Insights into the Fungicide Prothioconazole and Its Metabolite in Wheat: Residue Behavior and Risk Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Standard Solutions
2.3. Preparation and Cultivation of Wheat Seedlings
2.4. Prothioconazole Exposure Experiment
2.5. Wheat Grain and Flour Sample Collection
2.6. Sample Preparation
2.7. Sample Extraction
2.8. Clean-Up
2.9. Evaluation of Matrix Effects
2.10. Detection Testing Conditions
2.11. Data Statistics and Analysis
3. Results and Discussion
3.1. Reliability of Analytical Methods for Prothioconazole and Prothioconazole-Desthio in Wheat Substrates and Nutrient Solutions
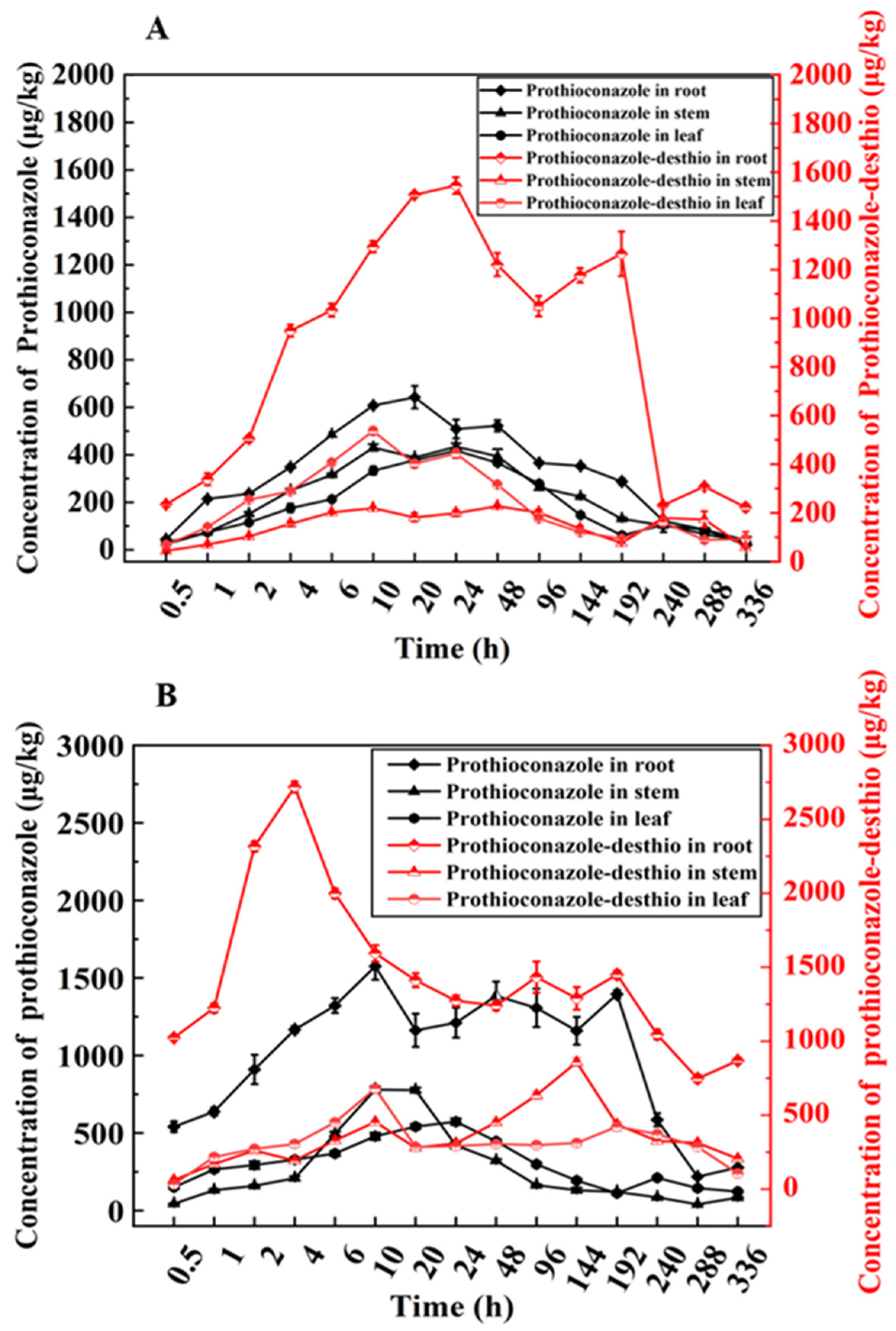
3.2. Uptake, Transport, and Metabolic Patterns of Prothioconazole in Wheat Plants by Nutrient Solution Culture
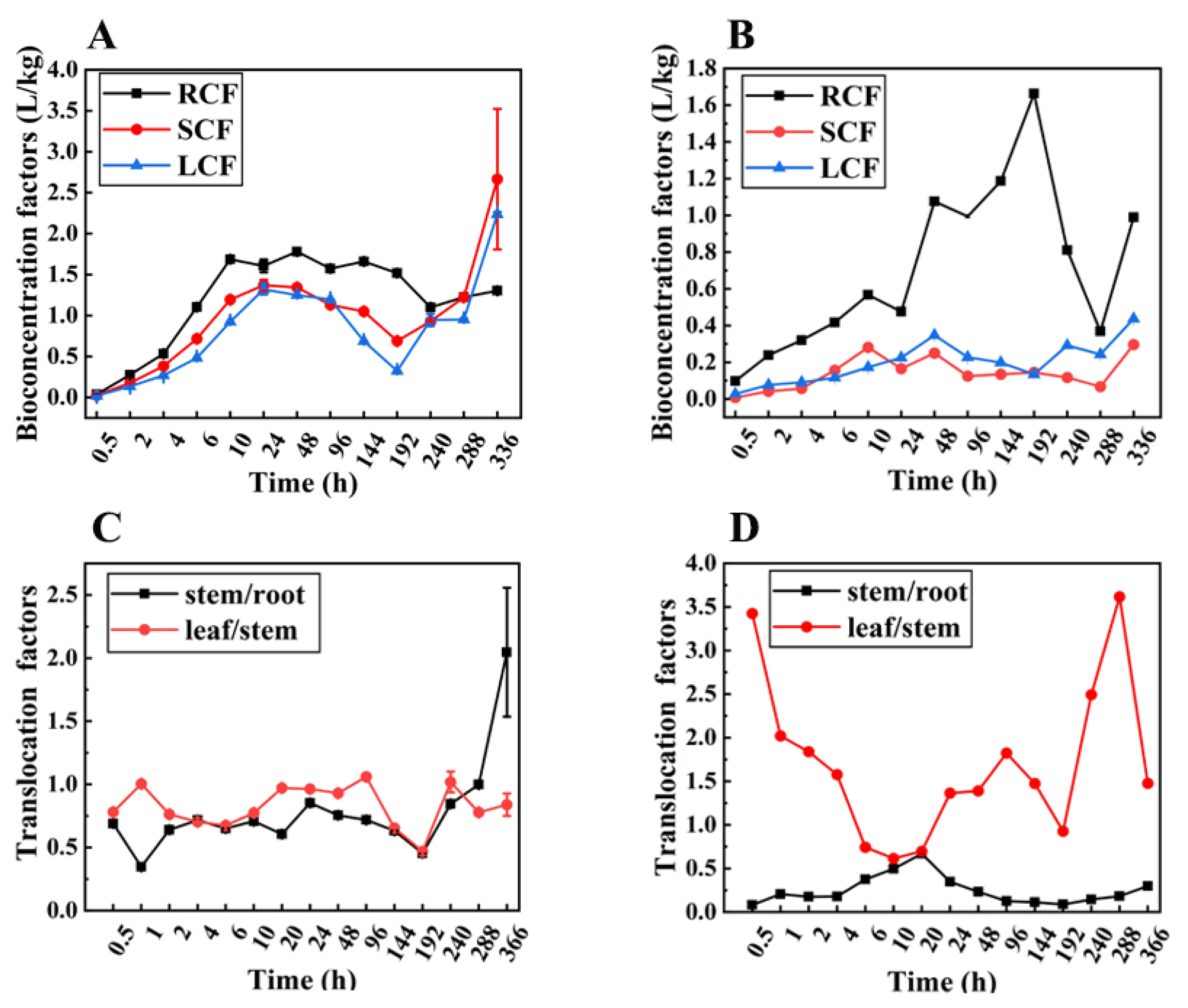
3.3. Bioconcentration and Transport of Prothioconazole in Wheat Plants by Nutrient Solution Culture
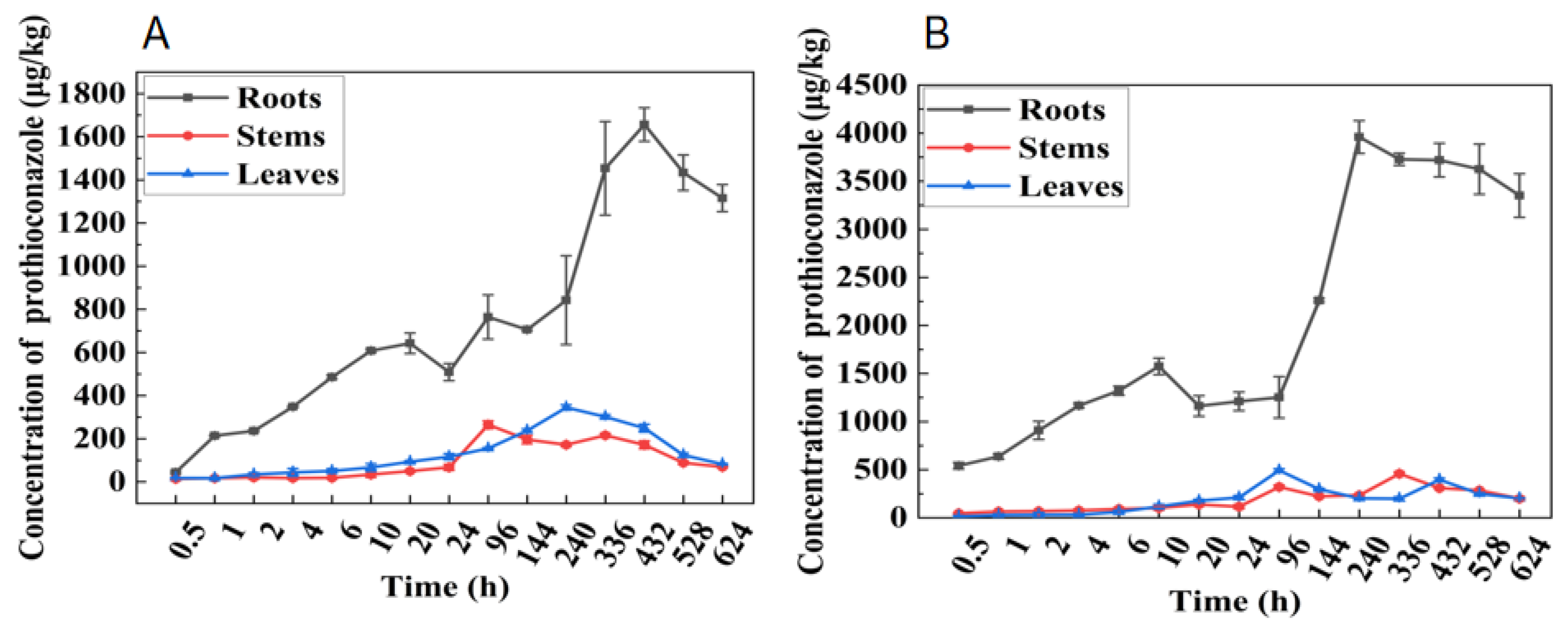
3.4. Uptake and Transport of Prothioconazole in Wheat under Replacement of Nutrient Solution
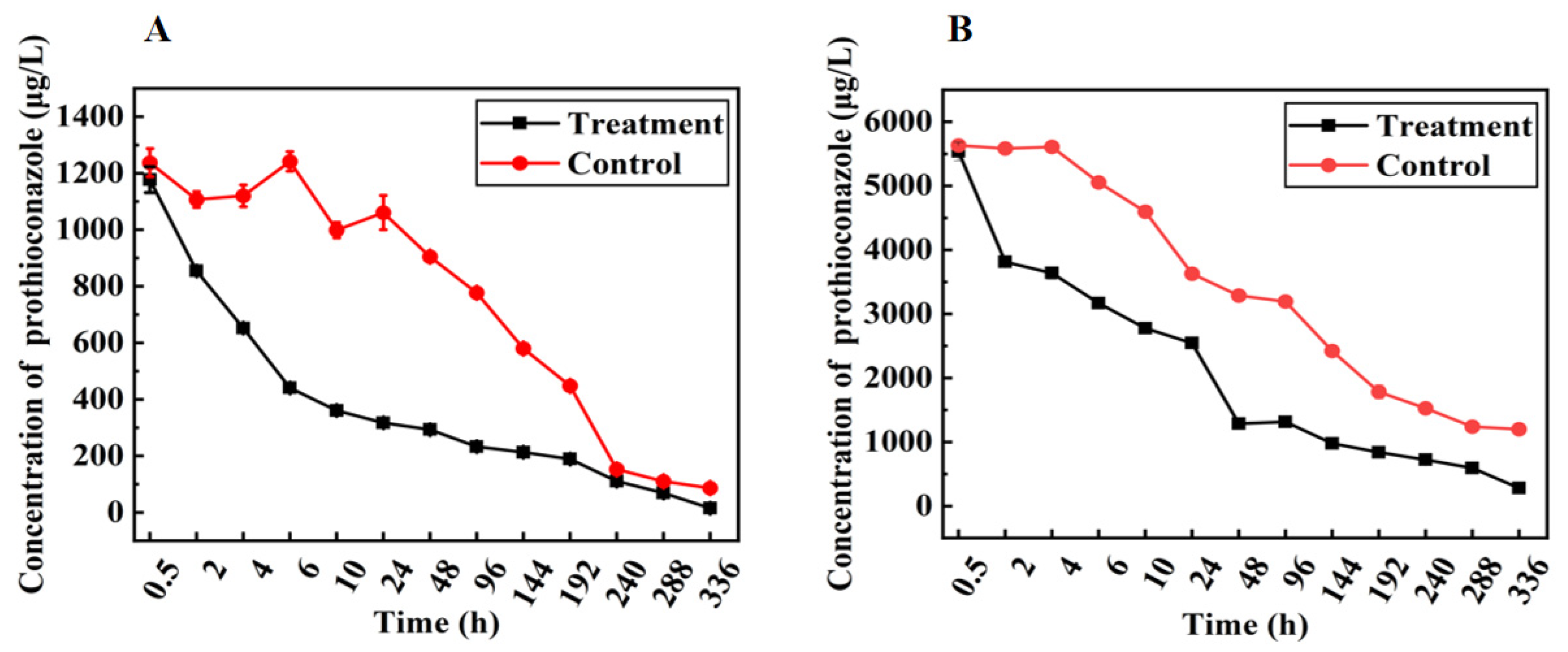
3.5. Residual Dynamics of Prothioconazole in Nutrient Solution Culture
3.6. Uptake, Transport, and Metabolism of Prothioconazole in Wheat Plants by Spraying Method
3.7. Dissipation of the Prothioconazole in Wheat Leaves of the Spraying Method
3.8. Residue Analysis of Prothioconazole and Its Metabolites in Wheat Grain and Flour Market Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fantke, P.; Charles, R.; Alencastro, L.F.D.; Friedrich, R.; Jolliet, O. Plant uptake of pesticides and human health: Dynamic modeling of residues in wheat and ingestion intake. Chemosphere 2011, 85, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Mendez, A.; Castillo, L.E.; Ruepert, C.; Hungerbuehler, K.; Ng, C.A. Tracking pesticide fate in conventional banana cultivation in Costa Rica: A disconnect between protecting ecosystems and consumer health. Sci. Total Environ. 2018, 613–614, 1250–1268. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Fantke, P. Considering degradation kinetics of pesticides in plant uptake models: Proof of concept for potato. Pest Manag. Sci. 2022, 79, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, S.; Li, B.; Liu, F.; Mu, W.; Pan, C.; Zou, N. Review on uptake and translocation behaviors of pesticides in plants and application technologies of pesticides. Chin. J. Pestic. Sci. 2021, 23, 607–616. [Google Scholar]
- Wu, X.; Qin, R.; Wu, H.; Yao, G.; Zhang, Y.; Li, P.; Xu, Y.; Zhang, Z.; Yin, Z.; Xu, H. Nanoparticle-immersed paper imprinting mass spectrometry imaging reveals uptake and translocation mechanism of pesticides in plants. Nano Res. 2020, 13, 611–620. [Google Scholar] [CrossRef]
- Niu, J.; Tang, J.; Tang, G.; Zhou, Z.; Tang, R.; Yang, J.; Jiang, N.; Li, J.; Cao, Y. Enhanced phototherapy activity by employing a nanosilica-coumarin-acifluorfen conjugate as the supplementary light source generator. ACS. Sustain. Chem. Eng. 2019, 7, 17706–17713. [Google Scholar] [CrossRef]
- Zhai, W.; Zhang, L.; Liu, H.; Zhang, C.; Liu, P.; Zhou, Z. Enantioselective degradation of prothioconazole in soil and the impacts on the enzymes and microbial community. Sci. Total Environ. 2022, 824, 153658. [Google Scholar] [CrossRef]
- Zhai, W.; Zhang, L.; Cui, J.; Wei, Y.; Wang, P.; Liu, D.; Zhou, Z. The biological activities of prothioconazole enantiomers and their toxicity assessment on aquatic organisms. Chirality 2019, 31, 468–475. [Google Scholar] [CrossRef]
- Shen, J.; Liu, P.; Sun, Y.; Xu, X.; Guo, L.; Rao, Q.; Minlan, C.; Liu, X. Embryonic exposure to prothioconazole induces oxidative stress and apoptosis in zebrafish (Danio rerio) early life stage. Sci. Total Environ. 2020, 756, 143859. [Google Scholar] [CrossRef]
- Xavier, S.A.; Mello, F.E.D.; Silva, H.P.D.; Canteri, M.G.; Koga, L.J.; Lopes, I.D.O.N.; Godoy, C.V. Microtiter method to monitor Corynespora cassiicola and sensitivity of the pathogen to carbendazim, prothioconazole and pyraclostrobin. Crop Prot. 2021, 144, 105554. [Google Scholar] [CrossRef]
- Audenaert, K.; Callewaert, E.; Höfte, M.; Saeger, D.S.; Haesaert, G. Hydrogen peroxide induced by the fungicide prothioconazole triggers deoxynivalenol (DON) production by Fusarium graminearum. BMC Microbiol. 2010, 10, 112. [Google Scholar] [CrossRef]
- Tarazona, A.; Mateo, E.M.; Gómez, J.V.; Romera, D.; Mateo, F. Potential use of machine learning methods in assessment of Fusarium culmorum and Fusarium proliferatum growth and mycotoxin production in treatments with antifungal agents. Fungal Biol. 2021, 125, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Tong, Z.; Chu, Y.; Sun, M.; Wang, M.; Gao, T.; Duan, J. Dissipation of Prothioconazole and Its Metabolite Prothioconazole-Desthio in Rice Fields and Risk Assessment of Its Dietary Intake. J. Agric. Food Chem. 2019, 67, 6458–6465. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.G. Pydiflumetofen Co-Formulated with Prothioconazole: A Novel Fungicide for Fusarium Head Blight and Deoxynivalenol Control. Toxins 2022, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Perovani, I.S.; Santos Barbetta, M.F.; da Silva, R.M.; Lopes, N.P.; de Oliveira, A.R.M. In vitro-in vivo correlation of the chiral pesticide prothioconazole after interaction with human CYP450 enzymes. Food Chem. Toxicol. 2022, 163, 112947. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gong, R.; Chu, Y.; Liu, S.; Xiang, D.; Li, C. Mechanistic Insights into Stereospecific Antifungal Activity of Chiral Fungicide Prothioconazole against Fusarium oxysporum F. sp. cubense. Int. J. Mol. Sci. 2022, 23, 2352. [Google Scholar] [CrossRef]
- Meng, Z.; Tian, S.; Sun, W.; Liu, L.; Yan, S.; Huang, S.; Zhu, W.; Zhou, Z. Effects of exposure to prothioconazole and its metabolite prothioconazole-desthio on oxidative stress and metabolic profiles of liver and kidney tissues in male mice. Environ. Pollut. 2021, 269, 116215. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, Y.; Ye, Y.; Yang, Y.; Hua, R.; Wu, X. Toxification metabolism and treatment strategy of the chiral triazole fungicide prothioconazole in water. J. Hazard. Mater. 2022, 432, 128650. [Google Scholar] [CrossRef]
- Liu, H.; Yao, G.; Liu, X.; Liu, C.; Zhan, J.; Liu, D.; Wang, P.; Zhou, Z. Approach for Pesticide Residue Analysis for Metabolite Prothioconazole-desthio in Animal Origin Food. J. Agric. Food Chem. 2017, 65, 2481–2487. [Google Scholar] [CrossRef]
- Solel, Z.; Schooley, J.M.; Edgington, L.V. Uptake and translocation of benomyl and carbendazim (methyl benzimidazol-2-yl carbamate) in the symplast. Pest Manag. Sci. 1973, 4, 713–718. [Google Scholar] [CrossRef]
- Chandler, J.; Basler, E.; Santelmann, P.W. Uptake and translocation of alachlor in soybean and wheat. Weed Sci. 1974, 22, 253–258. [Google Scholar] [CrossRef]
- Bouldin, J.L.; Farris, J.L.; Moore, M.T.; Smith, S., Jr.; Cooper, C.M. Hydroponic uptake of atrazine and lambda-cyhalothrin in Juncus effusus and Ludwigia peploides. Chemosphere 2006, 65, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Murano, H.; Otani, T.; Seike, N.; Sakai, M. Dieldrin uptake and translocation in plants growing in hydroponic medium. Environ. Toxicol. Chem. 2010, 29, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.; Tsuruta, H.; Inui, H. Different uptake pathways between hydrophilic and hydrophobic compounds in lateral roots of Cucurbita pepo. J. Pestic. Sci. 2015, 40, 99–105. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Wang, H.; Wang, Y.; Guo, W.; Yu, Z.; Ye, Q. Translocation and residue of 14C-benzene kresoxim-methyl in mature cucumber (Cucumis sativus L). Sci. Total Environ. 2021, 766, 144426. [Google Scholar] [CrossRef]
- Ju, C.; Dong, S.; Zhang, H.; Yao, S.; Wang, F.; Cao, D.; Xu, S.; Fang, H.; Yu, Y. Subcellular distribution governing accumulation and translocation of pesticides in wheat (Triticum aestivum L). Chemosphere 2020, 248, 126024. [Google Scholar] [CrossRef]
- Ju, C.; Zhang, H.; Yao, S.; Dong, S.; Wang, F.; Cao, D.; Fang, H.; Yu, Y. Uptake, Translocation, and Subcellular Distribution of Azoxystrobin in Wheat Plant (Triticum aestivum L). J. Agric. Food Chem. 2019, 67, 6691–6699. [Google Scholar] [CrossRef]
- Gu, Z.; Chen, X.; Rao, J.; Chen, B. Statistical evaluation to validate matrix-matched calibration for standardized beany odor compound quantitation in yellow pea flour using HS-SPME-GC-MS. Food Funct. 2022, 13, 3968–3981. [Google Scholar] [CrossRef]
- Xiao, S.; Gong, Y.; Li, Z.; Fantke, P. Improving Pesticide Uptake Modeling into Potatoes: Considering Tuber Growth Dynamics. J. Agric. Food Chem. 2021, 69, 3607–3616. [Google Scholar] [CrossRef]
- Hwang, J.I.; Zimmerman, A.R.; Kim, J.E. Bioconcentration factor-based management of soil pesticide residues: Endosulfan uptake by carrot and potato plants. Sci. Total Environ. 2018, 627, 514–522. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Shen, J.; Lang, H.; Dong, S.; Zhang, L.; Fang, H.; Yu, Y. Uptake, translocation, and metabolism of thiamethoxam in soil by leek plants. Environ. Res. 2022, 211, 113084. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; An, Q.; Hao, X.; Li, D.; Zhou, C.; Zhang, J.; Wei, X.; Pan, C. Dissipative behavior, residual pattern, and risk assessment of four pesticides and their metabolites during tea cultivation, processing and infusion. Pest Manag. Sci. 2022, 78, 3019–3029. [Google Scholar] [CrossRef] [PubMed]
- Hinman, M.L.; Klaine, S.J. Uptake and translocation of selected organic pesticides by the rooted aquatic plant Hydrilla verticillata Royle. Environ. Sci. Technol. 1992, 26, 609–613. [Google Scholar] [CrossRef]
- Miller, E.L.; Nason, S.L.; Karthikeyan, K.G.; Pedersen, J.A. Root Uptake of Pharmaceuticals and Personal Care Product Ingredients. Environ. Sci. Technol. 2016, 50, 525–541. [Google Scholar] [CrossRef]
- Trapp, S. Bioaccumulation of polar and ionizable compounds in plants. In Ecotoxicology Modeling; Springer: Boston, MA, USA, 2009; pp. 299–353. [Google Scholar]
- Dodgen, L.K.; Ueda, X.Q.; Parker, D.R.; Gan, J. Effect of transpiration on plant accumulation and translocation of PPCP/EDCs. Environ. Pollut. 2015, 198, 144–153. [Google Scholar] [CrossRef]
- Deletage-Grandon, C.; Chollet, J.F.; Faucher, M.; Rocher, F.; Komor, E.; Bonnemain, J.L. Carrier-Mediated Uptake and Phloem Systemy of a 350-Dalton Chlorinated Xenobiotic with an α-Amino Acid Function. Plant Physiol. 2001, 125, 1620–1632. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.W.H. Degradation of pesticides on plant surfaces and its prediction-a case study on tea plant. Environ. Monit. Assess. 1997, 44, 303–313. [Google Scholar]
- Katagi, T. Photodegradation of pesticides on plant and soil surfaces. In Reviews of Environmental Contamination and Toxicology; Springer: Berlin/Heidelberg, Germany, 2004; Volume 182, pp. 1–189. [Google Scholar]
- FAO/WHO. 5.19 Protoconazole (197) [R] Report of Joint FAO/WHO Meeting on Expertise in 2008; FAO/WHO: Geneva, Switzerland, 2008; pp. 197–326. [Google Scholar]
- González-Osnaya, L.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Dietary intake of ochratoxin A from conventional and organic bread. Int. J. Food Microbiol. 2007, 118, 87–91. [Google Scholar] [CrossRef]
- Fang, Q.; Wu, R.; Hu, G.; Lai, A.; Wu, K.; Zhang, L.; Feng, J.; Cao, H. Dissipation Behavior, Residue Distribution and Risk Assessment of Three Fungicides in Pears. J. Sci. Food Agric. 2020, 100, 1757–1763. [Google Scholar] [CrossRef]
- Fang, Q.; Yao, G.; Shi, Y.; Ding, C.; Wang, Y.; Wu, X.; Hua, R.; Cao, H. Residue Dynamics and Risk Assessment of Prochloraz and Its Metabolite 2,4,6-Trichlorophenol in Apple. Molecules 2017, 22, 1780. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Pesticide Residues in Food and Feed. 2010. Available online: http://www.codexalimentarius.net/mrls/pestdes/jsp/pest_qe.jsp (accessed on 26 September 2023).
- Yu, L.; Yu, D.; Zhang, M.; Li, X.; Zhu, X.; Xu, Z.; Wu, Q.; Li, J. Phloemmobility of metalaxyl in Ricinuscommunis seedling under different culture conditions. Chin. J. Pestic. Sci. 2018, 20, 500–505. [Google Scholar]
- Yu, Q.; Qin, S.; Wang, X.; Qiao, X. Dissipation of Acetamiprid and Imidacloprid under Different Temperature, Light and Biological Factors on Phyllosphere of Brassica chinensis. Chin. J. Pestic. Sci. 2006, 8, 147–151. [Google Scholar]

| Wheat Grain | Wheat Flour | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Num | Prothioconazole (μg/kg) | Prothioconazole-Desthio (μg/kg) | Num | Prothioconazole (μg/kg) | Prothioconazole-Desthio (μg/kg) | Num | Prothioconazole (μg/kg) | Prothioconazole-Desthio (μg/kg) | Num | Prothioconazole (μg/kg) | Prothioconazole-Desthio (μg/kg) |
| 1 | <LOD a | <LOD | 10 | <LOD | 2.4 | 20 | 12.5 | 5.7 | 30 | <LOD | 1.5 |
| 2 | <LOD | <LOD | 11 | <LOD | 2.3 | 21 | <LOD | 3.4 | 31 | <LOD | 3.2 |
| 3 | <LOD | 4.9 | 12 | <LOD | 3.0 | 22 | 3.8 | 2.0 | 32 | <LOD | 2.5 |
| 4 | 43.5 | 6.6 | 13 | <LOD | 3.1 | 23 | 18.8 | 1.8 | 33 | <LOD | 1.6 |
| 5 | 6.9 | 5.7 | 14 | <LOD | 3.4 | 24 | <LOD | 3.6 | 34 | <LOD | 2.2 |
| 6 | 86.3 | 8.8 | 15 | <LOD | 10.7 | 25 | <LOD | 2.3 | 35 | <LOD | 3.8 |
| 7 | 59.7 | 12.5 | 16 | <LOD | 7.5 | 26 | <LOD | 1.2 | 36 | <LOD | 2.0 |
| 8 | <LOD | <LOD | 17 | <LOD | 6.9 | 27 | <LOD | 1.3 | 37 | <LOD | 1.3 |
| 9 | 64.3 | 6.8 | 18 | 13.7 | 6.0 | 28 | <LOD | 1.8 | |||
| 19 | <LOD | 13.1 | 29 | 12.6 | 1.6 | ||||||
| Time (d) | Low-Concentration Group | High-Concentration Group | ||
|---|---|---|---|---|
| Residue Level (μg/kg) | Dissipation Rate (%) | Residue Level (μg/kg) | Dissipation Rate (%) | |
| 0 | 704.1 | —— | 1794.6 | —— |
| 1 | 234.3 | 66.7 | 787.1 | 56.1 |
| 2 | 187.3 | 73.4 | 501.8 | 72.1 |
| 4 | 121.0 | 82.8 | 410.1 | 77.2 |
| 6 | 95.2 | 86.5 | 302.5 | 83.1 |
| 8 | 68.5 | 90.3 | 208.0 | 88.4 |
| 10 | 59.8 | 91.5 | 151.3 | 91.6 |
| 12 | 28.7 | 95.9 | 114.7 | 93.6 |
| Dissolution equation | C = 0.3721e−0.009t | C = 1.0582e−0.008t | ||
| Correlation coefficient | R2 = 0.893 | R2 = 0.918 | ||
| Half-life (d) | t1/2 = 3.2 | t1/2 = 3.6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Q.; Yan, Z.; Zhang, C.; Shi, Y.; Zhang, Z.; Gao, Q.; Xiao, J.; Liao, M.; Qi, C.; Cao, H. Insights into the Fungicide Prothioconazole and Its Metabolite in Wheat: Residue Behavior and Risk Assessment. Agronomy 2023, 13, 2906. https://doi.org/10.3390/agronomy13122906
Fang Q, Yan Z, Zhang C, Shi Y, Zhang Z, Gao Q, Xiao J, Liao M, Qi C, Cao H. Insights into the Fungicide Prothioconazole and Its Metabolite in Wheat: Residue Behavior and Risk Assessment. Agronomy. 2023; 13(12):2906. https://doi.org/10.3390/agronomy13122906
Chicago/Turabian StyleFang, Qingkui, Zixuan Yan, Chengzhi Zhang, Yanhong Shi, Zhaoxian Zhang, Quan Gao, Jinjing Xiao, Min Liao, Chuanyong Qi, and Haiqun Cao. 2023. "Insights into the Fungicide Prothioconazole and Its Metabolite in Wheat: Residue Behavior and Risk Assessment" Agronomy 13, no. 12: 2906. https://doi.org/10.3390/agronomy13122906
APA StyleFang, Q., Yan, Z., Zhang, C., Shi, Y., Zhang, Z., Gao, Q., Xiao, J., Liao, M., Qi, C., & Cao, H. (2023). Insights into the Fungicide Prothioconazole and Its Metabolite in Wheat: Residue Behavior and Risk Assessment. Agronomy, 13(12), 2906. https://doi.org/10.3390/agronomy13122906






