Predicting the Base Neutralization Capacity of Soils Based on Texture, Organic Carbon and Initial pH: An Opportunity to Adjust Common Liming Recommendation Approaches to Specific Management and Climate Conditions
Abstract
:1. Introduction
- 10 kg acid deposition ammonium-N: 56 kg ha−1 CaO;
- 10 kg fertilizer ammonium-N: 40 kg ha−1 CaO;
- 10 kg fertilizer urea-N: 11 kg ha−1 CaO;
- 10 kg elemental S: 18 kg ha−1 CaO.
- Potatoes yielding 40 t ha−1: 10 kg ha−1 CaO;
- Cereal grain plus straw yielding 7 t ha−1: 30 kg ha−1 CaO;
- Grass (silage) yielding 40 t ha−1: 65 kg ha−1 CaO.
2. Materials and Methods
2.1. Site Description
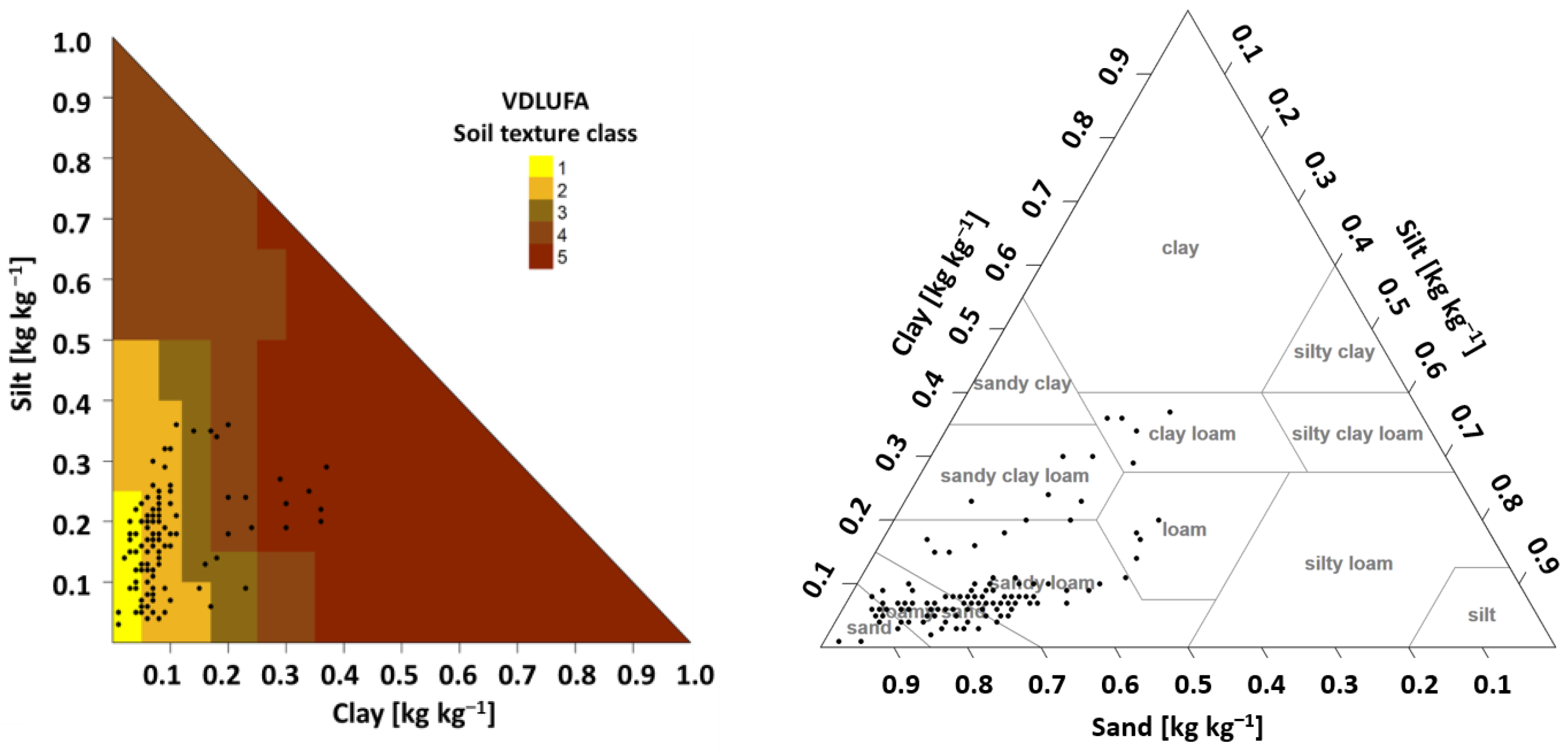
2.2. Soil Analyses
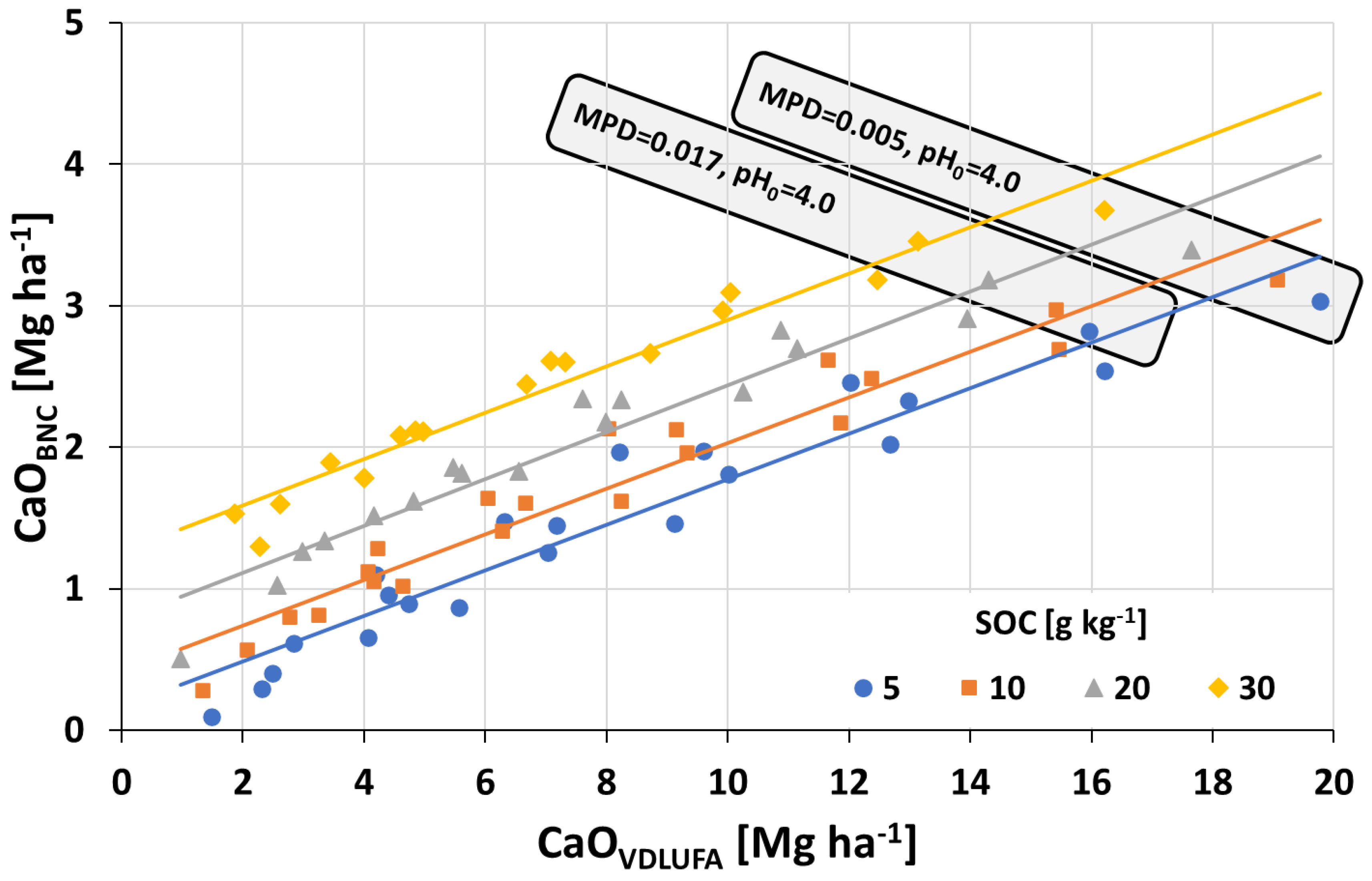
2.3. Creating the BNC Approach
2.4. Comparison of the CaO Amounts Necessary to Change pH0 to pHtarget as Calculated Using Both the VDLUFA and the BNC Approaches
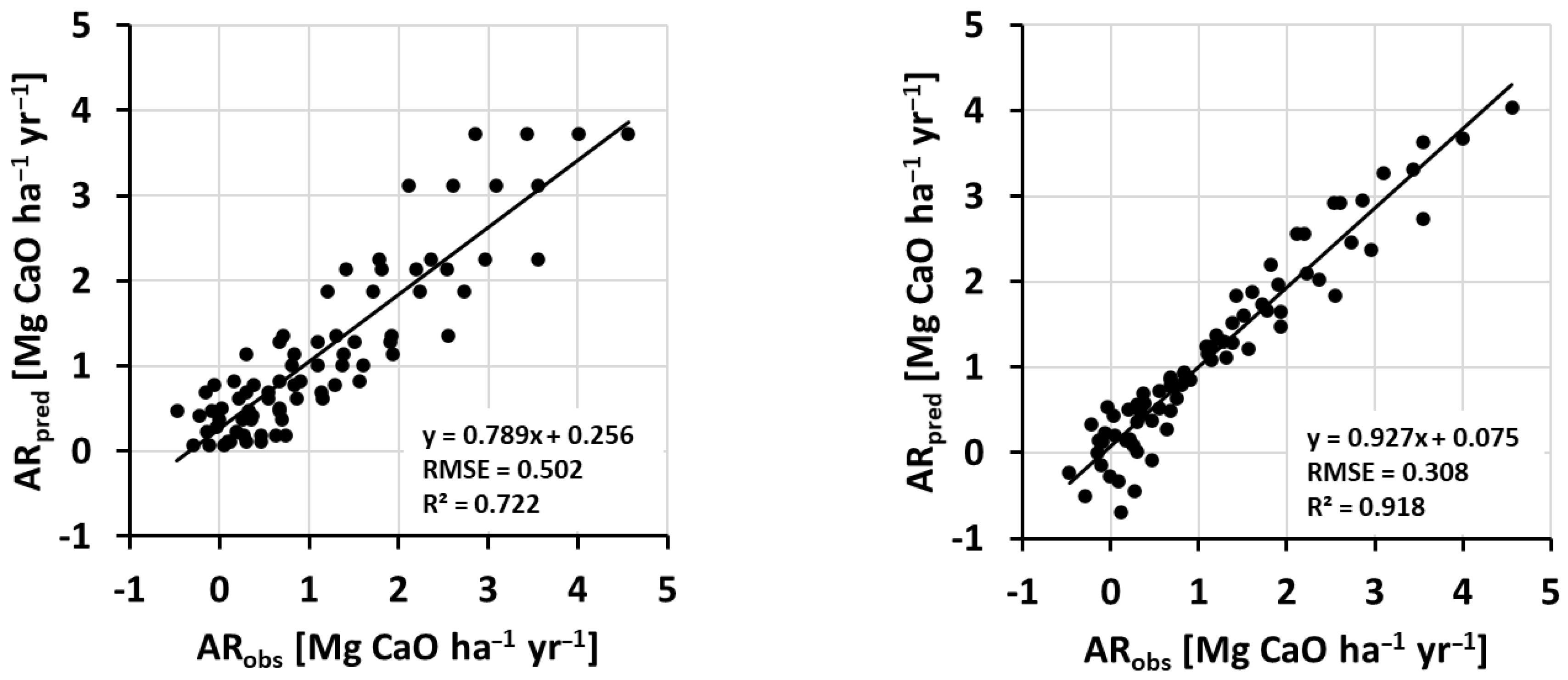
2.5. Statistics
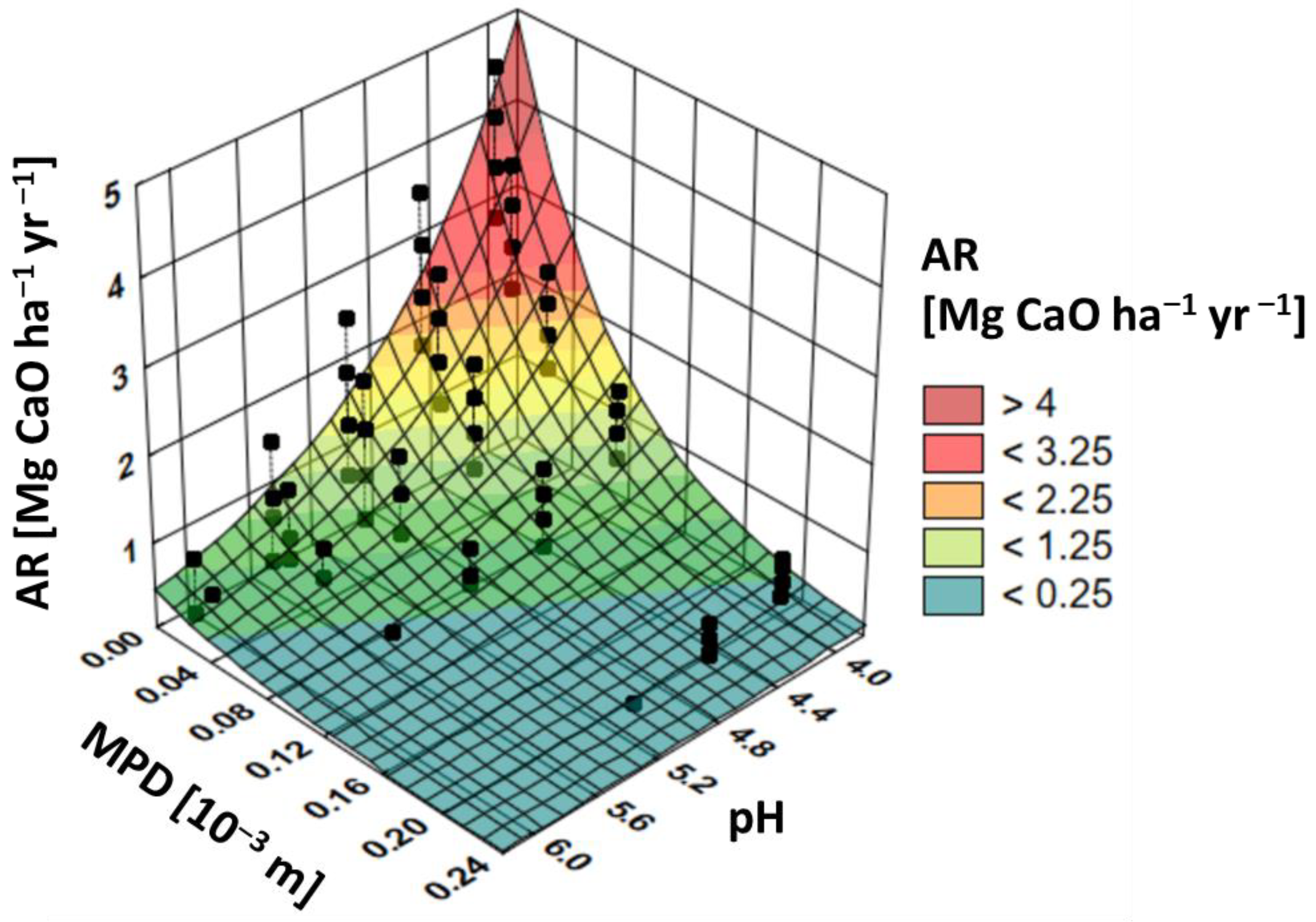
3. Results and Discussion
- Decreases if the SOC increases because the buffer capacity is linearly related to the SOC [36];
- Increases if the MPD increases because the buffer capacity is linearly related to the clay content [18] (which is inversely related to the MPD).
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chambers, B.J.; Garwood, T.W.D. Lime loss rates from arable and grassland soils. J. Agricult. Sci. 1998, 131, 455–464. [Google Scholar] [CrossRef]
- von Wulfen, U.; Roschke, M.; Kape, H.-E. Guide Values for the Investigation and Advice as well as for the Technical Implementation of the Fertilizer Ordinance (DüV): Common Information from the States of Brandenburg, Mecklenburg-Western Pomerania and Saxony-Anhalt, Güterfelde, Germany; Landesamt für Verbraucherschutz & Landwirtschaft und Flurneuordnung (LVLF): Güterfelde, Germany, 2008. (In German) [Google Scholar]
- de Haan, J.J.; van Geel, W.C.A.; van Asperen, P.; Postma, R.; Brinks, H.; Reijneveld, A.; Malda, J.T. Handboek Bodem en Bemesting. Web Publication/Site, WUR. 2014. Available online: http://www.handboekbodemenbemesting.nl (accessed on 24 August 2023).
- Sinclair, A.; Crooks, B.; Coull, M. Soils Information, Texture and Liming Recommendations. In Technical Note TN656; SRUC. Scotland’s Rural College: Aberdeen, UK, 2014; Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiCyreV6aKCAxXzh_0HHf0CChAQFnoECA0QAQ&url=https%3A%2F%2Fwww.sruc.ac.uk%2Fmedia%2F3acbc3rs%2Ftn656.pdf&usg=AOvVaw3eoj4iMfCYBWLvirPUmrvY&opi=89978449 (accessed on 24 August 2023).
- Söderström, M.; Sohlenius, G.; Rodhe, L.; Piikki, K. Adaptation of regional digital soil mapping for precision agriculture. Precis. Agric. 2016, 17, 588–607. [Google Scholar] [CrossRef]
- AHDB. Soil pH and Liming Recommendations for Arable and Grass Systems. Nutrient Management Guideline (RB209). Agriculture and Horticulture Development Board 2023, Coventry, UK. Available online: https://ahdb.org.uk/knowledge-library/soil-ph-and-liming-recommendations-for-arable-and-grass-systems (accessed on 30 August 2023).
- Tzilivakis, J.; Lewis, K.; Green, A.; Warner, D. ALA Lime Calculator [Software]; Agricultural Lime Association (ALA): London, UK, 2002. [Google Scholar]
- Vossen, P. Changing pH in Soil; University of California Cooperative Extension: Davis, CA, USA, 2002. [Google Scholar]
- Jacobs, A.; Flessa, H.; Don, A.; Heidkamp, A.; Prietz, R.; Dechow, R.; Gensior, A.; Poeplau, C.; Riggers, C.; Schneider, F.; et al. Arable Soils in Germany—Results of the Soil Status Survey; Johann Heinrich von Thünen-Institut: Braunschweig, Germany, 2018; 321p, Thünen Rep 64. (In German) [Google Scholar] [CrossRef]
- Bönecke, E.; Meyer, S.; Vogel, S.; Schröter, I.; Gebbers, R.; Kling, C.; Kramer, E.; Lück, K.; Nagel, A.; Philipp, G.; et al. Guidelines for precise lime management based on high-resolution soil pH, texture and SOM maps generated from proximal soil sensing data. Precis. Agric. 2020, 22, 493–523. [Google Scholar] [CrossRef]
- Meyer, S.; Kling, C.; Vogel, S.; Schröter, I.; Nagel, A.; Kramer, E.; Gebbers, R.; Philipp, G.; Lück, K.; Gerlach, F. Creating soil texture maps for precision liming using electrical resistivity and gamma ray mapping. In Precision Agriculture’19, Wageningen; Academic Publishers: Wageningen, The Netherlands, 2019; p. 92. [Google Scholar] [CrossRef]
- Ruehlmann, J.; Bönecke, E.; Meyer, S. Predicting the Lime Demand of Arable Soils from pH Value, Soil Texture and Soil Organic Matter Content. Agronomy 2021, 11, 785. [Google Scholar] [CrossRef]
- Vogel, S.; Bönecke, E.; Kling, C.; Kramer, E.; Lück, K.; Nagel, A.; Philipp, G.; Rühlmann, J.; Schröter, I.; Gebbers, R. Base Neutralizing Capacity of Agricultural Soils in a Quaternary Landscape of North-East Germany and Its Relationship to Best Management Practices in Lime Requirement Determination. Agronomy 2020, 10, 877. [Google Scholar] [CrossRef]
- Vogel, S.; Bönecke, E.; Kling, C.; Kramer, E.; Lück, K.; Philipp, G.; Rühlmann, J.; Schröter, I.; Gebbers, R. Direct prediction of site-specific lime requirement of arable fields using the base neutralizing capacity and a multi-sensor platform for on-the-go soil mapping. Precis. Agric. 2022, 23, 127–149. [Google Scholar] [CrossRef]
- Kerschberger, M.; Deller, B.; Hege, U.; Heyn, J.; Kape, H.E.; Krause, O.; Pollehn, J.; Rex, M.J.; Severin, K. Determination of the Lime Requirement of Arable and Grassland Soils; VDLUFA-Verlag: Darmstadt, Germany, 2000. (In German) [Google Scholar]
- Kerschberger, M.; Marks, G. Setting and maintaining a site-specific optimum pH value in the soil-a basic requirement for effective and environmentally compatible plant production. Berichte über Landwirtsch. 2007, 85, 56–77. (In German) [Google Scholar]
- Goulding, K.W. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef]
- Wang, X.; Tang, C.; Mahony, S.; Baldock, J.A.; Butterly, C.R. Factors affecting the measurement of soil pH buffer capacity: Approaches to optimize the methods. Eur. J. Soil Sci. 2015, 66, 53–64. [Google Scholar] [CrossRef]
- Bolton, J. Changes in magnesium and calcium in soils of the Broadbalk wheat experiment at Rothamsted from 1865 to 1966. J. Agric. Sci. 1972, 79, 217–223. [Google Scholar] [CrossRef]
- Bolton, J. Changes in soil pH and exchangeable calcium in two liming experiments on contrasting soils over 12 years. J. Agric. Sci. 1977, 89, 81–86. [Google Scholar] [CrossRef]
- Breemen, N.V.; Protz, R. Rates of calcium carbonate removal from soils. Can. J. Soil Sci. 1988, 68, 449–454. [Google Scholar] [CrossRef]
- Loide, V. About the effect of the contents and ratios of soil’s available calcium, potassium and magnesium in liming of acid soils. Agron. Res. 2004, 2, 71–82. [Google Scholar]
- BAD. Nutrient Losses from Agricultural Farms Managed According Good Agricultural Praxis; Bundesarbeitskreis Düngung: Frankfurt am Main, Germany, 2003. (In German) [Google Scholar]
- Archer, J. Crop Nutrition and Fertiliser Use; Farming Press Ltd.: Ipswich, MA, USA, 1985; p. 285. [Google Scholar]
- Slessarev, E.W.; Lin, Y.; Bingham, N.L.; Johnson, J.E.; Dai, Y.; Schimel, J.P.; Chadwick, O.A. Water balance creates a threshold in soil pH at the global scale. Nature 2016, 540, 567–569. [Google Scholar] [CrossRef]
- Brandenburg State Office for Mining, Geology and Raw Materials. Geological Map of the State of Brandenburg, Scale: 1:25,000 (GK 25). Cottbus, Brandenburg. Available online: https://geo.brandenburg.de/?page=Geologische-Karten&views=Ebenen----- (accessed on 1 August 2023).
- Shirazi, M.A.; Boersma, L.; Hart, J.W. A unifying quantitative analysis of soil texture: Improvement of precision and extension of scale. Soil Sci. Soc. Am. J. 1988, 52, 181–190. [Google Scholar] [CrossRef]
- DIN ISO 10694; Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis). Beuth: Berlin, Germany, 1996.
- DIN ISO 11277; Soil Quality—Determination of Particle Size Distribution in Mineral Soil Material—Method by Sieving and Sedimentation. Beuth: Berlin, Germany, 2009.
- Sparks, D. Environmental Soil Chemistry, 2nd ed.; Academic Press, an Imprint of Elsevier: San Diego, CA, USA, 2003. [Google Scholar]
- Sposito, G. The Chemistry of Soils, 3rd ed.; Oxford University Press: New York, NY, USA, 2016. [Google Scholar]
- Meiwes, K.J.; Koenig, N.; Khana, P.K.; Prenzel, J.; Ulrich, B. Chemical test methods for mineral soils, litter layers and roots to characterize and evaluate acidification in forest soils. In Die Erfassung des Stoffkreislaufs in Waldökosystemen—Konzeption und Methodik. Berichte des Forschungszentrums Waldökosysteme/Waldsterben, Bd. 7; Meiwes, K.-J., Hauhs, M., Gerke, H., Asche, N., Matzner, E., Lammersdorf, N., Eds.; Institut für Bodenkunde und Waldernährung der Universität Göttingen: Göttingen, Germany, 1984. (In German) [Google Scholar]
- Kvalseth, T.O. Cautionary Note about R2. Am. Stat. 1985, 39, 279–285. [Google Scholar]
- Alexander, D.L.; Tropsha, A.; Winkler, D.A. Beware of R 2: Simple, unambiguous assessment of the prediction accuracy of QSAR and QSPR models. J. Chem. Inf. Model. 2015, 55, 1316–1322. [Google Scholar] [CrossRef]
- Curtin, D.; Trolove, S. Predicting pH buffering capacity of New Zealand soils from organic matter content and mineral characteristics. Soil Res. 2013, 51, 494–502. [Google Scholar] [CrossRef]
- Magdoff, F.R.; Bartlett, R.J. Soil pH buffering revisited. Soil Sci. Soc. Am. J. 1985, 49, 145–148. [Google Scholar] [CrossRef]
- Magdoff, F.R.; Bartlett, R.J.; Ross, D.S. Acidification and pH buffering of forest soils. Soil Sci. Soc. Am. J. 1987, 51, 1384–1386. [Google Scholar] [CrossRef]
- Schilling, G. Plant Nutrition and Fertilization; Verlag Eugen Ulmer: Stuttgart, Germany, 2000; p. 464. (In German) [Google Scholar]
- Bloom, P.R.; McBride, M.B.; Weaver, R.M. Aluminum organic matter in acid soils: Buffering and solution aluminum activity. Soil Sci. Soc. Am. J. 1979, 43, 488–493. [Google Scholar] [CrossRef]
- Büchner, M.; Gerstengarbe, F.-W.; Gottschalk, P.; Gutsch, M.; Hattermann, F.F.; Huang, S.; Koch, H.; Lasch, P.; Lüttger, A.; Schellnhuber, H.J.; et al. Climate Impact on Germany; Potsdam-Institut für Klimafolgenforschung: Potsdam, Germany, 2012. (In German) [Google Scholar]
- Henrichs, M.; Langner, J.; Uhl, M. Development of a simplified urban water balance model (WABILA). Water Sci. Technol. 2016, 73, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Soil Science Division Staff. Soil Survey Manual; Ditzler, C., Scheffe, K., Monger, H.C., Eds.; USDA Handbook 18; Government Printing Office: Washington, DC, USA, 2017. Available online: https://www.nrcs.usda.gov/resources/guides-and-instructions/soil-survey-manual (accessed on 30 August 2023).

| Soil Texture | Land Use | Lime Equivalents to Compensate Acidification (kg CaO ha−1 yr−1) | ||
|---|---|---|---|---|
| Annual Precipitation | ||||
| <600 mm | 600–750 mm | >750 mm | ||
| sandy | Arable land | 300 | 400 | 500 |
| Grass land | 150 | 250 | 350 | |
| loamy | Arable land | 400 | 500 | 600 |
| Grass land | 200 | 300 | 400 | |
| clayey | Arable land | 500 | 600 | 700 |
| Grass land | 250 | 350 | 450 | |
| Farm I | Farm II | Farm III | |
|---|---|---|---|
| MPD [10−3 m] | |||
| Min | 0.01 | 0.05 | 0.01 |
| Max | 0.21 | 0.15 | 0.29 |
| SD | 0.05 | 0.03 | 0.09 |
| SOC (g kg−1) | |||
| Min | 4.40 | 6.40 | 5.75 |
| Max | 32.30 | 12.60 | 26.2 |
| SD | 5.11 | 1.25 | 6.66 |
| Initial pH | |||
| Min | 5.24 | 5.71 | 4.51 |
| Max | 7.55 | 7.32 | 7.24 |
| SD | 0.49 | 0.34 | 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruehlmann, J.; Bönecke, E.; Gebbers, R.; Gerlach, F.; Kling, C.; Lück, K.; Meyer, S.; Nagel, A.; Palme, S.; Philipp, G.; et al. Predicting the Base Neutralization Capacity of Soils Based on Texture, Organic Carbon and Initial pH: An Opportunity to Adjust Common Liming Recommendation Approaches to Specific Management and Climate Conditions. Agronomy 2023, 13, 2762. https://doi.org/10.3390/agronomy13112762
Ruehlmann J, Bönecke E, Gebbers R, Gerlach F, Kling C, Lück K, Meyer S, Nagel A, Palme S, Philipp G, et al. Predicting the Base Neutralization Capacity of Soils Based on Texture, Organic Carbon and Initial pH: An Opportunity to Adjust Common Liming Recommendation Approaches to Specific Management and Climate Conditions. Agronomy. 2023; 13(11):2762. https://doi.org/10.3390/agronomy13112762
Chicago/Turabian StyleRuehlmann, Joerg, Eric Bönecke, Robin Gebbers, Felix Gerlach, Charlotte Kling, Katrin Lück, Swen Meyer, Anne Nagel, Stefan Palme, Golo Philipp, and et al. 2023. "Predicting the Base Neutralization Capacity of Soils Based on Texture, Organic Carbon and Initial pH: An Opportunity to Adjust Common Liming Recommendation Approaches to Specific Management and Climate Conditions" Agronomy 13, no. 11: 2762. https://doi.org/10.3390/agronomy13112762
APA StyleRuehlmann, J., Bönecke, E., Gebbers, R., Gerlach, F., Kling, C., Lück, K., Meyer, S., Nagel, A., Palme, S., Philipp, G., Scheibe, D., Schröter, I., Vogel, S., & Kramer, E. (2023). Predicting the Base Neutralization Capacity of Soils Based on Texture, Organic Carbon and Initial pH: An Opportunity to Adjust Common Liming Recommendation Approaches to Specific Management and Climate Conditions. Agronomy, 13(11), 2762. https://doi.org/10.3390/agronomy13112762







