Comprehensive Metal-Based Nanopriming for Improving Seed Germination and Initial Growth of Field Pea (Pisum sativum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Priming Nanomaterial and Characterization of Nanoparticles
2.2. Seed Materials
2.3. Priming Treatments
2.4. Assessment of Seed Germination and Germination-Related Parameters of Field Pea Seeds
2.5. Assessment of Physiological Response of Pea Seeds to Priming
2.6. Statistical Analysis
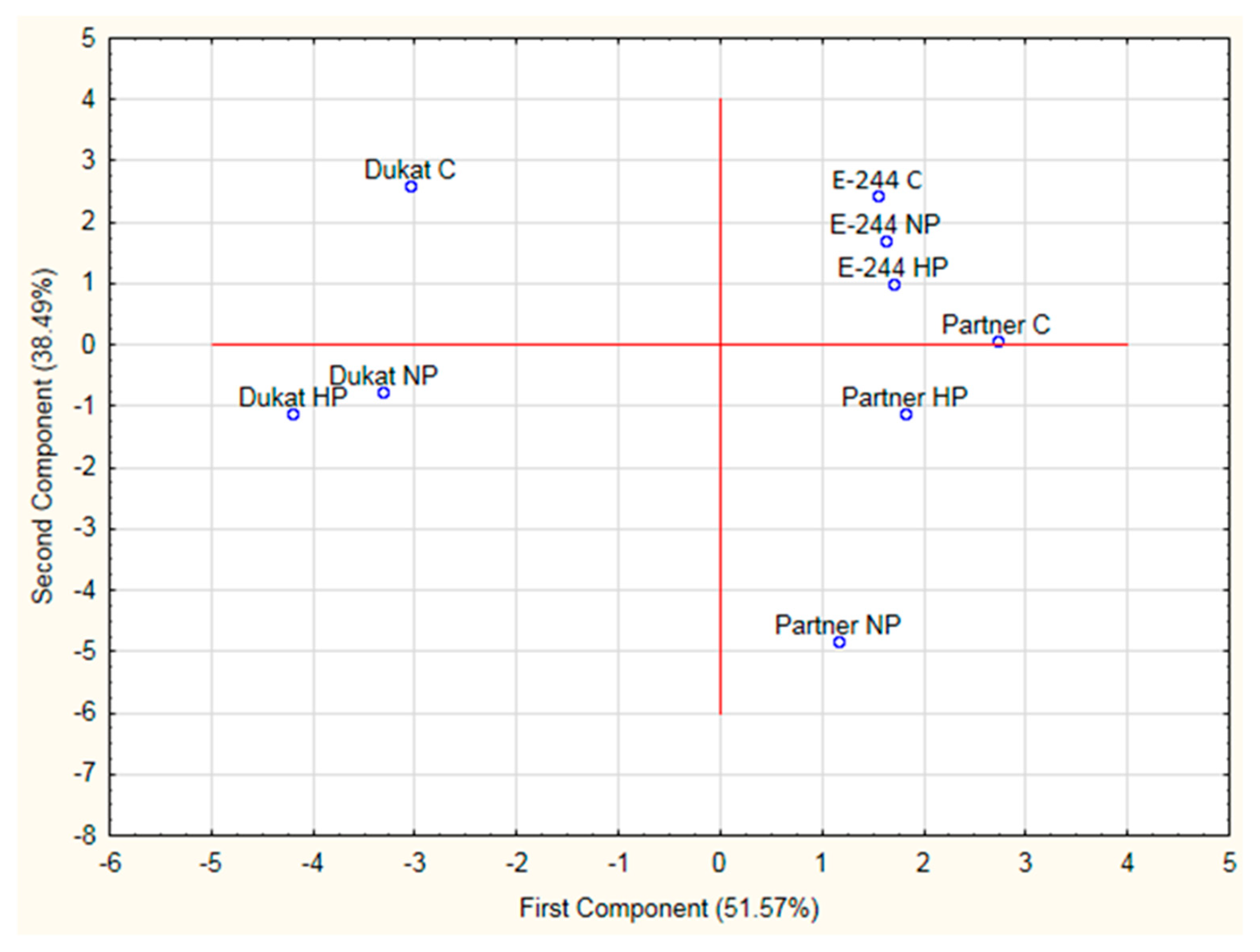
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, V.; Singh, C.M.; Chugh, V.; Kamaluddin, K.; Prajapati, P.K.; Mishra, A.; Kaushik, P.; Dhanda, P.S.; Yadav, A.; Satyendra. Morpho-physiological and Biochemical Responses of Field Pea Genotypes under Terminal Heat Stress. Plants 2023, 12, 256. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.A.; Gupta, M.; Ganai, M.A.; Ahanger, R.A.; Bhat, H.A. Yield, Soil Health and Nutrient Utilization of Field Pea (Pisum sativum L.) as Affected by Phosphorus and Biofertilizers under Subtropical Conditions of Jammu. Int. J. Mod. Plant Anim. Sci. 2013, 1, 1–8. [Google Scholar] [CrossRef]
- Khan, T.N.; Croser, J.S. Pea—Overview. Encycl. Grain Sci. 2004, 1, 418–427. [Google Scholar] [CrossRef]
- Krga, I. Yield and Quality of Field Peas and Oats Mixtures Depending on the Stage of Use and Nitrogen Fertilizer. Ph.D. Thesis, Faculty of Agriculture, University of Belgrade, Belgrade, Serbia, 2022; p. 408. [Google Scholar]
- FAOSTAT Database. Food and Agriculture Organization Statistics. Available online: https://www.fao.org/faostat/en/ (accessed on 14 February 2023).
- Abdelaal, K.; Alsubeie, M.S.; Hafez, Y.; Emeran, A.; Moghanm, F.; Okasha, S.; Omara, R.; Basahi, M.A.; Darwish, D.B.E.; Ibrahim, M.F.M.; et al. Physiological and Biochemical Changes in Vegetable and Field Crops under Drought, Salinity and Weeds Stresses: Control Strategies and Management. Agriculture 2022, 12, 2084. [Google Scholar] [CrossRef]
- Haddoudi, L.; Hdira, S.; Hanana, M.; Romero, I.; Haddoudi, I.; Mahjoub, A.; Ben Jouira, H.; Djébali, N.; Ludidi, N.; Sanchez-Ballesta, M.T.; et al. Evaluation of the Morpho-physiological, Biochemical and Molecular Responses of Contrasting Medicago truncatula Lines under Water Deficit Stress. Plants 2021, 10, 2114. [Google Scholar] [CrossRef] [PubMed]
- Shelar, A.; Nile, S.H.; Singh, A.V.; Rothenstein, D.; Bill, J.; Xiao, J.; Chaskar, M.; Kai, G.; Patil, R. Recent Advances in Nano-Enabled Seed Treatment Strategies for Sustainable Agriculture: Challenges, Risk Assessment, and Future Perspectives. Nano-Micro Lett. 2023, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- do Espirito Santo Pereira, A.; Caixeta Oliveira, H.; Fernandes Fraceto, L.; Santaella, C. Nanotechnology Potential in Seed Priming for Sustainable Agriculture. Nanomaterials 2021, 11, 267. [Google Scholar] [CrossRef]
- Tamindžić, G.; Ignjatov, M.; Miljaković, D.; Červenski, J.; Milošević, D.; Nikolić, Z.; Vasiljević, S. Seed Priming Treatments to Improve Heat Stress Tolerance of Garden Pea (Pisum sativum L.). Agriculture 2023, 13, 439. [Google Scholar] [CrossRef]
- Guo, X.; Zhi, W.; Feng, Y.; Zhou, G.; Zhu, G. Seed Priming Improved Salt-stressed Sorghum Growth by Enhancing Antioxidative Defense. PLoS ONE 2022, 17, e0263036. [Google Scholar] [CrossRef]
- Yavari, A.; Ghasemifar, E.; Shahgolzari, M. Seed Nanopriming to Mitigate Abiotic Stresses in Plants; Book Chapter; InotechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Salam, A.; Afridi, M.S.; Javed, M.A.; Saleem, A.; Hafeez, A.; Khan, A.R.; Zeeshan, M.; Ali, B.; Azhar, W.; Sumaira, U.Z.; et al. Nano-Priming against Abiotic Stress: A Way Forward towards Sustainable Agriculture. Sustainability 2022, 14, 14880. [Google Scholar] [CrossRef]
- Saha, D.; Choyal, P.; Mishra, U.N.; Dey, P.; Bandana Bose, B.; Prathibha, M.D.; Gupta, N.K.; Mehta, B.K.; Kumar, P.; Pandey, S.; et al. Drought Stress Responses and Inducing Tolerance by Seed Priming Approach in Plants. Plant Stress 2020, 4, 100066. [Google Scholar] [CrossRef]
- Kandhol, N.; Singh, V.P.; Ramawat, N.; Prasad, R.; Chauhan, D.K.; Sharma, S.; Grillo, R.; Sahi, S.; Peralta-Videa, J.; Tripathi, D.K. Nano-priming: Impression on the Beginner of Plant Life. Plant Stress 2023, 5, 100091. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Li, X.; Xin, C.; Si, J.; Li, S.; Li, Y.; Zheng, X.; Li, H.; Wei, X.; et al. Nano-ZnO Priming induces Salt Tolerance by Promoting Photosynthetic Carbon Assimilation in Wheat. Arch. Agron. Soil Sci. 2020, 66, 1259–1273. [Google Scholar] [CrossRef]
- Ye, Y.; Cota-Ruiz, K.; Hern’andez-Viezcas, J.A.; Vald´es, C.; Medina-Velo, I.A.; Turley, R.S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Manganese Nanoparticles control Salinity-modulated Molecular Responses in Capsicum annuum L. through Priming: A Sustainable Approach for Agriculture. ACS Sustain. Chem. Eng. 2020, 8, 1427–1436. [Google Scholar] [CrossRef]
- Khalaki, M.A.; Moameri, M.; Lajayer, B.A.; Astatkie, T. Influence of Nano-priming on Seed Germination and Plant Growth of Field and Medicinal Plants. Plant Growth Regul. 2021, 93, 13–28. [Google Scholar] [CrossRef]
- Azimi, F.; Oraei, M.; Gohari, G.; Panahirad, S.; Farmarzi, A. Chitosan-selenium Nanoparticles (Cs–Se NPs) Modulate the Photosynthesis Parameters, Antioxidant Enzymes Activities and Essential Oils in Dracocephalum moldavica L. under Cadmium Toxicity Stress. Plant Physiol. Biochem. 2021, 167, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-based Sustainable Agriculture and Food Science: Recent Advances and Future Outlook. Front. Bioeng. Biotechnol. 2020, 2, 9954. [Google Scholar] [CrossRef]
- Xin, X.; Zhao, F.; Rho, J.Y.; Goodrich, S.L.; Sumerlin, B.S.; He, Z. Use of Polymeric Nanoparticles to Improve Seed Germination and Plant Growth under Copper Stress. Sci. Total Environ. 2020, 745, 141055. [Google Scholar] [CrossRef]
- Szőllősi, R.; Molnár, Á.; Kondak, S.; Kolbert, Z. Dual Effect of Nanomaterials on Germination and Seedling Growth: Stimulation vs. Phytotoxicity. Plants 2020, 9, 1745. [Google Scholar] [CrossRef]
- Esper Neto, M.; Britt, D.W.; Lara, L.M.; Cartwright, A.; dos Santos, R.F.; Inoue, T.T.; Batista, M.A. Initial Development of Corn Seedlings after Seed Priming with Nanoscale Synthetic Zinc Oxide. Agronomy 2020, 10, 307. [Google Scholar] [CrossRef]
- González-García, Y.; López-Vargas, E.R.; Pérez-Álvarez, M.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Valdés-Reyna, J.; Pérez-Labrada, F.; Juárez-Maldonado, A. Seed Priming with Carbon Nanomaterials Improves the Bioactive Compounds of Tomato Plants under Saline Stress. Plants 2022, 11, 1984. [Google Scholar] [CrossRef] [PubMed]
- Acharya, P.; Jayaprakasha, G.K.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Nanoparticle-Mediated Seed Priming Improves Germination, Growth, Yield, and Quality of Watermelons (Citrullus lanatus) at Multi-locations in Texas. Sci. Rep. 2020, 10, 5037. [Google Scholar] [CrossRef]
- Bootz, A.; Vogel, V.; Schubert, D.; Kreuter, J. Comparison of scanning electron microsco-py, dynamic light scattering and analytical ultracentrifugation for the sizing of poly (butyl cyanoacry-late) nanoparticles. Eur. J. Pharm. Biopharm. 2004, 57, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Yan, K.; Li, J.; Zafar, S.; Hasnain, Z.; Aslam, N.; Iqbal, N.; Hussain, S.S.; Usman, M.; Abbas, M.; et al. AgriNanotechnology and Tree Nanobionics: Augmentation in Crop Yield, Biosafety, and Biomass Accumulation. Front. Bioeng. Biotechnol. 2022, 10, 853045. [Google Scholar] [CrossRef] [PubMed]
- Vasilyeva, M.M.; Iliykova, I.I.; Anisovich, M.V.; Hamolka, T.N.; Azizbekyan, S.G.; Yurkevich, H.S.; Ioda, V.I. Study of the toxicological properties of microfertilizers. Pub. Health Tox. 2021, 1 (Suppl. 1), A46. [Google Scholar] [CrossRef]
- Yurkevich, E.S.; Anisovich, M.V.; Azizbekyan, S.G. Study of Toxicological Properties of Microfertilizers “Nanoplant” in Experiments in Vitro. In Proceedings of the 9th International Conference Bionanotox 2018 “Biomaterials and Nanobiomaterials”, Heraklion, Greece, 6–13 May 2018; pp. 23–25. [Google Scholar]
- Arafa, S.A.; Attia, K.A.; Niedbała, G.; Piekutowska, M.; Alamery, S.; Abdelaal, K.; Alateeq, T.K.; Ali, M.; Elkelish, A.; Attallah, S.Y. Seed Priming Boost Adaptation in Pea Plants under Drought Stress. Plants 2021, 10, 2201. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; Seed Science and Technology: Zurich, Switzerland, 2022. [Google Scholar]
- Abdul-Baki, A.A.; Anderson, J.D. Vigour Determination in Soybean Seed by Multiple Criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Channaoui, S.; Idrissi, I.S.; Mazouz, H.; Nabloussi, A. Reaction of some Rapeseed (Brassica napus L.) Genotypes to Different Drought Stress Levels during Germination and Seedling Growth Stages. OCL 2019, 26, 23. [Google Scholar] [CrossRef]
- George, S.; Minha, N.M.; Jatoi, S.A.; Siddiqui, S.U.; Ghafoor, A.A. Impact of Polyethylene Glycol on Proline and Membrane Stability Index for Water Stress Regime in Tomato (Solanum lycopersicum). Pak. J. Bot. 2015, 47, 835–844. [Google Scholar]
- Guo, H.; Liu, Y.; Chen, J.; Zhu, Y.; Zhang, Z. The Effects of Several Metal Nanoparticles on Seed Germination and Seedling Growth: A Meta-Analysis. Coatings 2022, 12, 183. [Google Scholar] [CrossRef]
- Bayat, M.; Zargar, M.; Murtazova, K.M.-S.; Nakhaev, M.R.; Shkurkin, S.I. Ameliorating Seed Germination and Seedling Growth of Nano-Primed Wheat and Flax Seeds Using Seven Biogenic Metal-Based Nanoparticles. Agronomy 2022, 12, 811. [Google Scholar] [CrossRef]
- Rai-Kalal, P.; Jajoo, A. Priming with Zinc Oxide Nanoparticles Improve Germination and Photosynthetic Performance in Wheat. Plant Physiol. Biochem. 2021, 160, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Kornarzyński, K.; Sujak, A.; Czernel, G.; Wiącek, D. Effect of Fe3O4 Nanoparticles on Germination of Seeds and Concentration of Elements in Helianthus annuus L. under Constant Magnetic Field. Sci. Rep. 2020, 10, 8068. [Google Scholar] [CrossRef] [PubMed]
- Faraji, J.; Sepehri, A. Ameliorative Effects of TiO2 Nanoparticles and Sodium Nitroprusside on Seed Germination and Seedling Growth of Wheat under PEG Stimulated Drought Stress. J. Seed Sci. 2019, 41, 309–317. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Rehman, M.Z.; Waris, A.A. Zinc and Iron Oxide Nanoparticles Improved the Plant Growth and Reduced the Oxidative Stress and Cadmium Concentration in Wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef] [PubMed]
- El-Badri, A.M.; Maria Batool, M.; Wang, C.; Hashem, A.M.; Karim, M.; Tabl, K.M.; Nishawy, E.; Kuai, J.; Zhou, G.; Wang, B. Selenium and Zinc Oxide Nanoparticles Modulate the Molecular and Morpho-Physiological Processes during Seed Germination of Brassica napus under Salt Stress. Ecotoxicol. Environ. Saf. 2021, 225, 112695. [Google Scholar] [CrossRef]
- Wu, F.; Fang, Q.; Yan, S.; Pan, L.; Tang, X.; Ye, W. Effects of Zinc Oxide Nanoparticles on Arsenic Stress in Rice (Oryza sativa L.): Germination, Early Growth, and Arsenic Uptake. Environ. Sci. Pollut. Res. 2020, 27, 26974–26981. [Google Scholar] [CrossRef] [PubMed]
- Chau, N.H.; Doan, Q.H.; Chu, T.H.; Nguyen, T.T.; Dao Trong, H.; Ngo, Q.B. Effects of Different Nanoscale Microelement Containing Formulations for Presowing Seed Treatment on Growth of Soybean Seedlings. J. Chem. 2019, 2019, 8060316. [Google Scholar] [CrossRef]
- Shah, T.; Latif, S.; Saeed, F.; Ali, I.; Ullah, S.; Abdullah Alsahli, A.; Jan, S.; Ahmad, P. Seed Priming with Titanium Dioxide Nanoparticles Enhances Seed Vigour, Leaf Water Status, and Antioxidant Enzyme Activities in Maize (Zea mays L.) under Salinity Stress. J. King Saud Univ. Sci. 2021, 33, 101207. [Google Scholar] [CrossRef]
- Hojjat, S.S.; Kamyab, M. The Effect of Silver Nanoparticle on Fenugreek Seed Germination under Salinity Levels. Russ. Agric. Sci. 2017, 43, 61–65. [Google Scholar] [CrossRef]
- Chourasiya, V.K.; Nehra, A.; Shukla, P.S.; Singh, K.P.; Singh, P. Impact of Mesoporous Nano-Silica (SiO2) on Seed Germination and Seedling Growth of Wheat, Pea and Mustard Seed. J. Nanosci. Nanotechnol. 2021, 21, 3566–3572. [Google Scholar] [CrossRef]
- Pawar, V.A.; Ambekar, J.D.; Kale, B.B.; Apte, S.K.; Laware, S.L. Response in Chickpea (Cicer arietinum L.) Seedling Growth to Seed Priming with Iron Oxide Nanoparticles. Int. J. Biosci. 2019, 14, 82–91. [Google Scholar] [CrossRef]
- Chung, I.M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Influence of Silver Nanoparticles on the Enhancement and Transcriptional Changes of Glucosinolates and Phenolic Compounds in Genetically Transformed Root Cultures of Brassica rapa ssp. rapa. Bioprocess Biosyst. Eng. 2018, 41, 1665–1677. [Google Scholar] [CrossRef]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives. Nano-Micro Lett. 2020, 12, 45. [Google Scholar] [CrossRef]
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Dadpour, M.R.; Fotopoulos, V.; Kimura, S. Titanium Dioxide Nanoparticles (TiO2 NPs) Promote Growth and Ameliorate Salinity Stress Effects on Essential Oil Profile and Biochemical Attributes of Dracocephalum moldavica. Sci. Rep. 2020, 10, 912. [Google Scholar] [CrossRef]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A. Selenium (Se) Seed Priming Induced Growth and Biochemical Changes in Wheat under Water Deficit Conditions. Biol. Trace Elem. Res. 2013, 151, 284–293. [Google Scholar] [CrossRef]
- Garza-Alonso, C.A.; Gonzáles-García, Y.; Cadenas-Pliego, G.; Olivares-Sáenz, E.; Trejo-Téllez, L.I.; Benavides-Mendoza, A. Seed Priming with ZnO Nanoparticles Promotes Early Growth and Bioactive Compounds of Moringa oleifera. Not. Bot. Horti. Agrobot. Cluj Napoca 2021, 49, 12546. [Google Scholar] [CrossRef]
- Faraz, A.; Faizan, M.D.; Rajput, V.; Minkina, T.; Hayat, S.; Faisal, M.; Alatar, A.A.; Abdel-Salam, E.M. CuO Nanoparticle-Mediated Seed Priming Improves Physio-Biochemical and Enzymatic Activities of Brassica juncea. Plants 2023, 12, 803. [Google Scholar] [CrossRef]
- Waqas Mazhar, M.; Ishtiaq, M.; Maqbool, M.; Akram, R.; Shahid, A.; Shokralla, S.; Al-Ghobari, H.; Alataway, A.; Dewidar, A.Z.; El-Sabrout, A.M.; et al. Seed Priming with Iron Oxide Nanoparticles Raises Biomass Production and Agronomic Profile of Water-Stressed Flax Plants. Agronomy 2022, 12, 982. [Google Scholar] [CrossRef]
- Azizbekian, S.G.; Domash, V.I. Nanoplant–novoe Otechestvennoe Microudobrenie (Nanoplant–New Domestic Microfertilizers), Nashe Selskoe Khozyaistvo. Agron. Zemled. 2015, 7, 2–7. [Google Scholar]
- Abou-Zeid, H.M.; Ismail, G.S.M.; Abdel-Latif, S.A. Influence of Seed Priming with ZnO Nanoparticles on the Salt-induced Damages in Wheat (Triticum aestivum L.) Plants. J. Plant Nutr. 2021, 44, 629–643. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, S.; Alok, A.; Upadhyay, S.K.; Rawat, M.; Tsang, D.C.W.; Bolan, N.; Kim, K.H. The Potential of Green Synthesized Zinc Oxide Nanoparticles as Nutrient Source for Plant Growth. J. Clean. Prod. 2019, 214, 1061–1070. [Google Scholar] [CrossRef]
- Li, J.; Hu, J.; Ma, C.; Wang, Y.; Wu, C.; Huang, J.; Xing, B. Uptake, Translocation and Physiological Effects of Magnetic Iron Oxide (γ-Fe2O3) Nanoparticles in Corn (Zea mays L.). Chemosphere 2016, 159, 326–334. [Google Scholar] [CrossRef]
- Ahmed, B.; Rizvi, A.; Syed, A.; Elgorban, A.M.; Khan, M.S.; AL-Shwaiman, H.A.; Musarrat, J.; Lee, J. Differential Responses of Maize (Zea mays) at the Physiological, Biomolecular, and Nutrient Levels when Cultivated in the Presence of Nano or Bulk ZnO or CuO or Zn2+ or Cu2+ Ions. J. Hazard. Mater. 2021, 419, 126493. [Google Scholar] [CrossRef]
- Sun, Y.; Jing, R.; Zheng, F.; Zhang, S.; Jiao, W.; Wang, F. Evaluating Phytotoxicity of Bare and Starch-stabilized Zero-valent Iron Nanoparticles in Mung Bean. Chemosphere 2019, 236, 124336. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Sabaghnia, N. Effect of Pre-sowing Seed Treatments with Silicon Nanoparticles on Germinability of Sunflower (Helianthus annuus). Bot Lith. 2015, 21, 13–21. [Google Scholar] [CrossRef]
- Raja, K.; Sowmya, R.; Sudhagar, R.; Moorthy, P.S.; Govindaraju, K.; Subramanian, K.S. Biogenic ZnO and Cu Nanoparticles to Improve Seed Germination Quality in blackgram (Vigna mungo). Mater. Lett. 2019, 235, 164–167. [Google Scholar] [CrossRef]
- Prasad, T.N.V.K.V.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Sajanlal, P.R.; Pradeep, T. Effect of Nanoscale Zinc Oxide Particles on the Germination, Growth and Yield of Peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Dehkourdi, E.H.; Mosavi, M. Effect of Anatase Nanoparticles (TiO2) on Parsley Seed Germination (Petroselinum crispum) in Vitro. Biol. Trace Elem. Res. 2013, 155, 283–286. [Google Scholar] [CrossRef]
- Zheng, L.; Hong, F.; Lu, S.; Liu, C. Effect of Nano-TiO2 on Strength of Naturally Aged Seeds and Growth of Spinach. Biol. Trace Elem. Res. 2005, 104, 83–91. [Google Scholar] [CrossRef]
- Tolay, I. The Impact of Different Zinc (Zn) Levels on Growth and Nutrient Uptake of Basil (Ocimum basilicum L.) Grown under Salinity Stress. PLoS ONE 2021, 16, e0246493. [Google Scholar] [CrossRef]
- Kasote, D.M.; Lee, J.H.J.; Jayaprakasha, G.K.; Patil, B.S. Manganese Oxide Nanoparticles as Safer Seed Priming Agent to Improve Chlorophyll and Antioxidant Profiles in Watermelon Seedlings. Nanomaterials 2021, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Maswada, H.F.; Djanaguiraman, M.; Prasad, P.V.V. Seed Treatment with Nano-iron (III) Oxide enhances Germination, Seeding Growth and Salinity Tolerance of Sorghum. J. Agron. Crop Sci. 2018, 204, 577–587. [Google Scholar] [CrossRef]
- Messant, M.; Hani, U.; Hennebelle, T.; Guérard, F.; Gakière, B.; Gall, A.; Thomine, S.; Krieger-Liszkay, A. Manganese Concentration Affects Chloroplast Structure and the Photosynthetic Apparatus in Marchantia polymorpha. Plant Physiol. 2023, 192, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Palit, S.; Sharma, A.; Talukder, G. Effects of Cobalt on Plants. Bot. Rev. 1994, 60, 151–181. [Google Scholar] [CrossRef]
- Mohanty, N.; Vass, J.; Demeter, S. Impairment of Photosystem-II. Activity at the Level of Secondary Quinone Electron Acceptor in Chloroplasts Treated with Co, Ni and Zn Ions. Physiol. Pl. 1989, 76, 386–390. [Google Scholar] [CrossRef]
- Perez-Espinosa, A.; Moreno-Caselles, J.; Moral, R.; Perez-Murcia, M.D.; Gomez, I. Effect of Cobalt on Chlorophyll Contents in Tomato Plants. J. Plant Nutr. 2002, 25, 1933–1940. [Google Scholar] [CrossRef]
- Tripathy, B.C.; Mohanty, P. Stabilization by Glutaraldehyde Fixation by Chloroplast Membrane Structure and Function against Heavy Metal Ion Induced Damage. Pl. Sci. Lett. 1981, 22, 253–261. [Google Scholar] [CrossRef]
- Chen, J.; Yin, Y.; Zhu, Y.; Song, K.; Ding, W. Favorable Physiological and Morphological Effects of Molybdenum Nanoparticles on Tobacco (Nicotiana tabacum L.): Root Irrigation is Superior to Foliar Spraying. Front. Plant Sci. 2023, 14, 1220109. [Google Scholar] [CrossRef]
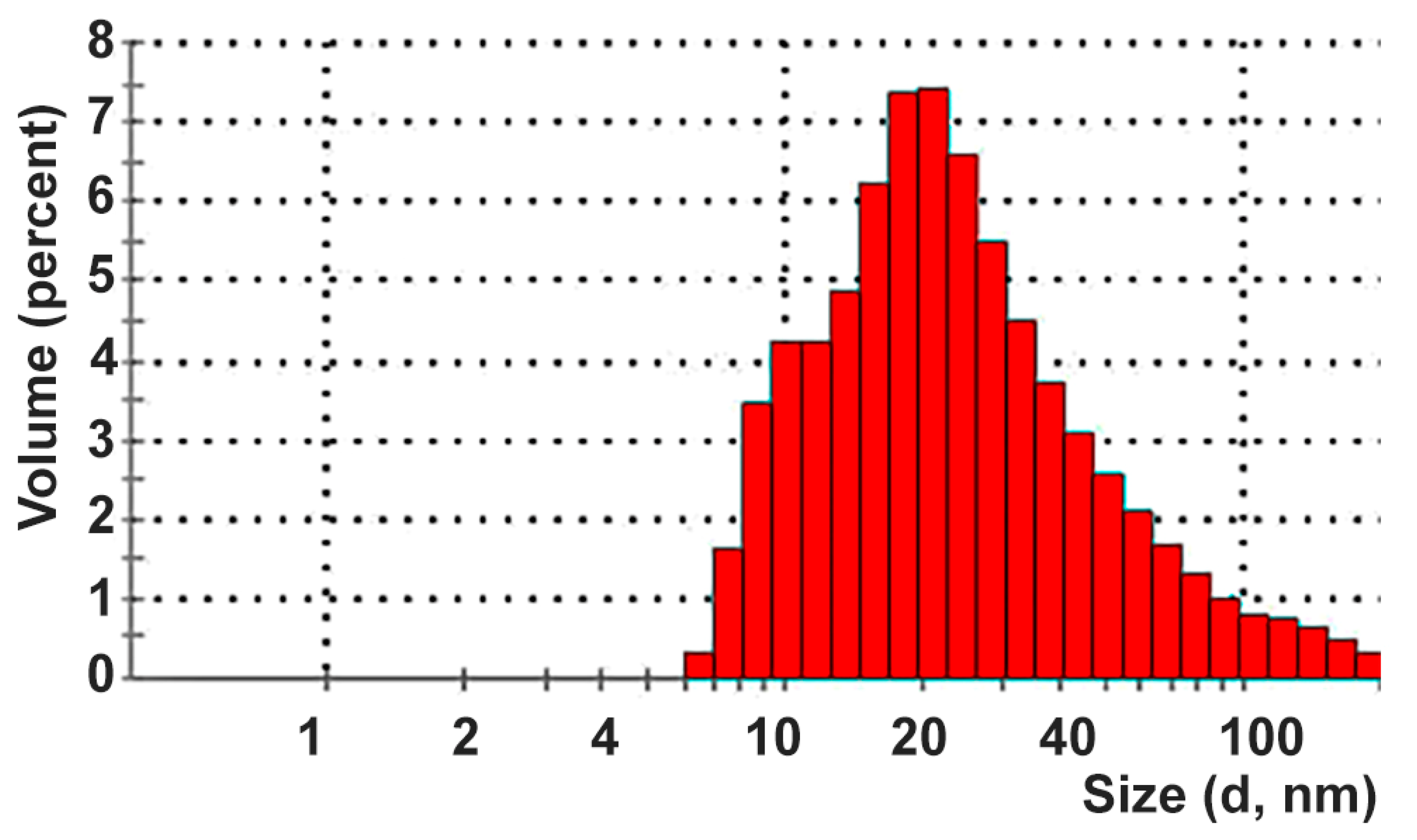
| Size d (nm) | 6.5 | 7.5 | 8.7 | 10.1 | 11.7 | 13.5 | 15.7 | 18.2 | 21.0 | 24.4 |
| Mean Volume (%) | 0.3 | 1.6 | 3.5 | 4.3 | 4.3 | 4.8 | 6.2 | 7.4 | 7.4 | 6.6 |
| Size d (nm) | 28.2 | 32.7 | 37.8 | 43.8 | 50.8 | 58.8 | 68.1 | 78.8 | 91.3 | 105.7 |
| Mean Volume (%) | 5.5 | 3.7 | 3.7 | 3.1 | 2.6 | 2.1 | 1.7 | 1.3 | 1.0 | 0.8 |
| Traits | Factors | ||
|---|---|---|---|
| Cultivar (C) | Treatment (T) | C × T | |
| Germination Energy | *** | *** | *** |
| Seed Germination | *** | *** | *** |
| Abnormal Seedlings | *** | ns | ns |
| Shoot Length | *** | *** | *** |
| Root Length | *** | *** | *** |
| Fresh Shoot Weight | *** | *** | *** |
| Fresh Root Weight | ** | * | ns |
| Dry Shoot Weight | ** | *** | ** |
| Dry Root Weight | *** | ns | ns |
| Root/Shoot Ratio | *** | *** | *** |
| Shoot Elongation Rate | *** | *** | *** |
| Root Elongation Rate | *** | *** | *** |
| Seedling Vigour Index | *** | *** | *** |
| Chlorophyll Content | *** | *** | *** |
| Pea Cultivar | Treatment | Germination Energy (%) | Seed Germination (%) | Abnormal Seedlings (%) | Shoot Length (mm) | Root Length (mm) |
|---|---|---|---|---|---|---|
| E-244 | Control | 73.00 ± 0.58 c | 91.67 ± 0.33 b | 2.00 ± 0.58 a | 43.63 ± 0.48 b | 85.93 ± 0.55 c |
| HP | 82.33 ± 0.33 b | 91.00 ± 0.58 b | 5.00 ± 1.53 a | 50.50 ± 0.25 a | 113.77 ± 0.50 b | |
| NP | 85.67 ± 0.33 a | 96.00 ± 0.58 a | 3.00 ± 1.15 a | 50.67 ± 0.44 a | 123.77 ± 0.69 a | |
| p value | 0.00000 | 0.00087 | 0.25193 | 0.00003 | 0.00000 | |
| Dukat | Control | 53.67 ± 0.33 a | 73.33 ± 0.33 c | 7.33 ± 0.33 a | 51.83 ± 0.17 c | 98.33 ± 0.44 b |
| HP | 47.33 ± 0.33 b | 78.33 ± 0.88 a | 7.67 ± 0.33 a | 78.00 ± 1.53 a | 102.67 ± 0.44 b | |
| NP | 53.67 ± 0.33 a | 75.67 ± 0.33 b | 7.00 ± 1.15 a | 71.00 ± 2.36 b | 104.00 ± 1.61 a | |
| p value | 0.00001 | 0.002614 | 0.81304 | 0.00007 | 0.01619 | |
| Partner | Control | 82.33 ± 0.67 a | 93.33 ± 0.33 a | 1.33 ± 0.67 b | 54.50 ± 0.29 c | 106.83 ± 1.17 c |
| HP | 82.00 ± 0.58 a | 94.33 ± 0.88 a | 2.33 ± 0.33 ab | 67.17 ± 0.93 b | 118.33 ± 0.67 b | |
| NP | 82.67 ± 0.67 a | 94.00 ± 0.58 a | 3.67 ± 0.33 a | 82.50 ± 0.58 a | 143.67 ± 1.09 a | |
| p value | 0.77026 | 0.56152 | 0.03505 | 0.00000 | 0.000001 |
| Pea Cultivar | Treatment | Fresh Shoot Weight (g) | Fresh Root Weight (g) | Dry Shoot Weight (g) | Dry Root Weight (g) |
|---|---|---|---|---|---|
| E-244 | Control | 1.93 ± 0.02 a | 2.10 ± 0.14 a | 0.178 ± 0.002 a | 0.178 ± 0.008 b |
| HP | 2.04 ± 0.06 a | 2.23 ± 0.04 a | 0.185 ± 0.005 a | 0.187 ± 0.003 ab | |
| NP | 1.91 ± 0.08 a | 2.37 ± 0.05 a | 0.177 ± 0.005 a | 0.204 ± 0.007 a | |
| p value | 0.38019 | 0.16797 | 0.57373 | 0.07827 | |
| Dukat | Control | 2.05 ± 0.02 b | 1.63 ± 0.08 a | 0.154 ± 0.007 b | 0.131 ± 0.008 a |
| HP | 2.70 ± 0.04 a | 1.71 ± 0.11 a | 0.199 ± 0.016 a | 0.151 ± 0.009 a | |
| NP | 2.74 ± 0.07 a | 1.83 ± 0.06 a | 0.214 ± 0.006 a | 0.148 ± 0.007 a | |
| p value | 0.0001 | 0.31085 | 0.018315 | 0.22694 | |
| Partner | Control | 2.22 ± 0.12 b | 2.40 ± 0.20 a | 0.191 ± 0.013 b | 0.198 ± 0.018 a |
| HP | 2.43 ± 0.16 b | 2.20 ± 0.03 a | 0.191 ± 0.011 b | 0.180 ± 0.002 a | |
| NP | 3.19 ± 0.05 a | 2.60 ± 0.06 a | 0.248 ± 0.008 a | 0.184 ± 0.007 a | |
| p value | 0.00283 | 0.15800 | 0.01484 | 0.53089 |
| Pea Cultivar | Treatment | Root/Shoot Ratio | Shoot Elongation Rate | Root Elongation Rate | Seedling Vigour Index | Chlorophyll Content (mg/g of FW) |
|---|---|---|---|---|---|---|
| E-244 | Control | 1.97 ± 0.03 c | 6.31 ± 0.37 a | 8.13 ± 0.37 c | 1187.7 ± 4.8 c | 1.61 ± 0.011 a |
| HP | 2.25 ± 0.00 b | 6.49 ± 0.23 a | 10.63 ± 0.34 b | 1494.9 ± 13.0 b | 1.65 ± 0.021 a | |
| NP | 2.44 ± 0.03 a | 7.32 ± 0.41 a | 14.31 ± 0.25 a | 1674.6 ± 12.6 a | 1.65 ± 0.033 a | |
| p value | 0.0001 | 0.1246 | 0.0000 | 0.0000 | 0.4212 | |
| Dukat | Control | 1.90 ± 0.00 a | 10.18 ± 0.33 c | 13.89 ± 0.11 b | 1101.2 ± 1.3 c | 1.93 ± 0.018 c |
| HP | 1.32 ± 0.03 c | 17.68 ± 0.42 a | 16.37 ± 0.37 a | 1415.3 ± 21.0 a | 2.05 ± 0.029 b | |
| NP | 1.47 ± 0.06 b | 15.47 ± 0.71 b | 11.70 ± 0.84 c | 1324.3 ± 21.9 b | 2.14 ± 0.033 a | |
| p value | 0.0001 | 0.0001 | 0.0025 | 0.0000 | 0.0027 | |
| Partner | Control | 1.96 ± 0.02 a | 10.79 ± 0.09 c | 8.78 ± 0.32 b | 1505.7 ± 7.7 c | 1.31 ± 0.027 c |
| HP | 1.76 ± 0.03 b | 12.91 ± 0.18 b | 7.50 ± 0.29 c | 1749.9 ± 14.7 b | 1.44 ± 0.022 b | |
| NP | 1.74 ± 0.01 b | 16.40 ± 0.03 a | 11.94 ± 0.24 a | 2126.2 ± 28.2 a | 1.63 ± 0.022 a | |
| p value | 0.0009 | 0.0000 | 0.0001 | 0.0000 | 0.0002 |
| Variable | GE | FG | AS | SL | RL | FSW | FRW | DSW | DRW | SER | RER | SVI | CHL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GE | 1.00 | 0.95 | −0.76 | −0.28 | 0.55 | −0.20 | 0.85 | 0.11 | 0.82 | −0.47 | −0.54 | 0.62 | −0.87 |
| FG | 1.00 | −0.81 | −0.20 | 0.49 | −0.15 | 0.82 | 0.17 | 0.83 | −0.39 | −0.51 | 0.65 | −0.85 | |
| AS | 1.00 | 0.30 | −0.17 | 0.21 | −0.64 | −0.03 | −0.64 | 0.38 | 0.67 | −0.36 | 0.82 | ||
| SL | 1.00 | 0.51 | 0.92 | 0.01 | 0.72 | −0.22 | 0.95 | 0.29 | 0.55 | 0.33 | |||
| RL | 1.00 | 0.50 | 0.64 | 0.55 | 0.40 | 0.29 | 0.12 | 0.94 | −0.27 | ||||
| FSW | 1.00 | 0.14 | 0.81 | −0.16 | 0.86 | 0.17 | 0.54 | 0.26 | |||||
| FRW | 1.00 | 0.36 | 0.84 | −0.18 | −0.38 | 0.71 | −0.71 | ||||||
| DSW | 1.00 | 0.20 | 0.60 | −0.03 | 0.63 | 0.04 | |||||||
| DRW | 1.00 | −0.37 | −0.37 | 0.50 | −0.72 | ||||||||
| SER | 1.00 | 0.32 | 0.33 | 0.42 | |||||||||
| RER | 1.00 | −0.07 | 0.68 | ||||||||||
| SVI | 1.00 | −0.42 | |||||||||||
| CHL | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamindžić, G.; Azizbekian, S.; Miljaković, D.; Turan, J.; Nikolić, Z.; Ignjatov, M.; Milošević, D.; Vasiljević, S. Comprehensive Metal-Based Nanopriming for Improving Seed Germination and Initial Growth of Field Pea (Pisum sativum L.). Agronomy 2023, 13, 2932. https://doi.org/10.3390/agronomy13122932
Tamindžić G, Azizbekian S, Miljaković D, Turan J, Nikolić Z, Ignjatov M, Milošević D, Vasiljević S. Comprehensive Metal-Based Nanopriming for Improving Seed Germination and Initial Growth of Field Pea (Pisum sativum L.). Agronomy. 2023; 13(12):2932. https://doi.org/10.3390/agronomy13122932
Chicago/Turabian StyleTamindžić, Gordana, Sergei Azizbekian, Dragana Miljaković, Jan Turan, Zorica Nikolić, Maja Ignjatov, Dragana Milošević, and Sanja Vasiljević. 2023. "Comprehensive Metal-Based Nanopriming for Improving Seed Germination and Initial Growth of Field Pea (Pisum sativum L.)" Agronomy 13, no. 12: 2932. https://doi.org/10.3390/agronomy13122932
APA StyleTamindžić, G., Azizbekian, S., Miljaković, D., Turan, J., Nikolić, Z., Ignjatov, M., Milošević, D., & Vasiljević, S. (2023). Comprehensive Metal-Based Nanopriming for Improving Seed Germination and Initial Growth of Field Pea (Pisum sativum L.). Agronomy, 13(12), 2932. https://doi.org/10.3390/agronomy13122932







