Agronomic and Physiological Traits Response of Three Tropical Sorghum (Sorghum bicolor L.) Cultivars to Drought and Salinity
Abstract
1. Introduction
2. Materials and Methods
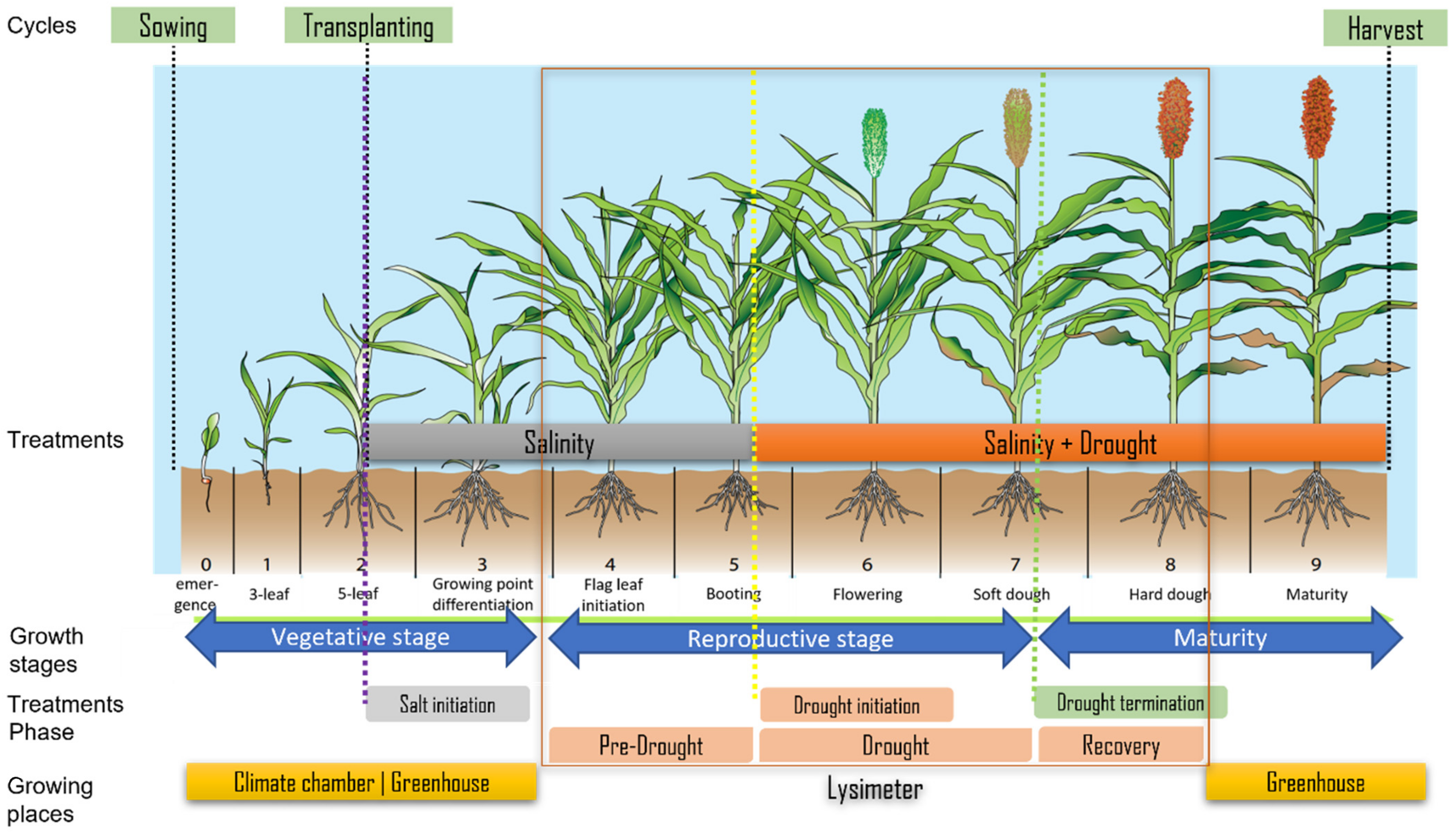
2.1. Plant Material and Treatments
2.2. Experimental Design
2.2.1. Experimental Set Up Using the High-Throughput Gravimetric Phenotyping (HTP) Platform (Plantarray)
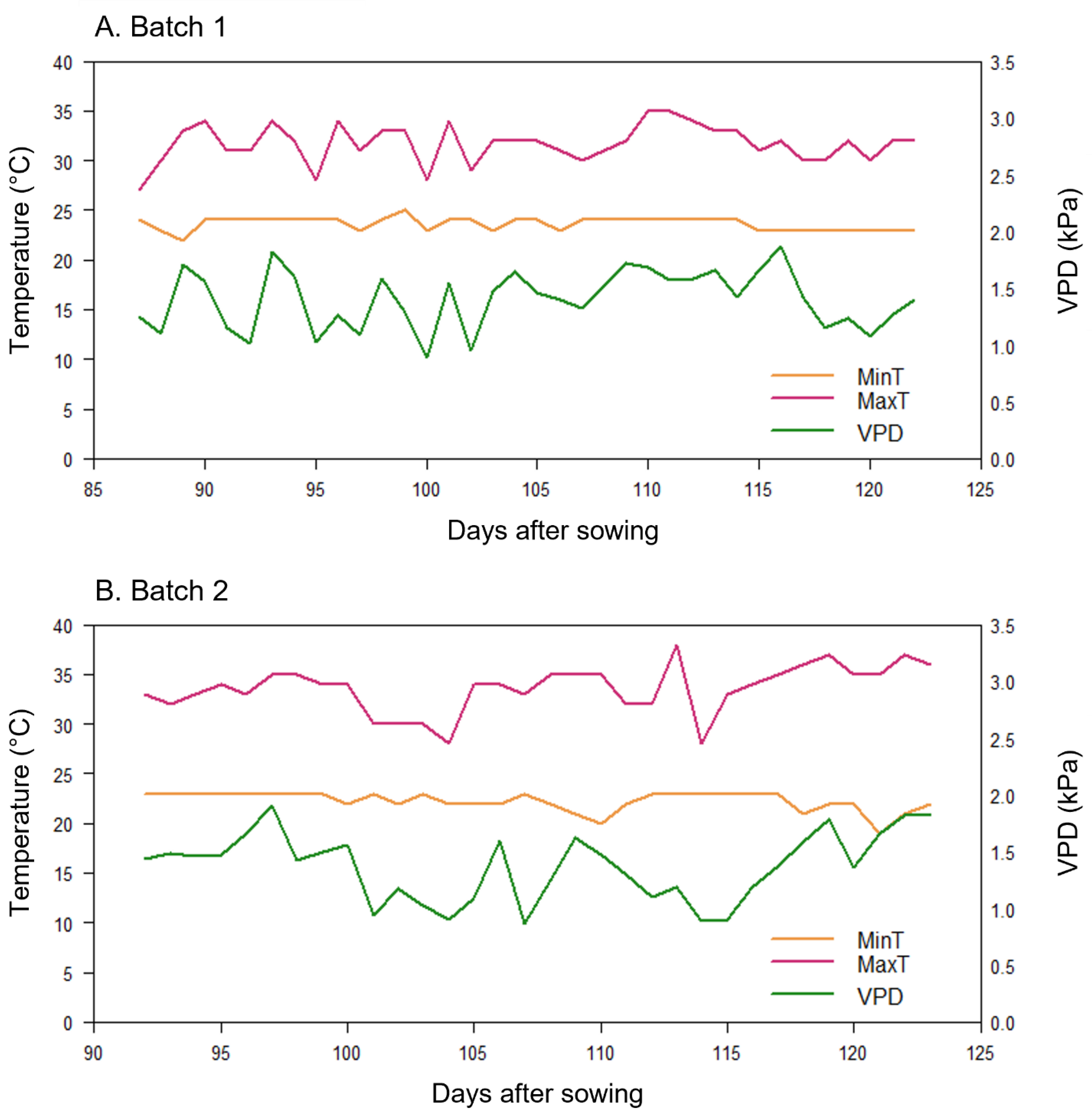
2.2.2. Details on Experimental Conditions
2.3. Data Collection and Statistical Analysis
3. Results
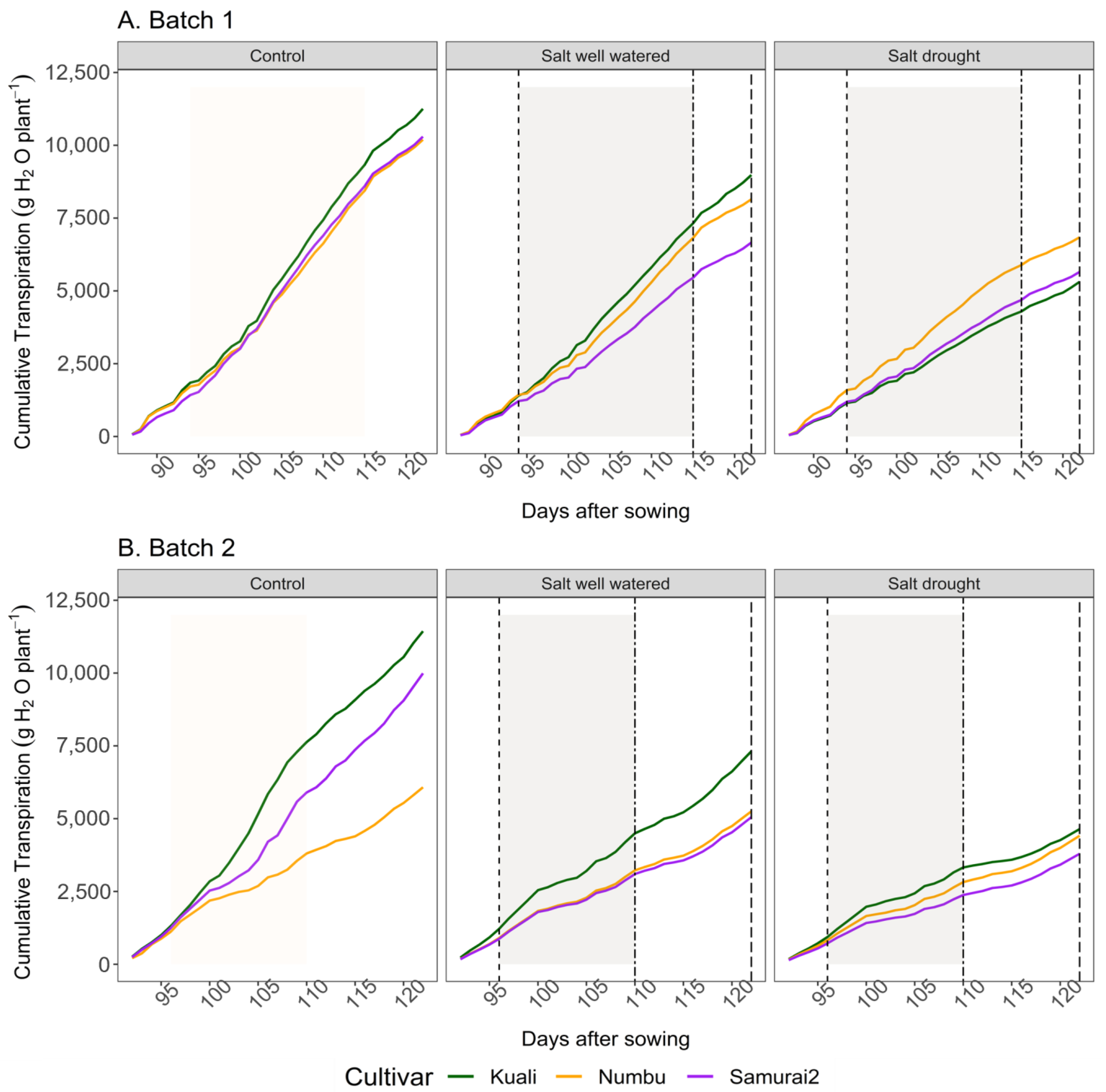
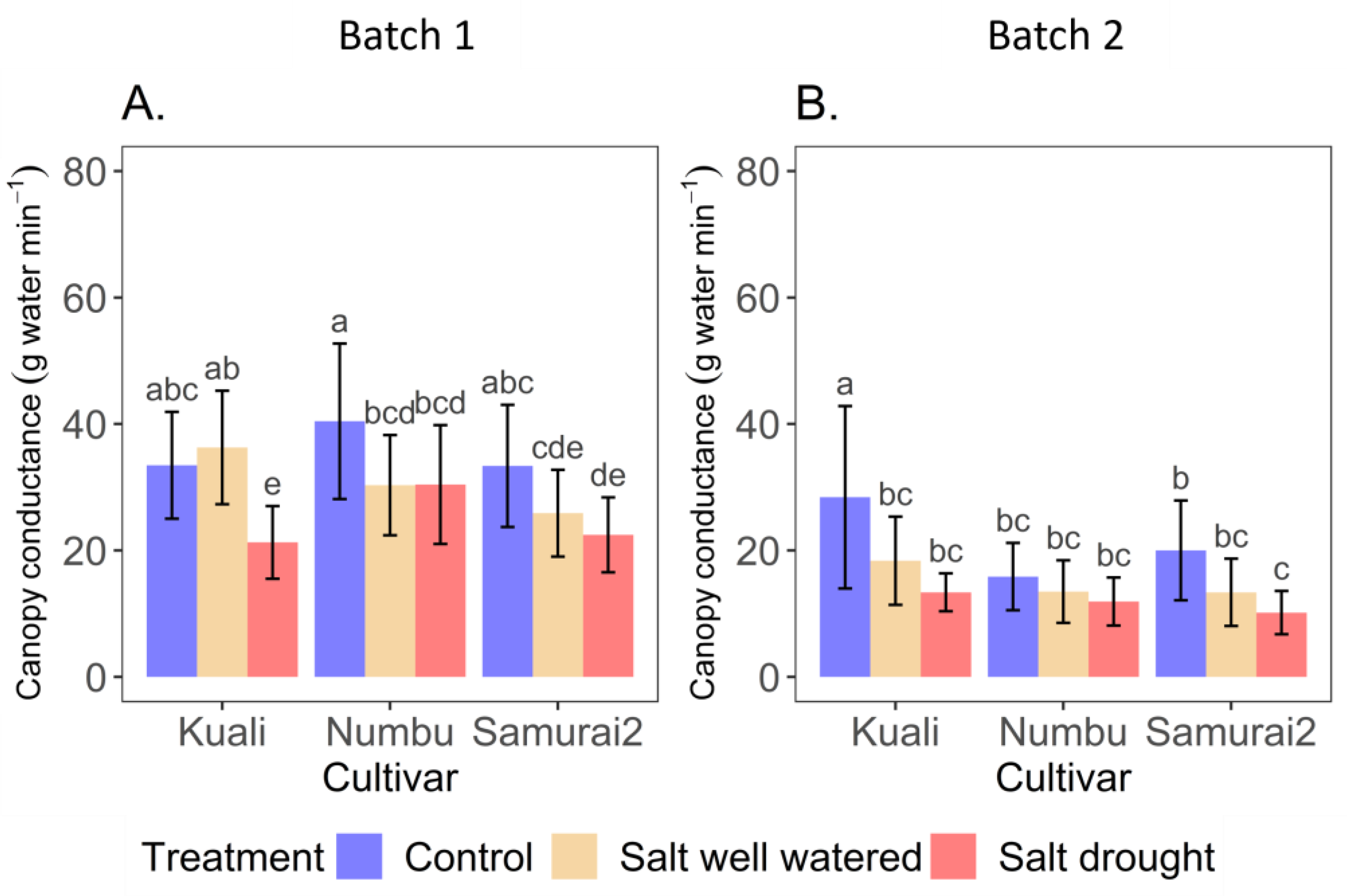
3.1. Effects of Salinity and Drought Stress on Transpiration
3.2. Effect of Salinity and Drought on Water Use Efficiency
3.3. Effect of Salinity and Drought on Yield Component
4. Discussion
4.1. Transpiration and Canopy Conductance in Response to Salinity and Salinity + Drought
4.2. Water Use Efficiency in Response to Salinity and Salinity + Drought
4.3. Yield Components Variation in Response to Salinity and Salinity + Drought
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talukder, B.; Ganguli, N.; Matthew, R.; van Loon, G.W.; Hipel, K.W.; Orbinski, J. Climate Change-Triggered Land Degradation and Planetary Health: A Review. Land Degrad. Dev. 2021, 32, 4509–4522. [Google Scholar] [CrossRef]
- Dai, A. Increasing Drought under Global Warming in Observations and Models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Trnka, M.; Feng, S.; Semenov, M.A.; Olesen, J.E.; Kersebaum, K.C.; Rötter, R.P.; Semerádová, D.; Klem, K.; Huang, W.; Ruiz-Ramos, M.; et al. Mitigation Efforts Will Not Fully Alleviate the Increase in Water Scarcity Occurrence Probability in Wheat-Producing Areas. Sci. Adv. 2019, 5, eaau2406. [Google Scholar] [CrossRef] [PubMed]
- Rötter, R.P.; Tao, F.; Höhn, J.G.; Palosuo, T. Use of Crop Simulation Modelling to Aid Ideotype Design of Future Cereal Cultivars. J. Exp. Bot. 2015, 66, 3463–3476. [Google Scholar] [CrossRef] [PubMed]
- Tester, M.; Langridge, P. Breeding Technologies to Increase Crop Production in a Changing World. Science 2010, 327, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Fita, A.; Rodríguez-Burruezo, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Breeding and Domesticating Crops Adapted to Drought and Salinity: A New Paradigm for Increasing Food Production. Front. Plant Sci. 2015, 6, 978. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hakeem, K.R.; Nahar, K.; Alharby, H.F. Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches. In Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–490. [Google Scholar] [CrossRef]
- Tari, I.; Laskay, G.; Takács, Z.; Poór, P. Response of Sorghum to Abiotic Stresses: A Review. J. Agron. Crop Sci. 2013, 199, 264–274. [Google Scholar] [CrossRef]
- Vasilakoglou, I.; Dhima, K.; Karagiannidis, N.; Gatsis, T. Sweet Sorghum Productivity for Biofuels under Increased Soil Salinity and Reduced Irrigation. Field Crop. Res. 2011, 120, 38–46. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, Y.; Zhao, Y.; Wang, Y.; Zou, F.; Huang, S.; Yang, X.; Xu, Z.; Hu, H. Physiological and Transcriptional Evaluation of Sweet Sorghum Seedlings in Response to Single and Combined Drought and Salinity Stress. S. Afr. J. Bot. 2022, 146, 459–471. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Cao, F.; Zhang, M.; Chen, X.; Zhang, G.; Wu, F. Difference in Yield and Physiological Features in Response to Drought and Salinity Combined Stress during Anthesis in Tibetan Wild and Cultivated Barleys. PLoS ONE 2013, 8, e77869. [Google Scholar] [CrossRef]
- Landi, S.; Hausman, J.F.; Guerriero, G.; Esposito, S. Poaceae vs. Abiotic Stress: Focus on Drought and Salt Stress, Recent Insights and Perspectives. Front. Plant Sci. 2017, 8, 1214. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic Stress, the Field Environment and Stress Combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity Induced Physiological and Biochemical Changes in Plants: An Omic Approach towards Salt Stress Tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Webber, H.; Rezaei, E.E.; Ryo, M.; Ewert, F. Framework to Guide Modeling Single and Multiple Abiotic Stresses in Arable Crops. Agric. Ecosyst. Environ. 2022, 340, 108179. [Google Scholar] [CrossRef]
- Punia, H.; Tokas, J.; Malik, A.; Singh, S.; Phogat, D.S.; Bhuker, A.; Mor, V.S.; Rani, A.; Sheokand, R.N. Discerning Morpho-Physiological and Quality Traits Contributing to Salinity Tolerance Acquisition in Sorghum [Sorghum bicolor (L.) Moench]. S. Afr. J. Bot. 2020, 140, 409–418. [Google Scholar] [CrossRef]
- Naim, A.M.E.; Jabereldar, A.A.; El Naim, A.M.; Abdalla, A.A.; Dagash, Y.M. Effect of Water Stress on Yield and Water Use Efficiency of Sorghum (Sorghum bicolor L. Moench) in Semi-Arid Environment. Int. J. Agric. For. 2017, 7, 1–6. [Google Scholar]
- Gano, B.; Dembele, J.S.B.; Tovignan, T.K.; Sine, B.; Vadez, V.; Diouf, D.; Audebert, A. Adaptation Responses to Early Drought Stress of West Africa Sorghum Varieties. Agronomy 2021, 11, 443. [Google Scholar] [CrossRef]
- Nxele, X.; Klein, A.; Ndimba, B.K. Drought and Salinity Stress Alters ROS Accumulation, Water Retention, and Osmolyte Content in Sorghum Plants. S. Afr. J. Bot. 2017, 108, 261–266. [Google Scholar] [CrossRef]
- Mansour, M.M.F.; Emam, M.M.; Salama, K.H.A.; Morsy, A.A. Sorghum under Saline Conditions: Responses, Tolerance Mechanisms, and Management Strategies. Planta 2021, 254, 24. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Niu, G.; Ganjegunte, G.K. Variability in Salt Tolerance of Sorghum bicolor L. Agric. Sci. 2014, 2, 9–21. [Google Scholar] [CrossRef][Green Version]
- Dewi, E.S.; Abdulai, I.; Bracho-Mujica, G.; Rötter, R.P. Salinity Constraints for Small-Scale Agriculture and Impact on Adaptation in North Aceh, Indonesia. Agronomy 2022, 12, 341. [Google Scholar] [CrossRef]
- Dalal, A.; Shenhar, I.; Bourstein, R.; Mayo, A.; Grunwald, Y.; Averbuch, N.; Attia, Z.; Wallach, R.; Moshelion, M. A High-Throughput Gravimetric Phenotyping Platform for Real-Time Physiological Screening of Plant–Environment Dynamic Responses. bioRxiv 2020. [Google Scholar] [CrossRef]
- Niu, G.; Xu, W.; Rodriguez, D.; Sun, Y. Growth and Physiological Responses of Maize and Sorghum Genotypes to Salt Stress. ISRN Agron. 2012, 2012, 145072. [Google Scholar] [CrossRef]
- Dalal, A.; Bourstein, R.; Haish, N.; Shenhar, I.; Wallach, R.; Moshelion, M. Dynamic Physiological Phenotyping of Drought-Stressed Pepper Plants Treated with “Productivity-Enhancing” and “Survivability-Enhancing” Biostimulants. Front. Plant Sci. 2019, 10, 905. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Haleem Abdul-Wajid, H.; Leonardo Battaglia, M. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Roozeboom, K.L.; Prasad, P.V.V. Sorghum Growth and Development; Wiley Online Library: Hoboken, NJ, USA, 2019; pp. 155–172. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; PBC: Boston, MA, USA, 2020. [Google Scholar]
- Ritchie, J.T.; Singh, U.; Godwin, D.C.; Bowen, W.T. Cereal Growth, Development and Yield. In Understanding Options for Agricultural Production; Springer: Dordrecht, The Netherlands, 1998; pp. 79–98. [Google Scholar]
- Van Oosterom, E.J.; Kulathunga, M.R.D.L.; Deifel, K.S.; McLean, G.B.; Barrasso, C.; Wu, A.; Messina, C.; Hammer, G.L. Dissecting and Modelling the Comparative Adaptation to Water Limitation of Sorghum and Maize: Role of Transpiration Efficiency, Transpiration Rate and Height. In Silico Plants 2021, 3, diaa012. [Google Scholar] [CrossRef]
- Sanon, M.; Hoogenboom, G.; Traoré, S.B.; Sarr, B.; Garcia Y Garcia, A.; Somé, L.; Roncoli, C. Photoperiod Sensitivity of Local Millet and Sorghum Varieties in West Africa. NJAS—Wagening. J. Life Sci. 2014, 68, 29–39. [Google Scholar] [CrossRef][Green Version]
- Borrell, A.; Van Oosterom, E.; George-Jaeggli, B.; Rodriguez, D.; Eyre, J.; Jordan, D.J.; Mace, E.; Singh, V.; Vadez, V.; Bell, M.; et al. Sorghum; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-819194-1. [Google Scholar]
- Alagarswamy, G.; Reddy, D.M.; Swaminathan, G. Durations of the Photoperiod-Sensitive and -Insensitive Phases of Time to Panicle Initiation in Sorghum. Field Crop. Res. 1998, 55, 1–10. [Google Scholar] [CrossRef]
- Xin, Z.; Wang, M.; Cuevas, H.E.; Chen, J.; Harrison, M.; Pugh, N.A.; Morris, G. Sorghum Genetic, Genomic, and Breeding Resources. Planta 2021, 254, 114. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Ranjbar, G.; Pakniyat, H.; Emam, Y. Physiological Mechanisms of Salt Stress Tolerance in Plants. In Plant-Environment Interaction; Wiley Online Library: Hoboken, NJ, USA, 2015; pp. 141–160. [Google Scholar] [CrossRef]
- Francois, L.E.; Donovan, T.; Maas, E.V. Salinity Effects on Seed Yield, Growth, and Germination of Grain Sorghum 1. Agron. J. 1984, 76, 741–744. [Google Scholar] [CrossRef]
- Talebi, B.; Heidari, M.; Ghorbani, H. Arsenic Application Changed Growth, Photo-Synthetic Pigments and Antioxidant Enzymes Activity in Sorghum (Sorghum bicolor L.) Under Salinity Stress. Sci. Agric. Bohem. 2019, 50, 155–163. [Google Scholar] [CrossRef]
- Wen, W.; Timmermans, J.; Chen, Q.; Van Bodegom, P.M. Monitoring the Combined Effects of Drought and Salinity Stress on Crops Using Remote Sensing in the Netherlands. Hydrol. Earth Syst. Sci. 2022, 26, 4537–4552. [Google Scholar] [CrossRef]
- Shelake, R.M.; Kadam, U.S.; Kumar, R.; Pramanik, D.; Singh, A.K.; Kim, J.Y. Engineering Drought and Salinity Tolerance Traits in Crops through CRISPR-Mediated Genome Editing: Targets, Tools, Challenges, and Perspectives. Plant Commun. 2022, 3, 100417. [Google Scholar] [CrossRef] [PubMed]
- Amombo, E.; Ashilenje, D.; Hirich, A.; Kouisni, L.; Oukarroum, A.; Ghoulam, C.; El Gharous, M.; Nilahyane, A. Exploring the Correlation between Salt Tolerance and Yield: Research Advances and Perspectives for Salt-Tolerant Forage Sorghum Selection and Genetic Improvement. Planta 2022, 255, 71. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Dourado, P.R.M.; de Souza, E.R.; Dos Santos, M.A.; Lins, C.M.T.; Monteiro, D.R.; Paulino, M.K.S.S.; Schaffer, B. Stomatal Regulation and Osmotic Adjustment in Sorghum in Response to Salinity. Agriculture 2022, 12, 658. [Google Scholar] [CrossRef]
- Sahin, U.; Ekinci, M.; Ors, S.; Turan, M.; Yildiz, S.; Yildirim, E. Effects of Individual and Combined Effects of Salinity and Drought on Physiological, Nutritional and Biochemical Properties of Cabbage (Brassica oleracea var. capitata). Sci. Hortic. 2018, 240, 196–204. [Google Scholar] [CrossRef]
- Guimarães, M.J.M.; Simões, W.L.; Salviano, A.M.; de Oliveira, A.R.; da Silva, J.S.; Barros, J.R.A.; Willadino, L. Management for Grain Sorghum Cultivation under Saline Water Irrigation. Rev. Bras. Eng. Agric. E Ambient. 2022, 26, 755–762. [Google Scholar] [CrossRef]
- Liao, Q.; Gu, S.; Kang, S.; Du, T.; Tong, L.; Wood, J.D.; Ding, R. Mild Water and Salt Stress Improve Water Use Efficiency by Decreasing Stomatal Conductance via Osmotic Adjustment in Field Maize. Sci. Total Environ. 2022, 805, 150364. [Google Scholar] [CrossRef]
- Roman, V.J.; van de Zedde, R.; Peller, J.; Visser, R.G.F.; van der Linden, C.G.; van Loo, E.N. High-Resolution Analysis of Growth and Transpiration of Quinoa Under Saline Conditions. Front. Plant Sci. 2021, 12, 634311. [Google Scholar] [CrossRef]
- Henderson, A.N.; Crim, P.M.; Cumming, J.R.; Hawkins, J.S. Phenotypic and Physiological Responses to Salt Exposure in Sorghum Reveal Diversity among Domesticated Landraces. Appl. Plant Sci. 2020, 107, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under Drought and Salt Stress: Regulation Mechanisms from Whole Plant to Cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A.; Gull, M. Influence of Salinity Stress on the Uptake of Magnesium, Phosphorus, and Yield of Salt Susceptible and Tolerant Sorghum Cultivars (Sorghum bicolor L.). J. Appl. Biol. Biotechnol. 2019, 7, 53–58. [Google Scholar] [CrossRef]
- Shokat, S.; Großkinsky, D.K. Tackling Salinity in Sustainable Agriculture-What Developing Countries May Learn from Approaches of the Developed World. Sustainability 2019, 11, 4558. [Google Scholar] [CrossRef]
- Aydinsakir, K.; Buyuktas, D.; Dinç, N.; Erdurmus, C.; Bayram, E.; Yegin, A.B. Yield and Bioethanol Productivity of Sorghum under Surface and Subsurface Drip Irrigation. Agric. Water Manag. 2021, 243, 106452. [Google Scholar] [CrossRef]



| Batch | Cultivars | Treatment | Batch | Cultivars | Treatment |
|---|---|---|---|---|---|
| 1 | Kuali | Control | 2 | Kuali | Control |
| EC 7 well watered | EC 14 well watered | ||||
| EC 7 drought | EC 14 drought | ||||
| Numbu | Control | Numbu | Control | ||
| EC 7 well watered | EC 14 well watered | ||||
| EC 7 drought | EC 14 drought | ||||
| Samurai2 | Control | Samurai2 | Control | ||
| EC 7 well watered | EC 14 well watered | ||||
| EC 7 drought | EC 14 drought |
| Cultivars | Treatment | Cumulative Transpiration per Phase (mL/Plant) | ||
|---|---|---|---|---|
| Pre Drought | Drought | Recovery | ||
| Kuali | Control | 1847 | 8997 a† | 11,256 a |
| Salt well watered | 1407 | 7048 ab | 8984 abc | |
| (24) | (22) | (20) | ||
| Salt–drought | 1153 | 4148 b | 5315 c | |
| (38) | (54) | (53) | ||
| Numbu | Control | 1718 | 8142 a | 10,196 ab |
| Salt well watered | 1432 | 6555 ab | 8150 abc | |
| (17) | (19) | (20) | ||
| Salt–drought | 1594 | 5757 ab | 6845 bc | |
| (16) | (29) | (33) | ||
| Samurai2 | Control | 1425 | 8280 a | 10,302 ab |
| Salt well watered | 1220 | 5258 ab | 6664 bc | |
| (14) | (36) | (35) | ||
| Salt–drought | 1198 | 4566 b | 5656 c | |
| (16) | (45) | (45) | ||
| Cultivars | Treatment | Cumulative Transpiration per Phase (mL/Plant) | ||
|---|---|---|---|---|
| Pre Drought | Drought | Recovery | ||
| Kuali | Control | 1003 | 7294 a† | 11,438 a |
| Salt well watered | 923 | 4195 bc | 7317 abc | |
| (8) | (42) | (36) | ||
| Salt–drought | 725 | 3156 bc | 4647 c | |
| (28) | (57) | (59) | ||
| Numbu | Control | 881 | 3557 bc | 6081 bc |
| Salt well watered | 698 | 3005 bc | 5255 c | |
| (21) | (16) | (14) | ||
| Salt–drought | 636 | 2642 c | 4417 c | |
| (28) | (26) | (14) | ||
| Samurai2 | Control | 952 | 5586 ab | 9994 ab |
| Salt well watered | 679 | 2883 bc | 5054 c | |
| (29) | (48) | (49) | ||
| Salt–drought | 548 | 2231 c | 3796 c | |
| (42) | (60) | (62) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewi, E.S.; Abdulai, I.; Bracho-Mujica, G.; Appiah, M.; Rötter, R.P. Agronomic and Physiological Traits Response of Three Tropical Sorghum (Sorghum bicolor L.) Cultivars to Drought and Salinity. Agronomy 2023, 13, 2788. https://doi.org/10.3390/agronomy13112788
Dewi ES, Abdulai I, Bracho-Mujica G, Appiah M, Rötter RP. Agronomic and Physiological Traits Response of Three Tropical Sorghum (Sorghum bicolor L.) Cultivars to Drought and Salinity. Agronomy. 2023; 13(11):2788. https://doi.org/10.3390/agronomy13112788
Chicago/Turabian StyleDewi, Elvira Sari, Issaka Abdulai, Gennady Bracho-Mujica, Mercy Appiah, and Reimund P. Rötter. 2023. "Agronomic and Physiological Traits Response of Three Tropical Sorghum (Sorghum bicolor L.) Cultivars to Drought and Salinity" Agronomy 13, no. 11: 2788. https://doi.org/10.3390/agronomy13112788
APA StyleDewi, E. S., Abdulai, I., Bracho-Mujica, G., Appiah, M., & Rötter, R. P. (2023). Agronomic and Physiological Traits Response of Three Tropical Sorghum (Sorghum bicolor L.) Cultivars to Drought and Salinity. Agronomy, 13(11), 2788. https://doi.org/10.3390/agronomy13112788








