13020370
Chicago/Turabian StyleMavromatis, Athanasios, Irini Nianiou-Obeidat, Alexios Polidoros, Zoi Parissi, Eleni Tani, Maria Irakli, Konstantinos A. Aliferis, Ioannis Zafeiriou, Photini V. Mylona, Efi Sarri, and et al. 2023. "Characterization of Lupin Cultivars Based on Phenotypical, Molecular and Metabolomic Analyses" Agronomy 13, no. 2: 370. https://doi.org/10.3390/agronomy13020370
APA StyleMavromatis, A., Nianiou-Obeidat, I., Polidoros, A., Parissi, Z., Tani, E., Irakli, M., Aliferis, K. A., Zafeiriou, I., Mylona, P. V., Sarri, E., Papadopoulou, E.-A., Tagiakas, R., Kougiteas, L., Kostoula, S., & Abraham, E. M. (2023). Characterization of Lupin Cultivars Based on Phenotypical, Molecular and Metabolomic Analyses. Agronomy, 13(2), 370. https://doi.org/10.3390/agronomy13020370









