Seasonal Changes in Amylose and Starch Compositions in ‘Ambrosia’ Apples Associated with Rootstocks and Orchard Climatic Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Orchard Profiles and Investigations
2.2. Sample Collections and Processes
2.3. Analysis of Amylose (AM), Amylopectin (AP), and Total Starch Content (TSC)
2.3.1. Reagents and Working Solutions
2.3.2. Starch Extraction and Purification
2.3.3. AM, AP Isolation, and Enzyme Hydrolyzation
2.3.4. Hydrolyzation and Determination
2.4. Prediction of Fruit Dry Matter Content
2.5. Blush Color Assessment
2.6. Harvest, Storage, and Quality Assessment of Fruit
2.7. Statistical Analyses
3. Results and Discussion
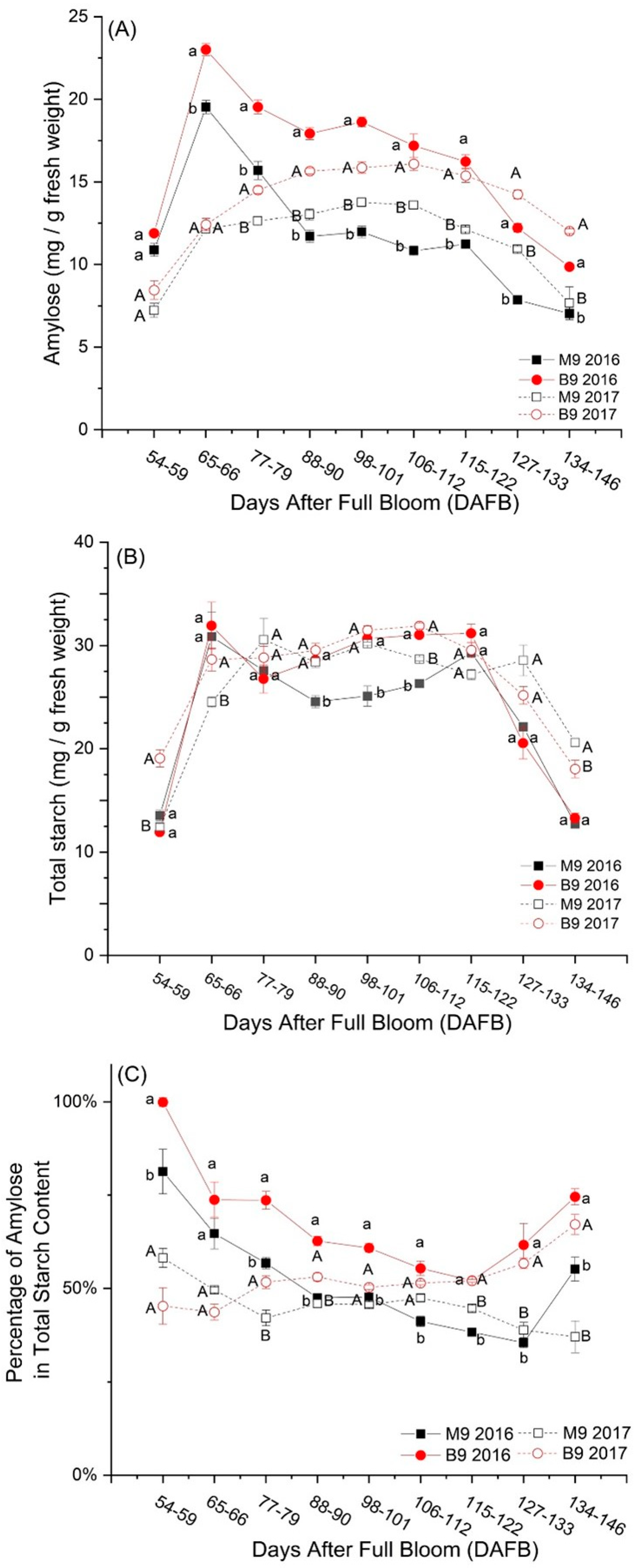
3.1. Changes in Starch Components under Rootstock Effects
3.2. Changes in Dry Matter Content (DMC) and Its Relation with Amylose (AM)
3.3. Seasonal Changes in Orchard Temperatures and Their Effects on Fruits
3.4. Effects of Orchard Temperatures, Rootstocks, and Starch Compositions on Fruit Quality
3.4.1. Blush Red Color
3.4.2. Fruit Quality Attributes after Storage
3.5. Limitations and Future Perspectives
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doerflinger, F.C.; Miller, W.B.; Nock, J.F.; Watkins, C.B. Relationships between starch pattern indices and starch concentrations in four apple cultivars. Postharvest Biol. Technol. 2015, 110, 86–95. [Google Scholar] [CrossRef]
- Fan, X.; Mattheis, J.P.; Patterson, M.E.; Fellman, J.K. Changes in amylose and total starch content in ‘Fuji’ apples during maturation. HortScience 1995, 30, 104–105. [Google Scholar] [CrossRef]
- Bekele, E.A.; Beshir, W.F.; Hertog, L.A.T.M.M.; Nicolai, B.M.; Geeraerd, A.H. Metabolic profiling reveals ethylene mediated metabolic changes and a coordinated adaptive mechanism of ‘Jonagold’ apple to low oxygen stress. Physio. Plant. 2015, 155, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Yadav, B.; Yadav, R.B. Resistant starch: Physiological roles and food applications. Food Rev. Int. 2008, 24, 193–234. [Google Scholar] [CrossRef]
- Topping, D. Cereal complex carbohydrates and their contribution to human health. J. Cereal. Sci. 2007, 46, 220–229. [Google Scholar] [CrossRef]
- Gupta, V.; Thakur, R.; Barik, M.; Das, A.B. Effect of high amylose starch-natural deep eutectic solvent based edible coating on quality parameters of strawberry during storage. J. Agric. Food Res. 2023, 11, 100487. [Google Scholar] [CrossRef]
- Calderón-Castro, A.; Odín Vega-García, M.; Zazueta-Morales, J.D.J.; Fitch-Vargas, P.R.; Carrillo-López, A.; Gutiérrez-Dorado, R.; Limón-Valenzuela, V.; Aguilar-Palazuelos, E. Effect of extrusion process on the functional properties of high amylose corn starch edible films and its application in mango (Mangifera indica L.) cv. Tommy Atkins. J. Food Sci. Technol. 2018, 55, 905–914. [Google Scholar] [CrossRef]
- Lu, C. Early summer deficit irrigation increases dry matter content and enhances quality of Ambrosia™ apple at- and post-harvest. Horticulturae 2022, 8, 571. [Google Scholar] [CrossRef]
- Palmer, J.; Diack, R.; Johnston, J.; Boldingh, H. Manipulation of fruit dry matter accumulation and fruit size in ‘Scifresh’apple through alteration of the carbon supply, and its relationship with apoplastic sugar composition. J. Hort. Sci. Biotech. 2013, 88, 483–489. [Google Scholar] [CrossRef]
- Toivonen, P.; Lannard, B. Dry matter content association with time of on-tree maturation, quality at harvest, and changes in quality after controlled atmosphere storage for ‘Royal Gala’ apples. Can. J. Plant Sci. 2021, 101, 98–106. [Google Scholar] [CrossRef]
- Lachapelle, M.; Bourgeois, G.; DeEll, J.R. Effects of preharvest weather conditions on firmness of ‘McIntosh’ apples at harvest time. HortScience 2013, 48, 474–480. [Google Scholar] [CrossRef]
- Warrington, I.J.; Fulton, T.A.; Halligan, E.A.; De Silva, H.N. Apple fruit growth and maturity are affected by early season temperature. J. Am. Soc. Hort. Sci. 1999, 124, 468–477. [Google Scholar] [CrossRef]
- Toivonen, P.M.A. Relation between preharvest conditions, harvest maturity and postharvest performance of apples. Acta Hortic. 2019, 1256, 469–480. [Google Scholar] [CrossRef]
- Xu, H.; Ediger, D. Rootstocks with different vigor influenced scion–water relations and stress responses in Ambrosia™ apple trees (Malus domestica var. Ambrosia). Plants 2021, 10, 614. [Google Scholar] [CrossRef]
- Lu, C.; Xu, H. Summer fruitlet thinning enhanced quality attributes of Ambrosia™ apple at harvest and after 4 months of cold air storage. Can. J. Plant Sci. 2021, 101, 1041–1050. [Google Scholar] [CrossRef]
- Xu, H.; Watanabe, Y.; Ediger, D.; Yang, X.; Iritani, D. Characteristics of sunburn browning fruit and rootstock-dependent damage-free yield of Ambrosia™ apple after sustained summer heat events. Plants 2022, 11, 1201. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Blatt, S.; Ediger, D. Tools for climate resilience in tree fruit I: Large-dwarfing rootstocks can alleviate sunburn damage in “Buckeye Gala” apple. Can. J. Plant Sci. 2022, 103, 128–132. [Google Scholar] [CrossRef]
- Jones, H.G. How do rootstocks control shoot water relations? New Phytol. 2012, 194, 301–303. [Google Scholar] [CrossRef]
- Xu, H.; Ediger, D.; Singh, A.; Pagliocchini, C. Rootstock–scion hydraulic balance influenced scion vigor and yield efficiency of Malus domestica cv. Honeycrisp on eight rootstocks. Horticulturae 2021, 7, 99. [Google Scholar] [CrossRef]
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci.Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Suni, M.; Nyman, M.; Eriksson, N.A.; Björk, L.; Björck, I. Carbohydrate composition and content of organic acids in fresh and stored apples. J. Sci. Food Agric. 2000, 80, 1538–1544. [Google Scholar] [CrossRef]
- Salo, M.L.; Korhonen, I. Carbohydrate and acid composition of some apple varieties. Agric. Food Sci. 1972, 44, 63–67. [Google Scholar] [CrossRef][Green Version]
- Thammawong, M.; Arakawa, O. Starch degradation of detached apple fruit in relation to ripening and ethylene. J. Jpn Soc. Hortic. Sci. 2007, 76, 345–350. [Google Scholar] [CrossRef]
- Neuwald, D.A.; Streif, J.; Kittemann, D. Fruit starch degradation patterns in apple cultivars on-tree and off-tree at different holding temperatures. In Proceedings of the ISHS Acta Horticulturae 858: III International Conference Postharvest Unlimited, Berlin, Germany, 5 November 2008; pp. 263–266. [Google Scholar]
- Thammawong, M.; Arakawa, O. Starch degradation characteristics in relation to physiological and biochemical properties during growth and maturation of apple fruit. J. Appl. Hortic. 2009, 11, 23–30. [Google Scholar] [CrossRef]
- Doerflinger, F.C. Starch Metabolism in Apple Fruit and Its Relationship with Maturation and Ripening. Ph.D. Thesis, Cornell University, Ithaca, NY, USA, 2015. Available online: https://hdl.handle.net/1813/40631 (accessed on 18 October 2023).
- Cliff, M.A.; Toivonen, P.M. Sensory and quality characteristics of ‘Ambrosia’apples in relation to harvest maturity for fruit stored up to eight months. Postharvest Biol. Technol. 2017, 132, 145–153. [Google Scholar] [CrossRef]
- Cliff, M.A.; Stanich, K.; Lu, R.; Hampson, C.R. Use of descriptive analysis and preference mapping for early-stage assessment of new and established apples. J. Sci. Food Agric. 2016, 96, 2170–2183. [Google Scholar] [CrossRef]
- Cline, J.A. Commercial Production of Ambrosia™ Apples in Ontario Agdex 211/32; Ontario Ministry of Agriculture, Food and Rural Affairs: Guelph, ON, Canada, 2009. Available online: http://www.omafra.gov.on.ca/english/crops/facts/09-041.htm (accessed on 19 November 2019).
- Toivonen, P.M.A. Comparison of IAD and starch-iodine indices at harvest and how they relate to post-storage firmness retention in Ambrosia™ apples over three growing seasons. Can. J. Plant Sci. 2015, 95, 1177–1180. [Google Scholar] [CrossRef]
- Smith, R.B.; Lougheed, E.C.; Franklin, E.W.; McMillan, I. The starch iodine test for determining stage of maturation in apples. Can. J. Plant Sci. 1979, 59, 725–735. [Google Scholar] [CrossRef]
- Brookfield, P.; Murphy, P.; Harker, R.; MacRae, E. Starch degradation and starch pattern indices; interpretation and relationship to maturity. Postharvest Biol. Technol. 1997, 11, 23–30. [Google Scholar] [CrossRef]
- Magel, E. Qualitative and quantitative determination of starch by a colorimetric method. Starch–Stärke 1991, 43, 384–387. [Google Scholar] [CrossRef]
- Magein, H.; Leurquin, D. Changes in amylose, amylopectin and total starch content in Jonagold apple fruit during growth and maturation. Acta Hortic. 2000, 517, 487–491. [Google Scholar] [CrossRef]
- Zhu, T.; Jackson, D.S.; Wehling, R.L.; Geera, B. Comparison of amylose determination methods and the development of a dual wavelength Iodine binding technique. Cereal Chem. 2008, 85, 51–58. [Google Scholar] [CrossRef]
- Gibson, T.S.; Solah, V.A.; McCleary, B.V. A procedure to measure amylose in cereal starches and flours with Concanavalin A. J. Cereal Sci. 1996, 25, 111–119. [Google Scholar] [CrossRef]
- Yun, S.H.; Matheson, N.K. Estimation of amylose content of starches after precipitation of amylopectin by concanavalin-A. Starch/Starke 1990, 42, 302–305. [Google Scholar] [CrossRef]
- Grabska, J.; Beć, K.B.; Ueno, N.; Huck, C.W. Analyzing the Quality Parameters of Apples by Spectroscopy from Vis/NIR to NIR Region: A Comprehensive Review. Foods 2023, 12, 1946. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Toivonen, P. Scheduling adequate irrigation mitigates postharvest soft scald disorder of Ambrosia™ apples grown in a semiarid eco-zone. Can. J. Plant Sci. 2022, 102, 884–890. [Google Scholar] [CrossRef]
- Gu, S. Growing degree hours—A simple, accurate, and precise protocol to approximate growing heat summation for grapevines. Int. J. Biometeorol. 2016, 60, 1123–1134. [Google Scholar] [CrossRef]
- Sugiura, T.; Ogawa, H.; Fukuda, N.; Moriguchi, T. Changes in the taste and textural attributes of apples in response to climate change. Sci. Rep. 2013, 3, 2418. [Google Scholar] [CrossRef]
- Belhassine, F.; Martinez, S.; Bluy, S.; Fumey, D.; Kelner, J.J.; Costes, E.; Pallas, B. August. Fruit growth, photosynthesis and starch accumulation are differentially affected by local variation in source/sink ratios. In Proceedings of the ISHS Acta Horticulturae 1281: XXX International Horticultural Congress IHC2018: International Symposium on Cultivars, Rootstocks and Management Systems of Deciduous Fruit and Fruit Tree Behaviour in Dynamic Environments, Istanbul, Turkey, 12–16 August 2018; pp. 455–462. [Google Scholar]




| 2016 | DAFB | 88 | 98 | 106 | 115 | 127 | 134 |
| r | 0.85 | 0.81 | 0.84 | 0.85 | 0.84 | 0.82 | |
| RSQ | 0.72 | 0.66 | 0.70 | 0.73 | 0.70 | 0.68 | |
| 2017 | DAFB | 90 | 101 | 112 | 122 | 133 | 143 |
| r | 0.80 | 0.77 | 0.84 | 0.88 | 0.93 | 0.83 | |
| RSQ | 0.64 | 0.59 | 0.71 | 0.77 | 0.86 | 0.70 |
| Year | Site | Rootstock | AM/AP Ratio | DMC (%) | Firmness (Newton) | TA (Malic Acid mg/100 mL) | SSC (%) |
|---|---|---|---|---|---|---|---|
| 2016 | N | M.9 | 0.93 ± 0.02 e | 14.64 ± 0.19 d | 72.38 ± 0.78 b | 140.33 ± 2.37 e | 11.54 ± 0.28 c |
| B.9 | 2.32 ± 0.07 c | 16.45 ± 0.04 b | 73.44 ± 0.70 b | 178.92 ± 4.63 bc | 13.08 ± 0.07 a | ||
| S | M.9 | 1.65 ± 0.04 d | 14.89 ± 0.13 cd | 72.54 ± 0.71 b | 160.50 ± 1.13 d | 11.70 ± 0.05 bc | |
| B.9 | 3.84 ± 0.14 a | 17.04 ± 0.15 a | 73.58 ± 0.64 b | 169.29 ± 2.83 cd | 13.53 ± 0.22 a | ||
| 2017 | N | M.9 | 0.42 ± 0.10 f | 13.63 ± 0.07 e | 69.56 ± 0.59 c | 143.64 ± 2.66 e | 12.18 ± 0.08 b |
| B.9 | 1.58 ± 0.05 d | 16.03 ± 0.14 b | 72.45 ± 0.23 b | 187.40 ± 1.03 b | 13.20 ± 0.04 a | ||
| S | M.9 | 0.83 ± 0.03 e | 13.18 ± 0.12 e | 71.52 ± 0.17 bc | 139.97 ± 1.73 e | 12.25 ± 0.10 b | |
| B.9 | 2.72 ± 0.11 b | 15.31 ± 0.13 c | 76.90 ± 0.58 a | 203.43 ± 1.54 a | 13.05 ± 0.06 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 His Majesty the King in Right of Canada, as represented by the Minister of Agriculture and Agri-Food Canada. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.; Xu, H.; Lannard, B.; Yang, X. Seasonal Changes in Amylose and Starch Compositions in ‘Ambrosia’ Apples Associated with Rootstocks and Orchard Climatic Conditions. Agronomy 2023, 13, 2923. https://doi.org/10.3390/agronomy13122923
Lu C, Xu H, Lannard B, Yang X. Seasonal Changes in Amylose and Starch Compositions in ‘Ambrosia’ Apples Associated with Rootstocks and Orchard Climatic Conditions. Agronomy. 2023; 13(12):2923. https://doi.org/10.3390/agronomy13122923
Chicago/Turabian StyleLu, Changwen, Hao Xu, Brenda Lannard, and Xiaotang Yang. 2023. "Seasonal Changes in Amylose and Starch Compositions in ‘Ambrosia’ Apples Associated with Rootstocks and Orchard Climatic Conditions" Agronomy 13, no. 12: 2923. https://doi.org/10.3390/agronomy13122923
APA StyleLu, C., Xu, H., Lannard, B., & Yang, X. (2023). Seasonal Changes in Amylose and Starch Compositions in ‘Ambrosia’ Apples Associated with Rootstocks and Orchard Climatic Conditions. Agronomy, 13(12), 2923. https://doi.org/10.3390/agronomy13122923







