Effects of Azotobacter and Carbon Dioxide Concentrations on the Growth and Yield of Rice Plants Grown in Two Paddy Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Effects of Azotobacter on Rice Growth and Yield under Elevated CO2
2.1.1. Soil Preparation and Property Analysis
2.1.2. Azotobacter Strains
2.1.3. Experimental Design
2.1.4. Rice Growth Parameters
2.1.5. Plant Nutrient Analysis
2.2. Statistical Analyses
3. Results
3.1. Effects of Azotobacter and CO2 Concentrations on Rice Growth
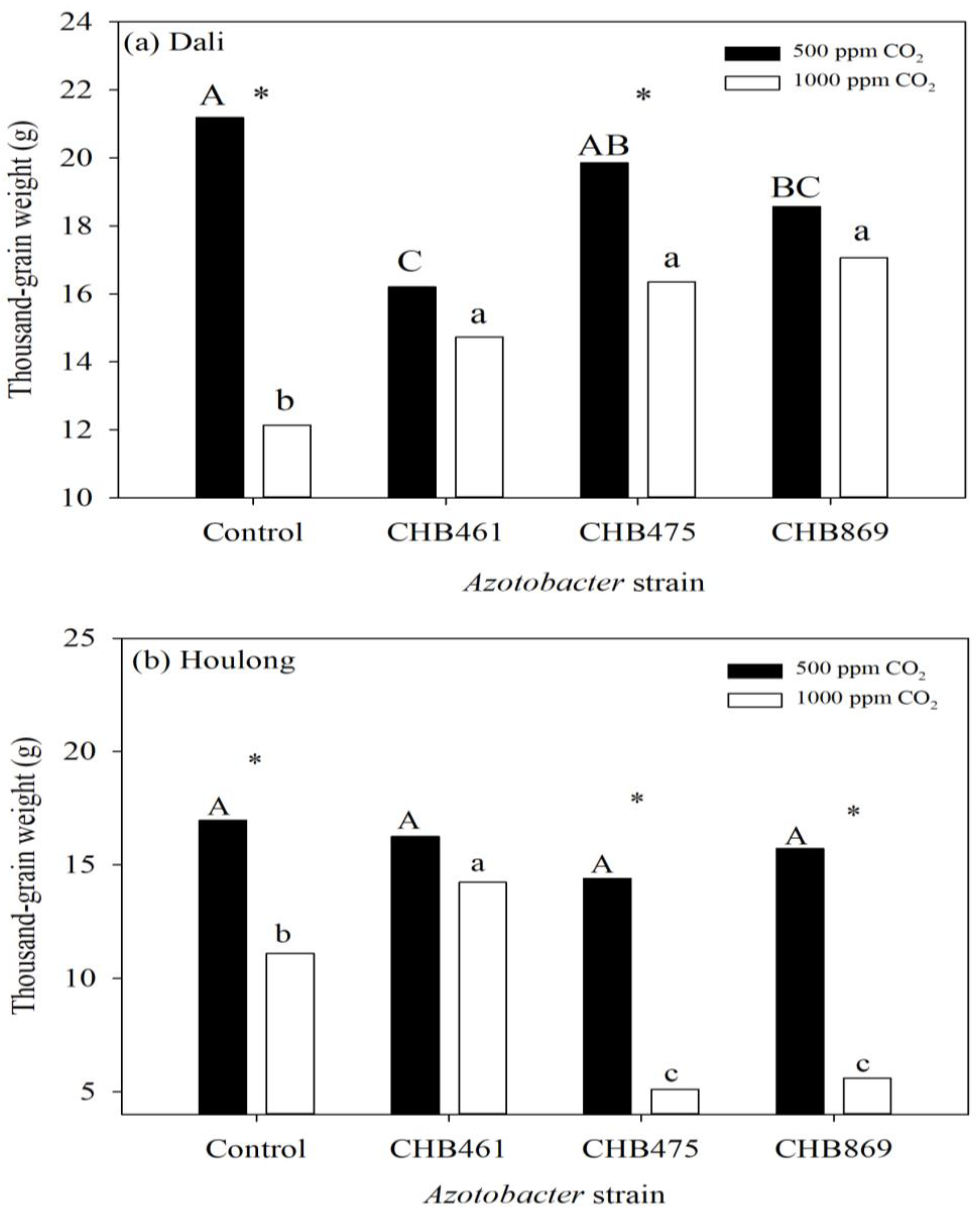
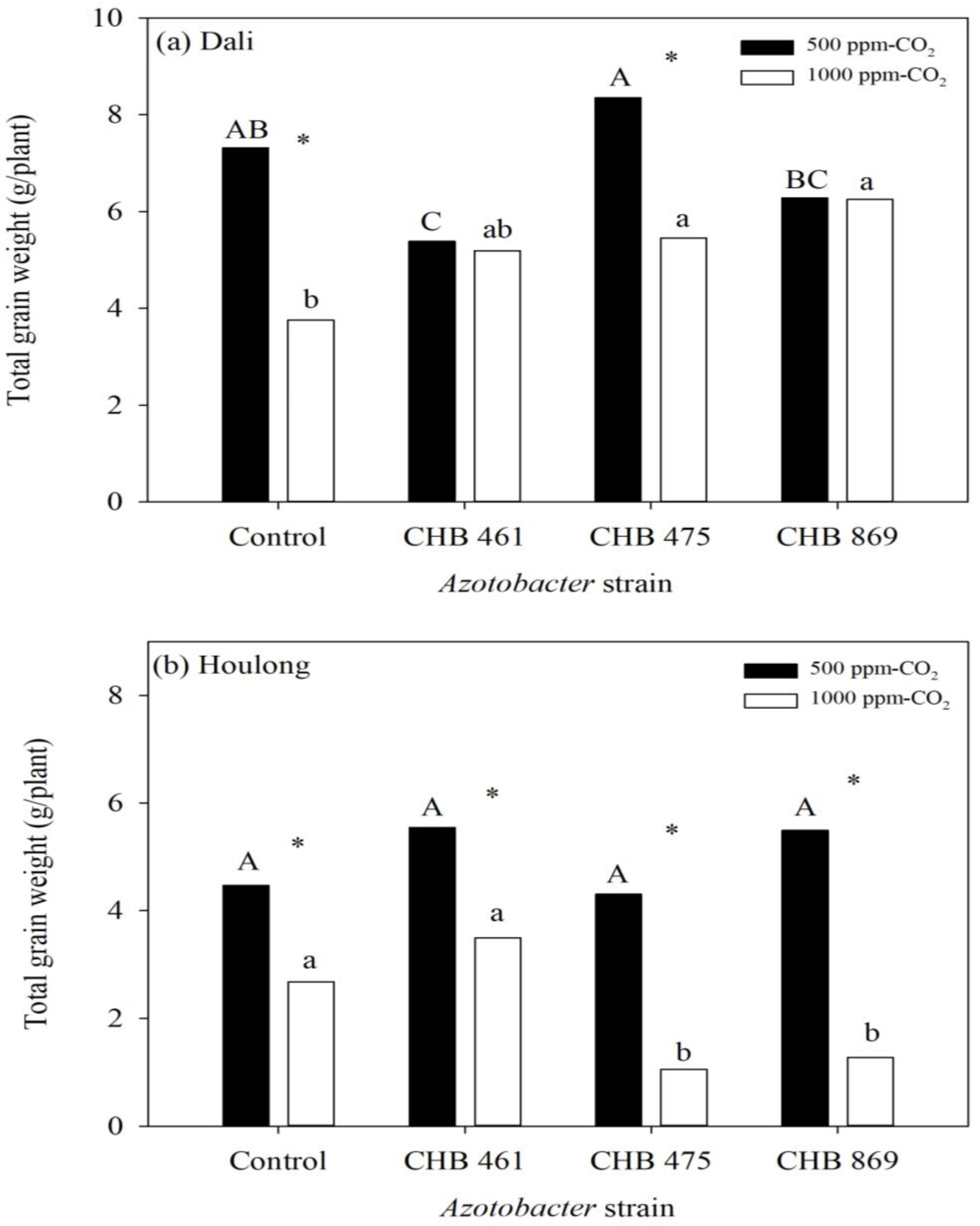
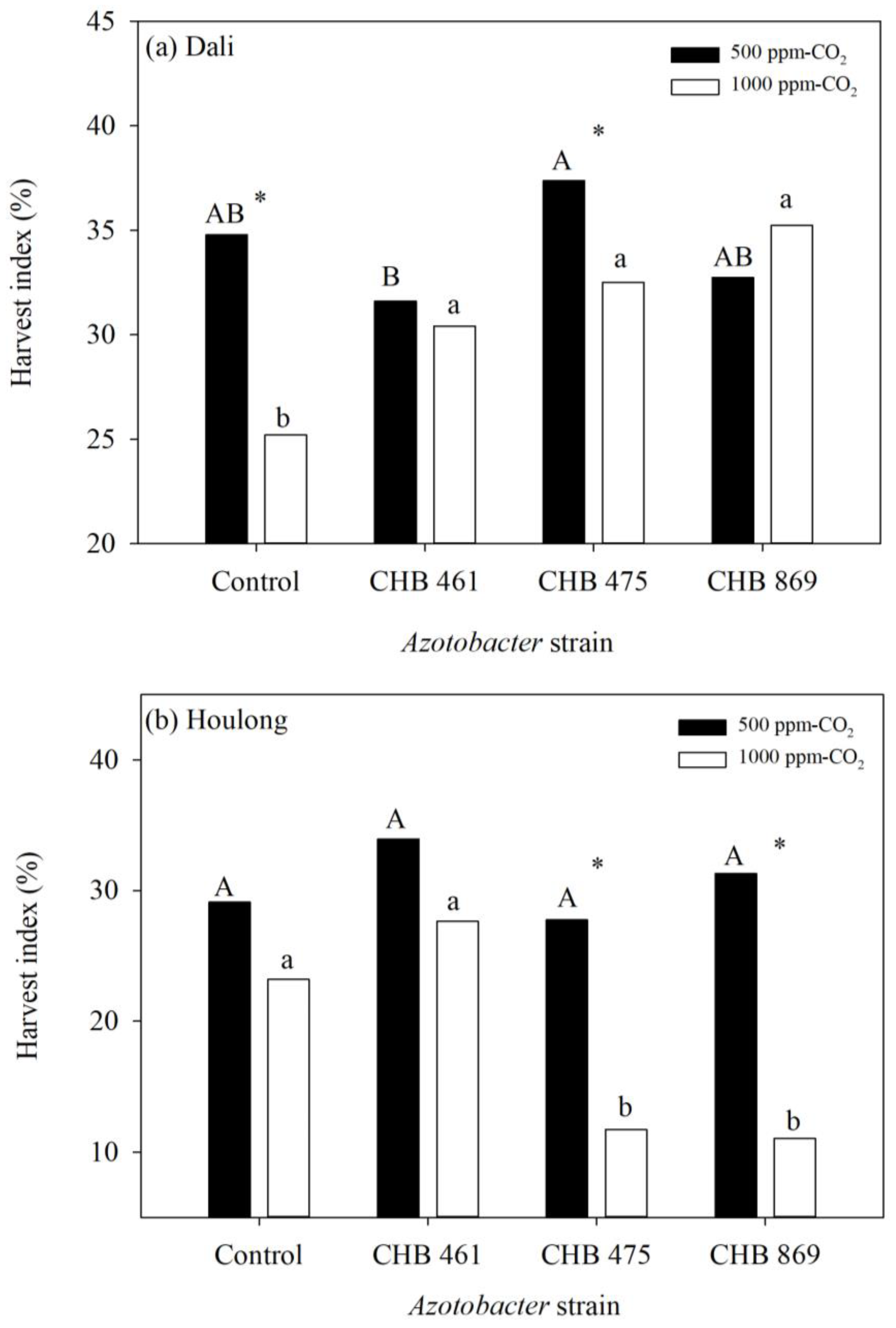
3.2. Effects of Azotobacter and CO2 Concentrations on Rice Yield
3.3. Effects of Azotobacter and CO2 Concentrations on Nutrient Uptake by Rice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, L.; Yang, Y.; Liu, J.; Hu, Z.; Liu, X.; Tian, M.; Yang, W.; Jin, J.; Wang, H.; Wang, Y.; et al. Different responses of ammonia-oxidizing archaea and bacteria in paddy soils to elevated CO2 concentration. Environ. Pollut. 2021, 286, 117558. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Chen, W.; Tong, K.; Wang, Y.; Jing, L.; Wang, Y.; Yang, L. Response of rice growth and leaf physiology to elevated CO2 concentrations: A meta-analysis of 20-year FACE studies. Sci. Total Environ. 2022, 807, 151017. [Google Scholar] [CrossRef] [PubMed]
- Salihi, M.S.; Ahmad-Hamdani, M.S.; Jusoh, M. The impact of carbon dioxide (CO2) enrichment on rice (Oryza sativa L.) production: A review. Pak. J. Bot. 2023, 55, 1197–1204. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, J.; Xu, X.; Liu, G.; Zhu, J.G. Effects of free-air CO2 enrichment (FACE) and nitrogen (N) supply on N uptake and utilization of indica and japonica cultivars (Oryza sativa L.). Ecol. Process. 2020, 9, 35. [Google Scholar] [CrossRef]
- Jing, L.; Wang, J.; Shen, S.; Wang, Y.; Zhu, J.; Wang, Y.; Yang, L. The impact of elevated CO2 and temperature on grain quality of rice grown under open-air field conditions. J. Sci. Food Agric. 2016, 96, 3658–3667. [Google Scholar] [CrossRef]
- Prusty, S.; Sahoo, R.K.; Sharaya, R.; Tuteja, N.; Gill, S.S. Unraveling the potential of native Azotobacter and Azospirillum spp. formulations for sustainable crop production of rice (Oryza sativa L. var. Khandagiri). S. Afr. J. Bot. 2023, 162, 10–19. [Google Scholar] [CrossRef]
- Nedunchezhiyan, V.; Velusamy, M.; Subburamu, K. Seed priming to mitigate the impact of elevated carbon dioxide associated temperature stress on germination in rice (Oryza sativa L.). Arch. Agron. Soil Sci. 2020, 66, 83–95. [Google Scholar] [CrossRef]
- Kumar, U.; Nayak, A.K.; Sahoo, S.; Kumar, A.; Kaviraj, M.; Shahid, M. Combined effects of elevated CO2, N fertilizer and water deficit stress on diazotrophic community in sub-humid tropical paddy soil. Appl. Soil Ecol. 2020, 155, 103682. [Google Scholar] [CrossRef]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.B.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef]
- Zaki, N.; Gomaa, A.M.; Galal, A.; Farrag, A.A. The associative impact of certain diazotrophs and farmyard manure on two rice varieties grown in a newly cultivated land. Res. J. Agric. Biol. Sci. 2009, 5, 185–190. [Google Scholar]
- Sahoo, R.K.; Ansari, M.W.; Dangar, T.K.; Mohanty, S.; Tuteja, N. Phenotypic and molecular characterisation of efficient nitrogen-fixing Azotobacter strains from rice fields for crop improvement. Protoplasma 2014, 251, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Minut, M.; Diaconu, M.; Rosca, M.; Cozma, P.; Bulgariu, L.; Gavrilescu, M. Screening of Azotobacter, Bacillus and Pseudomonas species as plant growth-promoting bacteria. Processes 2023, 11, 80. [Google Scholar] [CrossRef]
- Choudhury, A.T.M.A.; Kennedy, I.R. Prospects and potentials for systems of biological nitrogen fixation in sustainable rice production. Biol. Fertil. Soils 2004, 39, 219–227. [Google Scholar] [CrossRef]
- Piao, Z.; Cui, Z.J.; Yin, B.; Hu, J.; Zhou, C.H.; Xie, G.H.; Su, B.L.; Yin, S.X. Changes in acetylene reduction activities and effects of inoculated rhizosphere nitrogen-fixing bacteria on rice. Biol. Fertil. Soils 2005, 41, 371–378. [Google Scholar] [CrossRef]
- Das, A.C.; Saha, D. Effect of diazotrophs on the mineralization of organic nitrogen in the rhizosphere soils of rice (Oryza sativa). J. Crop Weed 2007, 3, 47–51. [Google Scholar]
- Sahoo, R.K.; Pradhan, M. Azotobacter vinellandii SINAz1 increases growth and productivity in rice under salinity stress. Legume Res. 2023, 46, 196–203. [Google Scholar] [CrossRef]
- Tejera, N.; Lluch, C.; Martínez-Toledo, M.V.; González-Lόpez, J. Isolation and characterization of Azotobacter and Azospirillum strains from the sugarcane rhizosphere. Plant Soil 2005, 270, 223–232. [Google Scholar] [CrossRef]
- Martinez-Toledo, M.V.; Gonzalez-Lopez, J.; de la Rubia, T.; Ramos-Cormenzana, A. Isolation and characterization of Azotobacter chroococcum from the roots of Zea mays. FEMS Microbiol. Ecol. 1985, 31, 197–203. [Google Scholar] [CrossRef]
- Kannan, T.; Ponmurugan, P. Response of paddy (Oryza sativa L.) varieties to Azospirillum brasilense inoculation. J. Phytol. 2010, 2, 8–13. [Google Scholar]
- Banik, A.; Dash, G.K.; Swain, P.; Kumar, U.; Mukhopadhyay, S.K.; Dangar, T.K. Application of rice (Oryza sativa L.) root endophytic diazotrophic Azotobacter sp. strain Avi2 (MCC 3432) can increase rice yield under green house and field condition. Microbiol. Res. 2019, 219, 56–65. [Google Scholar] [CrossRef]
- Smith, J.L.; Doran, J.W. Measurement and use of pH and electrical conductivity. In Methods for Assessing Soil Quality; Doran, J.W., Jones, A.J., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 169–185. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining organic carbon in soils: Effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 1934, 63, 251–263. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen–total. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Soil Science Society of America: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Tening, A.S.; Omueti, J.A.I. Suitability of extractants for predicting iron in soils of the humid zone of South-Western Nigeria. Agric. Biol. J. N. Am. 2011, 2, 1244–1250. [Google Scholar] [CrossRef]
- Chen, S.-L.; Tsai, M.-K.; Huang, Y.-M.; Huang, C.-H. Diversity and characterization of Azotobacter isolates obtained from rice rhizosphere soils in Taiwan. Ann. Microbiol. 2018, 68, 17–26. [Google Scholar] [CrossRef]
- Subba Rao, N.S. Biofertilizers in Argriculture; Taylor&Francis: New York, NY, USA, 1982; p. 200. [Google Scholar]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice; The International Rice Research Institute: Manila, Philippines, 1976. [Google Scholar]
- Newman, J.A.; Abner, M.L.; Dado, R.G.; Gibson, D.J.; Brookings, A.; Parsons, A.J. Effects of elevated CO2, nitrogen and fungal endophyte-infection on tall fescue: Growth, photosynthesis, chemical composition and digestibility. Glob. Chang. Biol. 2003, 9, 425–437. [Google Scholar] [CrossRef]
- Fageria, N.K.; Moreira, A.; Coelho, A.M. Yield and yield components of upland rice as influenced by nitrogen sources. J. Plant Nutr. 2011, 34, 361–370. [Google Scholar] [CrossRef]
- Wolf, B. A comprehensive system of leaf analysis and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Silva, H.; Cunha, A. Siderophore-producing rhizobacteria as a promising tool for empowering plants to cope with iron limitation in saline soils: A review. Pedosphere 2019, 29, 409–420. [Google Scholar] [CrossRef]
- Hider, R.C.; Kong, X.L. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010, 27, 637–657. [Google Scholar] [CrossRef]
- Pii, Y.; Marastoni, L.; Springeth, C.; Fontanella, M.C.; Beone, G.M.; Cesco, S.; Mimmo, T. Modulation of Fe acquisition process by Azospirillum brasilense in cucumber plants. Environ. Exp. Bot. 2016, 130, 216–225. [Google Scholar] [CrossRef]
- O’Toole, P.; Knoeles, R. Efficiency of acetylene reduction (nitrogen fixation) in soil: Effect of type and concentration of available carbohydrate. Soil Biol. Biochem. 1973, 5, 789–797. [Google Scholar] [CrossRef]
- Green, S.J.; Inbar, E.; Michel, F.C., Jr.; Hadar, Y.; Minz, D. Succession of bacterial communities during early plant development: Transition from seed to root and effect of compost amendment. Appl. Environ. Microbiol. 2006, 72, 3975–3983. [Google Scholar] [CrossRef]
- Poly, F.; Ranjard, L.; Nazaret, S.; Gourbière, F.; Monrozier, L.J. Comparison of nifH gene pools in soils and soil microenvironments with contrasting properties. Appl. Environ. Microbiol. 2001, 67, 2255–2262. [Google Scholar] [CrossRef]
- Prakamhang, J.; Minamisawa, K.; Teamtaisong, K.; Boonkerd, N.; Teaumroong, N. The communities of endophytic diazotrophic bacteria in cultivated rice (Oryza sativa L.). Appl. Soil Ecol. 2009, 42, 141–149. [Google Scholar] [CrossRef]
- Sylvia, D.M.; Fuhrmann, J.J.; Hartel, P.G.; Zuberer, D.A. Priciples and Applications of Soil Microbiology, 2nd ed.; Pearson Education Inc.: Upper Saddle River, NJ, USA, 2005; p. 640. [Google Scholar]
- Knowles, R.; Denike, D. Effect of ammonium-, nitrite- and nitrate nitrogen on anaerobic nitrogenase activity in soil. Soil Biol. Biochem. 1974, 6, 353–358. [Google Scholar] [CrossRef]
- Rao, V.R. Nitrogen fixation as influenced by moisture, ammonium sulfate, and organic sources in a paddy soil. Soil Biol. Biochem. 1976, 8, 445–448. [Google Scholar]
- Cvijanović, G.; Dozet, G.; Dukić, V.; Subić, J.; Cvijanović, D. Effects of nitrogen fertilising on the preceding crop and the application of Co and Mo on Azotobacter abundance in soya bean. Rom. Biotechnol. Lett. 2011, 16, 74–80. [Google Scholar]
- Kim, H.Y.; Lieffering, M.; Kobayashi, K.; Okada, M.; Miura, S. Seasonal changes in the effects of elevated CO2 on rice at three levels of nitrogen supply: A free air CO2 enrichment (FACE) experiment. Glob. Chang. Biol. 2003, 9, 826–837. [Google Scholar] [CrossRef]


| pH a | EC b | SOM c | Avail. N | Avail. P | Exch. K | Exch. Ca | Exch. Mg | Avail. Fe | Avail. Mn | Avail. Cu | Avail. Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil | dS/m | g/kg | ---------------------------------------------mg/kg------------------------------------------- | |||||||||
| Dali | 5.01 | 0.78 | 31.6 | 64.6 | 34.0 | 101 b | 930 | 170 | 507 | 3.96 | 10.1 | 8.83 |
| Houlong | 4.81 | 0.50 | 22.1 | 61.9 | 57.6 | 132 a | 576 | 118 | 543 | 4.40 | 3.75 | 3.06 |
| [CO2] | Inoculation | Dry Root Weight (g/Plant) | Dry Shoot Weight (g/Plant) | Total Dry Weight (g/Plant) |
|---|---|---|---|---|
| 500 ppm | Control | 4.46 bc * | 13.7 ab | 18.2 ab |
| A. beijerinckii CHB 461 | 5.66 ab | 11.8 bc | 17.5 bc | |
| A. vinelandii CHB 475 | 6.83 a | 13.9 a | 20.8 a | |
| A. chroococcum CHB 869 | 5.34 ab | 12.6 ab | 17.9 ab | |
| 1000 ppm | Control | 2.99 c | 10.7 c | 13.7 d |
| A. beijerinckii CHB 461 | 3.40 c | 11.2 c | 14.6 cd | |
| A. vinelandii CHB 475 | 3.29 c | 11.2 c | 14.5 cd | |
| A. chroococcum CHB 869 | 4.48 bc | 11.6 c | 16.1 b–d |
| [CO2] | Inoculation | Dry Root Weight (g/Plant) | Dry Shoot Weight (g/Plant) | Total Dry Weight (g/Plant) |
|---|---|---|---|---|
| 500 ppm | Control | 3.65 b * | 11.1 ab | 14.7 a |
| A. beijerinckii CHB 461 | 3.75 b | 11.0 ab | 14.8 a | |
| A. vinelandii CHB 475 | 2.67 c | 10.8 a–c | 13.4 ab | |
| A. chroococcum CHB 869 | 3.47 bc | 11.8 a | 15.3 a | |
| 1000 ppm | Control | 2.66 c | 8.55 c | 11.2 b |
| A. beijerinckii CHB 461 | 3.37 bc | 8.87 bc | 12.2 ab | |
| A. vinelandii CHB 475 | 4.76 a | 8.93 bc | 13.7 ab | |
| A. chroococcum CHB 869 | 3.86 ab | 10.7 a–c | 14.5 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-L.; Huang, C.-H. Effects of Azotobacter and Carbon Dioxide Concentrations on the Growth and Yield of Rice Plants Grown in Two Paddy Soils. Agronomy 2023, 13, 2998. https://doi.org/10.3390/agronomy13122998
Chen S-L, Huang C-H. Effects of Azotobacter and Carbon Dioxide Concentrations on the Growth and Yield of Rice Plants Grown in Two Paddy Soils. Agronomy. 2023; 13(12):2998. https://doi.org/10.3390/agronomy13122998
Chicago/Turabian StyleChen, Syuan-Lu, and Cheng-Hua Huang. 2023. "Effects of Azotobacter and Carbon Dioxide Concentrations on the Growth and Yield of Rice Plants Grown in Two Paddy Soils" Agronomy 13, no. 12: 2998. https://doi.org/10.3390/agronomy13122998
APA StyleChen, S.-L., & Huang, C.-H. (2023). Effects of Azotobacter and Carbon Dioxide Concentrations on the Growth and Yield of Rice Plants Grown in Two Paddy Soils. Agronomy, 13(12), 2998. https://doi.org/10.3390/agronomy13122998






