Effects of Three Long-Term Land Use Patterns on Soil Degradation in the Yellow River Delta: Evidence from Ecological Stoichiometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Sampling Method
2.3. Laboratory Analysis
2.4. Data Calculation
2.5. Statistical Analysis
3. Results
3.1. Effects of Different Land Use Patterns on Soil Physical and Chemical Properties
3.2. Effects of Different Land Use Patterns on Soil Stoichiometry
3.3. Effects of Different Land Use Patterns on Soil Enzyme Activity and Enzyme Stoichiometry
3.4. Factors Influencing Enzyme Stoichiometry in Three Land Use Patterns
4. Discussion
4.1. Ecological Stoichiometry Reveals the Nutrient Limitation of Three Land Use Patterns in the YRD
4.2. Response of Soil Nutrients and Their Availability to Land Use Patterns
4.3. Ecological Stoichiometry Guides Land Use Patterns in the YRD
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, C.J.; Song, J.R.; Wu, Q.; Shen, X.J.; Hu, Y.W.; Hu, C.H.; Li, W.H.; Wang, Z.H. Effects of applying river sediment with irrigation water on salinity leaching during wheat-maize rotation in the Yellow River Delta. Agric. Water Manag. 2023, 276, 108032. [Google Scholar] [CrossRef]
- Zhang, X.J.; Wang, G.Q.; Xue, B.L.; Zhang, M.X.; Tan, Z.X. Dynamic landscapes and the driving forces in the Yellow River Delta wetland region in the past four decades. Sci. Total Environ. 2021, 787, 147644. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Xia, J.B.; Yang, H.J.; Liu, J.T.; Shao, P.S. Biochar and effective microorganisms promote Sesbania cannabina growth and soil quality in the coastal saline-alkali soil of the Yellow River Delta, China. Sci. Total Environ. 2021, 756, 143801. [Google Scholar] [CrossRef]
- Li, Y.F.; Li, Q.Y.; Liu, H.S.; Li, S.J.; Wang, Y.J.; Liu, Y. Ecological stoichiometry-based study of the influence of soil saline-alkali stress on nutrient homeostasis in L. chinensis. Ecotoxicol. Environ. Saf. 2018, 165, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kang, E.; Song, B.; Wang, J.; Zhang, X.; Wang, J.; Li, M.; Yan, L.; Yan, Z.; Zhang, K.; et al. Soil salinity and nutrients availability drive patterns in bacterial community and diversity along succession gradient in the Yellow River Delta. Estuar. Coast. Shelf Sci. 2021, 262, 107621. [Google Scholar] [CrossRef]
- Tully, K.L.; Weissman, D.; Wyner, W.J.; Miller, J.; Jordan, T. Soils in transition: Saltwater intrusion alters soil chemistry in agricultural fields. Biogeochemistry 2019, 142, 339–356. [Google Scholar] [CrossRef]
- Zhai, Z.F.; Luo, M.; Yang, Y.; Liu, Y.X.; Chen, X.; Zhang, C.W.; Huang, J.F.; Chen, J. Trade-off between microbial carbon use efficiency and microbial phosphorus limitation under salinization in a tidal wetland. Catena 2022, 209, 105809. [Google Scholar] [CrossRef]
- Meng, L.; Qu, F.Z.; Bi, X.L.; Xia, J.B.; Li, Y.Z.; Wang, X.H.; Yu, J.B. Elemental stoichiometry (C, N, P) of soil in the Yellow River Delta nature reserve: Understanding N and P status of soil in the coastal estuary. Sci. Total Environ. 2021, 751, 141737. [Google Scholar] [CrossRef]
- Jin, Q.; Wang, C.; Sardans, J.; Vancov, T.; Fang, Y.Y.; Wu, L.Q.; Huang, X.T.; Gargallo-Garriga, A.; Wang, W.Q. Effect of soil degradation on the carbon concentration and retention of nitrogen and phosphorus across Chinese rice paddy fields. Catena 2022, 209, 105810. [Google Scholar] [CrossRef]
- Ma, D.L.; Huang, Q.J.; Liu, B.Z.; Zhang, Q. Analysis and Dynamic Evaluation of Eco-Environmental Quality in the Yellow River Delta from 2000 to 2020. Sustainability 2023, 15, 7835. [Google Scholar] [CrossRef]
- Muhammad, Q.; Huang, J.; Waqas, A.; Muhammad, A.; Li, D.C.; Zulqarnain, H.K.; Gao, J.S.; Liu, S.J.; Zhang, H.M. Linkages between ecoenzymatic stoichiometry and microbial community structure under long-term fertilization in paddy soil: A case study in China. Appl. Soil Ecol. 2021, 161, 103860. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, G.B.; Li, P.; Xue, S. Ecological stoichiometry of plant-soil-enzyme interactions drives secondary plant succession in the abandoned grasslands of Loess Plateau, China. Catena 2021, 202, 105302. [Google Scholar] [CrossRef]
- Shi, L.J.; Li, Q.K.; Fu, X.L.; Kou, L.; Dai, X.Q.; Wang, H.M. Foliar, root and rhizospheric soil C:N:P stoichiometries of overstory and understory species in subtropical plantations–ScienceDirect. CATENA 2020, 198, 105020. [Google Scholar] [CrossRef]
- Nannipieri, P.; Trasar-Cepeda, C.; Dick, R.P. Soil enzyme activity: A brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol. Fertil. Soils 2018, 54, 11–49. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Zeglin, L.H. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- He, Q.Q.; Wu, Y.H.; Bing, H.J.; Zhou, J.; Wang, J.P. Vegetation type rather than climate modulates the variation in soil enzyme activities and stoichiometry in subalpine forests in the eastern Tibetan Plateau. Geoderma 2020, 374, 114424. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, S. Responses of soil microbial community structure and greenhouse gas fluxes to crop rotations that include winter cover crops. Geoderma 2021, 385, 114843. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Xiao, T.H.; Hou, F.J. Different responses of soil C:N:P stoichiometry to stocking rate and nitrogen addition level in an alpine meadow on the Qinghai-Tibetan Plateau. Appl. Soil Ecol. 2021, 165, 103961. [Google Scholar] [CrossRef]
- Jiao, S.Y.; Zhang, M.; Wang, Y.M.; Liu, J.Q.; Li, Y.Q. Variation of soil nutrients and particle size under different vegetation types in the Yellow River Delta. Acta Ecol. Sin. 2014, 34, 148–153. [Google Scholar] [CrossRef]
- Jiao, S.Y.; Li, J.R.; Li, Y.Q.; Jia, J.W.; Xu, Z.Y. Soil C, N, and P distribution as affected by plant communities in the Yellow River Delta, China. PLoS ONE 2019, 14, e0226887. [Google Scholar] [CrossRef]
- Deforest, J.L. The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and l-DOPA. Soil Biol. Biochem. 2009, 41, 1180–1186. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.D.; Gao, D.X.; Wang, X.; Liu, W.C.; Deng, J.; Han, X.H.; Yang, G.H.; Feng, Y.Z.; Ren, G.; et al. Ecoenzymatic stoichiometry and nutrient dynamics along a revegetation chronosequence in the soils of abandoned land and Robinia pseudoacacia plantation on the Loess Plateau, China. Soil Biol. Biochem. 2019, 134, 1–14. [Google Scholar] [CrossRef]
- Bai, X.J.; Dippold, M.A.; An, S.S.; Wang, B.R.; Zhang, H.X.; Loeppmann, S. Extracellular enzyme activity and stoichiometry: The effect of soil microbial element limitation during leaf litter decomposition. Ecol. Indic. 2021, 121, 107200. [Google Scholar] [CrossRef]
- Guo, M.M.; Wang, W.L.; Kang, H.L.; Yang, B.; Li, J.M. Changes in soil properties and resistance to concentrated flow across a 25-year passive restoration chronosequence of grasslands on the Chinese Loess Plateau. Restor. Ecol. 2020, 28, 104–114. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Zheng, S.M.; Xia, Y.H.; Hu, Y.J.; Chen, X.B.; Rui, Y.C.; Gunina, A. Stoichiometry of carbon, nitrogen, and phosphorus in soil: Effects of agricultural land use and climate at a continental scale. Soil Tillage Res. 2021, 209, 104903. [Google Scholar] [CrossRef]
- Xi, G.; Jiang, Y.F. Spatial characteristics of ecological stoichiometry and their driving factors in farmland soils in Poyang Lake Plain, Southeast China. J. Soils Sediments 2019, 19, 263–274. [Google Scholar] [CrossRef]
- Zhang, K.; Xia, J.B.; Su, L.; Gao, F.L.; Cui, Q.; Xing, X.S.; Dong, M.M.; Li, C.R. Effects of microtopographic patterns on plant growth and soil improvement in coastal wetlands of the Yellow River Delta. Front. Plant Sci. 2023, 14, 1162013. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.C.; Xu, M.P.; Deng, J.; Han, X.H.; Yang, G.H.; Feng, Y.Z.; Ren, G.X. Response of forest growth to C:N:P stoichiometry in plants and soils during Robinia pseudoacacia afforestation on the Loess Plateau, China. Geoderma 2019, 337, 280–289. [Google Scholar] [CrossRef]
- Fei, C.; Zhang, S.R.; Feng, X.H.; Ding, X.D. Organic material with balanced C-nutrient stoichiometry and P addition could improve soil P availability with low C cost. J. Plant Nutr. Soil Sci. 2021, 184, 573–584. [Google Scholar] [CrossRef]
- Ji, L.; Tian, C.J.; Kuramae, E.E. Phosphorus-mediated succession of microbial nitrogen, carbon, and sulfur functions in rice-driven saline-alkali soil remediation. Soil Biol. Biochem. 2023, 184, 109125. [Google Scholar] [CrossRef]
- Fu, Z.Y.; Wang, P.; Sun, J.K.; Lu, Z.H.; Yang, H.J.; Liu, J.T.; Xia, J.B.; Li, T. Composition, seasonal variation, and salinization characteristics of soil salinity in the Chenier Island of the Yellow River Delta. Glob. Ecol. Conserv. 2020, 24, e01318. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, C.; Shao, S.; Li, J.; Xie, H.T.; Zhang, W.; Chen, F.S.; He, H.B.; Zhang, X.D. Interactive effects of elevated CO2 and nitrogen fertilization levels on photosynthesized carbon allocation in a temperate spring wheat and soil system. Pedosphere 2021, 31, 191–203. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Liu, Z.J.; Zhou, J.B.; Xu, X.P.; Zhu, Y.J. Long-term straw mulching with nitrogen fertilization increases nutrient and microbial determinants of soil quality in a maize–wheat rotation on China’s Loess Plateau. Sci. Total Environ. 2021, 775, 145930. [Google Scholar] [CrossRef]
- Wang, Y.B.; Wang, D.L.; Shi, B.K.; Sun, W. Differential effects of grazing, water, and nitrogen addition on soil respiration and its components in a meadow steppe. Plant Soil 2020, 447, 581–598. [Google Scholar] [CrossRef]
- Yu, P.J.; Liu, S.W.; Han, K.X.; Guan, S.C.; Zhou, D.W. Conversion of cropland to forage land and grassland increases soil labile carbon and enzyme activities in northeastern China. Agric. Ecosyst. Environ. 2017, 245, 83–91. [Google Scholar] [CrossRef]
- Topa, D.; Cara, I.G.; Jitareanu, G. Long term impact of different tillage systems on carbon pools and stocks, soil bulk density, aggregation and nutrients: A field meta-analysis. Catena 2021, 199, 105102. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, G.B.; Li, P.; Li, Q.; Xue, S. Ecoenzymatic stoichiometry and microbial nutrient limitation during secondary succession of natural grassland on the Loess Plateau, China. Soil Tillage Res. 2020, 200, 104605. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, D.W.; Denton, M.D.; Cong, S. Alfalfa monocultures promote soil organic carbon accumulation to a greater extent than perennial grass monocultures or grass-alfalfa mixtures. Ecol. Eng. 2019, 131, 53–62. [Google Scholar] [CrossRef]
- Sanginga, N. Role of biological nitrogen fixation in legume based cropping systems; a case study of West Africa farming systems. Plant Soil 2003, 252, 25–39. [Google Scholar] [CrossRef]
- Wang, L.L.; Xie, J.H.; Luo, Z.Z.; Niu, Y.N.; Coulter, J.A.; Zhang, R.Z.; Li, L.L. Forage yield, water use efficiency, and soil fertility response to alfalfa growing age in the semiarid Loess Plateau of China. Agric. Water Manag. 2021, 243, 106415. [Google Scholar] [CrossRef]
- Song, X.; Fang, C.; Yuan, Z.Q.; Li, F.M.; Sardans, J.; Penuelas, J. Long-term alfalfa (Medicago sativa L.) establishment could alleviate phosphorus limitation induced by nitrogen deposition in the carbonate soil. J. Environ. Manag. 2022, 324, 116346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.C.; Dippold, M.A.; Kuzyakov, Y.; Razavi, B.S. Spatial pattern of enzyme activities depends on root exudate composition. Soil Biol. Biochem. 2019, 133, 83–89. [Google Scholar] [CrossRef]
- Zhang, Y.; Shengzhe, E.; Wang, Y.N.; Su, S.M.; Bai, L.Y.; Wu, C.X.; Zeng, X.B. Long-term manure application enhances the stability of aggregates and aggregate-associated carbon by regulating soil physicochemical characteristics. Catena 2021, 203, 105342. [Google Scholar] [CrossRef]
- Haj-Amor, Z.; Araya, T.; Kim, D.G.; Bouri, S.; Lee, J.; Ghiloufi, W.; Yang, Y.R.; Kang, H.; Jhariya, M.K.; Banerjee, A.; et al. Soil salinity and its associated effects on soil microorganisms, greenhouse gas emissions, crop yield, biodiversity and desertification: A review. Sci. Total Environ. 2022, 843, 156946. [Google Scholar] [CrossRef] [PubMed]
- Hazra, K.K.; Nath, C.P.; Singh, U.; Praharaj, C.S.; Kumar, N.; Singh, N.P. Diversification of maize-wheat cropping system with legumes and integrated nutrient management increases soil aggregation and carbon sequestration. Geoderma 2019, 353, 308–319. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, W.J.; Jing, B. Optimizing brackish water and nitrogen application regimes for soil salinity, yield, fertilizer and water productivity of a mulched drip irrigated cotton cropping system. Field Crops Res. 2023, 302, 109097. [Google Scholar] [CrossRef]
- Kakeh, J.; Gorji, M.; Mohammadi, M.H.; Asadi, H.; Khormali, F.; Sohrabi, M. Effect of biocrusts on profile distribution of soil water content and salinity at different stages of evaporation. J. Arid Environ. 2021, 191, 104514. [Google Scholar] [CrossRef]
- Guo, R.; Bai, Z.Z.; Zhou, J.; Zhong, X.L.; Gu, F.X.; Liu, Q.; Li, H.R. Tissue physiological metabolic adaptability in young and old leaves of reed (Phragmites communis) in Songnen grassland. Plant Physiol. Biochem. 2018, 128, 99–105. [Google Scholar] [CrossRef]
- Qiu, X.C.; Peng, D.L.; Tian, H.X.; Wang, H.B.; Liu, X.; Cao, L.; Li, Z.; Cheng, S. Soil ecoenzymatic stoichiometry and microbial resource limitation driven by thinning practices and season types in Larix principis-rupprechtii plantations in North China. For. Ecol. Manag. 2021, 482, 118880. [Google Scholar] [CrossRef]
- He, H.H.; Peng, Q.; Wang, X.; Fan, C.B.; Pang, J.Y.; Lambers, H.; Zhang, X.C. Growth, morphological and physiological responses of alfalfa (Medicago sativa) to phosphorus supply in two alkaline soils. Plant Soil 2017, 416, 556–584. [Google Scholar] [CrossRef]
- Xia, J.B.; Ren, J.Y.; Zhang, S.Y.; Wang, Y.H.; Fang, Y. Forest and grass composite patterns improve the soil quality in the coastal saline-alkali land of the Yellow River Delta, China. Geoderma 2019, 349, 25–35. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Khomyakov, N.; Myachina, O.; Bogomolova, I.; Blagodatsky, S.; Kuzyakov, Y. Microbial interactions affect sources of priming induced by cellulose. Soil Biol. Biochem. 2014, 74, 39–49. [Google Scholar] [CrossRef]
- Shao, S.; Zhao, Y.; Zhang, W.; Hu, G.Q.; Xie, H.T.; Yan, J.H.; Han, S.J.; He, H.B.; Zhang, X.D. Linkage of microbial residue dynamics with soil organic carbon accumulation during subtropical forest succession. Soil Biol. Biochem. 2017, 114, 114–120. [Google Scholar] [CrossRef]
- Han, Y.W.; Jia, Y.F.; Wang, G.A.; Tan, Q.Q.; Liu, X.J.; Chen, C.J. Coupling of soil carbon and nitrogen dynamics in drylands under climate change. CATENA 2023, 221, 106735. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Rabbi, S.M.F.; Zhang, Y.Q.; Young, I.M.; Jones, A.R.; Dennis, P.G.; Menzies, N.W.; Kopittke, P.M.; Dalal, R.C. Soil organic carbon is significantly associated with the pore geometry, microbial diversity and enzyme activity of the macro-aggregates under different land uses. Sci. Total Environ. 2021, 778, 146286. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Liu, Y.L.; Hai, X.Y.; Shangguan, Z.P.; Deng, L. Dynamics of soil microbial C:N:P stoichiometry and its driving mechanisms following natural vegetation restoration after farmland abandonment. Sci. Total Environ. 2019, 693, 133613. [Google Scholar] [CrossRef] [PubMed]
- Morugán-Coronado, A.; Pérez-Rodríguez, P.; Insolia, E.; Soto-Gómez, D.; Fernández-Calviño, D.; Zornoza, R. The impact of intercropping, tillage and fertilizer type on soil and crop yield in fruit orchards under Mediterranean conditions: A meta-analysis of field studies. Agric. Ecosyst. Environ. 2020, 329, 107867. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, C.; Wang, Y.Q.; Cheng, H.; An, S.S.; Chang, S.X. Soil extracellular enzyme stoichiometry reflects the shift from P- to N-limitation of microorganisms with grassland restoration. Soil Biol. Biochem. 2020, 149, 107928. [Google Scholar] [CrossRef]
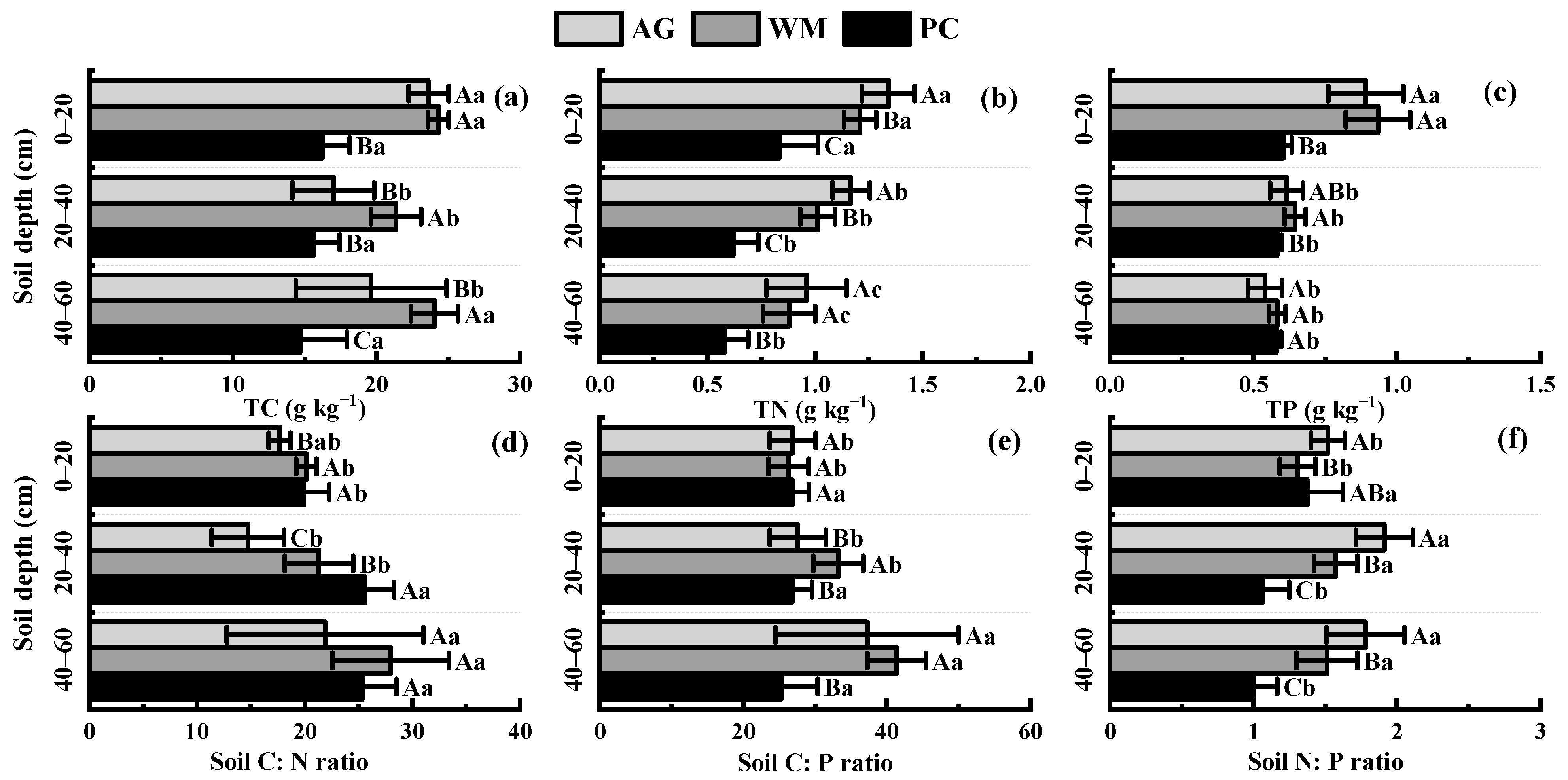


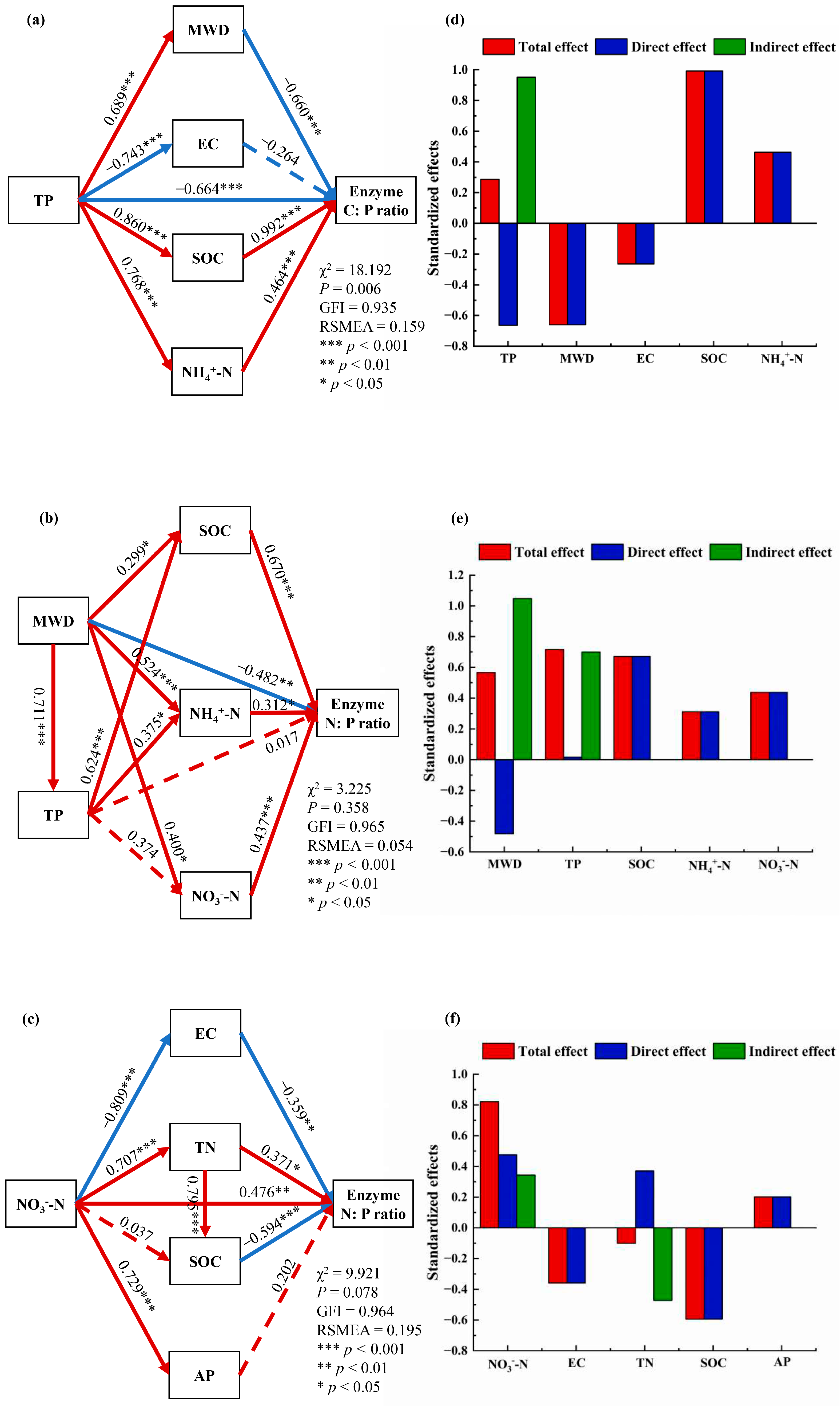
| Soil Depth (cm) | Land Use Patterns | BD (g cm−3) | Mass Fraction of WSA (%) | MWD (mm) | ||||
|---|---|---|---|---|---|---|---|---|
| >2 mm | 2–1 mm | 1–0.5 mm | 0.5–0.25 mm | <0.25 mm | ||||
| 0–20 | AG | 1.12 ± 0.10 Ba | 27.75 ± 6.57 Ab | 21.88 ± 1.51 Aa | 20.13 ± 2.47 Ba | 11.68 ± 4.45 Ba | 17.86 ± 4.33 Ba | 1.38 ± 0.19 Aa |
| WM | 1.12 ± 0.08 Ba | 12.27 ± 4.61 Ba | 16.71 ± 2.73 Bb | 26.13 ± 2.18 Aa | 20.69 ± 2.97 Aa | 24.20 ± 2.14 Ba | 0.92 ± 0.11 Bb | |
| PC | 1.46 ± 0.03 Aa | 20.88 ± 6.68 ABa | 14.71 ± 1.84 Bab | 12.87 ± 1.52 Ca | 13.48 ± 2.09 Ba | 38.07 ± 3.78 Aa | 1.04 ± 0.17 Ba | |
| 20–40 | AG | 1.12 ± 0.01 Ba | 36.48 ± 9.00 Aa | 22.33 ± 3.38 Aa | 15.40 ± 3.86 Bb | 10.44 ± 4.34 Ba | 15.36 ± 2.19 Cab | 1.60 ± 0.24 Aa |
| WM | 1.16 ± 0.02 Ba | 16.12 ± 6.55 Ba | 15.25 ± 1.61 Bb | 24.83 ± 4.43 Aa | 20.81 ± 3.84 Aa | 23.01 ± 3.24 Ba | 1.01 ± 0.18 Bab | |
| PC | 1.43 ± 0.02 Ab | 19.62 ± 3.73 Ba | 12.50 ± 1.99 Bb | 14.10 ± 1.77 Ba | 13.26 ± 4.22 ABa | 40.52 ± 2.10 Aa | 0.98 ± 0.10 Ba | |
| 40–60 | AG | 1.10 ± 0.04 Ba | 33.18 ± 9.61 Aab | 21.33 ± 2.90 Aa | 19.07 ± 5.82 Bab | 12.36 ± 3.02 Aa | 14.06 ± 3.00 Bb | 1.52 ± 0.26 Aa |
| WM | 1.11 ± 0.03 Ba | 15.76 ± 5.00 Ba | 22.13 ± 4.32 Aa | 27.78 ± 3.19 Aa | 16.81 ± 2.33 Ab | 17.51 ± 2.52 Bb | 1.10 ± 0.11 Ba | |
| PC | 1.47 ± 0.01 Aa | 18.35 ± 4.68 Ba | 16.25 ± 4.32 Aa | 14.65 ± 3.12 Ba | 14.83 ± 0.69 Aa | 35.92 ± 7.03 Aa | 1.00 ± 0.14 Ba | |
| Soil Depth (cm) | Land Use Patterns | pH | EC (μs cm−1) | SOC (g kg−1) | NH4+-N (mg kg−1) | NO3−-N (mg kg−1) | AP (mg kg−1) |
|---|---|---|---|---|---|---|---|
| 0–20 | AG | 8.51 ± 0.08 Aa | 343.11 ± 84.83 ABb | 11.13 ± 1.29 Aa | 13.12 ± 0.96 Aa | 28.57 ± 3.10 Aa | 32.27 ± 10.51 Aa |
| WM | 8.52 ± 0.15 Aa | 428.12 ± 104.06 Ac | 9.98 ± 0.75 Aa | 12.21 ± 1.34 Aa | 24.83 ± 1.13 Ba | 27.95 ± 10.46 Aa | |
| PC | 8.37 ± 0.13 Bb | 297.65 ± 43.79 Bc | 6.47 ± 1.30 Ba | 5.22 ± 0.32 Ba | 10.75 ± 0.35 Ca | 6.85 ± 2.48 Ba | |
| 20–40 | AG | 8.53 ± 0.12 Aa | 479.02 ± 115.97 Aa | 9.23 ± 1.02 Ab | 11.21 ± 1.03 Ab | 23.59 ± 3.07 Ab | 19.97 ± 8.48 Ab |
| WM | 8.56 ± 0.06 Aa | 511.89 ± 73.86 Ab | 7.57 ± 0.66 Ab | 10.23 ± 0.37 Bb | 20.96 ± 3.33 Ab | 17.15 ± 7.00 Ab | |
| PC | 8.64 ± 0.11 Aa | 425.34 ± 72.49 Ab | 4.64 ± 1.06 Bb | 4.63 ± 0.68 Cb | 7.60 ± 0.25 Bb | 3.60 ± 0.71 Bb | |
| 40–60 | AG | 8.56 ± 0.06 Aba | 535.49 ± 43.56 Ba | 7.50 ± 1.35 Ac | 9.67 ± 0.57 Ac | 17.12 ± 0.65 Bc | 10.44 ± 3.28 Ac |
| WM | 8.50 ± 0.07 Ba | 619.75 ± 75.88 Aa | 6.11 ± 0.89 Ac | 8.53 ± 0.33 Bc | 18.75 ± 1.67 Ac | 9.04 ± 2.92 Ac | |
| PC | 8.64 ± 0.13 Aa | 561.75 ± 88.78 ABa | 4.89 ± 0.61 Bb | 3.65 ± 0.71 Cc | 6.35 ± 0.35 Cc | 3.13 ± 0.50 Bb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, B.; Zhu, T.; Ming, Y.; Jia, S.; Li, C.; Wang, F.; Dong, Z.; Jiao, S.; Li, Y.; Shi, L. Effects of Three Long-Term Land Use Patterns on Soil Degradation in the Yellow River Delta: Evidence from Ecological Stoichiometry. Agronomy 2023, 13, 2744. https://doi.org/10.3390/agronomy13112744
Kong B, Zhu T, Ming Y, Jia S, Li C, Wang F, Dong Z, Jiao S, Li Y, Shi L. Effects of Three Long-Term Land Use Patterns on Soil Degradation in the Yellow River Delta: Evidence from Ecological Stoichiometry. Agronomy. 2023; 13(11):2744. https://doi.org/10.3390/agronomy13112744
Chicago/Turabian StyleKong, Baishu, Taochuan Zhu, Yufei Ming, Sen Jia, Chuanrong Li, Fenghua Wang, Zhi Dong, Shuying Jiao, Yongqiang Li, and Lianhui Shi. 2023. "Effects of Three Long-Term Land Use Patterns on Soil Degradation in the Yellow River Delta: Evidence from Ecological Stoichiometry" Agronomy 13, no. 11: 2744. https://doi.org/10.3390/agronomy13112744
APA StyleKong, B., Zhu, T., Ming, Y., Jia, S., Li, C., Wang, F., Dong, Z., Jiao, S., Li, Y., & Shi, L. (2023). Effects of Three Long-Term Land Use Patterns on Soil Degradation in the Yellow River Delta: Evidence from Ecological Stoichiometry. Agronomy, 13(11), 2744. https://doi.org/10.3390/agronomy13112744






