In Vitro Micropropagation of Endangered Achillea fragrantissima Forssk. Combined with Enhancement of Its Antihyperglycemic Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Micropropagation Protocol for Achillea fragrantissima Plantlets
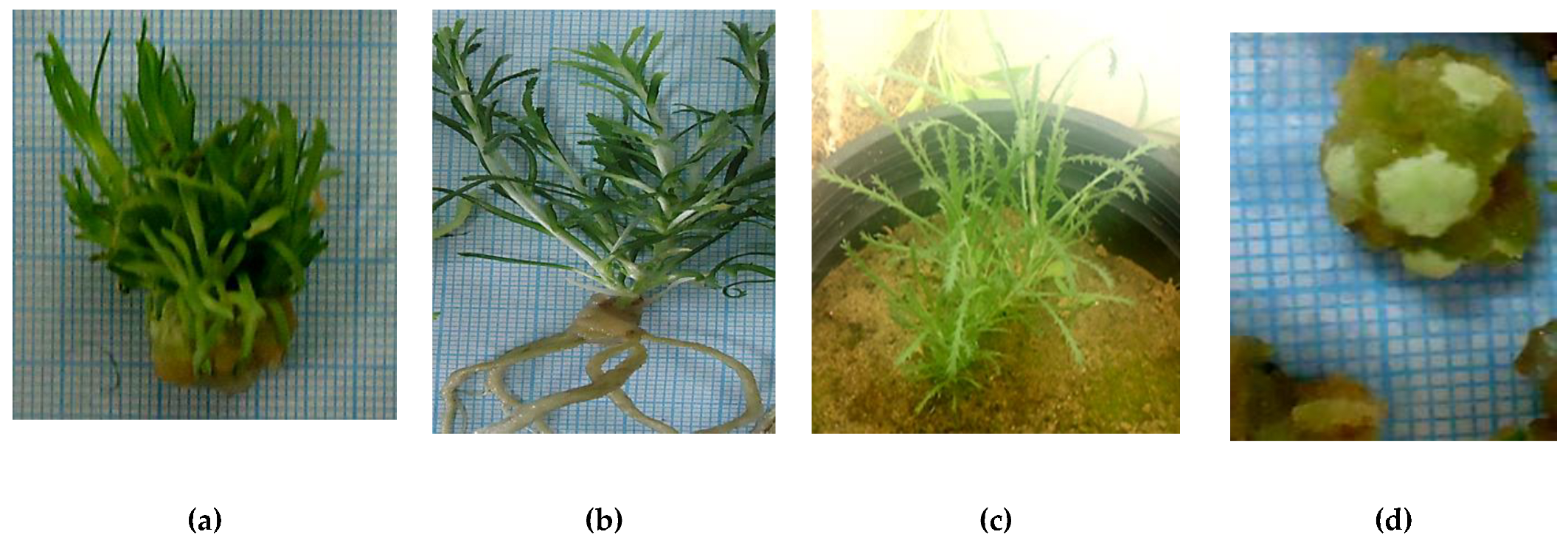
2.1.1. Establishment of In Vitro Achillea fragrantissima Plantlets
2.1.2. Shoot Proliferation of A. fragrantissima Plantlets
2.1.3. In Vitro Adventitious Root Formation and Ex Vitro Acclimatization
2.1.4. Callus Induction
2.1.5. Plant Regeneration and Organogenesis
2.2. In Vitro α-Amylase Inhibition Study of Ethanolic Extracts of Different Treatments
2.2.1. Ethanol Extraction of Bioactive Compounds from Different In Vitro Cultured Plants
2.2.2. α-Amylase Inhibition Assay
2.3. Quantitative Determination of Polysaccharides in Different Treatments
2.4. Screening of In Vivo Hypoglycemic Activity in Plant Extracts
2.4.1. Experimental Animals
2.4.2. Preparation of Aqueous Extracts of Different Samples
2.4.3. Induction of Hyperglycemia in Sprague-Dawley Rats Using Streptozotocin (STZ)
2.4.4. In Vivo Treatment Administration
2.4.5. Serum Sample Preparation and Biochemical Estimation of Serum Salivary α-Amylase Enzyme, Malondialdehyde (MDA), Reduced Glutathione (GSH), and Insulin
2.5. Statistical Analysis
3. Results and Discussion
3.1. In Vitro Micropropagation Protocol for A. fragrantissima
3.1.1. In Vitro Seed Germination and Establishment of Mother Stock
3.1.2. In Vitro Shoot Proliferation
3.1.3. In Vitro Root Formation and Acclimatization
3.1.4. Callus Induction
3.1.5. Plant Regeneration and Organogenesis
3.2. In Vitro α-Amylase Inhibition Assay of Different Treatments
3.3. Colorimetric Determination of Polysaccharides in Different Treatments
3.4. Screening of the In Vivo Hypoglycemic Activity of Some Selected Feedings and Estimations of Their Insulin Activity
3.5. Biochemical Estimation of Antioxidant Activity and In Vivo α-Amylase Inhibition Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaniv, Z.; Dafni, A.; Friedman, J.; Palevitch, D. Plants used for the treatment of diabetes in Palestine. J. Ethnopharmacol. 1987, 19, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Fattah, A.I.; Ali, S.A.; Aly, H.F.; Abd-Alla, H.I.; Shalaby, N.M.; Saleh, M.H. Therapeutic potential of Achillea fragrantissima extracts in amelioration of high-fat diet and low dose streptozotocin diabetic rats. J. Complement. Med. Res. 2018, 7, 115–130. [Google Scholar]
- Sultan, M.M.; Zaki, A.K. Antiviral screening of forty-two Egyptian medicinal plants. J. Ethnopharmacol. 2009, 126, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Elmann, A.; Mordechay, S.; Erlank, H.; Telerman, A.; Rindner, M.; Ofir, R. Anti-Neuroinflammatory effects of the extract of Achillea fragrantissima. BMC Complement. Altern. Med. 2011, 11, 1198–1208. [Google Scholar] [CrossRef]
- Alenad, A.M.; Al-Jaber, N.A.; Krishnaswamy, S.; Yakout, S.M.; Al-Daghri, N.M.; Alokail, M.S. Achillea fragrantissima extract exerts its anticancer effect via induction of differentiation, cell cycle arrest and apoptosis in chronic myeloid leukemia (CML) cell line K562. J. Med. Plants Res. 2013, 7, 1561–1567. [Google Scholar] [CrossRef]
- Hammad, H.M.; Matar, S.A.; Litescu, S.; Abuhamdah, S.; Al-Jaber, H.I.; Afifi, F.U. Biological activities of the hydro-alcoholic and aqueous extracts of Achillea fragrantissima (Forssk.) grown in Jordan. Nat. Sci. 2014, 6, 23–30. [Google Scholar] [CrossRef]
- Hijazi, M.A.; Jambi, H.A.; Aljehany, B.M.; Althaiban, M.A. Potential Protective Effect of Achillea fragrantissima against Adriamycin-Induced Cardiotoxicity in Rats via an Antioxidant and Anti-Inflammatory Pathway. BioMed Res. Int. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Mandour, M.A.; Al-Shami, S.A.; Al-Eknah, M.M.; Hussein, Y.A.; El-Ashmawy, I.M. The acute and long-term safety evaluation of aqueous, methanolic and ethanolic extracts of Achillea fragrantissima. Afr. J. Pharm. Pharmacol. 2013, 7, 2282–2290. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.F.; Alqasoumi, S.I.; El-Desoky, A.H.; Soliman, G.A.; Paré, P.W.; Hegazy, M.-E.F. Evaluation of the anti-inflammatory, analgesic and anti-ulcerogenic potentials of Achillea fragrantissima. S. Afr. J. Bot. 2015, 98, 122–127. [Google Scholar] [CrossRef]
- Patocka, J.; Navratilova, Z. Achillea fragrantissima: Pharmacology review. Clin. Oncol. 2019, 4, 1601. [Google Scholar]
- El-Absy, K.M.; Kasim, W.A.; El-Kady, H.F.; El-Shourbagy, M.N. Physiological studies on Achillea fragrantissima and Artemisia judaica in Saint Katherine, South Sinai, Egypt. Int. J. Sci. Res. Agric. Sci. 2015, 2, 127–136. [Google Scholar]
- Awad, B.M.; Habib, E.S.; Ibrahim, A.K.; Wanas, A.S.; Radwan, M.M.; Helal, M.A.; Elsohly, M.A.; Ahmed, S.A. Cytotoxic activity evaluation and molecular docking study of phenolic derivatives from Achillea fragrantissima (Forssk.) growing in Egypt. Med. Chem. Res. 2017, 26, 2065–2073. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Salama, M.M. A new α-glucosidase inhibitor from Achillea fragrantissima (Forssk.) Sch. Bip. growing in Egypt. Nat. Prod. Res. 2014, 28, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, A.F.; Richter, G. Chromatographic investigation of the essential oil of Achillea fragrantissima. J. Pharm. Sci. 1964, 53, 1502–1505. [Google Scholar] [CrossRef]
- Pais, M.; Rao, P. Biomolecules for Corrosion Mitigation of Zinc: A Short Review. J. Bio- Tribo-Corros. 2019, 5, 92. [Google Scholar] [CrossRef]
- Eltamany, E.E.; Radwan, M.M.; Ross, S.; Hassanean, H.; Khalifa, S.I.; Badr, G.M.; Ahmed, S. A New guaianolide with significant antioxidant activity isolated from Achillea fragrantissima and in vivo antidiabetic evaluation of different A. fragrantissima extracts. Planta Med. 2012, 78, 30. [Google Scholar] [CrossRef]
- El-Shatoury, S.; El-Kraly, O.; El-Kazzaz, W.; Dewedar, A. Antimicrobial Activities of Actinomycetes Inhabiting Achillea fragrantissima (Family: Compositae). Egypt. J. Nat. Toxins 2009, 6, 1–15. [Google Scholar]
- Alatar, A.A. Effect of temperature and salinity on germination of Achillea fragrantissima and Moringa peregrina from Saudi Arabia. Afr. J. Biotechnol. 2011, 10, 3393–3398. [Google Scholar] [CrossRef]
- Younes, L.S.; Shibli, R.A.; Al-Qudah, T.S. In vitro propagation and acclimatization of Achillea fragrantissima Frossk Sch. Bip. Jordan J. Agric. Sci. 2015, 11, 339–350. [Google Scholar]
- El Sherif, F.; Khattab, S.; Ibrahim, A.K.; Ahmed, S.A. Improved silymarin content in elicited multiple shoot cultures of Silybum marianum L. Physiol. Mol. Biol. Plants 2013, 19, 127–136. [Google Scholar] [CrossRef]
- Goda, S.M.; Ahmed, S.A.; El Sherif, F.; Hassanean, H.A.; Ibrahim, A.K. Genetically stable plants with boosted flavonoids content after in vitro regeneration of the endangered Capparis spinosa L. Glob. Drugs Ther. 2017, 2, 1–7. [Google Scholar] [CrossRef]
- Robinson, J.C.; Sáuco, V.G. Weaning (acclimatization) of in vitro-produced banana plants. Fruits 2009, 64, 325–332. [Google Scholar] [CrossRef]
- Khattab, S.; El Sherif, F. Effect of growth regulators on Carpobrotus edulis rapid micropropagation and molecular analysis. Am. J. Sci. 2011, 7, 511–520. [Google Scholar]
- Ali, H.; Houghton, P.J.; Soumyanath, A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J. Ethnopharmacol. 2006, 107, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Calorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bajpai, B.B. Biermann’s Handbook of Pulp and Paper—Carbohydrate Chemistry, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 363–371. [Google Scholar] [CrossRef]
- Kapoor, R.; Kakkar, P. Naringenin accords hepatoprotection from streptozotocin induced diabetes in vivo by modulating mitochondrial dysfunction and apoptotic signaling cascade. Toxicol. Rep. 2014, 1, 569–581. [Google Scholar] [CrossRef]
- Effiong, A.E.; Ebong, P.; Eseyin, O.A. Hypoglycemic Effect of Ethanol Extract and Fractions of Nauclea latifolium leaf on normal and alloxan-induced diabetic rats. Int. J. Biochem. Biotechnol. 2013, 2, 457–460. [Google Scholar]
- Vianna, R.; Brault, A.; Martineau, L.C.; Couture, R.; Arnason, J.T.; Haddad, P.S. In vivo anti-diabetic activity of the ethanolic crude extract of Sorbus decora C.K. Schneid. (Rosacea): A medicinal plant used by canadian james bay cree nations to treat symptoms related to diabetes. Evid. Based Complement. Altern. Med. 2011, 1, 1–7. [Google Scholar] [CrossRef]
- Premkumar, G.; Sankaranarayanan, R.; Jeeva, S.; Rajarathinam, K. Cytokinin induced shoot regeneration and flowering of Scoparia dulcis L. (Scrophulariaceae)–an ethnomedicinal herb. Asian Pac. J. Trop. Biomed. 2011, 1, 169–172. [Google Scholar] [CrossRef]
- Jana, S.; Sivanesan, I.; Jeong, B.R. Effect of cytokinins on in vitro multiplication of Sophora tonkinensis. Asian Pac. J. Trop. Biomed. 2013, 3, 549–553. [Google Scholar] [CrossRef]
- Traykova, B.; Bancheva, S.; Gorgorov, R.; Delcheva, M.; Stanilova, M. Ex situ and in situ conservation of Centaurea pseudaxillaris (Asteraceae) by means of plant biotechnology. J. BioSci. Biotechnol./online 2015, 137–145, 1314–6246. [Google Scholar]
- Shatnawi, M.A. Multiplication and Cryopreservation of Yarrow (Achillea millefolium L., Asteraceae). J. Agric. Sci. Technol. 2013, 15, 163–173. [Google Scholar]
- Sheelu, M.; Neeraj, S.; Sushila, K.; Milind, P.; Sarita, L. Effect of 6- Benzyl Amino purine hormone on the shooting growth of Ocimum gratissimum L. Int. Res. J. Pharm. 2014, 5, 106–108. [Google Scholar]
- Abd El-Kafie, O.M.; El-Banna, H.Y.; Elsharqawi, A.A. Effects of plant growth regulators on frequency shoot multiplication of an important ornamental and medicinal plant, snowbush (Breynia disticha). J. Plant Prod. 2018, 9, 515–520. [Google Scholar] [CrossRef]
- Cuce, M.; Inceer, H.; Imamoglu, K.V.; Ergin, T.; Ulcer, A.O. Towards ex situ conservation of globally rare Turkish endemic Tripleurospermum fissurale (Asteraceae). Vitr. Cell. Dev. Biol. Plant 2022, 58, 1002–1011. [Google Scholar] [CrossRef]
- Cui, S.; Ren, Y.; Hao, Y.; Zhang, J.; Chen, Z.; Zou, J.; Zhou, W.; Chen, X. An efficient protocol for regenerating shoots from paper mulberry (Broussonetia papyrifera) leaf explants. Open Life Sci. 2020, 15, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Chitra Devi, B.; Mahendran, P.; Servin Wesley, P.; Sahaya Shibu, B.; Vetrivel, P.; Malligavathi, D. Micropropagation of Acmella calva using encapsulated in vitro nodal explants. J. Trop. Med. Plants 2012, 13, 33–41. [Google Scholar]
- George, E.F.; Hall, M.A.; De Klerk, G.J.B. Plant Propagation by Tissue Culture, 3rd ed.; Springer Science & Business Media: Basingstoke, UK, 2008; pp. 1–28. [Google Scholar] [CrossRef]
- Florenta, D.V.; Simona, N.; Robert, R.; Madalin, E. Antioxidant and antimicrobial potential of Achillea oxyloba ssp. schurii (Sch. Bip.) Heimerl (Asteraceae) callus in vitro. Rom. J. Biol. Plant Biol. 2020, 36, 195–202. [Google Scholar]
- Moin, S.; Babu, S.S.; Mahalakshmipriya, A. In vitro callus production and antibacterial activity of Barleria lupulina Lindl. Asia Pac. J. Mol. Biol. Biotechnol. 2012, 20, 59–64. [Google Scholar]
- Sheeba, E.; Palanivel, S.; Parvathi, S. Effect of plant growth regulators on callus induction in Physalis minima Linn. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 4847–4851. [Google Scholar]
- De Silva, M.A.; Senarath, W.T. Development of a Successful Protocol for in vitro Mass Propagation of Celastrus paniculatus Willd.—A Valuable Medicinal Plant. Trop. Agric. Res. 2009, 21, 21–29. [Google Scholar] [CrossRef]
- Tippani, R.; Thammidala, C. TDZ induced plant regeneration from immature cotyledons of Pterocarpus marsupium Roxb. and validation of genetic homogeneity using ISSR markers. Vegetos 2021, 34, 144–152. [Google Scholar] [CrossRef]
- Karakas, F.P.; Turker, A.U. An efficient in vitro regeneration system for Bellis perennis L. and comparison of phenolic contents of field-grown and in vitro-grown leaves by LC-MS/MS. Ind. Crops Prod. 2013, 48, 162–170. [Google Scholar] [CrossRef]
- Faisal, M.; Anis, M. Shoot multiplication in Rauvolfia tetraphylla L. using thidiazuran. Plant Cell Tissue Organ Cult 2005, 80, 187–190. [Google Scholar] [CrossRef]
- El Sherif, F.; Khattab, S. Direct shoot regeneration from leaf, root and stem internode segments of male poplar trees and the molecular analysis of variant regenerated plants. Am. J. Sci. 2011, 7, 200–206. [Google Scholar]
- Chaâbani, G.; Tabart, J.; Kevers, C.; Dommes, J.; Ishfaq Khan, M.; Zaoui, S.; Chebchoub, L.; Lachaal, L.; Karray-Bouraoui, N. Effects of 2,4-dichlorophenoxyacetic acid combined to 6-benzylaminopurine on callus induction, total phenolic and ascorbic acid production, and antioxidant activities in leaf tissue cultures of Crataegus azarolus L. var. aronia. Acta Physiol. Plant. 2015, 37, 16. [Google Scholar] [CrossRef]
- Sun, L.; Warren, F.J.; Gidley, M.J. Natural products for glycaemic control: Polyphenols as inhibitors of alpha-amylase. Trends Food Sci. Technol. 2019, 91, 262–273. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Y.; Zhong, Z. Antihyperglycemic Effect of Ganoderma lucidum polysaccharides on streptozotocin-induced diabetic mice. Int. J. Mol. Sci. 2011, 12, 6135–6145. [Google Scholar] [CrossRef]
- Wu, J.; Shi, S.; Wang, H.; Wang, S. Mechanisms underlying the effect of polysaccharides in the treatment of type 2 diabetes: A review. Carbohydr. Polym. 2016, 144, 474–494. [Google Scholar] [CrossRef]
- Zhong, J.J. Production of ginseng saponin and polysaccharide by cell cultures of Panax notoginseng and Panax ginseng effects of plant growth regulators. Biotechnol. Appl. Biochem. 1998, 75, 261–268. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Piao, X.C.; Liu, J.S.; Jiang, J.; Lian, Z.X.; Kim, M.J.; Lian, M.L. Bioactive compound production by adventitious root culture of Oplopanax elatus in balloon-type airlift bioreactor systems and bioactivity property. Plant Cell Tissue Organ Cult 2015, 123, 413–425. [Google Scholar] [CrossRef]
- Rotshteyn, Y.; Zito, S.W. Application of modified in vitro screening procedure for identifying herbals possessing sulfonylurea-like activity. J. Ethnopharmacol. 2004, 93, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamed, A.R.; Mehanna, E.T.; Hazem, R.M.; Badr, J.M.; Abo-Elmatty, D.M.; Abdel-Kader, M.S.; Goda, M.S. Plicosepalus acacia Extract and Its Major Constituents, Methyl Gallate and Quercetin, Potentiate Therapeutic Angiogenesis in Diabetic Hind Limb Ischemia: HPTLC Quantification and LC-MS/MS Metabolic Profiling. Antioxidants 2021, 10, 1701. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.S.; Abdel-Moneim, A.A. Effect of chemicals on fungal alpha amylase activity. Zent. Mikrobiol. 1989, 144, 623–628. [Google Scholar] [CrossRef] [PubMed]
| Growth Media | BAP (µM/L) | IAA (µM/L) | |
|---|---|---|---|
| 1 | MS medium + 3% (w/v) sucrose + 0.6% (w/v) agar | 0 | 0 |
| 2 | 0.6 | 0.6 | |
| 3 | 1.2 | 0.6 | |
| 4 | 1.2 | 0.3 | |
| 5 | 2.4 | 0.6 | |
| 6 | 3 | 3 | |
| 7 | 6 | 6 |
| Time | Growth Regulator (µM/L) | Length of the Longest Shoot (cm) | No. of Shoots/Explant | Fresh Weight of Explant (g) | ||
|---|---|---|---|---|---|---|
| Kin | BAP | 2iP | ||||
| 1 month | 0.0 | 0.0 | 0.0 | 2.15 a | 1.41 a | 0.19 a |
| 1 | 0.0 | 0.0 | 2.86 ab | 4 ab | 0.55 b | |
| 2 | 0.0 | 0.0 | 2.72 abc | 3.70 ab | 0.35 bc | |
| 3 | 0.0 | 0.0 | 2.51 cde | 2.64 bc | 0.35 bc | |
| 0.0 | 1.2 | 0.0 | 2.17 cde | 5.11 bc | 0.79 bc | |
| 0.0 | 2.4 | 0.0 | 2.41 cde | 3.64 bcd | 0.48 bcd | |
| 0.0 | 3.6 | 0.0 | 2.95 cde | 3 bcde | 0.49 bcd | |
| 0.0 | 0.0 | 0.6 | 2.50 cde | 3.40 cde | 0.45 cd | |
| 0.0 | 0.0 | 1.2 | 2.61 bc | 2.13 de | 0.31 cd | |
| 0.0 | 0.0 | 1.8 | 3.06 c | 1.82 e | 0.31 d | |
| 2 months | 0.0 | 0.0 | 0.0 | 3.53 a | 5.17 a | 0.53 a |
| 1 | 0.0 | 0.0 | 3.44 ab | 10.33 ab | 1.79 a | |
| 2 | 0.0 | 0.0 | 3.44 ab | 7.50 ab | 0.53 ab | |
| 3 | 0.0 | 0.0 | 2.36 ab | 6 ab | 1.56 ab | |
| 0.0 | 1.2 | 0.0 | 2.75 ab | 10.13 bc | 2.05 ab | |
| 0.0 | 2.4 | 0.0 | 2.99 bc | 10.67 cd | 1.59 abc | |
| 0.0 | 3.6 | 0.0 | 3.74 bc | 12.33 cde | 2.45 bcd | |
| 0.0 | 0.0 | 0.6 | 4.29 c | 4.43 cde | 1.65 cd | |
| 0.0 | 0.0 | 1.2 | 2.88 c | 3.67 de | 0.96 cd | |
| 0.0 | 0.0 | 1.8 | 3.51 c | 2 e | 0.48 d | |
| Growth Regulators (µM/L) | No. of Roots/Explant | Length of the Longest Root (cm) | Rooting % | Fresh Weight of Plant (g) | ||
|---|---|---|---|---|---|---|
| IAA | IBA | NAA | ||||
| 0.0 | 0.0 | 0.0 | 0.25 a | 0.5 a | 15% | 0.19 a |
| 0.3 | 0.0 | 0.0 | 0 ab | 0 ab | 0% | 0.13 ab |
| 0.6 | 0.0 | 0.0 | 0 ab | 0 ab | 0% | 0.11 abc |
| 1.2 | 0.0 | 0.0 | 0 ab | 0 ab | 0% | 0.10 bc |
| 0.0 | 0.4 | 0.0 | 0.09 b | 0.46 b | 9.1% | 0.16 bc |
| 0.0 | 0.8 | 0.0 | 0.58 b | 1.35 b | 13% | 0.23 bc |
| 0.0 | 1.6 | 0.0 | 1.7 b | 2.05 b | 22% | 0.24 bc |
| 0.0 | 0 | 0.6 | 1.14 b | 0.75 b | 16.4% | 0.17 bc |
| 0.0 | 0 | 1.2 | 2.33 b | 1.67 b | 21.1% | 0.24 bc |
| 0.0 | 0 | 2.4 | 2.46 b | 3.4 b | 40.3% | 0.37 c |
| Explant | Treatment | Callus Weight (g) | Rooting | Nature |
|---|---|---|---|---|
| leaf/light | 0.6 µM/L BAP + 0.6 µM/L IAA | 0.17 a | 0.0 | hard |
| leaf/light | 3 µM/L BAP + 3 µM/L IAA | 0.64 ab | 0.0 | hard |
| leaf/light | 6 µM/L BAP + 6 µM/L IAA | 0.66 b | 0.0 | hard |
| leaf/light | 1.2 µM/L BAP + 0.6 µM/L IAA | 0.12 b | 0.0 | hard |
| leaf/light | 1.2 µM/L BAP + 0.3 µM/L IAA | 0.12 c | 0.0 | hard |
| leaf/light | 2.4 µM/L BAP + 0.6 µM/L IAA | 0.18 c | 0.0 | hard |
| leaf/dark | 0.6 µM/L BAP + 0.6 µM/L IAA | 0.22 cd | rooted | hard |
| leaf/dark | 3 µM/L BAP + 3 µM/L IAA | 1.06 cd | 0.0 | hard |
| leaf/dark | 6 µM/L BAP + 6 µM/L IAA | 0.79 cde | 0.0 | hard |
| leaf/dark | 1.2 µM/L BAP + 0.6 µM/L IAA | 0.32 cde | rooted | hard |
| leaf/dark | 1.2 µM/L BAP + 0.3 µM/L IAA | 0.16 cde | 0.0 | hard |
| leaf/dark | 2.4 µM/L BAP + 0.6 µM/L IAA | 0.17 cde | rooted | hard |
| root/light | 0.6 µM/L BAP + 0.6 µM/L IAA | 0.26 cde | 0.0 | soft |
| root/light | 3 µM/L BAP + 3 µM/L IAA | 0.36 cde | 0.0 | hard |
| root/light | 1.2 µM/L BAP + 0.6 µM/L IAA | 0.21 cde | rooted | soft |
| root/light | 1.2 µM/L BAP + 0.3 µM/L IAA | 0.19 cde | 0.0 | soft |
| root/light | 2.4 µM/L BAP + 0.6 µM/L IAA | 0.11 de | 0.0 | soft |
| root/dark | 0.6 µM/L BAP + 0.6 µM/L IAA | 0.14 de | rooted | soft |
| root/dark | 3 µM/L BAP + 3 µM/L IAA | 0.07 de | 0.0 | hard |
| root/dark | 6 µM/L BAP + 6 µM/L IAA | 0.01 de | 0.0 | hard |
| root/dark | 1.2 µM/L BAP + 0.6 µM/L IAA | 0.11 de | rooted | soft |
| root/dark | 1.2 µM/L BAP + 0.3 µM/L IAA | 0.04 de | rooted | soft |
| root/dark | 2.4 µM/L BAP + 0.6 µM/L IAA | 0.13 e | rooted | soft |
| TDZ (mM/L) | No. of Regenerated Plants | Length of Regenerated Plants (cm) |
|---|---|---|
| 0.5 | 6.67 a | 0.5 b |
| 1 | 7.75 a | 0.83 ab |
| 2 | 8 a | 1.5 a |
| Treatment | % Inhibition (n = 3) |
|---|---|
| Multiplication 1 month | |
| Control | 64 ± 0.03% |
| Kinetin | 14 ± 0.12% |
| BAP | NA |
| 2iP | NA |
| Multiplication 2 months | |
| Control | NA |
| Kinetin | NA |
| BAP | NA |
| 2iP | NA |
| Rooting 1 month | |
| Control | NA |
| IAA | NA |
| IBA | NA |
| NAA | 64 ± 0.21% |
| Leaf callus on MS medium with 3 µM/L BAP + 3 µM/L IAA at different ages | |
| 1 month | 37 ± 0.13% |
| 2 months | NA |
| 3 months | 23 ± 0.07% |
| 4 months | NA |
| 6 months | NA |
| Undifferentiated cells of different explants and light conditions | |
| LL-control | 79 ± 0.09% |
| LL BAP + IAA | 83 ± 0.11% |
| LD BAP + IAA | 85 ± 0.13% |
| RL BAP + IAA | 93 ± 0.32% |
| RD BAP + IAA | 93 ± 0.17% |
| Wild, Acclimated plants and standard | |
| Acclimated plant aged 7 months | 7 ± 0.04% |
| Wild | 53 ± 0.02% |
| Acarbose standard | 93 ± 0.15% |
| Treatment | Conc. (µg/100 mg Dry Weight) |
|---|---|
| Multiplication 1 month | |
| Control | 33.45 ± 0.4 * |
| Kinetin | 31.03 ± 1 * |
| BAP | 44.25 ± 0.4 * |
| 2iP | 44.39 ± 0.4 * |
| Multiplication 2 months | |
| Control | 49.46 ± 0.5 * |
| Kinetin | 61.30 ± 0.5 * |
| BAP | 66.55 ± 0.3 * |
| 2iP | 79.11 ± 0.6 * |
| Rooting 1 month | |
| Control | 47.22 ± 0.3 * |
| IAA | 62.59 ± 0.4 * |
| IBA | 94.92 ± 0.5 * |
| NAA | 100.2 ± 0.7 * |
| Leaf callus on MS medium with 3 µM/L BAP + 3 µM/L IAA at different ages | |
| 1 month | 65.54 ± 0.5 * |
| 2 months | 73.28 ± 0.5 * |
| 3 months | 68.46 ± 0.4 * |
| 4 months | 87.53 ± 0.3 * |
| 6 months | 88.07 ± 0.2 * |
| Undifferentiated cells of different explants and light conditions | |
| LL-control | 44.48 ± 0.5 * |
| LL BAP + IAA | 43.5 ± 0.4 * |
| LD BAP + IAA | 51.46 ± 0.5 * |
| RL BAP + IAA | 42.99 ± 0.6 * |
| RD BAP + IAA | 46.61 ± 0.3 * |
| Wild, Acclimated plants and standard | |
| Acclimated plant aged 7 months | 86.46 ± 0.5 * |
| Wild | 84.22 ± 0.4 |
| Groups | Blood Glucose Level (mg/dL) | Insulin Activity (µIU/mL) | |||
|---|---|---|---|---|---|
| After 1 h | After 2 h | After 4 h | After 2 h | After 4 h | |
| Normal rats | 113 ± 10.1 | 127.4 ± 9.3 | 130 ± 14.3 | 32.2 ± 0.3 | 31.3 ± 0.3 |
| Diabetic rats | 335 ± 25.6 # | 373 ± 20 # | 381.1 ± 35.5 # | 15.8 ± 0.2 # | 14.9 ± 0.4 # |
| Diabetic + metformin | 345 ± 31 # | 250 ± 29.4 * # | 201.5 ± 19.8 * # | 25.4 ± 0.2 * # | 39 ± 0.5 * # |
| Diabetic + wild plant | 321.9 ± 12.8 # | 290.3 ± 7.3 * # | 313.1 ± 7.4 * # | 16.2 ± 0.1 * # | 17.7 ± 0.2 * # |
| Diabetic + acclimated plant | 363 ± 57 # | 346.7 ± 55 * # | 301.6 ± 57.7 * # | 10 ± 0.3 # | 8.9 ± 0.3 # |
| Growth regulators | |||||
| Diabetic + IBA-fed plant | 373.5 ± 20.2 # | 322.3 ± 19.3 * # | 285.1 ± 15 * # | 24.9 ± 0.4 * # | 29.4 ± 0.1 * # |
| Diabetic + NAA-fed plant | 369.5 ± 15.7 # | 315.3 ± 18.3 * # | 289.5 ± 47.8 * # | 22.6 ± 0.1 * # | 26.5 ± 0.2 * # |
| Diabetic + 2iP-fed plant | 310.5 ± 12.1 # | 285.1 ± 12.8 * # | 308.1 ± 24.6 * # | 1.8 ± 0.2 # | 4.1 ± 0.2 # |
| Different ages of undifferentiated cells of 3 µM/L BAP + 3 µM/L IAA from leaf explants | |||||
| Diabetic + 4-month-old callus | 320.1 ± 16.3 # | 312.1 ± 16.4 * # | 260.8 ± 19.2 * # | 11 ± 0.2 # | 12.6 ± 0.2 # |
| Diabetic + 6-month-old callus | 349.3 ± 18.8 # | 324.8 ± 13.4 * # | 296.9 ± 22.5 * # | 7.9 ± 0.1 # | 9.9 ± 0.1 # |
| Undifferentiated cells of different explants and light conditions | |||||
| Diabetic + root callus of BAP and IAA/dark | 383.1 ± 9.5 # | 381.4 ± 12.3 # | 373.3 ± 10.2 # | 1.7 ± 0.2 # | 4 ± 0.2 # |
| Groups | Salivary α-Amylase Activity (U/L) | GSH (mg/dL) | MDA (nM/mL) | |||
|---|---|---|---|---|---|---|
| After 2 h | After 4 h | After 2 h | After 4 h | After 2 h | After 4 h | |
| Normal rats | 900 ± 15.3 | 910 ± 15.3 | 3.3± 0.2 | 3.2 ± 0.3 | 10.5 ± 0.3 | 11.2 ± 0.3 |
| Diabetic rats | 975.7 ± 5 # | 994 ± 9.5 # | 2.3 ± 0.19 # | 2 ± 0.15 # | 65.3 ± 0.3 # | 72.2 ± 0.2 # |
| Diabetic + metformin | 972 ± 8 # | 987 ± 7 # | 2.2 ± 0.18 # | 2.3 ± 0.2 * # | 62.5 ± 0.4 # | 70.5 ± 0.5 # |
| Diabetic + wild plant | 960 ± 9 * # | 974 ± 24 * # | 2.2 ± 0.17 # | 2.6 ± 0.2 * # | 17.5 ± 0.1 * # | 20.5 ± 0.1 * # |
| Diabetic + acclimated plant | 920 ± 7 * # | 864 ± 10.5 * # | 2.2 ± 0.2 * # | 2.7 ± 0.1 * # | 23.8 ± 0.3 * # | 18.5 ± 0.2 * # |
| Growth regulators | ||||||
| Diabetic + IBA-fed plant | 940 ± 2.9 * # | 960 ± 3 * # | 2.7 ± 0.3 * # | 2.9 ± 0.2 * # | 52.2 ± 0.2 * # | 48.8 ± 0.3 * # |
| Diabetic + NAA-fed plant | 526 ± 6 * # | 672 ± 12 * # | 2.1 ± 0.15 # | 1.8 ± 0.3 # | 72.3 ± 0.3 # | 76.1 ± 0.3 # |
| Diabetic + 2iP-fed plant | 861 ± 11.5 * # | 804 ± 13 * # | 0.8 ± 0.13 # | 2.1 ± 0.1 # | 60.2 ± 0.1 # | 65.6 ± 0.1 # |
| Different ages of undifferentiated cells of 3 µM/L BAP + 3 µM/L IAA from leaf explants | ||||||
| Diabetic + 4-month-old callus | 731 ± 10 * # | 648 ± 10.5 * # | 4.4 ± 0.2 * # | 5.6 ± 0.1 * # | 8.2 ± 0.1 * # | 9.4 ± 0.1 * # |
| Diabetic + 6-month-old callus | 658 ± 18.5 * # | 757 ± 25 * # | 3.2 ± 0.2 * | 4.6 ± 0.1 * # | 9.7 ± 0.1 * # | 10.7 ± 0.1 * # |
| Undifferentiated cells of different explants and light conditions | ||||||
| Diabetic + root callus of BAP and IAA/dark | 980 ± 18# | 997 ± 15 # | 0.2 ± 0.13 # | 0.2 ± 0.1 # | 75.1 ± 0.1 # | 71.3 ± 0.3 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goda, M.S.; Ahmed, S.A.; Sherif, F.E.; Khattab, S.; Hassanean, H.A.; Alnefaie, R.; Althumairy, D.; Abo-elmatty, D.M.; Ibrahim, A.K. In Vitro Micropropagation of Endangered Achillea fragrantissima Forssk. Combined with Enhancement of Its Antihyperglycemic Activity. Agronomy 2023, 13, 278. https://doi.org/10.3390/agronomy13020278
Goda MS, Ahmed SA, Sherif FE, Khattab S, Hassanean HA, Alnefaie R, Althumairy D, Abo-elmatty DM, Ibrahim AK. In Vitro Micropropagation of Endangered Achillea fragrantissima Forssk. Combined with Enhancement of Its Antihyperglycemic Activity. Agronomy. 2023; 13(2):278. https://doi.org/10.3390/agronomy13020278
Chicago/Turabian StyleGoda, Marwa S., Safwat A. Ahmed, Fadia El Sherif, Salah Khattab, Hashem A. Hassanean, Rasha Alnefaie, Duaa Althumairy, Dina M. Abo-elmatty, and Amany K. Ibrahim. 2023. "In Vitro Micropropagation of Endangered Achillea fragrantissima Forssk. Combined with Enhancement of Its Antihyperglycemic Activity" Agronomy 13, no. 2: 278. https://doi.org/10.3390/agronomy13020278
APA StyleGoda, M. S., Ahmed, S. A., Sherif, F. E., Khattab, S., Hassanean, H. A., Alnefaie, R., Althumairy, D., Abo-elmatty, D. M., & Ibrahim, A. K. (2023). In Vitro Micropropagation of Endangered Achillea fragrantissima Forssk. Combined with Enhancement of Its Antihyperglycemic Activity. Agronomy, 13(2), 278. https://doi.org/10.3390/agronomy13020278








