Plant Growth-Promoting Rhizobacteria Microbial Fertilizer Changes Soils’ Microbial Structure and Promotes Healthy Growth of Cigar Tobacco Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Species, Plant Material and Growing Conditions
2.2. Experimental Design
2.3. Determination of Plant Traits of Cigar Tobacco
2.4. Collection and Determination of Inter-Root Soil Samples and Tobacco Plant Roots
2.5. Analysis of 16S and ITS Full-Length Microbial Diversity in Inter-Root Soils
2.6. Statistical Analysis
3. Results
3.1. Effect of PGPR Microbial Fertilizer and Root Black Rot Pathogen Application on Soil Nutrients and Agronomic Traits of Cigar Plants
3.2. Effect of PGPR Microbial Fertilizer and Root Black Rot Pathogen Application on Inter-Root Soil Microorganisms
3.2.1. Microbial Diversity Characterization and Community Composition
3.2.2. Relative Abundance of Inter-Root Soil Bacterial and Fungal Communities
3.3. Correlation Analysis between Soil, Plants and Microorganisms
3.3.1. Correlation Analysis between Inter-Root Soil Microorganisms
3.3.2. Analysis of the Correlation between Soil Properties and Microorganisms
4. Discussion
4.1. Exogenous Microbial Agents Improve Soil Nutrients and Promote Plant Growth
4.2. Effect of Exogenous Microbial Agent Application on the Inter-Rhizosphere Soil Microbial Community of Cigar Plants
4.3. Correlation Analysis between Soils, Plants, and Microorganisms
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zheng, T.; Zhang, Q.; Li, P.; Wu, X.; Liu, Y.; Yang, Z.; Li, D.; Zhang, J.; Du, G. Analysis of Microbial Community, Volatile Flavor Compounds, and Flavor of Cigar Tobacco Leaves from Different Regions. Front. Microbiol. 2022, 13, 907270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lv, H.; Xiang, X.; Yang, A.; Feng, Q.; Dai, P.; Li, Y.; Jiang, X.; Liu, G.; Zhang, X. Construction of a SNP Fingerprinting Database and Population Genetic Analysis of Cigar Tobacco Germplasm Resources in China. Front. Plant Sci. 2021, 12, 618133. [Google Scholar] [CrossRef] [PubMed]
- Wilson, I.W.; Moncuquet, P.; Ellis, M.; White, R.G.; Zhu, Q.-H.; Stiller, W.; Llewellyn, D. Characterization and Genetic Mapping of Black Root Rot Resistance in Gossypium arboreum L. Int. J. Mol. Sci. 2021, 22, 2642. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Li, Y.; Ding, N.; Li, D.; Martinez, N.; Miller, R.; Zaitlin, D.; Yang, S. Development of user-friendly markers for disease resistance to black root rot of tobacco through genotyping by sequencing. Mol. Breed. 2018, 38, 76. [Google Scholar] [CrossRef]
- Wilpiszeski, R.L.; Aufrecht, J.A.; Retterer, S.T.; Sullivan, M.B.; Graham, D.E.; Pierce, E.M.; Zablocki, O.D.; Palumbo, A.V.; Elias, D.A. Soil Aggregate Microbial Communities: Towards Understanding Microbiome Interactions at Biologically Relevant Scales. Appl. Environ. Microbiol. 2019, 85, e00324-19. [Google Scholar] [CrossRef] [PubMed]
- Sangwongchai, W.; Tananuwong, K.; Krusong, K.; Thitisaksakul, M. Yield, Grain Quality, and Starch Physicochemical Properties of 2 Elite Thai Rice Cultivars Grown under Varying Production Systems and Soil Characteristics. Foods 2021, 10, 2601. [Google Scholar] [CrossRef] [PubMed]
- Miransari, M. Soil microbes and plant fertilization. Appl. Microbiol. Biotechnol. 2011, 92, 875–885. [Google Scholar] [CrossRef]
- Yu, G.-H.; Chen, C.-M.; He, X.-H.; Zhang, X.-Z.; Li, L.-N. Unexpected bulk density and microstructures response to long-term pig manure application in a Ferralic Cambisol Soil: Implications for rebuilding a healthy soil. Soil Tillage Res. 2020, 203, 104668. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Y.; Yao, T.; Ma, Y.; Zhang, H.; Yang, X. Effects of PGPR microbial inoculants on the growth and soil properties of Avena sativa, Medicago sativa, and Cucumis sativus seedlings. Soil Tillage Res. 2020, 199, 104577. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.-X.; Guo, X.; Wang, D.; Chu, H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Shang, X.; Zhang, M.; Zhang, Y.; Hou, X.; Yang, L. Waste seaweed compost and rhizosphere bacteria Pseudomonas koreensis promote tomato seedlings growth by benefiting properties, enzyme activities and rhizosphere bacterial community in coastal saline soil of Yellow River Delta, China. Waste Manag. 2023, 172, 33–42. [Google Scholar] [CrossRef]
- Yu, X.Q.; Zhang, Y.Z.; Shen, M.C.; Dong, S.Y.; Zhang, F.J.; Gao, Q.; He, P.L.; Shen, G.M.; Yang, J.M.; Wang, Z.B.; et al. Soil Conditioner Affects Tobacco Rhizosphere Soil Microecology. Microb. Ecol. 2023, 86, 460–473. [Google Scholar] [CrossRef]
- do Amaral, F.P.; Tuleski, T.R.; Pankievicz, V.C.S.; Melnyk, R.A.; Arkin, A.P.; Griffitts, J.; Tadra-Sfeir, M.Z.; Maltempi de Souza, E.; Deutschbauer, A.; Monteiro, R.A.; et al. Diverse Bacterial Genes Modulate Plant Root Association by Beneficial Bacteria. mBio 2020, 11, e03078-20. [Google Scholar] [CrossRef]
- Piromyou, P.; Buranabanyat, B.; Tantasawat, P.; Tittabutr, P.; Boonkerd, N.; Teaumroong, N. Effect of plant growth promoting rhizobacteria (PGPR) inoculation on microbial community structure in rhizosphere of forage corn cultivated in Thailand. Eur. J. Soil Biol. 2011, 47, 44–54. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Fathi Abd_Allah, E. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Wang, H.W.; Zhu, Y.X.; Xu, M.; Cai, X.Y.; Tian, F. Co-application of spent mushroom substrate and PGPR alleviates tomato continuous cropping obstacle by regulating soil microbial properties. Rhizosphere 2022, 23, 100563. [Google Scholar] [CrossRef]
- Tabassum, B.; Khan, A.; Tariq, M.; Ramzan, M.; Iqbal Khan, M.S.; Shahid, N.; Aaliya, K. Bottlenecks in commercialisation and future prospects of PGPR. Appl. Soil Ecol. 2017, 121, 102–117. [Google Scholar] [CrossRef]
- Ma, M.; Jiang, X.; Wang, Q.; Guan, D.; Li, L.; Ongena, M.; Li, J. Isolation and Identification of PGPR Strain and its Effect on Soybean Growth and Soil Bacterial Community Composition. Int. J. Agric. Biol. 2018, 20, 1289–1297. [Google Scholar] [CrossRef]
- Ahmad, A.-G.M.; Attia, A.-Z.G.; Mohamed, M.S.; Elsayed, H.E. Fermentation, formulation and evaluation of PGPR Bacillus subtilis isolate as a bioagent for reducing occurrence of peanut soil-borne diseases. J. Integr. Agric. 2019, 18, 2080–2092. [Google Scholar] [CrossRef]
- Chandra, P.; Khobra, R.; Sundha, P.; Sharma, R.K.; Jasrotia, P.; Chandra, A.; Singh, D.P.; Singh, G.P. Plant growth promoting Bacillus-based bio formulations improve wheat rhizosphere biological activity, nutrient uptake and growth of the plant. Acta Physiol. Plant. 2021, 43, 139. [Google Scholar] [CrossRef]
- Samaras, A.; Kamou, N.; Tzelepis, G.; Karamanoli, K.; Menkissoglu-Spiroudi, U.; Karaoglanidis, G.S. Root Transcriptional and Metabolic Dynamics Induced by the Plant Growth Promoting Rhizobacterium (PGPR) Bacillus subtilis Mbi600 on Cucumber Plants. Plants 2022, 11, 1218. [Google Scholar] [CrossRef]
- Samaras, A.; Roumeliotis, E.; Ntasiou, P.; Karaoglanidis, G. Bacillus subtilis MBI600 Promotes Growth of Tomato Plants and Induces Systemic Resistance Contributing to the Control of Soilborne Pathogens. Plants 2021, 10, 1113. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Shen, Z.Z.; Zhu, C.Z.; Jiao, Z.X.; Li, R.; Shen, Q.R. Pre-colonization of PGPR triggers rhizosphere microbiota succession associated with crop yield enhancement. Plant Soil 2019, 439, 553–567. [Google Scholar] [CrossRef]
- Xie, K.H.; Sun, M.T.; Shi, A.K.; Di, Q.H.; Chen, R.; Jin, D.; Li, Y.S.; Yu, X.C.; Chen, S.C.; He, C.X. The Application of Tomato Plant Residue Compost and Plant Growth-Promoting Rhizobacteria Improves Soil Quality and Enhances the Ginger Field Soil Bacterial Community. Agronomy 2022, 12, 1741. [Google Scholar] [CrossRef]
- Han, T.; You, C.; Zhang, L.; Feng, C.; Zhang, C.; Wang, J.; Kong, F. Biocontrol potential of antagonist Bacillus subtilis Tpb55 against tobacco black shank. Biocontrol 2016, 61, 195–205. [Google Scholar] [CrossRef]
- Lian, L.L.; Xie, L.Y.; Zheng, L.P.; Lin, Q.Y. Induction of systemic resistance in tobacco against Tobacco mosaic virus by Bacillus spp. Biocontrol Sci. Technol. 2011, 21, 281–292. [Google Scholar] [CrossRef]
- Guo, D.S.; Yuan, C.H.; Luo, Y.Y.; Chen, Y.H.; Lu, M.H.; Chen, G.C.; Ren, G.W.; Cui, C.B.; Zhang, J.T.; An, D.R. Biocontrol of tobacco black shank disease (Phytophthora nicotianae) by Bacillus velezensis Ba168. Pestic. Biochem. Physiol. 2020, 165, 104523. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.X.; Mo, W.D.; Yu, S.; Cheng, H.H.; Peng, L.J.; Liu, Z.Y. Whole Genome Sequence of Bacillus velezensis Strain GUMT319: A Potential Biocontrol Agent Against Tobacco Black Shank Disease. Front. Microbiol. 2021, 12, 658113. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, X.; Li, T.; Xie, Y.; Cao, Z.; Fang, P. Bio-organic Fertilizer with Bacillus subtilis F2 Promotes Strawberry Plant Growth and Changes Rhizosphere Microbial Community. J. Soil Sci. Plant Nutr. 2022, 22, 3045–3055. [Google Scholar] [CrossRef]
- Tao, S.Y.; Wu, Z.S.; Wei, M.M.; Liu, X.C.; He, Y.H.; Ye, B.C. Bacillus subtilis SL-13 biochar formulation promotes pepper plant growth and soil improvement. Can. J. Microbiol. 2019, 65, 333–342. [Google Scholar] [CrossRef]
- Gallart, M.; Paungfoo-Lonhienne, C.; Gonzalez, A.; Trueman, S.J. Nitrogen Source Influences the Effect of Plant Growth-Promoting Rhizobacteria (PGPR) on Macadamia integrifolia. Agronomy 2021, 11, 1064. [Google Scholar] [CrossRef]
- Ren, H.; Lv, C.Q.; Fernandez-Garcia, V.; Huang, B.L.; Yao, J.M.; Ding, W. Biochar and PGPR amendments influence soil enzyme activities and nutrient concentrations in a eucalyptus seedling plantation. Biomass Convers. Biorefin. 2021, 11, 1865–1874. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, P.; Jorquera, M.A.; Sangwan, P.; Kumar, P.; Verma, A.K.; Agrawal, S. Isolation of phytase-producing bacteria from Himalayan soils and their effect on growth and phosphorus uptake of Indian mustard (Brassica juncea). World J. Microbiol. Biotechnol. 2013, 29, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Cheng, K.K.; Huo, X.J.; Meng, P.P.; Cai, Z.H.; Wang, Z.K.; Zhou, J. Bioorganic fertilizer promotes pakchoi growth and shapes the soil microbial structure. Front. Plant Sci. 2022, 13, 1040437. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.K.; Yu, Q.Q.; Yang, K.; Yang, J.Y.; Li, C.Y.; Liu, X.L. Effects of Bacillus subtilis T6-1 on the Rhizosphere Microbial Community Structure of Continuous Cropping Poplar. Biology 2022, 11, 791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Wang, Y.C.; Han, X.B.; Gou, J.Y.; Li, W.; Zhang, C.S. A Novel Bio-Fertilizer Produced by Prickly Ash Seeds with Biochar Addition Induces Soil Suppressiveness against Black Shank Disease on Tobacco. Appl. Sci. 2021, 11, 7261. [Google Scholar] [CrossRef]
- Qi, Y.Q.; Liu, H.L.; Zhang, B.P.; Geng, M.X.; Cai, X.X.; Wang, J.H.; Wang, Y.P. Investigating the effect of microbial inoculants Frankia F1 on growth-promotion, rhizosphere soil physicochemical properties, and bacterial community of ginseng. Appl. Soil Ecol. 2022, 172, 104369. [Google Scholar] [CrossRef]
- Grobelak, A.; Napora, A.; Kacprzak, M. Using plant growth-promoting rhizobacteria (PGPR) to improve plant growth. Ecol. Eng. 2015, 84, 22–28. [Google Scholar] [CrossRef]
- Rani, R.; Kumar, V.; Gupta, P.; Chandra, A. Potential use of Solanum lycopersicum and plant growth promoting rhizobacterial (PGPR) strains for the phytoremediation of endosulfan stressed soil. Chemosphere 2021, 279, 130589. [Google Scholar] [CrossRef]
- Huang, Z.; Ruan, S.T.; Sun, Y.Y.; Cheng, X.Y.; Dai, J.H.; Gui, P.; Yu, M.J.; Zhong, Z.T.; Wu, J.Y. Bacterial inoculants improved the growth and nitrogen use efficiency of Pyrus betulifolia under nitrogen-limited conditions by affecting the native soil bacterial communities. Appl. Soil Ecol. 2022, 170, 104285. [Google Scholar] [CrossRef]
- Myresiotis, C.K.; Vryzas, Z.; Papadopoulou-Mourkidou, E. Enhanced root uptake of acibenzolar-S-methyl (ASM) by tomato plants inoculated with selected Bacillus plant growth-promoting rhizobacteria (PGPR). Appl. Soil Ecol. 2014, 77, 26–33. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Ye, C.; Su, Y.W.; Peng, W.C.; Lu, R.; Liu, Y.X.; Huang, H.C.; He, X.H.; Yang, M.; Zhu, S.S. Soil Acidification caused by excessive application of nitrogen fertilizer aggravates soil-borne diseases: Evidence from literature review and field trials. Agric. Ecosyst. Environ. 2022, 340, 108176. [Google Scholar] [CrossRef]
- Bai, Y.C.; Chang, Y.Y.; Hussain, M.; Lu, B.; Zhang, J.P.; Song, X.B.; Lei, X.S.; Pei, D. Soil Chemical and Microbiological Properties Are Changed by Long-Term Chemical Fertilizers That Limit Ecosystem Functioning. Microorganisms 2020, 8, 694. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Guo, S.; Jousset, A.; Zhao, Q.Y.; Wu, H.S.; Li, R.; Kowalchuk, G.A.; Shen, Q.R. Bio-fertilizer application induces soil suppressiveness against Fusarium wilt disease by reshaping the soil microbiome. Soil Biol. Biochem. 2017, 114, 238–247. [Google Scholar] [CrossRef]
- Chen, Y.H.; Li, S.S.; Liu, N.; He, H.; Cao, X.Y.; Lv, C.; Zhang, K.; Dai, J.L. Effects of different types of microbial inoculants on available nitrogen and phosphorus, soil microbial community, and wheat growth in high-P soil. Environ. Sci. Pollut. Res. 2021, 28, 23036–23047. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.S.; Hu, M.C.; Qian, D.; Xue, H.W.; Gao, N.; Lin, X.G. Microbial deterioration and restoration in greenhouse-based intensive vegetable production systems. Plant Soil 2021, 463, 1–18. [Google Scholar] [CrossRef]
- Liu, Z.S.; Xiao, J.W.; Zhang, X.C.; Dou, S.J.; Gao, T.G.; Wang, D.M.; Zhang, D.D. Influence of Bacillus subtilis strain Z-14 on microbial communities of wheat rhizospheric soil infested with Gaeumannomyces graminis var. tritici. Front. Microbiol. 2022, 13, 923242. [Google Scholar] [CrossRef]
- Iqbal, S.; Ullah, N.; Janjua, H.A. In Vitro Evaluation and Genome Mining of Bacillus subtilis Strain RS10 Reveals Its Biocontrol and Plant Growth-Promoting Potential. Agriculture 2021, 11, 1273. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, L.M.; Si, H.L.; Guo, H.; Liu, J.H.; Jia, J.; Su, Q.F.; Wang, Y.B.; Zang, J.P.; Xing, J.H.; et al. Maize stalk rot caused by Fusarium graminearum alters soil microbial composition and is directly inhibited by Bacillus siamensis isolated from rhizosphere soil. Front. Microbiol. 2022, 13, 986401. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Qin, X.Y.; Cao, A.C.; Lu, A.X. Impact of Biochar on Rhizosphere Bacterial Diversity Restoration Following Chloropicrin Fumigation of Planted Soil. Int. J. Environ. Res. Public. Health 2022, 19, 2126. [Google Scholar] [CrossRef]
- Ji, L.D.; Si, H.L.; He, J.Q.; Fan, L.Q.; Li, L. The shifts of maize soil microbial community and networks are related to soil properties under different organic fertilizers. Rhizosphere 2021, 19, 100388. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, A.N.; Zhou, J.N.; Zhang, W.F.; Li, P. Comparison of bacterial communities in soil samples with and without tomato bacterial wilt caused by Ralstonia solanacearum species complex. BMC Microbiol. 2020, 20, 89. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Schenk, P.M.; Wallenstein, M.D.; Singh, B.K. Tiny Microbes, Big Yields: Enhancing food crop production with biological solutions. Microb. Biotechnol. 2017, 10, 999–1003. [Google Scholar] [CrossRef]
- Araujo, R.; Dunlap, C.; Barnett, S.; Franco, C.M.M. Decoding Wheat Endosphere–Rhizosphere Microbiomes in Rhizoctonia solani–Infested Soils Challenged by Streptomyces Biocontrol Agents. Front. Plant Sci. 2019, 10, 1038. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.B.; Xiao, Y.H.; Yin, J.; Yi, T.Y.; Zhou, Z.C.; Hsiang, T.; Tang, Q.J.; Chen, W. Effects of Cultured Root and Soil Microbial Communities on the Disease of Nicotiana tabacum Caused by Phytophthora nicotianae. Front. Microbiol. 2020, 11, 929. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ghani, M.I.; Ding, H.Y.; Iqbal, M.; Cheng, Z.H.; Cai, Z.C. Garlic Substrate Induces Cucumber Growth Development and Decreases Fusarium Wilt through Regulation of Soil Microbial Community Structure and Diversity in Replanted Disturbed Soil. Int. J. Mol. Sci. 2020, 21, 6008. [Google Scholar] [CrossRef]
- Zhou, C.R.; Cheng, H.Y.; Wu, Y.L.; Zhang, J.B.; Li, D.; Pan, C.P. Bensulfuron-Methyl, Terbutylazine, and 2,4-D Butylate Disturb Plant Growth and Resistance by Deteriorating Rhizosphere Environment and Plant Secondary Metabolism in Wheat Seedlings. J. Agric. Food Chem. 2022, 70, 12796–12806. [Google Scholar] [CrossRef]
- Bakker, M.G.; Otto-Hanson, L.; Lange, A.J.; Bradeen, J.M.; Kinkel, L.L. Plant monocultures produce more antagonistic soil Streptomyces communities than high-diversity plant communities. Soil Biol. Biochem. 2013, 65, 304–312. [Google Scholar] [CrossRef]
- Yu, S.M.; Bai, X.; Liang, J.S.; Wei, Y.N.; Huang, S.Q.; Li, Y.; Dong, L.Y.; Liu, X.S.; Qu, J.J.; Yan, L. Inoculation of Pseudomonas sp. GHD-4 and mushroom residue carrier increased the soil enzyme activities and microbial community diversity in Pb-contaminated soils. J. Soils Sediments 2019, 19, 1064–1076. [Google Scholar] [CrossRef]
- Ju, W.L.; Jin, X.L.; Liu, L.; Shen, G.T.; Zhao, W.; Duan, C.J.; Fang, L.C. Rhizobacteria inoculation benefits nutrient availability for phytostabilization in copper contaminated soil: Drivers from bacterial community structures in rhizosphere. Appl. Soil Ecol. 2020, 150, 103450. [Google Scholar] [CrossRef]
- Ju, W.L.; Liu, L.; Fang, L.C.; Cui, Y.X.; Duan, C.J.; Wu, H. Impact of co-inoculation with plant-growth-promoting rhizobacteria and rhizobium on the biochemical responses of alfalfa-soil system in copper contaminated soil. Ecotoxicol. Environ. Saf. 2019, 167, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Zhou, G.S.; Yang, J.; Munir, S.; Ahmed, A.; Liu, Q.; Zhao, Z.X.; Ji, G.H. Bacillus amyloliquefaciens WS-10 as a potential plant growth-promoter and biocontrol agent for bacterial wilt disease of flue-cured tobacco. Egypt. J. Biol. Pest Control 2022, 32, 25. [Google Scholar] [CrossRef]
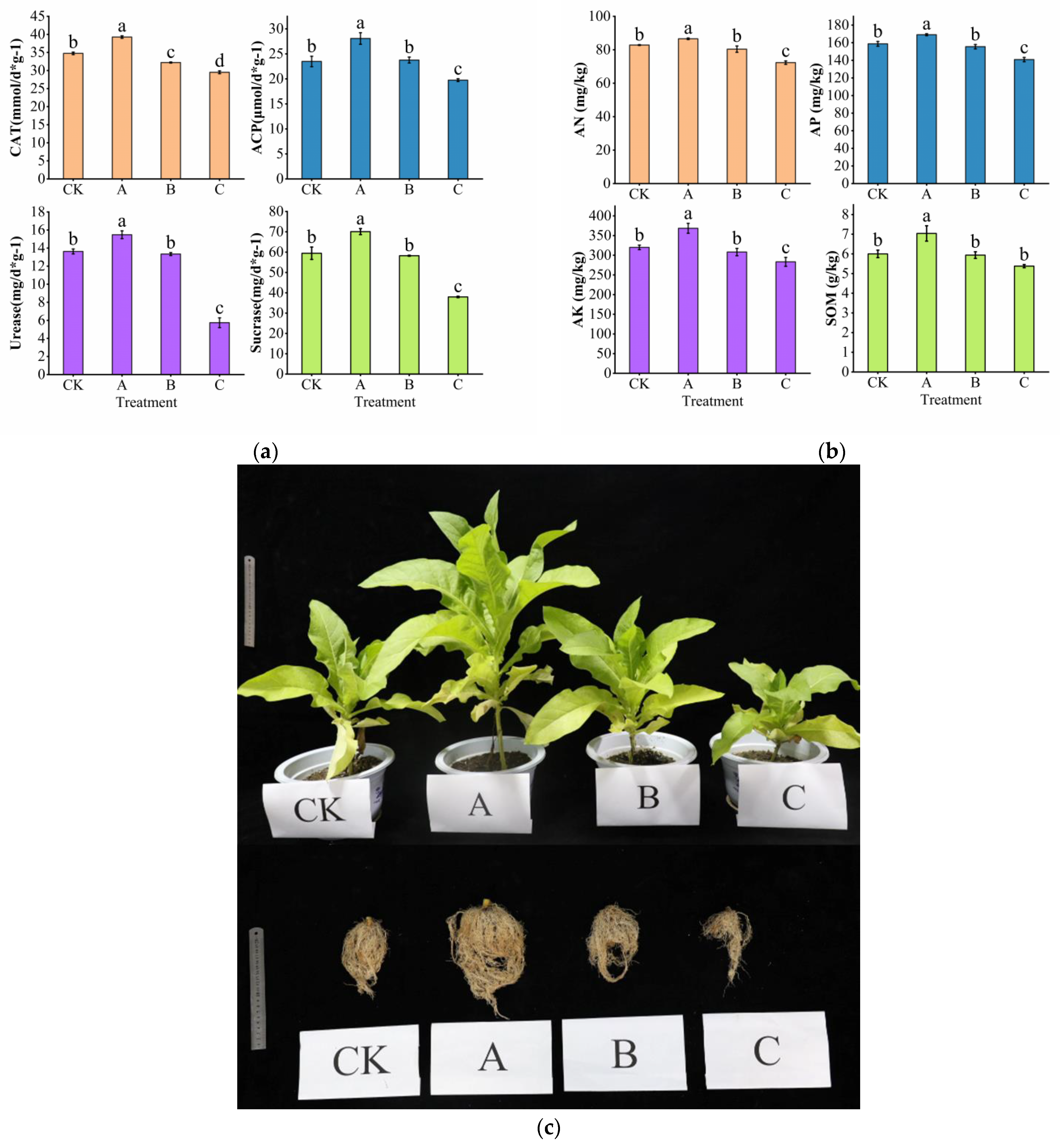





| Plant Trait | Treatments | |||
|---|---|---|---|---|
| CK | A | B | C | |
| Cigar Tobacco plant | ||||
| Plant height (cm) | 24.43 ± 0.23 b | 44.27 ± 0.43 a | 24.63 ± 0.37 b | 14.60 ± 0.46 c |
| Stem circumference (cm) | 2.67 ± 0.03 c | 4.20 ± 0.06 a | 3.23 ± 0.03 b | 2.43 ± 0.03 d |
| Number of leaves (pieces) | 17.00 ± 0.58 b | 22.67 ± 0.67 a | 16.00 ± 0.58 b | 13.33 ± 0.33 c |
| Leaf length (cm) | 28.50 ± 0.29 b | 34.77 ± 0.39 a | 28.53 ± 0.29 b | 24.70 ± 0.35 c |
| Leaf width (cm) | 9.83 ± 0.17 c | 14.03 ± 0.26 a | 11.60 ± 0.21 b | 9.43 ± 0.07 c |
| Fresh weight (g) | 6.18 ± 0.01 c | 10.39 ± 0.11 a | 6.71 ± 0.12 b | 5.44 ± 0.05 d |
| Dry weight (g) | 0.94 ± 0.01 c | 1.92 ± 0.01 a | 1.19 ± 0.02 b | 0.64 ± 0.02 d |
| Root system | ||||
| Root length (cm) | 16.87 ± 0.26 b | 27.07 ± 0.22 a | 16.83 ± 0.18 b | 10.10 ± 0.23 c |
| Root vitality (μg/g ∗ h−1) | 130.75 ± 2.45 b | 202.58 ± 1.80 a | 133.47 ± 2.04 b | 94.69 ± 3.54 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, X.; Fu, S.; Guo, X.; Sun, Z.; Liu, F.; Chen, Q.; Yu, T.; Gao, Y.; Zhang, L.; Yang, L.; et al. Plant Growth-Promoting Rhizobacteria Microbial Fertilizer Changes Soils’ Microbial Structure and Promotes Healthy Growth of Cigar Tobacco Plants. Agronomy 2023, 13, 2895. https://doi.org/10.3390/agronomy13122895
Shang X, Fu S, Guo X, Sun Z, Liu F, Chen Q, Yu T, Gao Y, Zhang L, Yang L, et al. Plant Growth-Promoting Rhizobacteria Microbial Fertilizer Changes Soils’ Microbial Structure and Promotes Healthy Growth of Cigar Tobacco Plants. Agronomy. 2023; 13(12):2895. https://doi.org/10.3390/agronomy13122895
Chicago/Turabian StyleShang, Xianchao, Sha Fu, Xiaomeng Guo, Zheng Sun, Fangyu Liu, Qian Chen, Tao Yu, Yun Gao, Li Zhang, Long Yang, and et al. 2023. "Plant Growth-Promoting Rhizobacteria Microbial Fertilizer Changes Soils’ Microbial Structure and Promotes Healthy Growth of Cigar Tobacco Plants" Agronomy 13, no. 12: 2895. https://doi.org/10.3390/agronomy13122895
APA StyleShang, X., Fu, S., Guo, X., Sun, Z., Liu, F., Chen, Q., Yu, T., Gao, Y., Zhang, L., Yang, L., & Hou, X. (2023). Plant Growth-Promoting Rhizobacteria Microbial Fertilizer Changes Soils’ Microbial Structure and Promotes Healthy Growth of Cigar Tobacco Plants. Agronomy, 13(12), 2895. https://doi.org/10.3390/agronomy13122895





