Exogenous GR24 Inhibits Strawberry Tillering by Affecting the Phytohormone Signaling and Sugar Metabolism Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Measurement of Plant Growth Indexes
2.3. Determination of the Endogenous Phytohormone Content
2.4. Determination of Sucrose, Fructose, and Glucose Contents
2.5. Transcriptome Analysis
2.6. Real-Time Fluorescence Quantification (RT-qPCR)
2.7. Statistical Analysis
3. Results
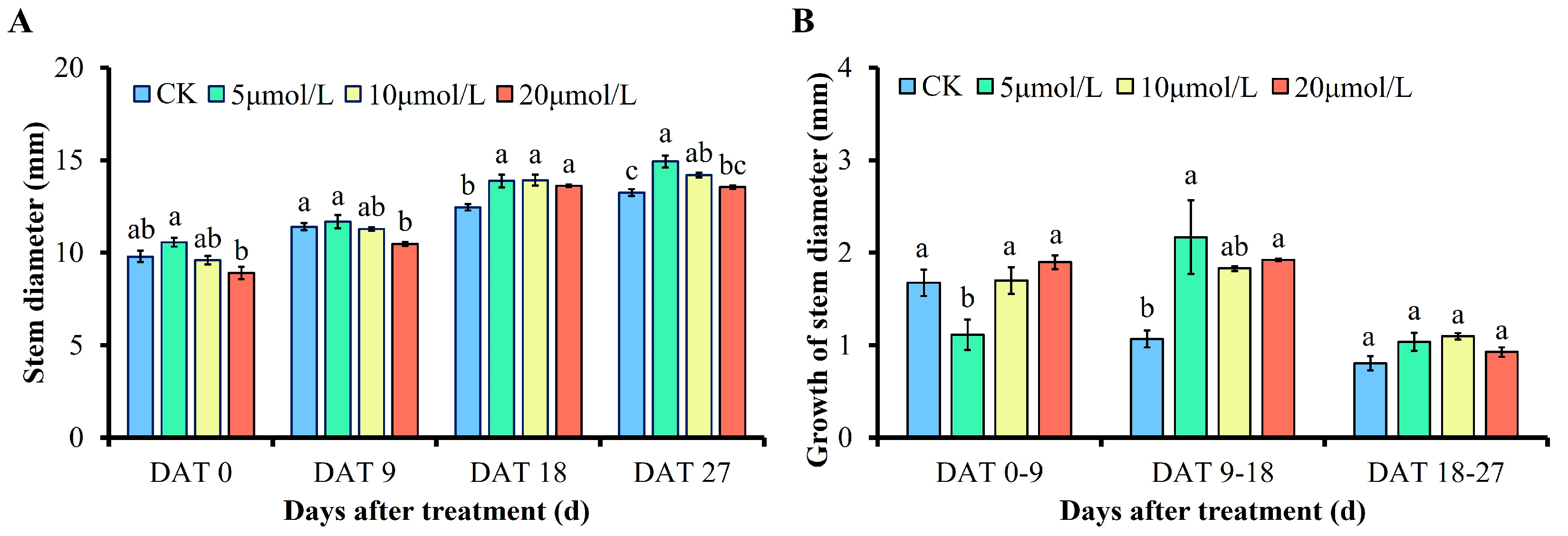
3.1. Effects of Different Concentrations of GR24 on Stem Diameter and Its Growth
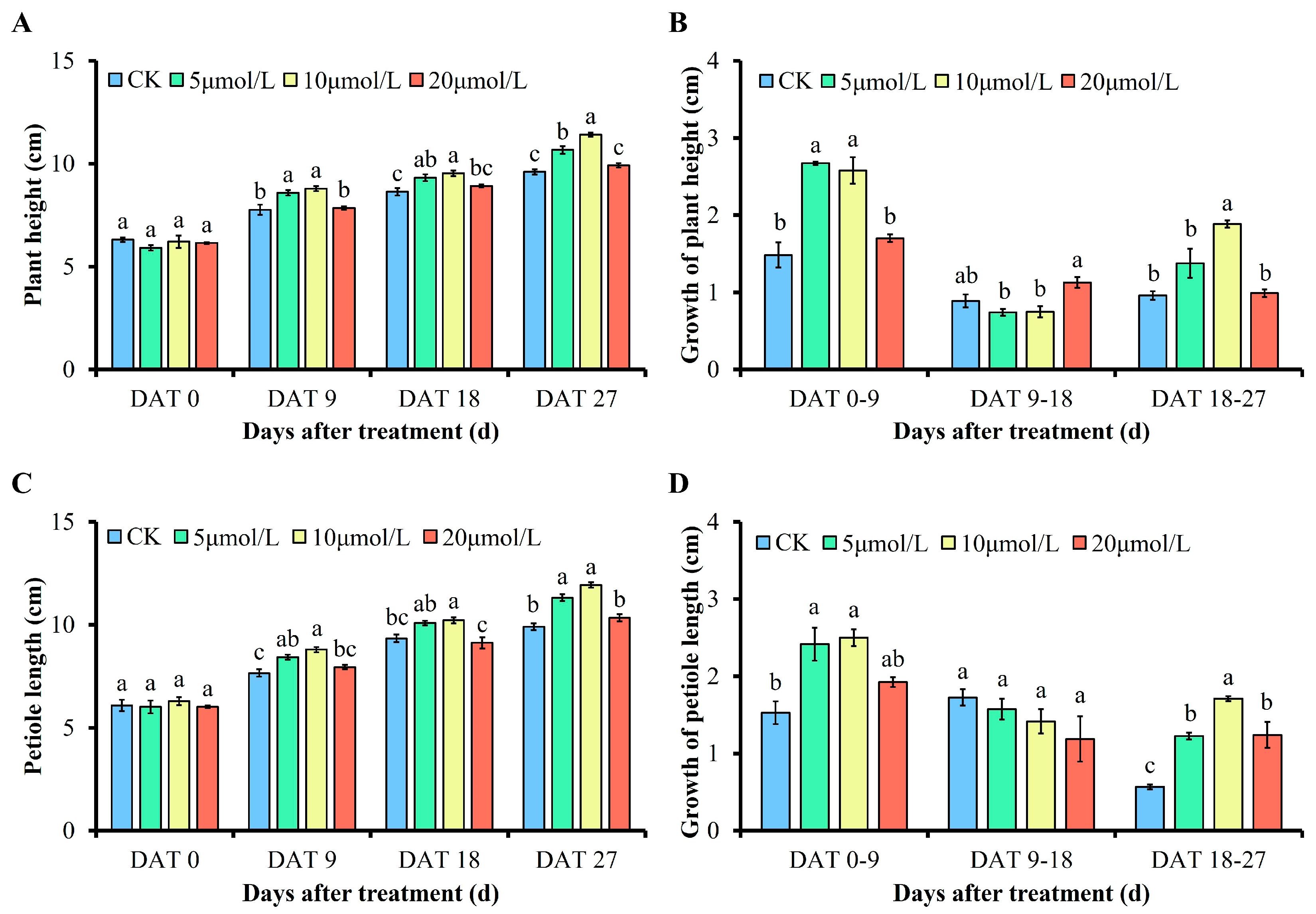
3.2. Effects of Different Concentrations of GR24 on Plant Height, Petiole Length, and Their Growth
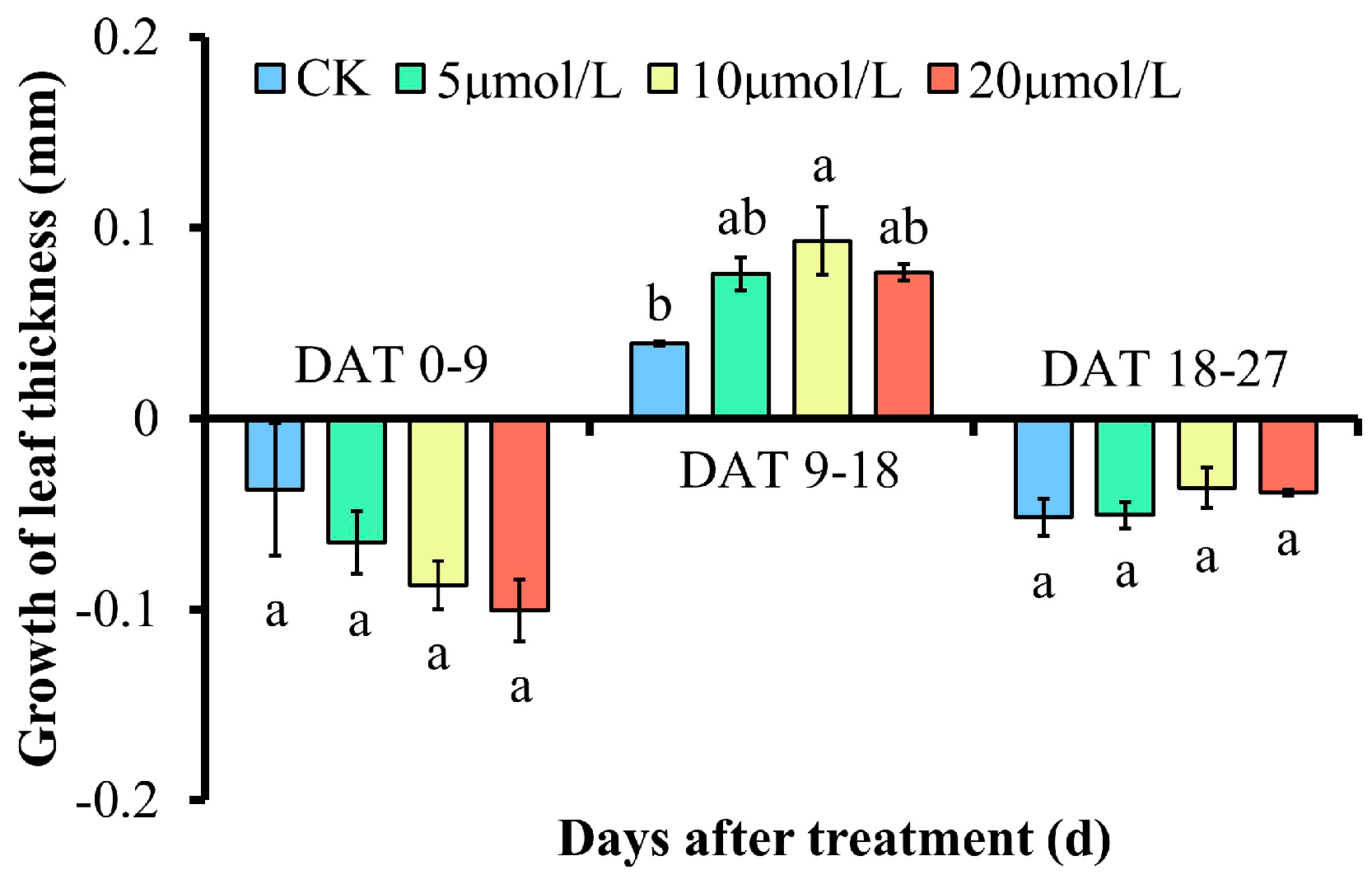
3.3. Effect of Different Concentrations of GR24 on Leaf Thickness and Its Growth
3.4. Effect of Different Concentrations of GR24 on Strawberry Stolons and Flowering Branches
3.5. Effect of GR24 on the Sugar Content of the Strawberry Crowns
3.6. Effect of GR24 on the Content of Endogenous Phytohormones
3.7. Transcriptome Analysis of the Cytokinin Anabolic and Signaling Pathways
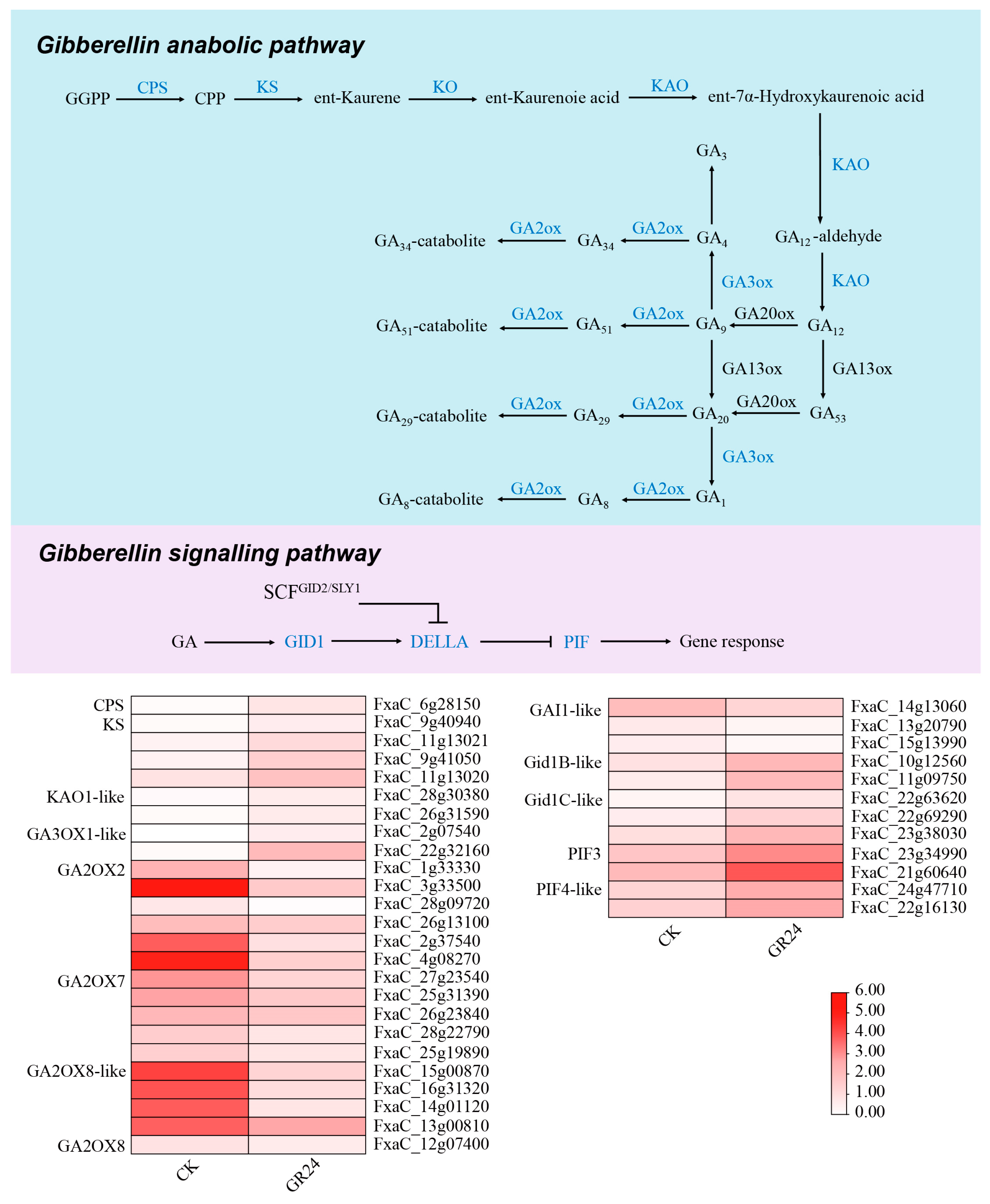
3.8. Transcriptome Analysis of the GA Anabolic and Signaling Pathways
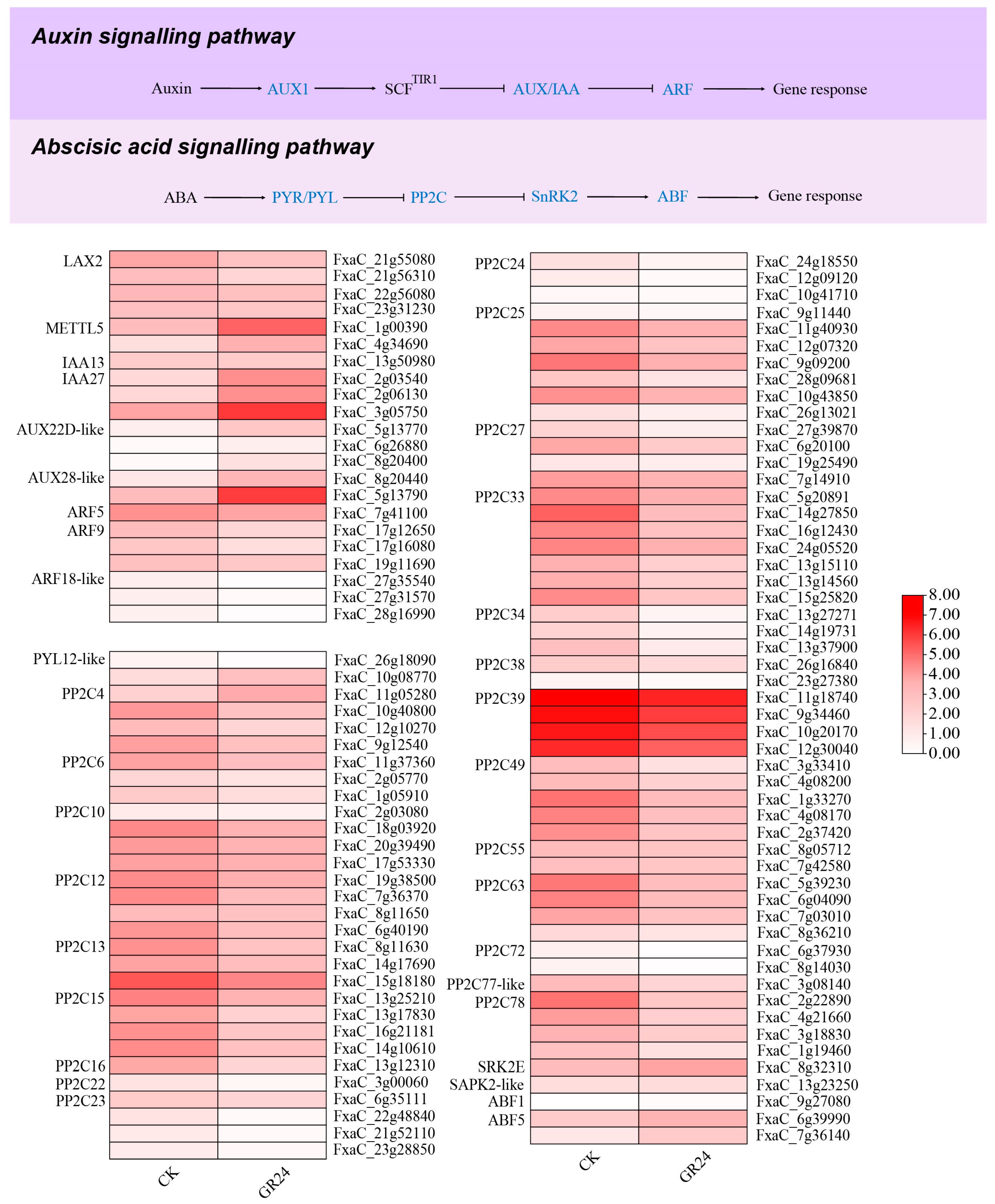
3.9. Transcriptome Analysis of the Auxin Signaling Pathway
3.10. Transcriptome Analysis of the ABA Signaling Pathway
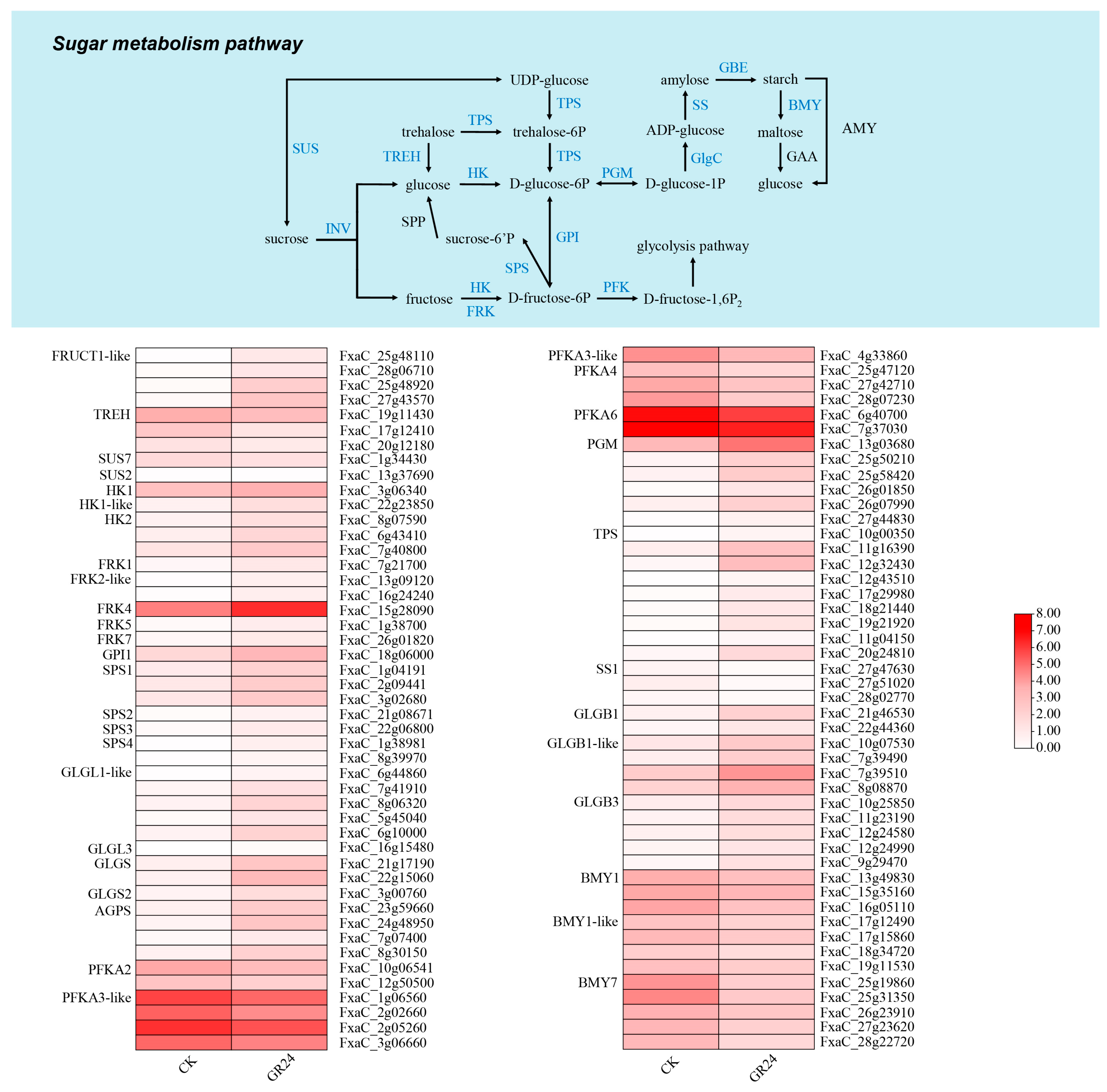
3.11. Transcriptome Analysis of the Sugar Metabolism Pathway
3.12. Real-Time Quantitative PCR of the Key Genes
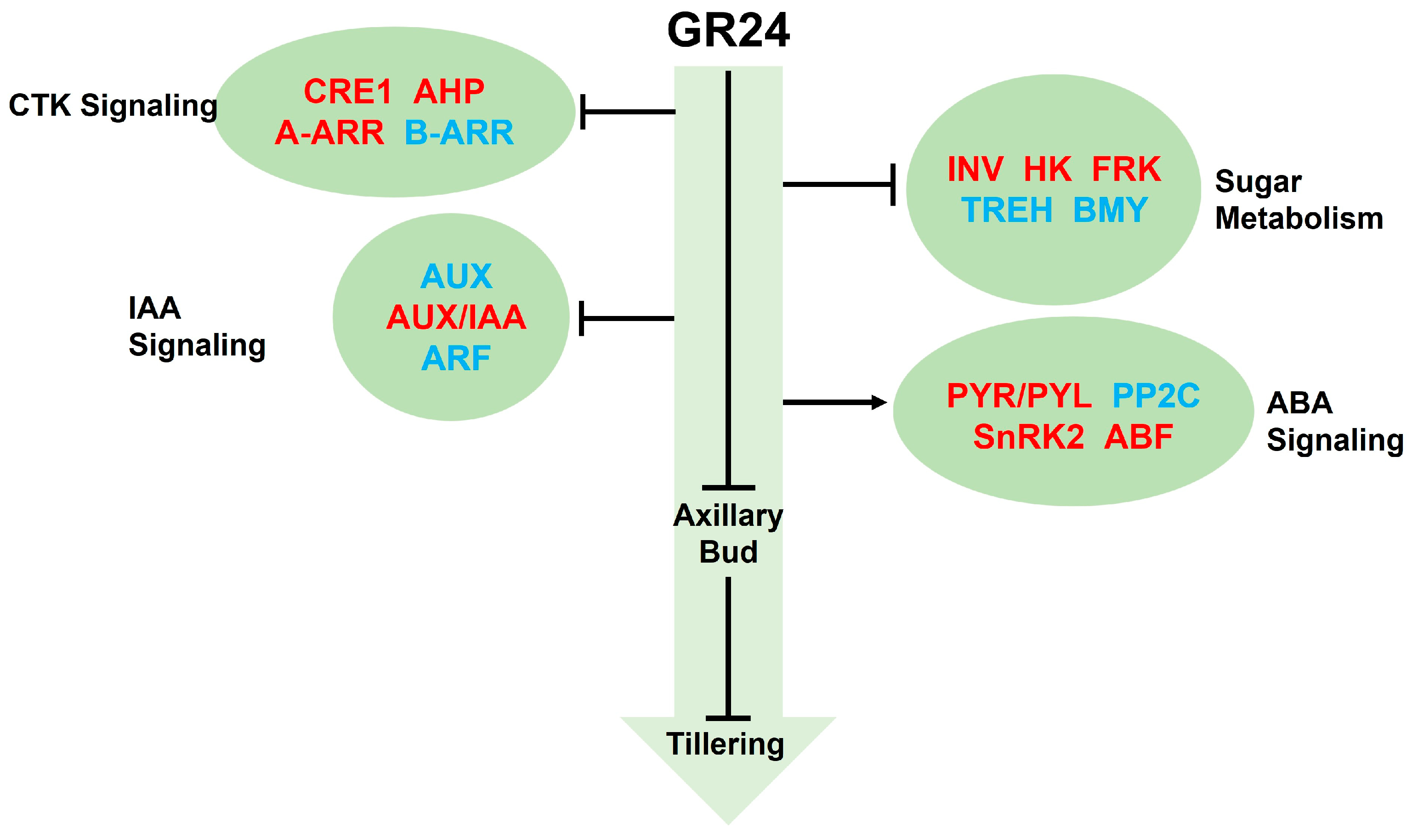
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gasparrini, M.; Giampieri, F.; Forbes-Hernandez, T.Y.; Afrin, S.; Cianciosi, D.; Reboredo-Rodriguez, P.; Varela-Lopez, A.; Zhang, J.J.; Quiles, J.L.; Mezzetti, B.; et al. Strawberry extracts efficiently counteract inflammatory stress induced by the endotoxin lipopolysaccharide in Human Dermal Fibroblast. Food Chem. Toxicol. 2018, 114, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Zhao, M.Z.; Qian, Y.M.; Yu, H.M.; Xia, J.; Wu, E.J. Phenotypic analysis combined with tandem mass tags (TMT) labeling reveal the heterogeneity of strawberry stolon buds. BMC Plant Biol. 2020, 19, 505. [Google Scholar] [CrossRef] [PubMed]
- Tenreira, T.; Lange, M.J.P.; Lange, T.; Bres, C.; Labadie, M.; Monfort, A.; Hernould, M.; Rothan, C.; Denoyes, B. A specific gibberellin 20-oxidase dictates the flowering-runnering decision in diploid strawberry. Plant Cell 2017, 29, 2168–2182. [Google Scholar] [CrossRef] [PubMed]
- Hytönen, T.; Elomaa, P.; Moritz, T.; Junttila, O. Gibberellin mediates daylength-controlled differentiation of vegetative meristems in strawberry (Fragaria × ananassa Duch). BMC Plant Biol. 2009, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Konsin, M.; Voipio, I.; Palonen, P. Influence of photoperiod and duration of short-day treatment on vegetative growth and flowering of strawberry (Fragaria × ananassa Duch.). J. Hort. Sci. Biotech. 2001, 76, 77–82. [Google Scholar] [CrossRef]
- Hytönen, T.; Palonen, P.; Mouhu, K.; Junttila, O. Crown branching and cropping potential in strawberry (Fragaria × ananassa Duch.) can be enhanced by daylength treatments. J. Hort. Sci. Biotech. 2004, 79, 466–471. [Google Scholar] [CrossRef]
- Hytönent, T.; Kurokura, T. Control of flowering and runnering in strawberry. Hortic. J. 2020, 89, 96–107. [Google Scholar] [CrossRef]
- Guo, L.; Plunkert, M.; Luo, X.; Liu, Z.C. Developmental regulation of stolon and rhizome. Curr. Opin. Plant Biol. 2021, 59, 101970. [Google Scholar] [CrossRef]
- Andres, J.; Caruana, J.; Liang, J.H.; Samad, S.; Monfort, A.; Liu, Z.C.; Hytonen, T.; Koskela, E.A. Woodland strawberry axillary bud fate is dictated by a crosstalk of environmental and endogenous factors. Plant Physiol. 2021, 187, 1221–1234. [Google Scholar] [CrossRef]
- Li, Y.L.; Hu, J.T.; Wei, H.; Jeong, B.R. A long-day photoperiod and 6-benzyladenine promote runner formation through upregulation of soluble sugar content in strawberry. Int. J. Mol. Sci. 2020, 21, 4917. [Google Scholar] [CrossRef]
- Yu, J.J.; Li, M.; Li, Q.G.; Wang, R.Y.; Li, R.N.; Yang, Z.M. Reallocation of soluble sugars and IAA regulation in association with enhanced stolon growth by elevated CO2 in creeping bentgrass. Plants 2022, 11, 1500. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.; Yuan, Y.J.; Bai, X.M.; Li, C.N.; Li, J.X.; Chen, H. Carbon and nitrogen metabolism affects kentucky bluegrass rhizome expansion. BMC Plant Biol. 2023, 23, 221. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.T.; Guan, S.C.; Wen, C.J.; Li, P.; Gao, Z.; Chen, X. Auxin and cytokinin coordinate the dormancy and outgrowth of axillary bud in strawberry runner. BMC Plant Biol. 2019, 19, 528. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.Q.; Tan, Q.W.; Zhu, L.Q.; Xu, L.B.; Yang, S.K.; Hu, J.L.; Gao, L.J.; Hou, P.; Shao, Y.H.; Jiang, D.; et al. Low red/far-red ratio can induce cytokinin degradation resulting in the inhibition of tillering in wheat (Triticum aestivum L.). Front. Plant Sci. 2022, 13, 971003. [Google Scholar] [CrossRef] [PubMed]
- Djennane, S.; Hibrand-Saint, O.L.; Kawamura, K.; Lalanne, D.; Laffaire, M.; Thouroude, T.; Chalain, S.; Sakr, S.; Boumaza, R.; Foucher, F.; et al. Impacts of light and temperature on shoot branching gradient and expression of strigolactone synthesis and signalling genes in rose. Plant Cell Environ. 2014, 37, 742–757. [Google Scholar] [CrossRef] [PubMed]
- Evers, J.B.; van der Krol, A.R.; Vos, J.; Struik, P.C. Understanding shoot branching by modelling form and function. Trends Plant Sci. 2011, 16, 464–467. [Google Scholar] [CrossRef]
- Bhoi, A.; Yadu, B.; Chandra, J.; Keshavkant, S. Contribution of strigolactone in plant physiology, hormonal interaction and abiotic stresses. Planta 2021, 254, 28. [Google Scholar] [CrossRef]
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of witchweed (Striga lutea Lour.): Isolation and properties of a potent stimulant. Science 1966, 154, 1189–1190. [Google Scholar] [CrossRef]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pages, V.; Dun, E.A.; Pillot, J.P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–1894. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Beveridge, C.A. Roles for auxin, cytokinin, and strigolactone in regulating shoot branching. Plant Physiol. 2009, 149, 1929–1944. [Google Scholar] [CrossRef]
- Dun, E.A.; de Saint Germain, A.; Rameau, C.; Beveridge, C.A. Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol. 2012, 158, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Yamagami, D.; Umehara, M.; Hanada, A.; Yoshida, S.; Sasaki, Y.; Yajima, S.; Kyozuka, J.; Ueguchi-Tanaka, M.; Matsuoka, M.; et al. Regulation of strigolactone biosynthesis by gibberellin signaling. Plant Physiol. 2017, 174, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Koltai, H.; Prandi, C. Strigolactones: Biosynthesis, synthesis and functions in plant growth and stress responses. In Phytohormones: A Window to Metabolism, Signaling and Biotechnological Applications; Springer: Berlin/Heidelberg, Germany, 2014; pp. 265–288. [Google Scholar]
- Zwanenburg, B.; Mwakaboko, A.S.; Reizelman, A.; Anilkumar, G.; Sethumadhavan, D. Structure and function of natural and synthetic signalling molecules in parasitic weed germination. Pest. Manag. Sci. 2009, 65, 478–491. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Yu, H.; Guo, H.Y.; Lin, T.; Kou, L.Q.; Wang, A.Q.; Shao, N.; Ma, H.Y.; Xiong, G.S.; et al. Transcriptional regulation of strigolactone signalling in Arabidopsis. Nature 2021, 158, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Xu, Y.; Sun, W.X.; Wang, J.Y.; Gao, Y.X.; Wang, L.; Xu, W.P.; Wang, S.P.; Jiu, S.; Zhang, C.X. Strigolactones modulate stem length and diameter of cherry rootstocks through interaction with other hormone signaling pathways. Front. Plant Sci. 2023, 14, 1092654. [Google Scholar] [CrossRef] [PubMed]
- Vogel, D.; Hills, P.; Moore, J.P. Strigolactones GR-24 and Nijmegen applications result in reduced susceptibility of tobacco and grapevine plantlets to Botrytis cinerea infection. Plants 2023, 12, 3202. [Google Scholar] [CrossRef]
- Huang, D.D.; Wang, Y.Y.; Zhang, D.C.; Dong, Y.F.; Meng, Q.X.; Zhu, S.H.; Zhang, L.L. Strigolactone maintains strawberry quality by regulating phenylpropanoid, NO, and H2S metabolism during storage. Postharvest Biol. Tech. 2021, 178, 111546. [Google Scholar] [CrossRef]
- Wu, H.; Li, H.H.; Chen, H.; Qi, Q.; Ding, Q.Q.; Xue, J.; Ding, J.; Jiang, X.N.; Hou, X.L.; Li, Y. Identification and expression analysis of strigolactone biosynthetic and signaling genes reveal strigolactones are involved in fruit development of the woodland strawberry (Fragaria vesca). BMC Plant Biol. 2019, 19, 73. [Google Scholar] [CrossRef]
- Hu, P.P.; Zhang, X.F.; Zhao, X.; Li, G.; Zhao, F.L.; Li, L.J.; Zhou, H.C. Relationship between strigolactones and branching in strawberry. J. Fruit. Sci. 2019, 36, 578–589. [Google Scholar]
- Aksic, M.F.; Tosti, T.; Sredojevic, M.; Milivojevic, J.; Meland, M.; Natic, M. Comparison of sugar profile between leaves and fruits of blueberry and strawberry cultivars grown in organic and integrated production system. Plants 2019, 8, 205. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, H.W.; Wang, X.R.; Xie, X.L.; Yue, X.Y.; Tang, H.R. An alternative cetyltrimethylammonium bromide-based protocol for RNA isolation from blackberry (Rubus L.). Genet. Mol. Res. 2012, 11, 1773–1782. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Lee, D.K.; Choi, Y.D.; Kim, J.K. OsIAA6, a member of the rice Aux/IAA gene family, is involved in drought tolerance and tiller outgrowth. Plant Sci. 2015, 236, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Kishor, P.B.K.; Tiozon, R.N.; Fernie, A.R..; Sreenivasulu, N. Abscisic acid and its role in the modulation of plant growth, development, and yield stability. Trends Plant Sci. 2022, 27, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Agusti, J.; Herold, S.; Schwarz, M.; Sanchez, P.; Ljung, K.; Dun, E.A.; Brewer, P.B.; Beveridge, C.A.; Sieberer, T.; Sehr, E.M.; et al. Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc. Natl. Acad. Sci. USA 2011, 108, 20242–20247. [Google Scholar] [CrossRef] [PubMed]
- Screpanti, C.; Fonne-Pfister, R.; Lumbroso, A.; Rendine, S.; Lachia, M.; De Mesmaeker, A. Strigolactone derivatives for potential crop enhancement applications. Bioorg. Med. Chem. Lett. 2016, 26, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- White, A.R.F.; Mendez, J.A.; Khosla, A.; Nelson, D.C. Rapid analysis of strigolactone receptor activity in a Nicotiana benthamiana dwarf14 mutant. Plant Direct. 2022, 6, e389. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.H.; Wu, Z.; Zheng, J.; Koskela, E.A.; Fan, L.J.; Fan, G.X.; Gao, D.H.; Dong, Z.F.; Hou, S.F.; Feng, Z.K.; et al. The GATA factor HANABA TARANU promotes runner formation by regulating axillary bud initiation and outgrowth in cultivated strawberry. Plant J. 2022, 110, 1237–1254. [Google Scholar] [CrossRef]
- Liu, Y.H.; Yu, L.; Ding, J.H.; Wuang, R.Z.; Huang, Z.G.; Xiao, L.T. Research progress in synergistic regulatory roles of phytohormones in shoot branching. Plant Physiol. J. 2012, 48, 941–948. [Google Scholar]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef]
- Arite, T.; Umehara, M.; Ishikawa, S.; Hanada, A.; Maekawa, M.; Yamaguchi, S.; Kyozuka, J. d14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol. 2008, 50, 1416–1424. [Google Scholar] [CrossRef]
- Su, S.Y.; Luo, W.G.; Xu, M.; Yuan, F.; Li, L.X.; Xu, F.; Su, Y.; Xiao, L.T. Effects of strigolactone on the branching, growth and development in Brassica napus. Mol. Plant Breed. 2020, 18, 6822–6827. [Google Scholar]
- Ni, J.; Gao, C.C.; Chen, M.S.; Pan, B.Z.; Ye, K.Q.; Xu, Z.F. Gibberellin promotes shoot branching in the perennial woody plant Jatropha curcas. Plant Cell Physiol. 2015, 56, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vera, R.; Garcia, J.M.; Pozo, M.J.; Lopez-Raez, J.A. Do strigolactones contribute to plant defence? Mol. Plant Pathol. 2014, 15, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Brewer, P.B.; Dun, E.A.; Ferguson, B.J.; Rameau, C.; Beveridge, C.A. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol. 2009, 150, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.C.; Zhang, Y.; Wang, S.; Wang, W.N.; Xu, X.L.; Wu, J.R.; Fang, Y.L.; Ju, Y.L. Effects of strigolactone and abscisic acid on the quality and antioxidant activity of grapes (Vitis vinifera L.) and wines. Food Chem. X 2022, 16, 100496. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.M.; Cao, X.W.; Wang, J.J.; Xiong, A.S.; Hou, X.L.; Li, Y. Effects of exogenous GR24 on the growth of axillary bud of non- heading Chinese cabbage. J. Nanjing Univ. 2016, 39, 366–372. [Google Scholar]
- Zha, M.; Wang, Y.; Chen, B.X.; Tan, Z.C. Strigolactones and cytokinin interaction in buds in the control of rice tillering. Front. Plant Sci. 2022, 13, 837136. [Google Scholar] [CrossRef]
- Zha, M.; Imran, M.; Wang, Y.; Xu, J.; Ding, Y.; Wang, S.H. Transcriptome analysis revealed the interaction among strigolactones, auxin, and cytokinin in controlling the shoot branching of rice. Plant Cell Rep. 2019, 38, 279–293. [Google Scholar] [CrossRef]
- Waldie, T.; Leyser, O. Cytokinin targets auxin transport to promote shoot branching. Plant Physiol. 2018, 177, 803–818. [Google Scholar] [CrossRef]
- Mauriat, M.; Sandberg, L.G.; Moritz, T. Proper gibberellin localization in vascular tissue is required to control auxin-dependent leaf development and bud outgrowth in hybrid aspen. Plant J. 2011, 67, 805–816. [Google Scholar] [CrossRef]
- Rameau, C.; Bertheloot, J.; Leduc, N.; Andrieu, B.; Foucher, F.; Sakr, S. Multiple pathways regulate shoot branching. Front. Plant Sci. 2015, 5, 741. [Google Scholar] [CrossRef] [PubMed]
- Rinne, P.L.H.; Welling, A.; Vahala, J.; Ripel, L.; Ruonala, R.; Kangasjärvi, J.; van der Schoot, C. Chilling of dormant buds hyperinduces FLOWERING LOCUS T and recruits GA-inducible 1,3-β-Glucanases to reopen signal conduits and release dormancy in Populus. Plant Cell. 2011, 23, 130–146. [Google Scholar] [CrossRef]
- Li, W.J.; Zhang, J.X.; Sun, H.Y.; Wang, S.M.; Chen, K.Q.; Liu, Y.X.; Li, H.; Ma, Y.; Zhang, Z.H. FveRGA1, encoding a DELLA protein, negatively regulates runner production in Fragaria vesca. Planta 2018, 247, 941–951. [Google Scholar] [CrossRef]
- Salehin, M.; Bagchi, R.; Estelle, M. SCFTIR1/AFB-based auxin perception: Mechanism and role in plant growth and development. Plant Cell. 2015, 27, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Pattison, R.J.; Catala, C. Evaluating auxin distribution in tomato (Solanum lycopersicum) through an analysis of the PIN and AUX/LAX gene families. Plant J. 2012, 70, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.L.; Wang, J.L.; Yang, H.Q.; Yuan, C.; Niu, Y.; Tang, Q.L.; Wei, D.Y.; Tian, S.B.; Yang, Y.; Wang, Z.M. Cloning and functional analysis of auxin response factor gene SmARF5 in Solanum melongena. Hortic. Plant J. 2021, 49, 1895–1906. [Google Scholar]
- Yao, C.; Finlayson, S.A. Abscisic acid is a general negative regulator of Arabidopsis axillary bud growth. Plant Physiol. 2015, 169, 611–626. [Google Scholar] [CrossRef]
- Ortiz-Morea, F.A.; Vicentini, R.; Silva, G.F.F.; Silva, E.M.; Carrer, H.; Rodrigues, A.P.; Nogueira, F.T.S. Global analysis of the sugarcane microtranscriptome reveals a unique composition of small RNAs associated with axillary bud outgrowth. J. Exp. Bot. 2013, 64, 2307–2320. [Google Scholar] [CrossRef]
- Guo, J.J.; Yang, X.H.; Weston, D.J.; Chen, J.G. Abscisic acid receptors: Past, present and future. J. Integr. Plant Biol. 2011, 53, 469–479. [Google Scholar] [CrossRef]
- Wei, X.Y.; Yang, J.; Lei, D.; Feng, H.; Yang, Z.N.; Wen, G.Q.; He, Z.Y.; Zeng, W.J.; Zou, J. The SlTCP26 promoting lateral branches development in tomato. Plant Cell Rep. 2021, 40, 1115–1126. [Google Scholar] [CrossRef]
- Ge, H.J.; Li, G.F.; Wan, S.W.; Zhao, A.H.; Huang, Y.; Ma, R.Q.; Zhang, R.F.; Song, Y.J.; Sha, G.L. Whole genome re-sequencing and transcriptome reveal an alteration in hormone signal transduction in a more-branching mutant of apple. Gene 2022, 818, 146214. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.J.; Xie, L.L.; Zhao, Q.; Sun, Y.T.; Zhang, D. Cyclanilide induces lateral bud outgrowth by modulating cytokinin biosynthesis and signalling pathways in apple identified via transcriptome analysis. Int. J. Mol. Sci. 2023, 23, 581. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Perez-Garcia, M.D.; Daviere, J.M.; Barbier, F.; Oge, L.; Gentilhomme, J.; Voisine, L.; Peron, T.; Launay-Avon, A.; Clement, G.; et al. Outgrowth of the axillary bud in rose is controlled by sugar metabolism and signalling. J. Exp. Bot. 2021, 72, 3044–3060. [Google Scholar] [CrossRef] [PubMed]
- Tarancón, C.; González-Grandío, E.; Oliveros, J.C.; Nicolas, M.; Cubas, P. A conserved carbon starvation response underlies bud dormancy in woody and herbaceous species. Front. Plant Sci. 2017, 8, 788. [Google Scholar] [CrossRef]
- Fan, Z.Q.; Huang, G.W.; Fan, Y.R.; Yang, J.Y. Sucrose facilitates rhizome development of perennial rice (Oryza longistaminata). Int. J. Mol. Sci. 2022, 23, 13396. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Jiang, Y.; He, C.; She, M.; Li, M.; Chen, Q.; Zhang, Y.; Lin, Y.; Zhang, Y.; Wang, Y.; et al. Exogenous GR24 Inhibits Strawberry Tillering by Affecting the Phytohormone Signaling and Sugar Metabolism Pathways. Agronomy 2023, 13, 3078. https://doi.org/10.3390/agronomy13123078
Peng Y, Jiang Y, He C, She M, Li M, Chen Q, Zhang Y, Lin Y, Zhang Y, Wang Y, et al. Exogenous GR24 Inhibits Strawberry Tillering by Affecting the Phytohormone Signaling and Sugar Metabolism Pathways. Agronomy. 2023; 13(12):3078. https://doi.org/10.3390/agronomy13123078
Chicago/Turabian StylePeng, Yuting, Yuyan Jiang, Caixia He, Musha She, Mengyao Li, Qing Chen, Yong Zhang, Yuanxiu Lin, Yunting Zhang, Yan Wang, and et al. 2023. "Exogenous GR24 Inhibits Strawberry Tillering by Affecting the Phytohormone Signaling and Sugar Metabolism Pathways" Agronomy 13, no. 12: 3078. https://doi.org/10.3390/agronomy13123078
APA StylePeng, Y., Jiang, Y., He, C., She, M., Li, M., Chen, Q., Zhang, Y., Lin, Y., Zhang, Y., Wang, Y., He, W., Wang, X., Tang, H., & Luo, Y. (2023). Exogenous GR24 Inhibits Strawberry Tillering by Affecting the Phytohormone Signaling and Sugar Metabolism Pathways. Agronomy, 13(12), 3078. https://doi.org/10.3390/agronomy13123078







