Abstract
On a daily basis, a wide range of materials including inorganic nanoparticles (NPs) inadvertently find their way into the environment. Meanwhile, intentionally used NPs, such as the new generation of nanofertilizers (NFs) are designed to enhance agronomic production. However, their physicochemical properties and not-so-well understood effects raise potential risks to the plant reproductive cycle, specifically pollen development, a subject largely absent in academic research. Even slight contamination, deformation, or aberration of pollen could have enormous impacts on the ecosystem. Thus, our objective was to evaluate the influence of various metal-based NPs on sunflower pollen morphology and its yield. Nano-formulations were applied during the 2019–2021 agronomic seasons on two sunflower hybrids, Neostar and Edison, in Dolná Malanta, near Nitra, Slovak Republic. Pollen morphology findings indicated that conventional ZnSO4 had the most positive impact on the size of pollen grains compared to ZnO-NPs, Fe3O4-NPs, and the NP-free control. Gold-NPs on SiO2 mesoporous silica (AuSi-NPs) showed a statistically insignificant impact, while the use of TiO2-NPs in agriculture remained a topic of debate. Surprisingly, pollen characteristics did not fully correspond to crop yields. Despite causing a reduction in pollen grain size, the TiO2-NPs consistently showed the highest yield compared to other variants. Employing low concentrations of NFs did not notably alter pollen morphology, reinforcing our commitment to eco-friendly, precise, and sustainable agriculture.
1. Introduction
Nanotechnology finds diverse applications in agriculture, including nanosensors, nanopesticides, and nanofertilizers [1,2]. A nanoparticle is typically defined as a material with dimensions up to 100 nm in one of the three vector directions [3].
Following the “essentiality” concept for plant nutrition proposed by Liu and Lal [4], different NFs are categorized as (i) macro-NFs containing elements such as Ca, C, N, P, K; (ii) micro-NFs (e.g., Zn, Fe, Cu); (iii) nanomaterial-enhanced fertilizers (e.g., SiO2 or silicates with nutrient-doped porous diatoms); and (iv) nanoparticulate plant-growth enhancers, such as carbon nanotubes, TiO2-NPs, and Au-NPs, with undetermined modes of actions.
The ZnO-NP fertilizer shows promise as an alternative to conventional ZnSO4 or macro-sized ZnO fertilizers due to its unique properties [5,6,7]. Generally, Zn, an essential micronutrient, plays diverse roles in plant physiology, including chlorophyll synthesis, enzyme cofactor functions, stress tolerance, and the regulation of plant hormones like auxin and cytokinin [8,9,10].
Iron is a crucial micronutrient for plants, indispensable for protein and enzyme formation, electron transport in cells, chlorophyll synthesis, nucleic acid production, ATP synthesis, atmospheric nitrogen fixation, and stress mitigation [11]. Hence, Fe deficiency can result in leaf chlorosis [12,13]. In flowers, anthers serve as major Fe reservoirs [14], and Fe is vital for proper pollen development. Deficiency during the reproductive stage can lead to distorted pollen due to tapetum dysfunction and ultimately, as a result of developmental disorders, pollen grain exine defects occur [15].
TiO2, originating from various industrial activities, including agriculture, has become a notable environmental factor [16]. While not essential for plant growth, nano-sized TiO2 positively influences plant physiology by enhancing enzyme activity [17,18], chlorophyll content, photosynthetic efficiency [19], drought tolerance [20], nutrient uptake and absorption [21], plant morphology [22], seedling development, pollen germination, DNA protection, and reducing heavy metal toxicity [23]. Titanium-based organic complexes have shown positive effects on apple pollination [24] and strawberry fruit development [25]. On the other hand, several adverse impacts on plants have been described, such as a reduction in the initial germination stages of Vicia narbonensis and Zea mays at higher doses [26]. Additionally, the application of TiO2-NPs in soybean plants (Glycine max) led to a significant reduction in the colonization of arbuscular mycorrhizal fungi, plant growth, and leaf phosphorus levels [27].
Silica, although not traditionally considered essential, significantly influences plant growth and development. It strengthens cell walls, enhances stress resistance, stimulates the immune system, promotes antioxidant production, and supports photosynthesis by reducing transpiration [28,29,30]. However, the role of SiO2 mesoporous silica in anchoring Au-NPs remains unclear. Gold, typically insignificant to plant growth, has limited physiological or biochemical functions in NP form. Positive effects are observed at ultra-low concentrations during germination and early development [31]. Gold NPs obtained by green synthesis induce cell division and increase pollen germination [32,33].
In the realm of applying NPs to plants from an ecotoxicological perspective, diverse tests evaluate their environmental effects, including assessments for acute toxicity, chronic toxicity, reproductive toxicity, genotoxicity, and more [34].
Pollen, with its unique capacity to absorb and accumulate a diverse range of pollutants such as heavy metals, pesticides, toxins, and other contaminants, could serve as a valuable biomarker for assessing the impact of environmental pollution [35,36] or for environmental conditions biomonitoring [37,38]. Examination of the reproductive structures of a flower reveals that pollen, as the male gametophyte, is more sensitive compared to ovules of the female gametophyte [39]. Pollen plays a critical role in fertilizing the female gamete to develop a plant embryo. Yet, this phase in the plant life cycle is notably vulnerable due to the heightened sensitivity of the male haploid phase to various environmental conditions, including the fertilization method [40,41].
In the current academic literature, the morphological alterations of pollen affected by diverse NFs are not comprehensively documented. Only a few studies have described variable negative effects of NPs at high concentrations. For example, the application of Ag-NPs led to decreased pollen viability in Arabidopsis [42], and a toxic impact on the pollen structure of kiwifruit was reported [43]. Furthermore, Dutta Gupta et al. [44] observed changes in exine structure and pollen grain morphology in Peltophorum due to Ag-NPs, while Pd-NPs resulted in pore penetration and alterations in ultrastructure and pollen grain shape [36]. Conversely, a few positive effects of Ag-NPs on the size and viability of peach pollen grains were demonstrated by Mosa et al. [45]. In this specific instance, there is a notable absence of information regarding the influence on plant development and the pollen morphology associated with nano-domain effects of micronutrient-based Zn, Fe NFs, or growth stimulants such as Si and Au, as well as the potential consequences for the final crop yield.
Changes in the morphological attributes of pollen grains, including size, shape, exine structure, sculpture, polar axis length, equatorial diameter, colpi characteristics, and spines, can provide direct or indirect insights into various environmental and ecological changes [46] or serve as significant diagnostic features for the Asteraceae family [47].
In general, the pollen grain coat plays a crucial role in protection, facilitating effective pollen transfer and enabling interactions with stigmas [48].
Additionally, the size, structure, and density of spines influence the accessibility of pollen for pollinators and contribute to pollinator diversity [49]. Moreover, the intricate ornamentation of the exine offers a greater surface area for interactions with the environment. Gametogenesis affects the resultant shape and size variations of pollen [50], which in turn, determine pollen functionality [51].
In this context, sunflower (Helianthus annuus L.) is one of the most widely cultivated oil-bearing crops worldwide with significant agronomic perspective [52,53]. Sunflower pollen holds promise as a valuable nutritional resource, being rich in lipids, antioxidants, and essential nutrients such as Na and K [54]. These attributes make it a vital source for bees and other pollinators that play crucial roles in supporting ecological communities [55]. Additionally, sunflower pollen exhibits noteworthy antiseptic effects on pollinators [55,56].
Current studies on changes in pollen morphology primarily focus on ecotoxicological in vitro investigations conducted under strict laboratory conditions, usually using higher concentrations of NPs [36,43]. Surprisingly, academic outcomes lack real-world agronomic research addressing the implications of environmentally sustainable NP concentrations compared to conventional fertilizers.
The aim of the current study was to evaluate selected micro-based and plant-enhanced NPs at eco-friendly concentrations. This involved examining their chemical reactivity, physical stability, as well as their influence on pollen morphology in association with their agronomical yield. The research was conducted in field conditions over three growing seasons, employing two sunflower hybrids.
2. Materials and Methods
2.1. Origin and Characterization of Metal-Related Nanoparticles Applied on Sunflower
A spray dispersion of ZnO-NPs for agronomic purposes was commercially obtained from Sigma-Aldrich (Saint Louis, MO, USA) and applied at a concentration of 10 mg·L−1. Morphological, size, and crystallographic details of these NPs are discussed in Kolenčík et al. [57], while their colloid dispersion properties are outlined in Kolenčík et al. [58]. Conventional agronomic fertilizer with the same Zn concentration (10 mg·L−1) in a water-soluble form (ZnSO4) was obtained from A.F.T. Bratislava s. r. o. (Bratislava, Slovakia).
Synthetic Fe3O4-NPs were prepared and stabilized in a citrate buffer as magnetite mineral, with an applied concentration of 76 mg·L−1, following the methodology of Domingo et al. [59]. Physico-chemical and crystallographic characteristics of Fe3O4-NPs are detailed in a study performed by Ernst et al. [60].
The formation of gold NPs was carried out on silica-based materials, specifically mesoporous algae cells of Mallomonas kalinae, following the methodology of Holišová et al. [61]. In the solution applied to sunflower leaves, the concentrations of gold and silicon were 0.1 mg·L−1 and 10 mg·L−1, respectively.
For TiO2, the concentration was set at 10 mg·L−1, using nano-powder with a metal basis of ≥99.5% from Sigma-Aldrich (Saint Louis, MO, USA). Additionally, all applied NP solutions were diluted in water as an aqueous medium and subjected to ultrasonication before use to enhance colloidal dispersion. The utilization of NPs and agrochemicals in combination with specific sunflower hybrids during selected growing seasons is detailed in Table 1.

Table 1.
Various types of NPs employed as “next generation” nanofertilizers along with control experiments using an aqueous medium which were applied to two different sunflower hybrids and examined over the course of three growing seasons.
2.2. Plant Material
The field experiment employed the sunflower (Helianthus annuus L.) hybrid SY Neostar, modified by Syngenta in Basel, Switzerland. This hybrid is a two-line imidazoline-resistant variety, ideally suited for the ClearField Plus® system. On the other hand, SY Edison is a two-line, early sunflower hybrid [62]. Both the SY Edison and SY Neostar hybrids exhibit very high resistance to typical sunflower pathogens [63].
2.3. Site Description
The collection and study of pollen morphology were conducted at a single experimental site of the Slovak University of Agriculture (SUA) in Dolná Malanta near Nitra, Slovakia, Central Europe. This location is situated at an elevation of 250 m above sea level within the Danubian Lowland. To the north, the Tribeč mountain range is located, the Žitavská Lowland to the east, and the Danubian Lowland extends to the south. In terms of its petrological composition, the nearby area is characterized by the presence of leucocratic granites, Mesozoic limestones, Neogene sediments, and Quaternary alluvial sediments [64]. The soil mineral composition, determined through X-ray diffraction analysis, is predominantly composed of quartz, muscovite, and anorthite [60]. Soils are classified as silt loam haplic Luvisols [65]. The locality is characterized by intensive agricultural activity and is part of a region known for its typical maize crops, with sunflowers being incorporated into the seven-plot crop rotation.
2.4. Monitoring Climate-Seasonal Variation
The variability of precipitation (Table 2) and the average monthly temperature (Table 3) during the three vegetation seasons from 2019–2021 were monitored at the meteorological station of SUA in Nitra.

Table 2.
Monthly variations in precipitation during the vegetation seasons from 2019 to 2021 at the experimental field in Dolná Malanta, Nitra district, Slovakia.

Table 3.
Monthly variations in air temperature during the vegetation seasons from 2019 to 2021 at the experimental field in Dolná Malanta, Nitra district, Slovakia.
2.5. Field Experimental Setup
Field experiments on sunflowers were conducted over three vegetation seasons (2019–2021). The experiments included four treatments, organized in randomly allocated blocks, covering an area of 60 m2, and repeated three times [66]. Prior to the field experiments, deep plowing was consistently performed before each vegetation season using a Zetor Forterra tractor (Zetor Tractors, a.s. Brno, Czech Republic). In all cases, winter wheat (Triticum aestivum L.) was cultivated as the forecrop.
Before sowing sunflowers, key nutrients such as P, K, Ca, and Mg were applied to the soil during the autumn. Nitrogen, in the forms of N-NO3 and N-NH4+, was applied in the spring to ensure compliance with legally defined limits [67]. To achieve this, the 15-15-15 DUSLOFERT fertilizer (Duslo, a. s., Šaľa, Slovakia) was applied at a rate of approximately 400 kg·ha−1 using machinery, specifically a tractor equipped with the Ferti fertilizer applicator (Agromehanica, Boljerac, Serbia), as part of the pre-sowing soil preparation.
Following the guidelines of Alberio et al. [68], sunflower seeds were sown in rows at a depth of 60 mm, with a seed-to-seed spacing of 220 mm and a 700 mm gap between rows, using the Monosem NG Plus 3 planter (Monosem, Largeasse, France). Standard procedures were followed in all experimental trials, including the application of herbicidal and fungicidal formulations. Specifically, the Wing® herbicide (BASF, Ludwigshafen am Rhein, Germany) was applied at a concentration of 4 dm3·ha−1, and the Pictor® fungicide at a rate of 0.4 dm3·ha−1 (BASF, Ludwigshafen am Rhein, Germany) using an AGT 865T/S sprayer (Agromehanica, Boljerac, Serbia). During each season, NP solutions were applied to sunflower leaves using a handheld pressure sprayer (Mythos Di Martino, Mussolente, Italy). The application process was carried out in the early morning hours, taking advantage of favorable wind conditions, until the sunflower leaves were thoroughly moistened. The initial spray application was conducted on the 40th day after sowing, coinciding with the leaf development stage, while the subsequent spray event took place on the 80th day after sowing, aligning with the stages of stem elongation and flower bud formation (Figure 1). Prior to each foliar deposition onto sunflower plants, the NP samples underwent a 15 min ultrasonic treatment to enhance their colloidal dispersion effects.
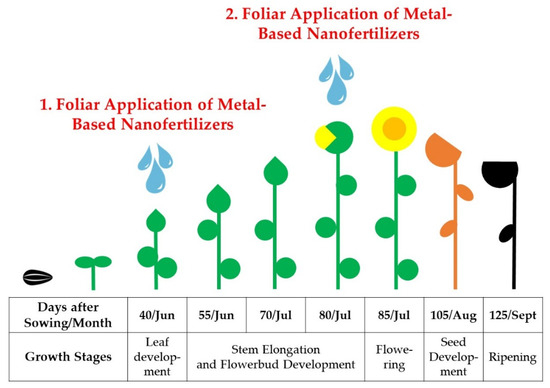
Figure 1.
Schematic figure of the development stages of sunflowers with settled foliar application of NPs during the 2019–2021 growing seasons.
2.6. Assessment of Sunflower Yield
After the sunflowers had reached full seed maturity, harvesting was performed using a small combine harvester, the Claas Dominator 38 (CLAAS KGaA mbH, Harsewinkel, Germany). Seed moisture levels were determined through analysis using the He Lite device (Pfeuffer GmbH, Kitzingen, Germany). Yields for each year were assessed under each treatment using the Kern PCB3500-2 laboratory scale (KERN & Sohn GmbH, Balingen, Germany) and the Numirex seed counter (MEZOS spol. s.r.o., Hradec Králové, Czech Republic). Sunflower yield was conveniently calculated in tons per hectare (t·ha−1) according to the methodology proposed by Ernst et al. [60]. Statistical analysis of sunflower yields for all variants was conducted using TIBCO Statistica®, version 14.0 (TIBCO Software Inc., Palo Alto, CA, USA) [69]. Before performing the multifactorial analysis of variance (ANOVA), the normality of the experimental data was assessed at significance levels of p ≤ 0.05 and p ≤ 0.01 using the Student’s t-test, Shapiro–Wilk test for experiments, and Fisher’s least significant difference (LSD).
2.7. Examination of Critical Pollen Morphological Attributes Using Scanning Electron Microscopy
The pollen grain morphology was evaluated using a scanning electron microscope (SEM) JEOL JSM-7610F Plus (Tokyo, Japan) with a Schottky cathode at 25 keV in a high vacuum chamber. Pollen grain samples were prepared on brass stubs with carbon tape and then coated with gold to avoid sample charging (Quorum Q150T, Quorum Technologies, Laughton, UK).
Pollen grains were gathered from fully blooming flowers, and morphological characteristics were evaluated for fully developed pollen grains. The collected pollen was air-dried in laboratory conditions for 24–48 h and then prepared for SEM analysis. The following characteristics of pollen grains were measured: polar length (P), equatorial length (E), the P/E ratio, area without spines (APGa), area with spines (APGb), and spine length (S). The shape of the pollen grains was classified according to the Erdtman scale [70] based on the ratio of P to E (Figure 2).
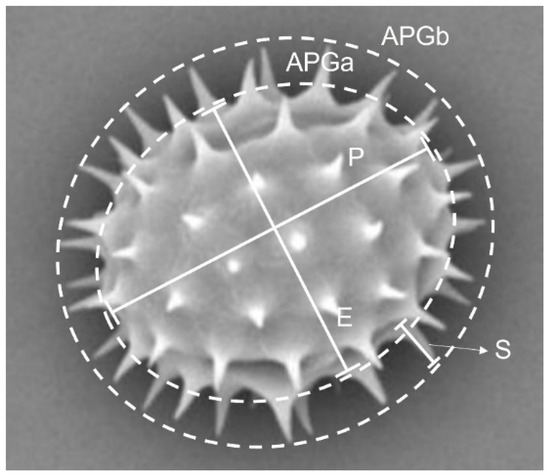
Figure 2.
The parameters of sunflower pollen grains which were considered and analyzed with NP application. Note: P: polar length, E: equatorial length, the P/E ratio, APGa: area without spines, APGb: area with spines, S: spine length.
An average of 100 pollen grains from each variant was evaluated. Palynological terminology followed that of Halbritter et al. [71]. The data obtained were statistically analyzed using Tukey’s HSD test at a significance level of p ≤ 0.01 (Statistica software, version 10, StatSoft, Inc., Tulsa, OK, USA).
3. Results
3.1. Analysis of Sunflower Pollen Morphology under the Influence of Nanoparticle Application in Field Conditions
The findings from the vegetation season in 2019 revealed notable distinctions among variants in two key parameters. Primarily, a significant variance was identified in the equatorial diameter between the NP-free control and the AuSi-NP-treated variant (Figure 3c,h). Additionally, significant differences were observed in the spine length, both between the ZnO-NPs variant (Figure 3g,h) and the control one, and between the Fe3O4-NPs variant (Figure 3d,h) and the control without NP application. Additional statistical data from 2019 are provided in Table 4.
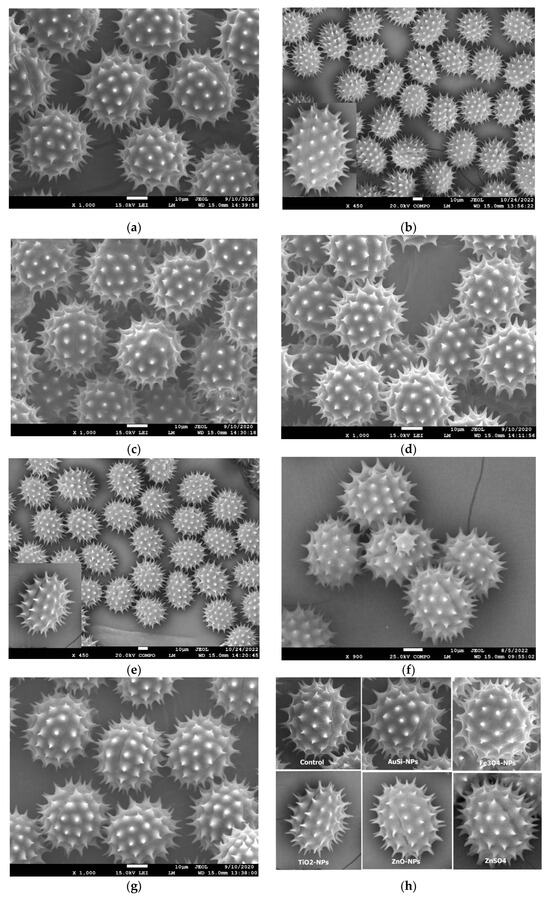
Figure 3.
Typical SEM images visualizing changes in the morphology or morphological parameters of sunflower pollen after the real-word application of various types of metallic-based nanoparticles: (a) typical shape and size of the Neostar variety pollen; (b) typical shape and size of the Edison variety pollen; (c) typical elongation in equatorial diameter with AuSi-NPs treatment; (d) reduced spine length with Fe3O4-NPs application; (e) smaller area of pollen grains without spines and equatorial diameter with TiO2-NPs treatment; (f) larger area of pollen grains without spines with ZnSO4 application; (g) increased spine length with ZnO-NPs treatment; (h) details of pollen grains investigated.

Table 4.
Pollen characteristics of the Neostar hybrid after AuSi-NPs, Fe3O4-NPs, and ZnO-NPs foliar applications compared to NP-free control (Min—minimum, Max—maximum, Cv—coefficient of variation, P—length of polar axis, E—equatorial diameter, APG a—area of pollen grains without spine, APG b—area of pollen grains with spine, S—length of spine). The results from the Tukey HSD test (p ≤ 0.01) are indicated with letters “a” and “b”.
Evidently, in the year 2020, marked distinctions were observed across various parameters in the studied variants. Particularly, a statistically significant dissimilarity in negative connotation was identified in the area of pollen grains without spines, equatorial diameter, and the P/E parameter between the TiO2-NPs variant and the NP-free control. Conversely, the spine length exhibited significant variances across all variants, with the most notable positive response observed in the longest spine, specifically in the instance of the TiO2-NPs application. A notable contrast was reported between the ZnSO4 variant and the control without NP treatment, wherein the ZnSO4 application beneficially resulted in a significant increase in both the length of the polar axis and the equatorial diameter compared to the control variant (Figure 3f,h). Additional findings from the vegetation season 2020 are found in Table 5.

Table 5.
Pollen characteristics of the Neostar hybrid after the TiO2-NPs, ZnO-NPs, and ZnSO4 foliar applications compared to NP-free control (Min—minimum, Max—maximum, Cv—coefficient of variation, P—length of polar axis, E—equatorial diameter, APG a—area of pollen grains without spine, APG b—area of pollen grains with spine, S—length of spine). The results from the Tukey HSD test (p ≤ 0.01) are indicated with letters “a”, “b”, “c”, and “d”.
The results from 2021 in the Edison hybrid revealed a significant difference in the length of the polar axis in the TiO2-NPs variant in comparison with NP-free control, which demonstrated a higher value. Moreover, the TiO2-NPs variant displayed a significant adverse response in the equatorial diameter when compared to the control variant (Figure 3e,h). Additionally, an observable contrast was identified in the P/E parameter, where the TiO2-NPs treatment exhibited a lower value compared to the control one. This was consistent with the recorded differences in the length of the spine, where the TiO2-NPs variant demonstrated a statistically larger size in contrast to the NP-free variant. Conversely, in the AuSi-NPs variant, a notable distinction was observed in the spine length, which was demonstrably smaller than the control variant. The rest of the 2021 results are listed in Table 6.

Table 6.
Pollen characteristics of the Edison hybrid after AuSi-NPs, TiO2-NPs, and ZnO-NPs foliar applications compared to NP-free control (Min—minimum, Max—maximum, Cv—coefficient of variation, P—length of polar axis, E—equatorial diameter, APG a—area of pollen grains without spine, APG b—area of pollen grains with spine, S—length of spine). The results from the Tukey HSD test (p ≤ 0.01) are indicated with letters “a”, “b” and “c”.
The pollen grains of the Neostar and Edison hybrids are categorized as medium-sized pollen grains (25–50 µm). Based on polar axes, in general, the Neostar hybrid exhibited smaller grains in both vegetative seasons (Table 4 and Table 5) than the Edison hybrid (Table 6).
In terms of the shapes of the sunflower pollen grains, the Neostar hybrid exhibited more variability than the Edison hybrid. Based on the P/E ratio, both types of pollen grains belong to the category of prolate spheroidal to subprolate in shape (Figure 3a,b), with a slight preference towards oblate spheroidal pollen grains for the Neostar hybrid. Prolate pollen grains were common in both hybrids.
In 2019, the control variant of the Neostar hybrid had 62.71% subprolate pollen grains. The experimental variants with AuSi-NPs, Fe2O3-NPs, and ZnO-NPs showed higher proportions of prolate spheroidal pollen grains, ranging from 53.23% to 60.34%. In 2020, the Neostar variety had nearly equal proportions of prolate spheroidal (50.68%) and subprolate (48.63%) pollen grains, except for the TiO2-NPs variant, which had generally a higher proportion of subprolate pollen grains at 65.73% (Table 7). This suggests a sensitivity of the Neostar variety to both year-to-year changes and experimental treatments. The Edison hybrid did not exhibit changes in pollen grain shape among all experimental applications compared to the control, with subprolate grains prevailing in all cases.

Table 7.
Percentage of pollen shape classes (P/E ratio) of sunflower cultivars according to Erdtman’s [70] classification: peroblate < 0.5; oblate 0.5–0.75; suboblate 0.75–0.88; oblate spheroidal 0.88–1; spheroidal 1; prolate spheroidal 1–1.14; subprolate 1.14–1.33; prolate 1.33–2; perprolate ˃ 2.
3.2. Evaluation of Sunflower Yield with Metal-Based Nanoparticle Application
In the 2019 growing season at the experimental site, a statistically significant increase in yield was observed with the application of ZnO-NPs compared to the NP-free control (Table 8). While the other applied variants, specifically AuSi-NPs and Fe3O4-NPs, showed a positive trend toward higher yields, these differences did not reach statistical significance [60]. In the 2020 growing season, a statistically significant increase in yield was recorded with the application of TiO2-NPs and ZnSO4 compared to the control (Table 8). For ZnO-NPs, the yield was higher than the NP-free control but did not reach statistical significance. In the 2021 season, application of TiO2-NPs, AuSi-NPs, and ZnO-NPs displayed significant impacts on grain yield compared to the NP-free control variant.

Table 8.
Comparison of sunflower yields with the application of AuSi-NPs, ZnO-NPs, Fe3O4-NPs, TiO2-NPs, ZnSO4, and NP-free control variant over three vegetation seasons (2019–2021) at the experimental site in Dolná Malanta, Nitra district, Slovak Republic.
4. Discussion
Metal-based NPs enter the environment through various anthropogenic pathways, including agricultural activities [1]. In the form of soluble ion-metals, they naturally contribute to plant metabolism, growth, and development [8]. However, at higher concentrations, NPs can often have negative or toxic effects on plants [72]. The effects of metallic-based NPs and ionic components in plants have been extensively studied, particularly concerning physiological and biochemical aspects such as enzymatic activity [73], photosynthesis and transpiration [74], metal accumulation affecting plant tissue [75], or the development of reproductive organs [76]. However, only a few studies have investigated the direct impact of NPs on pollen or pollen morphology in real agronomic conditions. Most research has focused on atmospheric environmental pollution [37] or the immediate effects on pollen viability and other in vitro functional attributes [36,43]. Hence, our study provides valuable insights into the understanding of the effects of diverse NP foliar applications on sunflower pollen morphology and yield, filling a notable gap in this research area.
In this context, sunflowers serve as excellent models for both agronomic and environmental research.
4.1. The Effect of ZnO-NPs and Conventional Agrochemical ZnSO4 on Sunflower Pollen Morphology and Their Impact on Yield
Our findings indicate a positive impact of ZnO-NPs foliar application on sunflower yield (Table 8), yield-related parameters, and seasonal physiology during the 2019 season, as previously described in a study by Ernst et al. [60].
For the Neostar hybrid, a demonstrable alteration in pollen morphology was observed, specifically in the spine parameter. In 2019, it exhibited a lower value, while in 2020, it showed a higher value compared to the control. Notably, this parameter displayed significant variability, especially in 2020, among all the NP-treated variants.
In the context of sunflower cultivation, the correlation between temperature and annual precipitation, as highlighted by Astiz and Hernández [77], may have subsequent implications for nutrient uptake, absorption, or the response to NP foliar deposition. Our results clearly show that during pollen maturation in July, there was a marked absence of rainfall, combined with normal temperatures. In contrast, the vegetation season of 2020 was characterized by minimal precipitation and exceptionally cold temperatures (see Table 2 and Table 3). In addition, in 2020, statistically significant enhancements were noted in pollen morphology parameters, specifically in the elongation of polar axes and the APG parameter.
The Edison hybrid exhibited no statistically significant differences in pollen grain morphology and its morphological parameters compared to the control variant. In fact, it displayed slightly negative characteristics, though not statistically significant (see Table 6). This could be attributed to the inherent variability of the Edison hybrid [62], which shows lower sensitivity to metal-based NP foliar application or other external abiotic-induced stressors, including seasonal–climatic fluctuations.
Nevertheless, in these practical, in-field experiments where ZnO-NPs were applied to sunflowers, we have, for the first time, confirmed that despite the varied responses in pollen morphology, the sunflower yield consistently demonstrated a positive trend over three growing seasons for both sunflower hybrids evaluated. In this context, Zn appears to play a significant role in the development of male reproductive organs and gametes. Zinc deficiency results in delayed flowering, reduced size of the anthers, pollen production, decreased size of pollen grains, and causes morphological changes in pollen, especially in the exine structure of lentils [40] and black gram [41]. Additionally, reduced pollen viability has been found in maize [78], lentils [40] and black gram [41], and chickpeas [79].
Considering the sunflower plant response, including pollen morphology, we can anticipate two effects after the application of ZnO-NPs. First, as we know, when colloidal dispersion is applied, ZnO-NPs undergo photodegradation, leading to Zn assimilation and subsequent redistribution by the leaves/plant to various geochemical species [80]. This process could potentially enhance other physiological, metabolic, and biochemical pathways in the plant [8]. In terms of physiology and higher yields, we have previously observed such effects at low, comparable concentrations in the case of Setaria italica [57], lentils [58], and sunflowers [81]. The interaction with ZnO-NPs could have a direct impact on the morphology, structure, and function of pollen, as outlined by Yoshihara et al. [82]. This interaction resulted in reduced pollen grain germination and tube elongation in the case of Lilium longiflorum. Moreover, the application of commercial nanofertilizer ZnO-NPs at a concentration of 7.5 mL·L−1 caused pollen aberrations and structural changes in the sporoderm, along with degenerative changes in the content of peach pollen grains [45].
On the other side, the negative impact of Zn deficiency on the morphology and viability of pollen grains can be mitigated by adding ZnSO4 [79]. Although Zn supplementation at a dose of 500 mg/pot ZnSO4 has a positive effect on the size of pea pollen, a high dose of 1000 mg/pot of this element causes abnormalities in sporoderm of pollen grains and deformation of the pollen [83]. Our result confirmed that after the application of ZnSO4, sunflower pollen grains clearly reached the highest values for several evaluated size parameters, reflecting the positive influence of Zn and S on pollen development.
A remarkable finding is the change in the shape of the pollen of the Neostar variety after the application of ZnO-NPs in 2019, when all the treated variants exhibited a shape modification of the pollen grains compared to the control. While we defined the pollen grains in the control variant as subprolate based on the ratio of the polar and equatorial diameter, in the treated variants including ZnO-NPs, they were prolate spheroidal (Table 7). However, in the following year, this change in shape occurred only in the variant with ZnSO4 treatment.
This shape modification can be associated with seasonal weather fluctuations (see Table 2 and Table 3) and could potentially impact pollen collection and distribution by sunflower pollinators [84]. In this context, the use of agronomically and conventionally applied ZnSO4 appears to be more suitable than treatment with “pure” ZnO-NPs. ZnSO4 is readily bioavailable and mobile in an ion-soluble form. Additionally, the SO4 anion may function as a macronutrient [79], potentially leading to a more substantial agronomic impact (Table 8).
This was further confirmed at a statistically more significant level in terms of yield (refer to Table 8) and all relevant parameters of pollen morphology, namely the length of polar axis, equatorial diameter, and area of pollen grains (APGa) (see Table 5). In addition, since SO4 is a biologically available form of sulphur, it can participate in the formation of pectins [85], which are polymers integrated into the intine of pollen grains [86], naturally supporting the function of pollen grain germination. It can also contribute to the production and regulation of phytohormones, especially cytokinin, which could be reflected in the development of sunflower pollen [87]. In this scenario, larger pollen grains have an advantage in germination and growth towards fertilizing ovules when compared to smaller pollen grains, as demonstrated in Hibiscus syriacus with improved fruit quality [88].
4.2. The Impact of Iron Nanoparticles on Pollen Morphology and Their Associations with Sunflower Yield
In terms of pollen morphology, the application of Fe3O4-NPs appears to have a statistically insignificant impact. This is evident from the smaller pollen grains (APGb) and shorter spines compared to the other variants, including the NP-free control. These findings may be linked to plain seasonal physiological responses and relatively indistinct yield (see Table 8) and yield-related parameters, including oil content, which are comparable to the control without NP application, as documented in the study by Ernst et al. [60].
In heavy metal-contaminated soils, with Zn and Fe concentrations sometimes up to seven times higher where Chenopodium botrys L. was grown, Yousefi et al. [89] observed other development irregularities that affected plant fertility and survival. These irregularities also led to changes in anther morphology and a reduced pollen count in plants from the contaminated site.
In our study, one of the stress-inducing factors associated with the properties of Fe3O4-NPs, particularly concerning pollen morphology, is their small size, which does not exceed 5 nm. In this size range, we can expect a nano-domain effect, resulting in a certain level of metastability and an associated increase in the reactivity of these NPs, as pointed out by Lin et al. [90]. Assuming monodisperse NPs with high crystallinity and a higher concentration (76 mg·L−1), a hypothesis could be formulated regarding the increased proliferation, translocation, and enormous reactivity of Fe3O4-NPs or their residues into pollen grains during the flowering phenophase. However, this hypothesis is challenged by the overall lower Fe concentrations in final-quality sunflower grains, as reported by Ernst et al. [60].
While soluble Fe is recognized as a micronutrient, the transport, mobility, and effects, as well as the destiny of Fe in the NP nature within plants or their structures, may differ. This variance has been demonstrated in pumpkin plants (Cucurbita maxima L.) [91] and is evident in its negative impact on the overall development of tobacco (Nicotiana tabacum) [92].
If Fe3O4-NPs could directly impact pollen grains, we should take into consideration their higher mechanical and chemical stability, which could modify various pollen properties, such as a reduction in pollen viability or the abortiveness of Arabidopsis with a subsequent reduction in seed yield [93].
4.3. The Examined Impact of AuSi NPs on Pollen Morphology and Their Correlation with Sunflower Crop Yield
The evaluation of the AuSi-NP variant after application at the same concentration (0.1 mg·L−1 Au and 10 mg·L−1 SiO2) in two growing seasons and with two hybrids, Neostar and Edison, yielded somewhat ambiguous results. In 2019, for the Neostar hybrid, when considering pollen morphology, statistically significant differences were observed only in the case of an elongated equatorial diameter. There were also indications of potential changes in polar diameter and spine length, but these were statistically insignificant. The other parameters favored the control variant, but with insignificant differences (see Table 4).
In the case of the Edison hybrid, during the 2021 vegetation season (see Table 6), the morphological parameters, when compared to the control, were statistically insignificant. Considerable differences from the NP-free variant were observed only in terms of shorter spine length or smaller equatorial diameter, which does not correspond to the results of the Neostar hybrid. Here, spines were the shortest among all variants, including the control. This paradoxical variation in spine length could be seen as an indicator of differential protection against potential environmental effects between the two hybrids.
Like pollen spines, shorter equatorial diameters may also represent a type of plant adaptation mechanism in response to specific environments or preferences for pollinators, aiding the plant in facilitating pollination by certain types of insects or other pollinators [94]. Surprisingly, it is worth noting that the AuSi nano-composite exhibited effects on pollen morphology like ZnO-NPs in both applied hybrids and growing seasons. However, it demonstrated a significant increasing trend in yield (refer to Table 8), even in the case of the Edison hybrid, which is known to be less responsive to external factors, and this was at a higher statistical level.
The Au-NPs at a size of approximately 10 nm, with excellent crystallinity, monodispersity, and a dominant spherical shape [61], along with unique biocompatibility and stability [95], theoretically suggest that Au NPs could pass through plant membranes and tissues relatively easily [31], potentially negatively affecting the final quality and content of sunflower achenes. However, this was not chemically and analytically confirmed in our case [60]. It may be related to the very low concentration we applied the NPs (0.1 mg·L−1), which was best reflected in terms of physiological parameters, yield, and yield-related parameters [60]. Mahakham et al. [96] found in maize that Au-NPs preferentially entered the grains, but translocation into vegetative organs or roots was not recorded.
Hypothetically, Au-NPs may directly affect pollen morphology, potentially causing disruptions in internal and external structures and functionality. Surprisingly, our findings during the 2019 vegetation season demonstrated a higher agro-ecological terrestrial insect population, optimal living conditions, and functionality [60].
Since Au-NPs were applied together with SiO2 mesoporous silica Mallomonas kaline, we also expected a synergistic effect due to the presence of silica as a plant micronutrient [97] and a kind of plant growth stimulator in NP form [4]. This could possibly explain the observed twofold increase in Si concentrations in the final seed quality compared to the control [60]. This form of SiO2 (mesoporous silica Mallomonas kaline) is relatively more soluble, more bioavailable, and more mobile than crystallographically structurally more stable and inert nanoforms of SiO2. This form, in the context of negative effects on selected terrestrial communities, was confirmed by Hussein et al. [98] with the potential of being used as a nanopesticide.
An alternative hypothesis suggests that the pollen or other generative parts of the plant might incorporate silica due to its functional sorption complex, relative smaller size, porous structure, chemical inertness, or surface modification [61].
4.4. The Effect of TiO2-NPs Treatment on Sunflower Pollen Morphology and Yield
From our results, it is evident that sunflower yields for both hybrids were significantly higher when applying TiO2-NPs, not only in comparison with the control but also with all other NP-treated variants (see Table 8). These findings are quite surprising because, when we consider the relatively negative impact on the pollen size of both the Neostar and Edison hybrids, they showed the lowest values for the polar axis, equatorial diameter, and other related parameters (see Table 5 and Table 6). Paradoxically, although smaller pollen grains are generally characterized by lower germination, they may show a higher rate of pollen tube growth. From this point view, even small pollen can produce more vigorous progeny, which can pose a certain selective advantage [99].
Concurrently, pollen grains in the variant with Ti in both varieties exhibited statistically the highest values for spines compared to other variants. This can be seen as a kind of adaptive mechanism in response to Ti-induced stress, which could, on the other hand, significantly benefit the visitation preferences of individual pollinator types. In effect, some pollinators visit species with large echinate pollen grains less frequently, such as Knautia arvensis and some Malvaceae, but also collect similar pollen from Cucurbita pepo [100].
In the case of the application of TiO2-NPs, positive, negative, or no effects are elaborated in connection with plant response in the academic literature [72]. Here Ti is not presumed to have an essential character but is rather considered as a plant stimulator [4,72].
Evidently, our results showed a relatively negative impact on the morphological features of sunflower pollen with TiO2-NPs interaction, while crop yields consistently remained excellent (see Table 8). In this context, Vear et al. [101] hypothesize that the energy saved in pollen production can be used to increase the oil content. There are indications of the impact of TiO2-NPs, evident in the intensified physiology of the same hybrid, Neostar, during the 2018 growing season, along with yield and yield-related parameters, as well as oil content [81]. These outcomes are comparable to the improvements in yield and oil content seen in Glycine max, as published by Rezaei et al. [102].
In our study, we applied the anatase-rutile form of TiO2-NPs with structural modifications, higher chemical and mechanical stability, and a size corresponding to ~30 nm [81]. The potential positive impact of our applied concentration range of TiO2-NPs has been reflected in several studies. Larue et al. [103] observed increased uptake and translocation of Ti into leaves, as well as root elongation in Triticum aestivum L., albeit at similar sizes but slightly higher concentrations. Similarly, Servin et al. [104] noted enhanced root growth in Cucumis sativus, which also exhibited a strong correlation with their nitrogen content. It is worth noting that in both cases, TiO2-NPs were applied via the root system rather than through leaf deposition.
Additionally, when considering the analytically determined total content of Ti affecting the final quality of sunflower grains, where TiO2 is one of the most resistant materials, the NPs have a relatively small size and ability to transfer within the plant. It is surprising that an increased total content was not found in them compared to the NP-free control [81]. Thus, we can indirectly assert that the transfer of Ti or TiO2-NPs into the reproductive organs, which affects seed quality, was limited, even though there are certainly indications of modifications in pollen morphology (see Table 5 and Table 6).
On the contrary, we postulated that TiO2-NPs might exert adverse effects due to the pronounced chemical and mechanical stability of NPs associated with the potential negative photocatalytic activity of TiO2-NPs, generating reactive oxygen species (ROS) when exposed to sunlight, as elucidated by Lei et al. [19]. Nevertheless, the study conducted by Huang et al. [105] highlights their beneficial impact in improving pollen germination rates and pollen tube length in litchi male flowers through the application of TiO2-NPs.
5. Conclusions
In summary, our study found that the application of metal-based NPs as NFs had variable effects on sunflower pollen morphology, but only at non-critical levels, and they did not significantly impact crop yields in field experiments conducted over three vegetative seasons with two hybrids of sunflower. We credit this outcome to the application of eco-friendly, low-concentration NFs, ensuring precise and sustainable agriculture.
(i) There was a relative “negative” trend in the application of TiO2-NPs concerning sunflower pollen morphology; however, this did not manifest in the form of reduced crop yields for both sunflower hybrids, Neostar and Edison. It may be interpreted as an adaptive mode of actions in response to induced Ti or TiO2-NP stress during sunflower development, targeting more to yield production with anatase-rutile minerals at the ~30 nm size.
(ii) At the same Zn concentration corresponding to 10 mg·L−1, the most favorable response to sunflower pollen morphology was observed with the agronomically conventional ionically soluble fertilizer ZnSO4, followed by ZnO-NPs. Furthermore, the use of both NFs led to higher yields compared to the control, where yields were consistently diminished.
(iii) When we consider Au-NPs as a plant-growth stimulator affecting plant morphology corresponding to 10 nm Au-NPs anchored to SiO2 mesoporous silica, there were no clear differences observed in the examined parameters. This might be related to the applied hybrids, with Neostar appearing to be more sensitive than the Edison hybrid, as well as in the control experiments (NP-free). However, the AuSi-NPs variant unexpectedly achieved higher yields, where surprisingly the Edison hybrid showed a more beneficial response to yield at a statistically significant level, even though it is considered to be less sensitive.
(iv) In this study, no statistically significant differences in pollen morphology were observed when magnetite (Fe3O4-NPs), classified as a micro-based nanoform, was utilized at its highest concentration with the smallest particle size. Contrary to our initial hypothesis, which posited higher reactivity due to its relative stability and active surface, this postulate was not confirmed. However, the agronomic yield exhibited a slight improvement when compared to the NP-free control.
Author Contributions
Investigation, writing—original draft preparation, supervision, proposed the topic, Ľ.Ď. and M.K.; obtained formal analysis, figures, tables, and software—statistical implementations, and validation, V.Ž.Č. and V.S.; writing—review and editing, and supervision, P.E., Y.Q. and H.F.; writing—review and editing, checked grammar, review and editing whole concept of manuscript, M.Š. and H.Ď.; performed agronomical experiments and yield determination, D.E.; performed microscopic investigation of sunflower pollen, Ľ.Ď., N.K., L.T. and S.K.; performed SEM analysis, G.K.; funding acquisition and project administration Ľ.Ď. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Grant Agency of the Slovak Republic Ministry of Education and the Slovak Academy of Sciences under contract VEGA 1/0655/23, VEGA 1/0359/22, VEGA 1/0331/23.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Šebesta, M.; Ďurišová, Ľ.; Ernst, D.; Kšiňan, S.; Illa, R.; Sunil, B.R.; Ingle, A.P.; Qian, Y.; Urík, M.; Kolenčík, M. Foliar application of metallic nanoparticles on crops under field conditions. In Plant and Nanoparticles; Chen, J.-T., Ed.; Springer Nature Singapore: Singapore, 2022; pp. 171–215. [Google Scholar]
- Seleiman, M.F.; Ahmad, A.; Alhammad, B.A.; Tola, E. Exogenous application of zinc oxide nanoparticles improved antioxidants, photosynthetic, and yield traits in salt-stressed maize. Agronomy 2023, 13, 2645. [Google Scholar] [CrossRef]
- Kolenčík, M.; Nemček, L.; Šebesta, M.; Urík, M.; Ernst, D.; Kratošová, G.; Konvičková, Z. Effect of TiO2 as plant-growth stimulating nanomaterial on crop production. In Plant Responses to Nanomaterials, Recent Interventions, and Physiological and Biochemical Responses, 1st ed.; Singh, V.P., Singh, S., Prasad, S.M., Chauhan, D.K., Tripathi, D.K., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 129–144. [Google Scholar]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Salehi, H.; De Diego, N.; Chehregani Rad, A.; Benjamin, J.J.; Trevisan, M.; Lucini, L. Exogenous application of ZnO nanoparticles and ZnSO4 distinctly influence the metabolic response in Phaseolus vulgaris L. Sci. Total Environ. 2021, 778, 146331. [Google Scholar] [CrossRef] [PubMed]
- Mousavi Kouhi, S.M.; Lahouti, M.; Ganjeali, A.; Entezari, M.H. Comparative phytotoxicity of ZnO nanoparticles, ZnO microparticles, and Zn2+ on rapeseed (Brassica napus L.): Investigating a wide range of concentrations. Toxicol. Environ. Chem. 2014, 96, 861–868. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Ahmad, A.; Battaglia, M.L.; Bilal, H.M.; Alhammad, B.A.; Khan, N. Zinc oxide nanoparticles: A unique saline stress mitigator with the potential to increase future crop production. S. Afr. J. Bot. 2023, 159, 208–218. [Google Scholar] [CrossRef]
- Sturikova, H.; Krystofova, O.; Huska, D.; Adam, V. Zinc, zinc nanoparticles and plants. J. Hazard. Mater. 2018, 349, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Al-Selwey, W.A.; Alsadon, A.A.; Alenazi, M.M.; Tarroum, M.; Ibrahim, A.A.; Ahmad, A.; Osman, M.; Seleiman, M.F. Morphological and biochemical response of potatoes to exogenous application of ZnO and SiO2 nanoparticles in a water deficit environment. Horticulturae 2023, 9, 883. [Google Scholar] [CrossRef]
- Ahmad, A.; Tola, E.; Alshahrani, T.S.; Seleiman, M.F. Enhancement of morphological and physiological performance of Zea mays L. under saline stress using ZnO nanoparticles and 24-Epibrassinolide seed priming. Agronomy 2023, 13, 771. [Google Scholar] [CrossRef]
- Ranieri, A.; Castagna, A.; Baldan, B.; Soldatini, G.F. Iron deficiency differently affects peroxidase isoforms in sunflower. J. Exp. Bot. 2001, 52, 25–35. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Sun, W.; Wang, T. The adaptive mechanism of plants to iron deficiency via iron uptake, transport, and homeostasis. Int. J. Mol. Sci. 2019, 20, 2424. [Google Scholar] [CrossRef]
- Zia-ur-Rehman, M.; Naeem, A.; Khalid, H.; Rizwan, M.; Ali, S.; Azhar, M. Responses of plants to iron oxide nanoparticles. In Nanomaterials in Plants, Algae, and Microorganisms; Tripathi, D.K., Ahmad, P., Sharma, S., Chauhan, D.K., Dubey, N.K., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 221–238. [Google Scholar]
- Roschzttardtz, H.; Conéjéro, G.; Divol, F.; Alcon, C.; Verdeil, J.-L.; Curie, C.; Mari, S. New insights into Fe localization in plant tissues. Front. Plant Sci. 2013, 4, 350. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-H.; Suen, D.-F. Iron insufficiency in floral buds impairs pollen development by disrupting tapetum function. Plant J. 2021, 108, 244–267. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.Y.; Gottschalk, F.; Hungerbühler, K.; Nowack, B. Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ. Pollut. 2014, 185, 69–76. [Google Scholar] [CrossRef]
- Hong, F.; Yang, F.; Liu, C.; Gao, Q.; Wan, Z.; Gu, F.; Wu, C.; Ma, Z.; Zhou, J.; Yang, P. Influences of nano-TiO2 on the chloroplast aging of spinach under light. Biol. Trace Elem. Res. 2005, 104, 249–260. [Google Scholar] [CrossRef]
- Hong, F.; Zhou, J.; Liu, C.; Yang, F.; Wu, C.; Zheng, L.; Yang, P. Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol. Trace Elem. Res. 2005, 105, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Mingyu, S.; Chao, L.; Liang, C.; Hao, H.; Xiao, W.; Xiaoqing, L.; Fan, Y.; Fengqing, G.; Fashui, H. Effects of nanoanatase TiO2 on photosynthesis of spinach chloroplasts under different light illumination. Biol. Trace Elem. Res. 2007, 119, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Jaberzadeh, A.; Moaveni, P.; Moghadam, H.R.T.; Zahedi, H. Influence of bulk and nanoparticles titanium foliar application on some agronomic traits, seed gluten and starch contents of wheat subjected to water deficit stress. Not. Bot. Horti. Agrobot. Cluj Napoca. 2013, 41, 201–207. [Google Scholar] [CrossRef]
- Lyu, S.; Wei, X.; Chen, J.; Wang, C.; Wang, X.; Pan, D. Titanium as a beneficial element for crop production. Front. Plant Sci. 2017, 8, 597. [Google Scholar] [CrossRef]
- Kamali, M.; Shoor, M.; Feizi, H. Impacts of nanosized and bulk titanium dioxide on flowering and morphophysiological traits of petunia (Petunia hybrida) under salinity stress. J. Hortic. Sci. 2018, 32, 199–212. [Google Scholar] [CrossRef]
- Abdelhaliem, E.; Al-Shalawi, J. Attenuation of lead genotoxicity in Glycine max by adsorbent nanosized titanium dioxide using phenotypic, cytogenetic and DNA status bioassays. Genet. Mol. Res. 2019, 8, GMR18350. [Google Scholar]
- Bieniasz, M.; Konieczny, A. The effect of titanium organic complex on pollination process and fruit development of apple cv. topaz. Agronomy 2021, 11, 2591. [Google Scholar] [CrossRef]
- Bieniasz, M.; Konieczny, A.; Błaszczyk, J.; Nawrocki, J.; Kopeć, M.; Mierzwa-Hersztek, M.; Gondek, K.; Zaleski, T.; Knaga, J.; Pniak, M. Titanium organic complex improves pollination and fruit development of remontant strawberry cultivars under high-temperature conditions. Agriculture 2022, 12, 1795. [Google Scholar] [CrossRef]
- Ruffini Castiglione, M.; Giorgetti, L.; Geri, C.; Cremonini, R. The effects of nano-TiO2 on seed germination, development and mitosis of root tip cells of Vicia narbonensis L. and Zea mays L. J. Nanopart. Res. 2011, 13, 2443–2449. [Google Scholar] [CrossRef]
- Burke, D.J.; Pietrasiak, N.; Situ, S.F.; Abenojar, E.C.; Porche, M.; Kraj, P.; Lakliang, Y.; Samia, A.C.S. Iron oxide and titanium dioxide nanoparticle effects on plant performance and root associated microbes. Int. J. Mol. Sci. 2015, 16, 23630–23650. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Roy, S. Nanosilica facilitates silica uptake, growth and stress tolerance in plants. Plant Physiol. Biochem. 2020, 157, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Esmaili, S.; Tavallali, V.; Amiri, B. Nano-silicon complexes enhance growth, yield, water relations and mineral composition in tanacetum parthenium under water deficit stress. Silicon 2021, 13, 2493–2508. [Google Scholar] [CrossRef]
- Al-Selwey, W.A.; Alsadon, A.A.; Ibrahim, A.A.; Labis, J.P.; Seleiman, M.F. Effects of zinc oxide and silicon dioxide nanoparticles on physiological, yield, and water use efficiency traits of potato grown under water deficit. Plants 2023, 12, 218. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Engineered gold nanoparticles and plant adaptation potential. Nanoscale Res. Lett. 2016, 11, 400. [Google Scholar] [CrossRef]
- Gopinath, K.; Venkatesh, K.S.; Ilangovan, R.; Sankaranarayanan, K.; Arumugam, A. Green synthesis of gold nanoparticles from leaf extract of Terminalia arjuna, for the enhanced mitotic cell division and pollen germination activity. Ind. Crops Prod. 2013, 50, 737–742. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Bhakyaraj, K.; Gopinath, K.; Govindarajan, M.; Kumuraguru, S.; Mohan, S.; Kaleeswarran, P.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Gum-mediated fabrication of eco-friendly gold nanoparticles promoting cell division and pollen germination in plant cells. J. Clust. Sci. 2017, 28, 507–517. [Google Scholar] [CrossRef]
- Handy, R.D.; Cornelis, G.; Fernandes, T.; Tsyusko, O.; Decho, A.; Sabo-Attwood, T.; Metcalfe, C.; Steevens, J.A.; Klaine, S.J.; Koelmans, A.A.; et al. Ecotoxicity test methods for engineered nanomaterials: Practical experiences and recommendations from the bench. Environ. Toxicol. Chem. 2012, 31, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Pfahler, P.L. Analysis of ecotoxic agents using pollen tests. In Plant Toxin Analysis; Linskens, H.F., Jackson, J.F., Eds.; Springer: Berlin/Heidelberg, Germnay, 1992; pp. 317–331. [Google Scholar]
- Speranza, A.; Leopold, K.; Maier, M.; Taddei, A.R.; Scoccianti, V. Pd-nanoparticles cause increased toxicity to kiwifruit pollen compared to soluble Pd(II). Environ. Pollut. 2010, 158, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Sénéchal, H.; Visez, N.; Charpin, D.; Shahali, Y.; Peltre, G.; Biolley, J.-P.; Lhuissier, F.; Couderc, R.; Yamada, O.; Malrat-Domenge, A.; et al. A Review of the effects of major atmospheric pollutants on pollen grains, pollen content, and allergenicity. Sci. World J. 2015, 2015, 940243. [Google Scholar] [CrossRef] [PubMed]
- Mičieta, K.; Murín, G. Microspore analysis for genotoxicity of a polluted environment. Environ. Exp. Bot. 1996, 36, 21–27. [Google Scholar] [CrossRef]
- Salehi, H.; Chehregani Rad, A.; Raza, A.; Chen, J.-T. Foliar application of CeO2 nanoparticles alters generative components fitness and seed productivity in bean crop (Phaseolus vulgaris L.). Nanomaterials 2021, 11, 862. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Pathak, G.C.; Sharma, C.P. Zinc is critically required for pollen function and fertilisation in lentil. J. Trace Elem. Med. Biol. 2006, 20, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Pathak, G.C.; Sharma, C.P. Impairment in reproductive development is a major factor limiting yield of black gram under zinc deficiency. Biol. Plant. 2009, 53, 723–727. [Google Scholar] [CrossRef]
- Ke, M.; Li, Y.; Qu, Q.; Ye, Y.; Peijnenburg, W.J.G.M.; Zhang, Z.; Xu, N.; Lu, T.; Sun, L.; Qian, H. Offspring toxicity of silver nanoparticles to Arabidopsis thaliana flowering and floral development. J. Hazard. Mater. 2020, 386, 121975. [Google Scholar] [CrossRef]
- Speranza, A.; Crinelli, R.; Scoccianti, V.; Taddei, A.R.; Iacobucci, M.; Bhattacharya, P.; Ke, P.C. In vitro toxicity of silver nanoparticles to kiwifruit pollen exhibits peculiar traits beyond the cause of silver ion release. Environ. Pollut. 2013, 179, 258–267. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Saha, N.; Agarwal, A.; Venkatesh, V. Silver nanoparticles (AgNPs) induced impairment of in vitro pollen performance of Peltophorum pterocarpum (DC.) K. Heyne. Ecotoxicology 2020, 29, 75–85. [Google Scholar] [CrossRef]
- Mosa, W.F.A.; El-Shehawi, A.M.; Mackled, M.I.; Salem, M.Z.M.; Ghareeb, R.Y.; Hafez, E.E.; Behiry, S.I.; Abdelsalam, N.R. Productivity performance of peach trees, insecticidal and antibacterial bioactivities of leaf extracts as affected by nanofertilizers foliar application. Sci. Rep. 2021, 11, 10205. [Google Scholar] [CrossRef] [PubMed]
- Ejsmond, M.J.; Wrońska-Pilarek, D.; Ejsmond, A.; Dragosz-Kluska, D.; Karpińska-Kołaczek, M.; Kołaczek, P.; Kozłowski, J. Does climate affect pollen morphology? Optimal size and shape of pollen grains under various desiccation intensity. Ecosphere 2011, 2, art117. [Google Scholar] [CrossRef]
- Eftekharian, R.; Sheidai, M.; Attar, F.; Noormohammadi, Z. Pollen morphology of Senecio L. and Iranecio B. Nord. (Asteraceae: Senecioneae) in Iran. Acta Biol. Szeged. 2017, 61, 157–162. [Google Scholar]
- Wang, R.; Dobritsa, A.A. Exine and aperture patterns on the pollen surface: Their formation and roles in plant reproduction. Annu. Plant Rev. Online 2018, 1, 589–628. [Google Scholar]
- Vaissière, B.E.; Vinson, S.B. Pollen morphology and its effect on pollenl collection by honey bees, Apis Mellifera L. (Hymenoptera: Apidae), with special reference to upland cotton, Gossypium Hirsutum L. (Malvaceae). Grana 1994, 33, 128–138. [Google Scholar] [CrossRef]
- Laveau, J.H.; Schneider, C.; Berville, A. Microsporogenesis abortion in cytoplasmic male sterile plants from H. petiolaris or H. petiolaris fallax crossed by sunflower (Helianthus annuus). Ann. Bot. 1989, 64, 137–148. [Google Scholar] [CrossRef]
- Atlagić, J.; Dozet, B.; ŠKorić, D. Meiosis and pollen viability in Helianthus tuberosus L. and its hybrids with cultivated sunflower. Plant Breed. 1993, 111, 318–324. [Google Scholar] [CrossRef]
- Pal, D. Sunflower (Helianthus annuus L.) seeds in health and nutrition. In Nuts and Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 1097–1105. [Google Scholar]
- Muhlbauer, W.; Muller, J. Drying Atlas: Drying Kinetics and Quality of Agricultural Products; Woodhead Publishing: Sawston, UK, 2020. [Google Scholar]
- Filipiak, M.; Shields, M.W.; Cairns, S.M.; Grainger, M.N.C.; Wratten, S.D. The conserved and high K-to-Na ratio in sunflower pollen: Possible implications for bee health and plant-bee interactions. Front. Plant Sci. 2022, 13, 1042348. [Google Scholar] [CrossRef]
- Giacomini, J.J.; Leslie, J.; Tarpy, D.R.; Palmer-Young, E.C.; Irwin, R.E.; Adler, L.S. Medicinal value of sunflower pollen against bee pathogens. Sci. Rep. 2018, 8, 14394. [Google Scholar] [CrossRef]
- LoCascio, G.M.; Aguirre, L.; Irwin, R.E.; Adler, L.S. Pollen from multiple sunflower cultivars and species reduces a common bumblebee gut pathogen. R. Soc. Open Sci. 2019, 6, 190279. [Google Scholar] [CrossRef]
- Kolenčík, M.; Ernst, D.; Komár, M.; Urík, M.; Šebesta, M.; Dobročka, E.; Černý, I.; Illa, R.; Kanike, R.; Qian, Y.; et al. Effect of foliar spray application of zinc oxide nanoparticles on quantitative, nutritional, and physiological parameters of foxtail millet (Setaria italica L.) under field conditions. Nanomaterials 2019, 9, 1559. [Google Scholar] [CrossRef] [PubMed]
- Kolenčík, M.; Ernst, D.; Komár, M.; Urík, M.; Šebesta, M.; Ďurišová, Ľ.; Bujdoš, M.; Černý, I.; Chlpík, J.; Juriga, M.; et al. Effects of foliar application of ZnO nanoparticles on lentil production, stress level and nutritional seed quality under field conditions. Nanomaterials 2022, 12, 310. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.C.; Mercadal, M.; Petriz, J.; De Madariaga, M.A. Preparation of PEG-grafted immunomagnetoliposomes entrapping citrate stabilized magnetite particles and their application in CD34+ cell sorting. J. Microencapsul. 2001, 18, 41–54. [Google Scholar] [PubMed]
- Ernst, D.; Kolenčík, M.; Šebesta, M.; Ďurišová, Ľ.; Ďúranová, H.; Kšiňan, S.; Illa, R.; Safarik, I.; Černý, I.; Kratošová, G.; et al. Agronomic investigation of spray dispersion of metal-based nanoparticles on sunflowers in real-world environments. Plants 2023, 12, 1789. [Google Scholar] [CrossRef] [PubMed]
- Holišová, V.; Urban, M.; Kolenčík, M.; Nemcová, Y.; Schrofel, A.; Peikertová, P.; Slabotinský, J.; Kratošová, G. Biosilica-nanogold composite: Easy-to-prepare catalyst for soman degradation. Arab. J. Chem. 2019, 12, 262–271. [Google Scholar] [CrossRef]
- Syngenta, E. Sy Edison. Available online: https://www.deltaagrar.rs/agrotrade_and_distribution/seeds/sunflower_seeds/syngenta.443.html (accessed on 16 November 2023).
- Syngenta Neostar. Available online: https://agriline.net/-/sale/field-crop-seeds/nasinnya-sonyashniku-si-neostar-klp-SY-Neostar-CLP--23022317535434656200 (accessed on 16 November 2023).
- Harčár, J.; Priechodská, Z.; Karolus, K.; Karolusová, E.; Remšík, K.; Šucha, P. Vysvetlivky ku geologickej mape severovýchodnej časti Podunajskej nižiny; Geologický ústav Diolíza Štúra: Bratislava, Slovakia, 1988. [Google Scholar]
- Šimanský, V.; Kovačik, P. Long-term effects of tillage and fertilization on pH and sorption parameters of haplic Luvisol. J. Elem. 2015, 20, 1033–1040. [Google Scholar] [CrossRef]
- Duflo, E.; Banerjee, A. Handbook of Field Experiments, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1. [Google Scholar]
- MPSR Vyhláška MP SR č. 338/2005 Z. z. Available online: https://www.mpsr.sk/vyhlaska-mp-sr-c-338-2005-z-z/29-23-29-1845/ (accessed on 18 September 2009).
- Alberio, C.; Izquierdo, N.G.; Aguirrezábal, L.A.N. Sunflower crop physiology and agronomy. In Sunflower; Martínez-Force, E., Dunford, N.T., Salas, J.J., Eds.; AOCS Press: Urbana, IL, USA, 2015; pp. 53–91. [Google Scholar]
- TIBCO Software Inc. (Data Analysis Software System), Version 14.0. Available online: https://www.tibco.com/ (accessed on 5 November 2023).
- Erdtman, G. Polen Morphology and Plant Taxonomy: Angiosperm. (An Introduction a Palynology); E.J. Brill.: Leiden, The Netherlands, 1986. [Google Scholar]
- Halbritter, H.; Ulrich, S.; Grímsson, F.; Weber, M.; Zetter, R.; Hesse, M.; Buchner, R.; Svojtka, M.; Frosch-Radivo, A. Illustrated Pollen Terminology; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Cox, A.; Venkatachalam, P.; Sahi, S.; Sharma, N. Silver and titanium dioxide nanoparticle toxicity in plants: A review of current research. Plant Physiol. Biochem. 2016, 107, 147–163. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Plant response to engineered metal oxide nanoparticles. Nanoscale Res. Lett. 2017, 12, 92. [Google Scholar] [CrossRef]
- Su, Y.; Ashworth, V.; Kim, C.; Adeleye, A.S.; Rolshausen, P.; Roper, C.; White, J.; Jassby, D. Delivery, uptake, fate, and transport of engineered nanoparticles in plants: A critical review and data analysis. Environ. Sci. Nano 2019, 6, 2311–2331. [Google Scholar] [CrossRef]
- Shukla, P.K.; Misra, P.; Kole, C. Uptake, translocation, accumulation, transformation, and generational transmission of nanoparticles in plants. In Plant Nanotechnology: Principles and Practices; Kole, C., Kumar, D.S., Khodakovskaya, M.V., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 183–218. [Google Scholar]
- Malayeri, B.; Chehregani, A.; Mohsenzadeh, F.; Golmohammadi, R. Effect of heavy metals on the development stages of ovule and embryonic sac in Euphorbia cheiradenia. Pak. J. Biol. Sci. 2005, 8, 622–625. [Google Scholar]
- Astiz, V.; Hernández, L.F. Pollen production in sunflower (Helianthus annuus L.) is affected by air temperature and relative humidity during early reproductive growth. Phyton 2013, 82, 297–302. [Google Scholar]
- Sharma, P.N.; Chatterjee, C.; Sharma, C.P.; Agarwala, S.C. Zinc deficiency and anther development in maize. Plant Cell Physiol. 1987, 28, 11–18. [Google Scholar]
- Pathak, G.C.; Gupta, B.; Pandey, N. Improving reproductive efficiency of chickpea by foliar application of zinc. Braz. J. Plant Physiol. 2012, 24, 173–180. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; van der Ent, A.; Cheng, M.; Jiang, H.; Lund Read, T.; Lombi, E.; Tang, C.; de Jonge, M.D.; Menzies, N.W.; et al. Absorption of foliar-applied Zn in sunflower (Helianthus annuus): Importance of the cuticle, stomata and trichomes. Ann. Bot. 2019, 123, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Kolenčík, M.; Ernst, D.; Urík, M.; Ďurišová, Ľ.; Bujdoš, M.; Šebesta, M.; Dobročka, E.; Kšiňan, S.; Illa, R.; Qian, Y. Foliar application of low concentrations of titanium dioxide and zinc oxide nanoparticles to the common sunflower under field conditions. Nanomaterials 2020, 10, 1619. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, S.; Hirata, S.; Yamamoto, K.; Nakajima, Y.; Kurahashi, K.; Tokumoto, H. ZnO nanoparticles effect on pollen grain germination and pollen tube elongation. PCTOC 2021, 145, 405–415. [Google Scholar] [CrossRef]
- Weryszko-Chmielewska, E.; Stpiczyńska, M.; Michońska, M. Morphological anomalies in pea (Pisum sativum L. cv. Hamil.) pollen grains under high doses of zinc. Acta Soc. Bot. Pol. 1991, 60, 259–272. [Google Scholar] [CrossRef][Green Version]
- Puškadija, Z.; Štefanić, E.; Mijić, A.; Zdunić, Z.; Parađiković, N.; Florijančić, T.; Opačak, A. Influence of weather conditions on honey bee visits (Apis mellifera carnica) during sunflower (Helianthus annuus L.) blooming period. Poljoprivreda 2007, 13, 230–233. [Google Scholar]
- Thakur, B.R.; Singh, R.K.; Handa, A.K.; Rao, M.A. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef]
- Jaffri, S.R.F.; MacAlister, C.A. Sequential Deposition and Remodeling of Cell Wall Polymers During Tomato Pollen Development. Front. Plant Sci. 2021, 12, 703713. [Google Scholar] [CrossRef]
- Hirose, N.; Takei, K.; Kuroha, T.; Kamada-Nobusada, T.; Hayashi, H.; Sakakibara, H. Regulation of cytokinin biosynthesis, compartmentalization and translocation. J. Exp. Bot. 2008, 59, 75–83. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, X.; Jiang, Y.; Chen, C.; Chen, J.; Zhang, J.; Wen, Y. Combined morphological and palynological classification for Hibiscus syriacus L. (Malvaceae): Construction of the diagnostic classification framework and implications of pollen morphological variation on fruiting. Agronomy 2023, 13, 828. [Google Scholar] [CrossRef]
- Yousefi, N.; Chehregani, A.; Malayeri, B.; Lorestani, B.; Cheraghi, M. Investigating the effect of heavy metals on developmental stages of anther and pollen in Chenopodium botrys L. (Chenopodiaceae). Biol. Trace Elem. Res. 2011, 140, 368–376. [Google Scholar] [PubMed]
- Lin, M.; Huang, H.; Liu, Z.; Liu, Y.; Ge, J.; Fang, Y. Growth–dissolution–regrowth transitions of Fe3O4 nanoparticles as building blocks for 3D magnetic nanoparticle clusters under hydrothermal conditions. Langmuir 2013, 29, 15433–15441. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Han, J.; Xiao, J.Q.; Jin, Y. Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J. Environ. Monit. 2008, 10, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, R.; Alkhatib, B.; Abdo, N.; Al-Eitan, L.; Creamer, R. Physio-biochemical and ultrastructural impact of (Fe3O4) nanoparticles on tobacco. BMC Plant Biol. 2019, 19, 253. [Google Scholar] [CrossRef] [PubMed]
- Bombin, S.; LeFebvre, M.; Sherwood, J.; Xu, Y.; Bao, Y.; Ramonell, K.M. Developmental and reproductive effects of iron oxide nanoparticles in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 24174–24193. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.M.; Itagaki, T.; Sakai, S. Intraspecific variation in morphology of spiny pollen grains along an altitudinal gradient in an insect-pollinated shrub. Plant Biol. 2023, 25, 287–295. [Google Scholar] [CrossRef]
- Shah, M.; Badwaik, V.; Kherde, Y.; Waghwani, H.; Modi, T.; Aguilar, Z.P.; Rodgers, H.; Hamilton, W.; Marutharaj, T.; Webb, C.; et al. Gold nanoparticles: Various methods of synthesis and antibacterial applications. Front. Biosci. 2014, 19, 1320–1344. [Google Scholar] [CrossRef]
- Mahakham, W.; Theerakulpisut, P.; Maensiri, S.; Phumying, S.; Sarmah, A.K. Environmentally benign synthesis of phytochemicals-capped gold nanoparticles as nanopriming agent for promoting maize seed germination. Sci. Total Environ. 2016, 573, 1089–1102. [Google Scholar] [CrossRef]
- Hernandez-Apaolaza, L. Can silicon partially alleviate micronutrient deficiency in plants? a review. Planta 2014, 240, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.S.; Tawfeek, M.E.; Eldesouky, S.E. Toxicity and biochemical effects of spirotetramat and its binary mixtures with nanosilica against Aphis gossypii glover, Bemisia tabaci gennadius and the earthworm, Eisenia fetida. Alex. Sci. Exch. 2022, 43, 107–119. [Google Scholar] [CrossRef]
- Tejaswini. Variability of pollen grain features: A plant strategy to maximize reproductive fitness in two species of Dianthus? Sex. Plant Reprod. 2002, 14, 347–353. [Google Scholar]
- Konzmann, S.; Koethe, S.; Lunau, K. Pollen grain morphology is not exclusively responsible for pollen collectability in bumble bees. Sci. Rep. 2019, 9, 4705. [Google Scholar] [CrossRef] [PubMed]
- Vear, F.; Pham-Delegue, M.; de Labrouhe, D.D.T.; Marilleau, R.; Loublier, Y.; Le Metayer, M.; Douault, P.; Philippon, J. Genetical studies of nectar and pollen production in sunflower. Agronomie 1990, 10, 219–231. [Google Scholar] [CrossRef]
- Rezaei, F.; Moaveni, P.; Mozafari, H. Effect of Different Concentrations and Time of Nano TiO2 Spraying on Quantitative and Qualitative Yield of Soybean (Glycine max L.) at Shahr-e-Qods, Iran; Biological Forum; Satya Prakashan: Delhi, India, 2015; pp. 957–964. [Google Scholar]
- Larue, C.; Veronesi, G.; Flank, A.-M.; Surble, S.; Herlin-Boime, N.; Carrière, M. Comparative uptake and impact of TiO2 nanoparticles in wheat and rapeseed. J. Toxicol. Environ. Health Part A 2012, 75, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Servin, A.D.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; Diaz, B.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Synchrotron micro-XRF and micro-XANES confirmation of the uptake and translocation of TiO2 nanoparticles in cucumber (Cucumis sativus) plants. Environ. Sci. Technol. 2012, 46, 7637–7643. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, Y.; Ding, X.; Ning, Z.; Shen, J.; Chen, H.; Su, Z. Effect of nano-TiO2 composite on the fertilization and fruit-setting of litchi. Nanomaterials 2022, 12, 4287. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).