Abstract
The pear pest, Cacopsylla jukyungi (Hemiptera: Psyllidae), is the most damaging insect to commercial pears in South Korea. An assessment of the population genetic characteristics of the species will raise the potential of effective control. In the present study, we developed eight microsatellite markers specific to C. jukyungi and genotyped 132 individuals collected from 11 localities throughout South Korea. Populations showed lower observed heterozygosity than expected heterozygosity and slightly or highly positive values of inbreeding coefficients, suggesting that C. jukyungi is subjected to inbreeding. A strong dependence on commercial pear trees throughout the whole life cycle, even during overwintering, and a nationwide targeting of the overwintered adults are likely contributors to such heterozygosity deficiency. On the other hand, population analyses consistently indicated strong gene flow among populations, implying the presence of persistent factors that have facilitated this process. The nationwide expansion of pear orchards and the replacement with a popular new cultivar during the last 50 years, which may have accompanied the spread of C. jukyungi-bearing pear grafts and scions, are likely causes of such facilitated dispersal. Thus, a management strategy against unintended anthropogenic dispersal of the pear psyllid will be required for better control of C. jukyungi.
1. Introduction
Pears rank fifth in production quantity and first in export quantity among fruits cultured in South Korea [1]. Cacopsylla jukyungi (Kwon, 1983) (Hemiptera: Psyllidae) [2] is the most damaging insect pest to commercial pears [3,4]. The species was once misidentified as C. pyricola (Foerster, 1848) [3,5,6] until Cho et al. [7] corrected psyllid taxonomy. C. jukyungi, which undergoes 4–5 generations a year [3,4] displays seasonal dimorphism, converting from a light-colored summer form to a dark overwintering winter form in late summer and autumn when the photoperiod shortens [8]. In the late fall, C. jukyungi winter form adults move underneath coarse bark scales closer to the bottom of pear trees for overwintering, and during February and March of the subsequent year, the winter forms move upward to tree branches when daily maximum air temperature increases to a certain level [3,4,9]. Right after the upward movement, the insects mate and females deposit eggs on flower buds, leaf buds, and crevices on and around flowers and leaf buds, and then year-round outbreak starts if not properly controlled (Figure 1) [3,4,10]. Both nymphs and adults damage leaves and fruits by sucking the sap and by secreting honeydew that causes sooty mold, marks the fruits, and inhibits photosynthesis [11].

Figure 1.
Cacopsylla jukyungi at different stages. (A) Overwintering winter-form adults underneath bark scale (after removing bark scale covering C. jukyungi); (B) winter-form adults on the branch; (C) winter-form adults mating; (D) eggs on flower bud; (E) eggs on crevice around flower bud; (F) nymphs on twigs; and (G) summer-form adults.
The most effective control method that is recommended and practiced nationwide is spraying commercial machine oil emulsion when overwintered C. jukyungi adults have fully climbed up to the branches from the overwintering places and their eggs start to hatch [3,4,9]. Additional sprays also are administered depending on the severity of the outbreak, but these two periods are the most extensively practiced against C. jukyungi nationwide [11].
Despite the species being the most damaging insect pest to commercial pears, little is known about its behavioral characteristics related to habitats and pattern of dispersal on a regional scale, which could be aided by molecular data in terms of genetic diversity, population genetic structure, and gene flow. Previously, diverse aspects of population genetics of several species of Cacopsylla Ossiannilsson, 1970, found in other countries have been studied using co-dominant microsatellite DNA. These include marker development for C. melanoneura (Foerster, 1848) [12], understanding genetic differentiation, gene flow, and allocation of genetic diversity in C. chinensis (Yang and Li, 1981) [13], host plant-dependent population differentiation in C. melanoneura [14], detection of biotypes in C. pruni (Scopoli, 1763) [15], and detection of lower heterozygosity in C. pruni [16], but no such study has been conducted in C. jukyungi.
In this study, we developed eight microsatellite markers specific to C. jukyungi. The markers were used to genotype 11 C. jukyungi populations (132 individuals) in South Korea and gain an understanding of the insect’s genetic diversity, inbreeding, gene flow, and population structure.
2. Materials and Methods
2.1. Sampling and DNA Extraction
One-hundred and thirty-two C. jukyungi adults were sampled throughout 11 South Korean localities from October 2011 to March 2022 (Figure 2; Table 1). Each sample was collected at a distance of at least 3 m to minimize the chance of capturing littermates. Field-collected individuals were subjected to morphological examination following Cho et al. [7]. Total DNA was extracted from −70 °C-stored whole body, excluding the midgut, using a Wizard Genomic DNA Purification Kit following the manufacturer’s instructions (Promega, WI, USA) and was stored at −20 °C until molecular analysis. For species identification, a DNA barcode region (658 bp of the mitochondrial COI gene) was sequenced in all samples, and the estimated sequence divergence was determined following Kang et al. [6] (Tables S1 and S2). Furthermore, phylogenetic analysis using the 658 bp of COI was performed with the neighbor-joining method with the Kimura 2-parameter option [17] using MEGA ver. 11.09 [18]. For this, all haplotypes of C. jukyungi from the current study and public databases, along with several species of Cacopsylla, were included to confirm the species identity of C. jukyungi samples (Figure S1). To root the tree, two intrafamilial species of Acizzia (Heslop-Harrison, 1961) were utilized as outgroups. The reliability of the trees was tested with 1000 bootstrapping iterations [19]. Comparison of the seven haplotypes obtained in this study and the two haplotypes used for the identification of C. jukyungi by Cho et al. [7] showed that the sequence diverged only 0–2 bp (0–0.3%) (Table S2), and phylogenetic analysis confirmed that all C. jukyungi haplotypes form a single group with the highest nodal support, separate from other species of Cacopsylla (Figure S1).
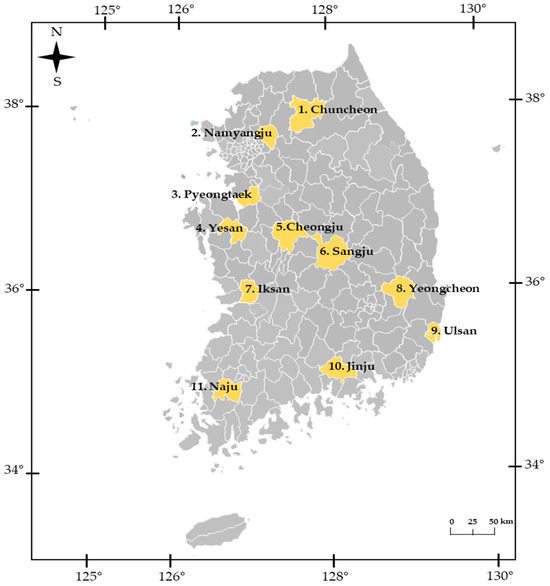
Figure 2.
Sampling locations of Cacopsylla jukyungi in South Korea. General locality names are provided in the map. See Table 1 for site details.

Table 1.
Cacopsylla jukyungi samples used in this study.
2.2. Next-Generation Sequencing
For the construction of a DNA library, DNA was extracted from one specimen collected at Daap-myeon, Gwangyang City, Jeollanamdo Province, South Korea (35°04′00.5″ N, 127°44′02.5″ E) in April 2021. This specimen was also previously used to obtain the mitochondrial genome sequence of the species (GenBank accession no. ON553958) [20]. Leftover specimens and DNA were deposited at Chonnam National University with the accession number CNU15412.
DNA quality and concentration were assessed using a NanoDrop Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Approximately 50 ng of genomic DNA was sheared into fragments of approximately 300 bp using a Covaris S220 ultrasonicator (Covaris, Woburn, MA, USA) and then processed to produce a paired-end library using a TruSeq Nano DNA Library Kit, following the manufacturer’s instructions (Illumina, San Diego, CA, USA). The size and concentration of the prepared library were confirmed using an Agilent 2100 Bioanalyzer system (Agilent Technologies, Santa Clara, CA, USA), and the library was quantified by qPCR using the CFX96 Real-Time System (BioRad, Hercules, CA, USA). The library was sequenced on an MGISEQ2000 platform (MGI Tech, Shenzhen, China) using 150 bp paired-end reads, resulting in 266,128,190 reads (Table S3). After sequencing errors were discarded using the error correction module of ALLPATHS-LG [21], genome assembly was performed using IDBA_UD with the pre-correction option [22]. Reliability of assembly was further confirmed by remapping the short reads to the assembled sequences using Bowtie2 [23]; only assembled scaffolds with an average depth > 10× were retained for microsatellite marker identification, resulting in 967,146 scaffolds with an average length of 904 bp (Table S3). Scaffolds were then converted into a single FASTQ format file; the microsatellite sequences, consisting of mono- to hexa-nucleotides, were searched using the Msatcommander program [24], resulting in 630,655 microsatellite motives (Table S4). Excluding mono-nucleotide repeats (48.8%), tri-nucleotide repeats were the most abundant class of microsatellites (21.76%, 137,256 regions) detected in the C. jukyungi genome, followed by di-nucleotides (16.66%, 105,092 regions), tetra-nucleotides (10.03%, 63,228 regions), penta-nucleotides (1.7%, 10,728 regions), and hexa-nucleotides (1.05%, 6596 regions) (Table S4). Sequencing data were deposited in the Sequence Read Archive of GenBank with the accession number SRX16308894.
2.3. Development of Microsatellite Markers and Genotyping
Primers were designed using Primer 3 [25] from over 30 randomly selected candidate microsatellites for preliminary screening. Of these, 11 were eventually selected after a preliminary screening, which considered amplification efficiency, the degree of polymorphism, and specificity for target loci (Table 2). PCR was carried out using 25 μL reaction volumes with a reaction mixture containing 30 ng of DNA, 1 × PCR buffer [50 mM KCl, 10 mM Tris–HCl (pH 8.8), and 1.5 mM MgCl2], 2.5 mM dNTPs, 200 nM of each primer, and 1 unit of Pure Speed PFU DNA polymerase (Smarteome, Seoul, Republic of Korea). One of the primer pairs for each locus was labeled with 6-carboxyfluorescein fluorescent dye (Gencube, Seoul, Republic of Korea) [26]. PCR amplification was performed using the following conditions: an initial denaturation step at 95 °C for 3 min, 30 amplification cycles (denaturation at 94 °C for 30 s, annealing at 47–48 °C for 30 s, and extension at 72 °C for 1 min), and a final extension step at 72 °C for 5 min using AccuPower® PCR PreMix (Bioneer, Daejeon, Republic of Korea) in an ABI 2720 Thermocycler (PE Applied Biosystems, Foster City, CA, USA). Aliquots (0.2 μL) of the amplified PCR products were mixed with 9.8 μL of Hi-Di Formamide (PE Applied Biosystems, Carlsbad, CA, USA) and 0.2 μL of Liz-500 size standards (PE Applied Biosystems). The mixture was then denatured at 95 °C for 5 min, placed on ice, and separated in an ABI 3730xl sequencer (PE Applied Biosystems). GeneMapper Software ver. 4.1 (PE Applied Biosystems) was used to analyze the allele sizes and genotypes. Leftover DNA was deposited at Chonnam National University with accession numbers (=sample numbers) shown in Table 1.

Table 2.
Eleven microsatellite loci identified in Cacopsylla jukyungi.
2.4. Microsatellite Data Analysis
To validate the markers, the number of alleles (a), observed heterozygosity (HO) [27], and expected heterozygosity (HE) [27] were calculated for each locus using GenAlEx ver. 6.5 [28]. In addition, this software was used to determine FIS [29], a measure of the deficiency in heterozygosity resulting from non-random mating, for each locus. Polymorphic information content (PIC) [30] was calculated using FSTAT ver. 2.9.3 [31].
Micro-Checker ver.2.2.3 [32] was used to evaluate the microsatellite data by identifying genotyping errors, allelic dropouts, and the presence of null alleles. Frequencies of null alleles (Nu) at each locus were estimated in FreeNa [33] using the Expectation Maximization (EM) algorithm [34] with 10,000 bootstrap replicates. Mean and standard error (SE) of Weir and Cockerham’s F-statistics were calculated using the jackknife method over all loci and delete-1 loci, and a 95% confidence interval (CI) was generated by bootstrapping with 10,000 replications using FSTAT ver. 2.9.3 [31]. Among eleven loci, Micro-Checker flagged eight loci to have ‘true’ null alleles (p < 0.05; Table S5), with the estimates of null allele frequency ranging between 0.05845 (ID-9837) and 0.263988 (ID-2165) (Table S6). Among them, three loci (ID-753, ID-2165, and ID-8889) had a null allele frequency higher than 0.2 (0.225664 in ID-753, 0.263988 in ID-2165, and 0.232819 in ID-8889) and were removed from subsequent analysis (Table S6). The remaining eight loci with null allele frequency lower than 0.2 (0.007244–0.156898) were retained owing to their small influence on the average genetic diversity and structure. Indeed, jackknife analysis revealed no outliers outside of the 95% CI in these eight loci (Table S7). The GenBank accession numbers of these eight loci are provided in Table 3.

Table 3.
Characteristics of eight microsatellite loci in Cacopsylla jukyungi.
Genotypic linkage disequilibrium (LD) between all pairs of loci, as well as the deviation of genotypic frequencies from the Hardy–Weinberg equilibrium (HWE) per locus, were tested using GENEPOP Web ver. 4.2 [35,36], with the Markov chain approach modified from Guo and Thompson [37] using 10,000 steps of dememorization and iteration. The 95% significance levels for HWE and LD tests were adjusted using a Bonferroni correction [38].
Genetic diversity within the population was measured as the number of alleles, the number of private alleles, HO, and HE using GenAlEx ver. 6.5 [28]. Pairwise estimate of the fixation index FST [39] was calculated to measure the degree of correlation between population pairs based on the infinite allele model of mutation using Arlequin ver. 3.5 [40]. In addition, this software was used to calculate the significance of the FST using a Fisher’s exact test based on 10,000 permutations.
Principal coordinates analysis (PCoA) was performed via covariance with standardization of the genetic distances to detect and plot the relationships among populations and among individuals using GenAlEx ver. 6.5 with default parameters [28]. STRUCTURE ver. 2.3.1 [41] was used to assess the most probable number of genetically distinct clusters (K) in the populations. An admixture model with correlated allele frequencies was used, with the K-value ranging from 1 to 10. For each K-value, 10 independent runs were performed with a burn-in period of 10,000 iterations, followed by 500,000 iterations for data collection. The structure result was visualized using the web-based tool STRUCTURE HARVESTER ver. 0.6.94 [42].
To test for correlation between geographic and genetic distances, a Mantel test [43] using isolation by distance (IBD), which compares the matrices of pairwise genetic distance [FST/(1 − FST)] and the logarithms of geographical distance data (ln km), was performed, with the significance test conducted over 10,000 randomizations [43]. The analysis was conducted using the IBD software package ver 1.52, with the negative genetic distance set to zero [44].
The software BOTTLENECK ver. 1.2 [45,46] was used to detect whether there was a recent reduction in effective population size. Populations that have experienced a significant reduction in size are expected to have excess heterozygosity because rare alleles are lost [45]. We tested for a significant departure from mutation–drift equilibrium with an excess of heterozygotes under three different models of microsatellite mutation: the infinite allele model (IAM), the stepwise mutation model (SMM), and the two-phase model (TPM) based on 10,000 iterations. The TPM incorporates elements of SMM and IAM (variance = 12, SMM = 70%). We evaluated the significance of sign tests and the Wilcoxon signed-rank test, the latter being the most appropriate test when fewer than 20 microsatellite loci are used [46]. In addition, a mode-shift distortion test to determine the allele frequency distribution was performed. A population that has not experienced a bottleneck is expected to show an L-shaped distribution (many low-frequency alleles and few high-frequency alleles), whereas bottlenecked populations exhibit a mode shift [47].
3. Results
3.1. Characteristics of Microsatellite Loci
The eight microsatellite markers contained either di- or tri-nucleotide repeat motifs, with the expected size ranging from 162 bp (locus ID-10656) to 226 bp (locus ID-8470), including the primer sequences (Table 2). The eight microsatellite markers showed high genotyping success, ranging from 0.795 (ID-3900) to 0.992 (ID-10656), with an average availability of 0.927 (Table 3). For each locus, the allele numbers ranged from 9.0 (ID-8470, ID-10656, and ID-10751) to 30 (ID-9837), with a mean value of 17.5. The major allele frequency ranged from 0.109 (ID-9837) to 0.686 (ID-5968), with a mean value of 0.368. The number of genotypes ranged from 16 (ID-8470) to 72 (ID-10585), with an average of 36.25. The PIC results showed that seven loci were highly informative (PIC > 0.5), and one locus was moderately informative (0.25 < PIC < 0.5). HO and HE ranged from 0.264 to 0.882 (mean = 0.648) and 0.504 to 0.940 (mean = 0.774), respectively, and seven loci, excluding ID-10656, showed higher HE than HO. A positive FIS value, which indicates heterozygote deficiency, was observed in most loci, except for ID-10656 at −0.063. No locus deviated from HWE after applying the Bonferroni correction (p = 0.05/8 ≤ 0.005). The tests for genotypic LD showed no significant allele associations among the eight loci after applying the Bonferroni correction, suggesting that all loci can be considered independent markers.
3.2. Genetic Diversity
The allelic patterns across populations showed no obvious difference overall (Figure 3, Table S8). The number of alleles (Na) per population ranged from 6.75 (standard error (SE) = 0.881; locality 5, Cheongju) to 8.375 (SE = 1.295; locality 1, Chuncheon). The Na did not significantly vary among populations when SE was considered. The number of effective alleles (Ne) ranged from 4.106 (SE = 0.731; locality 3, Pyeongtaek) to 5.451 (SE = 1.138; Chuncheon), without statistical significance. The Chuncheon population had the highest Na and Ne. The number of private alleles, which corresponded to the number of alleles unique to a single population, ranged from 0.125 (SE = 0.125; Pyeongtaek and Cheongju) to 1.0 (SE = 0.378; locality 7, Iksan), without statistical significance, suggesting that population-specific alleles are uncommon. The within-population gene diversity, which corresponds to HE in the diploid data, ranged from 0.667 (SE = 0.065; locality 4, Yesan) to 0.767 (SE = 0.037; Chuncheon), with a mean value of 0.721 and without statistical significance among populations when SE was considered. Similarly, HO did not show significant differences among populations, ranging from 0.546 (SE = 0.077; Yesan) to 0.730 (SE = 0.078; Chuncheon), with a mean value of 0.649. All populations showed higher HE than HO values, although the difference was not significant when SE was considered. All populations showed positive FIS, ranging from 0.011 (SE = 0.059; Cheongju) to 0.243 (SE = 0.112; locality 9, Ulsan), indicating that they were subjected to inbreeding. The FIS values were not substantially different among populations when SE was considered.
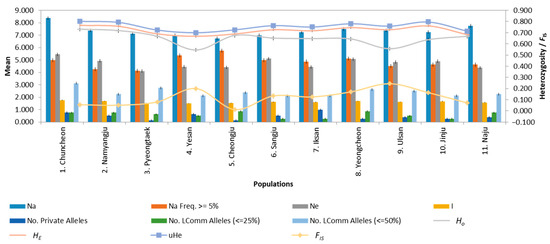
Figure 3.
Allelic patterns across 11 populations of Cacopsylla jukyungi. Na, number of different alleles; Na (Freq. ≥ 5%), number of alleles with frequency greater than 5%; Ne, number of effective alleles; I, Shannon’s Information Index; No. Private Alleles, number of alleles unique to a single population; No. LComm Alleles (≤25%), number of locally common alleles occurring in 25% or less of the populations; No. LComm Alleles (≤50%), number of locally common alleles occurring in 50% or less of the populations; HE, expected heterozygosity; HO, observed heterozygosity; and FIS, inbreeding coefficient. Vertical bars represent the standard error.
3.3. Bottleneck Test
The results of BOTTLENECK tests, which are used to detect a recent population decline, showed a significant excess of heterozygosity in populations 3 and 1 under the assumption of the SMM and TPM models, respectively, in the sign test (Table 4). In the Wilcoxon signed-rank test, arguably the most appropriate test for current analysis, which used fewer than 20 microsatellite loci [46], three populations (Namyangju, Yeongcheon, and Jinju) showed a significant excess of heterozygosity only under the IAM model. As a second method to detect potential bottlenecks, the mode-shift distortion test showed all populations to have a normal L-shaped distribution of allele frequency, suggesting an absence of recent population bottlenecks. Collectively, depending on the assumed models, the results showed that a recent bottleneck occurred in some populations, but none of the populations simultaneously displayed a significant excess of heterozygosity, particularly under different models of the Wilcoxon signed-rank test and the mode-shift distortion test.

Table 4.
Probability results of BOTTLENECK analysis of Cacopsylla jukyungi populations using different models.
3.4. Population Genetic Analyses
FST analysis of C. jukyungi populations showed a total of 18 statistically significant estimates among 55 comparisons at the level of p < 0.05 or p < 0.01, but no population pair showed a significance level of p < 0.001 (Table 5). Namyangju (locality 2) had the highest number of populations that were significantly divergent at ten populations, and other populations ranged from one to six. The only population that showed no significant divergence from those in Namyangju was geographically the closest Chuncheon. Thus, genetic distance appears to reflect geographic distance in this case. However, other population pairs at varying geographic distances showed a jagged genetic distance inconsistent with geographic distance. For example, Chuncheon and Cheongju populations, which are ~140 km apart, had a statistically significant genetic distance (FST = 0.06120; p < 0.05), whereas Chuncheon population and Jinju population (locality 10), which are ~300 km apart, showed no statistically significant genetic distance (FST = 0.01695; p > 0.05). Similarly, Ulsan and Jinju populations, which are ~50 km apart, had a statistically significant genetic distance (FST = 0.05357; p < 0.05), whereas Ulsan and Pyeongtaek populations, which are ~250 km apart, did not show any statistically significant genetic distance (FST = 0.02343; p > 0.05). IBD analysis results support a weak isolation by distance, providing only a marginally significant association and a very low coefficient of determination (r2 = 0.082; p = 0.047; Figure 4).

Table 5.
Fixation indices (FST) between pairs of populations of Cacopsylla jukyungi.
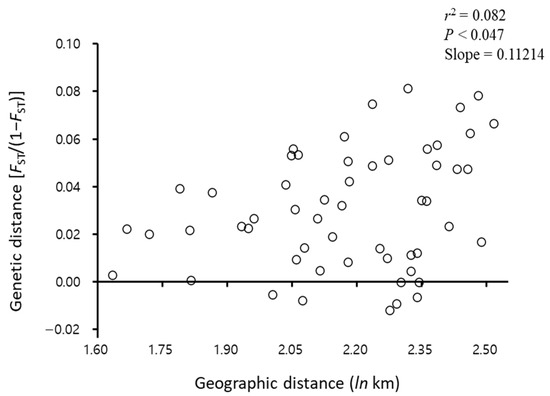
Figure 4.
Isolation by distance accessed by plotting FST/1 − FST against geographic distance (ln), along with corresponding coefficient of determination (r2), p value, and slope of the regression line using eight microsatellite loci.
The PCoA results showed the genetic relationships among individuals and populations (Figure 5). The first two principal coordinates of individual genotypes explained only 13.27% of the total genetic variance (6.93% and 6.34%, respectively; Figure 5A). Most individuals had overlapped and clustered positions in relation to each other, excluding only a few individuals that were located at each margin of the first or second coordinates, indicating that most individuals of C. jukyungi are genetically related to each other. In contrast, population-based analysis showed a more distinct separation, dividing the eleven populations roughly into two main groups (seven in the left group and four in the right group), based on the first principal coordinate, which accounted for 43.53% (Figure 5B). However, this division appears not to be decisive considering substantially larger within-group distances (Figure 5B). The second component, which accounted for 27.35% of the variation, did not clearly divide populations into any discernable groups, although a couple of populations (e.g., Namyangju and Yesan) were placed at the margins by the second component. Collectively, PCoA analysis did not provide a clear pattern of separation in terms of individuals and populations.
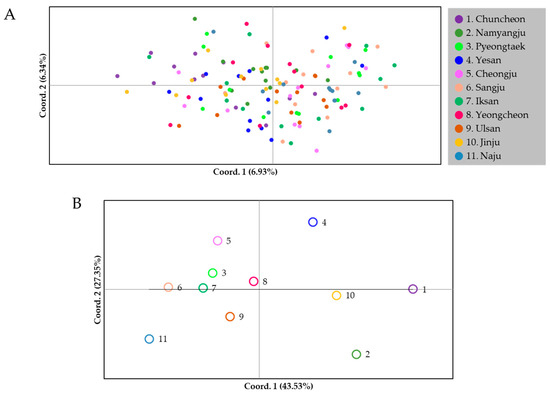
Figure 5.
Results of the principal coordinate analysis (PCoA) depicting allelic variance in 132 individuals from 11 populations. (A) Individual-based analysis. (B) Population-based analysis. The percentages variation explained by the 1st and 2nd axes are indicated.
To investigate the population structure and admixture patterns among the C. jukyungi populations, STRUCTURE analysis was performed (Figure 6). An examination of the likelihood scores from 10 replicate runs across K-values from 1 to 10 suggested K = 5 as the most likely number of distinct gene pools (Figure 6A). At K = 5, all populations consistently possessed the five gene pools, and no population had an independent gene pool (Figure 6B). Although no notable difference in gene pool frequency among populations was detectable upon visual inspection, Namyangju and Chuncheon at K = 5 showed a slightly higher frequency of the yellow gene pool. At a suboptimal K = 4 and 6, all populations still consistently had four or six gene pools, respectively, and no population had an independent gene pool (Figure 6B). The only detectable difference was the higher frequency of the yellow gene pool in the Namyangju and Chuncheon populations at K = 5. In the FST analysis, the former population had the highest number of statistically divergent populations, and the latter was the only population that did not show statistically significant divergence from the former (Table 5). The results of STRUCTURE analysis, FST, and PCoA (Table 5, Figure 5 and Figure 6) collectively suggest that there is some genetic differentiation among populations, but no population is completely different or isolated, and gene flow among populations is substantial. Moreover, the presence of IBD, which is observed when local genetic drift surpasses gene flow, was only marginally supported (p < 0.047), with a notably lower coefficient of determination (r2 = 0.082; Figure 4), suggesting that some factors facilitated rather prevented gene flow.
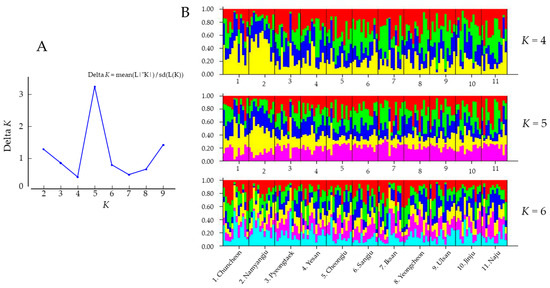
Figure 6.
Clustering analysis of multilocus microsatellite data of Cacopsylla jukyungi performed using STRUCTURE software. (A), plot of Delta K calculated with the formula Delta K = mean (|L″(K)|)/sd(L(K)), n = 132. (B), bar plots of estimated membership of each individual in K = 4, 5, and 6 clusters. Black bars separate the 11 populations. Different colors represent different gene pools.
4. Discussion
Our microsatellite analyses of C. jukyungi populations in South Korea showed somewhat incompatible results among analyses, particularly considering the year-round dependence of the species on commercial pear trees as the sole host. Thus, a careful inspection of the ecological characteristics of C. jukyungi and the nationwide expansion history of pear orchards may be needed for a comprehensive understating of the population genetic data and the rampant spread of C. jukyungi. Specifically, all populations showed higher HE than HO and positive values of the inbreeding coefficients (Figure 3, Table S8), which can be interpreted as a certain level of inbreeding occurring in C. jukyungi populations [48]. In contrast, FST, IBD, PCoA, and STRUCTURE (Figure 4, Figure 5 and Figure 6, Table 5) suggest a substantial level of gene flow among populations.
4.1. Higher HE and Positive FIS in All Populations
We attribute inbreeding to the year-round dependency on the host plant and pest control practices against C. jukyungi. The adult winter form of C. jukyungi overwinters mainly under the bark scale of pear trees from late fall; adults start the upward movement to branches mostly during February and March; and mating and egg deposits occur right after reaching the upper fruiting twigs [3,4]. Once eggs are laid, year-round occurrence continues to produce 4–5 generations in a year, causing damage to leave and fruits until overwintering (Figure 1) [3,4,11]. Thus, C. jukyungi spends its whole life cycle of feeding, development, reproduction, and overwintering from eggs to adults on commercial pear trees as the sole host [3,4]. The species may therefore have a high chance of mating closer to the natal sites, not looking for available mates by dispersal to distant pear orchards or to other hosts.
The year-round pervasiveness of the pear psyllid is highly dependent on the density of overwintered populations and their eggs in early spring; thus one of the first and major control strategies focuses on the control of overwintered adults [11]. For this, a prediction model, which incorporates ecological data and effective accumulated temperature, has been developed to forecast the date of upward movement on the pear tree from overwintering places [3]. According to the model, upward movement is completed when the daily maximum air temperature higher than 6 °C reaches 16–21 days from February 1st [3]. Thus, this is the recommended period for machine oil spraying nationwide, which blocks the spiracle of C. jukyungi directly by spraying on the body or indirectly by contact with leftover oil emulsion on the tree for pear psyllid control. The second recommended control period based on the prediction model is when ~50% of eggs are hatched, which corresponds to approximately one week after the first control practice [11]. These two periods are relatively well practiced nationwide. Thus, such control practices targeting overwintered adults and their eggs should substantially reduce mating choice.
Similar to the results of the current study, microsatellite DNA analyses of other Cacopsylla species often shows a higher HE than HO, albeit the proposed explanation varies among studies and the ecology of Cacopsylla differs in some respects from that of C. jukyungi. For example, the population genetic structure of C. chinensis [49], which also severely damages commercial pears in China, has been studied using seven microsatellite loci in 16 Chinese populations [13]. The study showed substantially higher HE than HO (0.5646–0.9018 vs. 0.0940–0.2534) and positive FIS values in all loci [14]. C. chinensis, which is oligophagous, including pears, produces six to seven generations per year on pear trees, but returns to these trees in the spring after overwintering in other host plants [50]. Thus, this species may have more mating choices than C. jukyungi, which spends its whole life cycle only on commercial pears; however, C. chinensis shows a higher HE than HO and positive FIS estimates [13]. Cacopsylla melanoneura is found in Europe as a univoltine; it moves to the overwintering host plants around mid-June, and overwintered adults reach apple and hawthorn plants around the end of January in northern Italy [51]. The species also has a heterozygote deficit and positive FIS values in most samples, and the results are explained by the combined effect of habit specificity and control practice, rather than being a result of strong null allele pervasiveness [14]. These results are similar to those of the current study, even though C. melanoneura differs from C. jukyungi, having overwintering host plants, along with more than one feeding host. C. pruni, which is widespread in Europe, the Caucasus, and Siberia, and is univoltine [52], develops on several species of Prunus (Linnaeus, 1737); the main host is blackthorn P. spinosa (Linnaeus, 1753), it overwinters on conifers at an altitude of 700–1300 m, and it returns to the plains via long-distance migration [53]. Allelic variability assessed in a collection of 149 females obtained from five localities covering a large geographical area in France using nine microsatellite markers showed a significant deficit in heterozygotes for most loci (HE = 0.68–0.81 vs. HO = 0.39–0.55) [16]. However, this result was interpreted mainly as a result of true null alleles found in all localities for almost all loci (p < 0.05), with estimates of null allele frequency ranging between 0.05 and 0.40.
Considering the interpretation of C. melanoneura results [16], we cannot completely exclude the possibility that the observed heterozygote deficit and positive FIS values in our data may have been caused by null alleles. However, a null allele frequency of <0.2 is often used considering its small influence on diversity and structures [33,54,55]. Moreover, five of the eight loci selected had a null allele frequency lower than 0.1 (0.007244 to 0.156898; Table S6), and no locus had an outlier outside the 95% CI in jackknife analysis (Table S7), even when the three loci discarded because of a null allele frequency higher than 0.2 were included (Table S6). Thus, the positive FIS and higher HE observed are highly unlikely to be caused solely by null alleles. Instead, the high degree of dependence of C. jukyungi on commercial pears and the nationwide control practices right after its upward movement to tree branches from overwintering places could be the main factors underlying the observed allele characteristics. Indeed, the control practice based on temperature and life stage congruency has been the recommended procedure nationwide for more than a decade and is perceived as an effective control strategy [11].
4.2. Overall Absence of Population Structure
It has often been suggested that inbreeding is a cause of decreased growth rate, fertility, fecundity, and offspring viability [56]. If the reduction in such population characteristics is sufficiently severe, limited gene flow and a consequent population structure is expected to be observed in isolated small populations [57,58]. For C. jukyungi, inbreeding should be particularly evident considering its whole life stage dependence on commercial pears as the sole host plant and the control practice targeting the initial overwintered populations, which are the sole source of year-round growth.
Nevertheless, FST, PCoA, STRUCTURE, and IBD (Figure 4, Figure 5 and Figure 6, Table 5) results did not compromise the result of heterozygote deficiency and positive FIS, showing instead a substantial gene flow among most populations (FST), somewhat randomized relationships among most populations (PCoA), and consequent admixture in gene pools (STRUCTURE). Moreover, the Mantel test only marginally supported correlations between geographic and genetic distances, with a notably lower coefficient of determination (Figure 4), suggesting that gene flow among populations is substantial compared to random genetic drift occurring locally [59]. These results collectively imply that some factors must facilitate a high gene flow and the resulting population genetic characteristics.
One factor could be insufficient nationwide control practice, allowing rampant growth in local populations that bolsters subsequent dispersal. This is likely because none of the populations showed a clear recent decrease in BOTTLENECK analyses, although a recent bottleneck was sometimes detected in a few populations depending on the analysis model (Table 4). Pear orchards in South Korea covered approximately 12,000 ha in 2015, accounting for 15,229 farm households [60]; thus, the actual control practice targeting overwintered adults and their eggs/nymphs during a limited time period (e.g., 6 days for overwintered adults and about one week for 50% hatched eggs) may not have been well practiced unanimously, even with a nationwide active recommendation. Furthermore, geographic differences in the date of upward movement owing to temperature variations may have weakened the efficacy of the nationwide control effort. Indeed, a study to validate the prediction model that forecasts the upward movement of overwintered adults has found an approximately one-week difference between the central region, from which the prediction model was developed, and the southern region of South Korea, which is warmer than the central region [4]. It is possible that current rapid global warming may have confounded the dates of upward movement and subsequent development. Moreover, although it could be negligible, neglected pear patches without control practice, which are found infrequently, may also have served as sources of local population growth, increasing the chance of gradual extension to neighboring orchards and eventually to distant orchards. For genetically homogeneous populations, it has been theorized that a small number of migrants may be sufficient to prevent drift [29].
Another probable factor is artificial transportation, which we think it as a major factor. Considering the high dependence of C. jukyungi on pear trees, nationwide genetic similarities do not appear to result solely from their active dispersal. In particular, oil emulsion sprayed using a high-speed concentrate sprayer produces wind, which facilitates the dispersal of C. jukyungi to closer weeds and even to neighboring orchards, but they are often observed to return after spraying is finished [11]. This suggests that the pear psyllid may not be a very mobile insect. Thus, we speculate that such nationwide genetic similarities may be related to unintended, human-mediated dispersal during the expansion of the pear orchard area in South Korea.
Cacopsylla jukyungi was first reported in the late 1970s [2], but damage by the species was not reported until the early 1990s. However, subsequent to the first damage report in 1993, damage by C. jukyungi was continuously reported, and it became a nationwide insect pest, ranking as the most damaging insect pest to pear trees after 1998 [61]. Such recognition appears to be highly correlated with the expansion history of pear orchards, which started at ~4000 ha in 1946 and culminated in 27,146 ha in 2002, although it subsequently decreased (e.g., 11,200 ha in 2016) [11,62]. During this explosive expansion over approximately 50 years, pear orchards reached nationwide coverage, excluding only mountainous, high-elevation areas; chief producing districts changed, and pre-existing varieties were replaced with a new variety, Niitaka, which represented 75% of pear cultivating areas in 2002 [11]. During this expansion, inadvertent delivery of various life stages of pear psyllids to previously unoccupied places may have occurred.
Replacement of a pre-existing cultivar with a new one in an orchard is performed either by planting pear grafts, which are prepared by grafting a scion from a parent stock, or by renewal through top-working. To create a new orchard, commercial pear grafts are generally planted. To shorten the non-fruiting age of pears, pear grafts are transported to the final destination after grafts are grown for at least one year. The most suitable period recommended for planting grafts in pear orchards is from February to March, before germination [11]. This period partially overlaps with adult upward movement to tree branches, egg deposition, and nymph hatching of the first generation of C. jukyungi, although some regional variation exists. Such practice has long been performed to replace pear cultivars and create new pear orchards and is still practiced. During this course, scion and grafts contaminated with the pear psyllid are a highly likely cause of artificial transportation to new areas nationwide, causing a relatively high gene flow, marginal lack of IBD, admixture of gene pools, and shallow population structure, even though the pear psyllid is not a mobile insect. Indeed, the grafting of pear scions by farmers has also been reported as a possible source of unexpected distribution of one C. chinensis lineage in Taiwan [63,64].
We have previously analyzed the gene flow of C. jukyungi populations in South Korea, in which we misidentified the pear psyllid as C. pyricola, using 712–716 bp of the complete internal transcribed spacer 2 (ITS-2) [6]. We found only a single population pair with statistically significant genetic distance in the comparisons among six populations. Based on the findings, we suggested that the pear psyllid populations in South Korea are neither genetically isolated nor hampered for gene flow, although the use of ITS-2 provided limited variation and lacked heterozygosity information necessary to infer inbreeding [6].
Based on these population genetic results, we propose the following improvements related to the control of C. jukyungi: (1) for the control of overwintered populations, further scrutinized nationwide control practice could be required, without any neglected pear orchards. (2) For further precise control practice, the prediction model that forecasts the date of upward movement of overwintered pear psyllid requires an improvement, particularly for local populations under the observed climate change. (3) When pear grafts are transported between regions or orchards, thorough sterilization could be essential to avoid an unintentional transfer of the grafts contaminated with the pear psyllid.
5. Conclusions
In conclusion, analysis of the genetic variability and population genetic structure of C. jukyungi showed heterozygosity deficiency and positive inbreeding coefficients in all populations; however, these populations revealed almost no structure, possibly resulting from an effective nationwide gene flow. Considering the year-round heavy dependence of the pear psyllid on commercial pear trees and the nationwide targeted control practice, previously neglected factors such as transportation of pear psyllid-contaminated grafts in the explosive expansion of pear orchards and replacement of pear cultivars could be responsible for such genetic interrelationships of the pear psyllid in South Korea. Therefore, strict control of contaminated graft transportation could be one way to alleviate pear psyllid rampant growth. Moreover, nationwide control recommendations based on a prediction model that more thoroughly reflects regional temperature variations could be helpful for better control of overwintered C. jukyungi, particularly under the rapid climate change of recent years.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13112710/s1, Figure S1: Neighbor-Joining tree for genetic relationships among 658 bp of COI haplotypes of Cacopsylla species including those of C. jukyungi.; Table S1: samples, COI haplotypes, and GenBank accession numbers of Cacopsylla jukyungi sequences.; Table S2: pairwise comparisons of COI haplotypes of Cacopsylla jukyungi sequences.; Table S3: summary statistics of MGISEQ2000 paired-end (2 × 150) read sequence data and de novo assembly of the Cacopsylla jukyungi genome.; Table S4: summary statistics of filtered scaffolds of the Cacopsylla jukyungi genome for microsatellite marker identification.; Table S5: null allele analysis using Micro-Checker software.; Table S6: null allele frequencies for 11 microsatellite loci in Cacopsylla jukyungi populations detected using FreeNa software (https://www1.montpellier.inra.fr/CBGP/software/FreeNA/, accessed on 4 July 2023); Table S7: jackknifing of eight microsatellite loci at 95% confidence with 10,000 bootstraps.; Table S8: source data for the calculation of allelic patterns across 11 populations of Cacopsylla jukyungi. References [6,20] are cited in both the main text and Supplementary Materials and references [65,66,67,68] are cited in Supplementary Materials.
Author Contributions
Conceptualization, A.R.K., M.J.K. and I.K.; methodology, J.S.P., M.J.K. and J.-Y.P.; software, J.S.P. and J.-Y.P.; validation, J.-H.S.; writing—original draft preparation, A.R.K. and I.K.; writing—review and editing, J.S.P. and I.K.; project administration, A.R.K. and I.K.; funding acquisition, A.R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01509505)” Rural Development Administration, Republic of Korea.
Data Availability Statement
The data presented in this study are available in the text and supplementary material here.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, W.T.; Choi, K.R.; Lee, H.I.; Kim, J.H.; Kim, J.Y. Agricultural Outlook 2023 Korea, Chapter 14: Trends and Prospects of Fruit Supply and Demand; Korea Rural Economic Institute Press: Naju, Republic of Korea, 2023; pp. 533–618. [Google Scholar]
- Kwon, Y.J. Psylloidea of Korea (Homoptera: Sternorrhyncha); Editorial Committee of Insecta Koreana: Seoul, Republic of Korea, 1983; pp. 1–181. [Google Scholar]
- Kim, D.S.; Yang, C.Y.; Jeon, H.Y. An empirical model for the prediction of the onset of upward-movement of overwintered Cacopsylla pyricola (Homoptera: Psyllidae) in pear orchards. Korean J. Agric. For. Meteorol. 2007, 9, 228–233. [Google Scholar] [CrossRef]
- Park, J.S.; Park, J.W.; Kang, A.R.; Lee, S.H.; Yang, K.-Y.; Kim, W.S.; Kim, I. Analysis of occurrence pattern of the pear psylla; Cacopsylla pyricola; in the pear exporting complex. Trends Agric. Life Sci. 2014, 48, 1–8. [Google Scholar]
- Foerster, A. Uebersicht der Gattungen und Arten in der Familie der Psylloden. Verhandlungen des Naturhistorischen. Ver. Preuss. Rheinl. 1848, 5, 65–98. [Google Scholar]
- Kang, A.R.; Baek, J.Y.; Lee, S.H.; Cho, Y.S.; Kim, W.S.; Han, Y.S.; Kim, I. Geographic homogeneity and high gene flow of the pear psylla; Cacopsylla pyricola (Hemiptera: Psyllidae); detected by mitochondrial COI gene and nuclear ribosomal internal transcribed spacer 2. Anim. Cells Syst. 2012, 16, 145–153. [Google Scholar] [CrossRef]
- Cho, G.; Burckhardt, D.; Inoue, H.; Luo, X.; Lee, S. Systematics of the east Palaearctic pear psyllids (Hemiptera: Psylloidea) with particular focus on the Japanese and Korean fauna. Zootaxa 2017, 4362, 75–98. [Google Scholar] [CrossRef]
- An, J.H.; Yiem, M.S.; Kim, D.S. Effects of photoperiod and temperature on formation and fecundity of two seasonal forms of Psylla pyricola (Homoptera: Psyllidae). Korean J. Appl. Entomol. 1996, 35, 205–208. [Google Scholar]
- Park, J.W.; Park, J.S.; Kang, A.R.; Na, I.S.; Cha, G.H.; Oh, H.J.; Lee, S.H.; Yang, K.Y.; Kim, W.S.; Kim, I. Establishment of pest forecasting management system for the improvement of pass ratio of Korean exporting pears. Int. J. Ind. Entomol. 2012, 25, 163–169. [Google Scholar] [CrossRef]
- Rural Development Administration. Nongsaro: Agricultural Technology Information. 2015. Available online: http://www.nongsaro.go.kr (accessed on 20 August 2023).
- Rural Development Administration. Agriculture Technique Guide 013: Pear; Rural Development Administration; Seungil Media Group: Daejeon City, Republic of Korea, 2020. [Google Scholar]
- Malagnini, V.; Pedrazzoli, F.; Forno, F.; Komjanc, M.; Ioriatti, C. Characterization of microsatellite loci in Cacopsylla melanoneura Förster (Homoptera: Psyllidae). Mol. Ecol. Notes 2007, 7, 495–497. [Google Scholar] [CrossRef]
- Sun, J.R.; Li, Y.; Yan, S.; Zhang, Q.; Xu, H. Microsatellite marker analysis of genetic diversity of Cacopsylla chinensis (Yang et Li)(Hemiptera: Psyllidae) populations in China. Acta Entomol. Sin. 2011, 54, 820–827. [Google Scholar]
- Malagnini, V.; Pedrazzoli, F.; Papetti, C.; Cainelli, C.; Zasso, R.; Gualandri, V.; Pozzebon, A.; Ioriatti, C. Ecological and genetic differences between Cacopsylla melanoneura (Hemiptera; Psyllidae) populations reveal species host plant preference. PLoS ONE 2013, 8, e69663. [Google Scholar] [CrossRef][Green Version]
- Sauvion, N.; Lachenaud, O.; Genson, G.; Rasplus, J.; Labonne, G. Are there several biotypes of Cacopsylla pruni? Bull. Insectology 2007, 60, 185–186. [Google Scholar]
- Sauvion, N.; Lachenaud, O.; Mondor-Genson, G.; Easplus, J.Y.; Labonne, G. Nine polymorphic microsatellite loci from the psyllid Cacopsylla pruni (Scopoli); the vector of European stone fruit yellows. Mol. Ecol. Resour. 2009, 9, 1196–1199. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rate of base substitution through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenics: An approach using the bootstrap. Evolution 1985, 29, 783–791. [Google Scholar] [CrossRef]
- Kang, A.R.; Kim, M.J.; Park, J.S.; Seo, H.J.; Song, J.H.; Won, K.H.; Choi, E.D.; Kim, I. Comparative analysis of two pear pests, Cacopsylla jukyungi and Cacopsylla burckhardti (Hemiptera: Psyllidae), based on complete mitochondrial genomes and comparison to confamilial species. Agronomy 2022, 12, 2037. [Google Scholar] [CrossRef]
- Gnerre, S.; MacCallum, I.; Przybylski, D.; Ribeiro, F.J.; Burton, J.N.; Walker, B.J.; Sharpe, T.; Hall, G.; Shea, T.P.; Sykes, S.; et al. High-quality draft assemblies of mammalian genomes from massively parallel sequence data. Proc. Natl. Acad. Sci. USA 2011, 108, 1513–1518. [Google Scholar] [CrossRef]
- Peng, Y.; Leung, H.C.; Yiu, S.M.; Chin, F.Y. IDBA-UD: A de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics 2012, 28, 1420–1428. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar]
- Faircloth, B.C. MSATCOMMANDER: Detection of microsatellite repeat arrays and automated, locus-specific primer design. Mol. Ecol. Resour. 2008, 8, 92–94. [Google Scholar] [CrossRef]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Bioinform. Methods Protoc. 2000, 132, 365–386. [Google Scholar]
- Yue, G.H.; Chen, F.; Orban, L. Rapid isolation and characterization of microsatellites from the genome of Asian arowana (Scleropages formosus, Osteoglossidae, Pisces). Mol. Ecol. 2000, 9, 1007–1009. [Google Scholar] [CrossRef]
- Weir, B.S. Genetic Data Analysis II; Sinauer Associates, Inc.: Sunderland, MA, USA, 1990; pp. 1–445. [Google Scholar]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Hartl, D.L.; Clark, A.G. Principles of Population Genetics, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 1997; pp. 1–568. [Google Scholar]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar] [PubMed]
- Goudet, J. FSTAT, a Program to Estimate and Test Gene Diversities and Fixation Indices. Version 2.9.3. 2001. Available online: http://www2.unil.ch/popgen/softwares/fstat.htm (accessed on 15 January 2023).
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.M.; Shipley, P. Microchecker: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Chapuis, M.-P.; Estoup, A. Microsatellite null alleles and estimation of population differentiation. Mol. Biol. Evol. 2007, 24, 621–631. [Google Scholar] [PubMed]
- Dempster, A.P.; Laird, N.M.; Rubin, D.B. Maximum likelihood from incomplete data via the EM algorithm. J. R. Stat. Soc. Ser. B Methodol. 1977, 39, 1–22. [Google Scholar] [CrossRef]
- Raymond, M.; Rousset, F. GENEPOP (ver. 1.2): Population genetics software for exact tests and ecumenicism. J. Hered. 1995, 86, 248–249. [Google Scholar] [CrossRef]
- Rousset, F. Genepop’007: A complete reimplementation of the Genepop software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef]
- Guo, S.W.; Thompson, E.A. Performing the exact test of Hardy-Weinberg proportions for multiple alleles. Biometrics 1992, 48, 361–372. [Google Scholar] [CrossRef]
- Rice, W.R. Analyzing tables of statistical tests. Evolution 1989, 43, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Weir, B.S.; Cockerham, C.C. Estimating F-statistics for the analysis of population structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [PubMed]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Earl, D.A.; vonHoldt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- Bohonak, A.J. IBD (Isolation by Distance): A program for analyses of isolation by distance. J. Hered. 2002, 93, 153–154. [Google Scholar] [CrossRef]
- Cornuet, J.M.; Luikart, G. Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 1996, 144, 2001–2014. [Google Scholar] [CrossRef]
- Piry, S.; Luikart, G.; Cournet, J.M. BOTTLENECK: A computer program for detecting recent reductions in the effective population size using allele frequency data. J. Hered. 1999, 90, 502–503. [Google Scholar] [CrossRef]
- Luikart, G.; Allendorf, F.W.; Cornuet, J.M.; Sherwin, W.B. Distortion of allele frequency distributions provides a test for recent population bottlenecks. J. Hered. 1998, 89, 238–247. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; White, V.L.; Jasper, M.; Yagui, H.; Sinclair, S.J.; Kearney, M.R. An endangered flightless grasshopper with strong genetic structure maintains population genetic variation despite extensive habitat loss. Ecol. Evol. 2021, 11, 5364–5380. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.K.; Li, F. The Pear psylla (Homoptera) of China with descriptions of seven new species. Entomotaxonomia 1981, 3, 35–47. [Google Scholar]
- Zhao, N.N.; Zhang, H.; Zhang, X.C.; Luan, X.B.; Zhou, C.; Liu, Q.Z.; Shi, W.P.; Liu, Z.L. Evaluation of acute toxicity of essential oil of garlic (Allium sativum) and its selected major constituent compounds against overwintering Cacopsylla chinensis (Hemiptera: Psyllidae). J. Econ. Entomol. 2013, 106, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, R.; Bosco, D.; Alma, A. Population dynamics of Cacopsylla melanoneura (Homoptera: Psyllidae); a vector of apple proliferation phytoplasma in Northwestern Italy. J. Econ. Entomol. 2002, 95, 544–551. [Google Scholar] [CrossRef]
- Ossiannilsson, F. The Psylloidea (Homoptera) of Fennoscandia and Denmark; Brill: Leiden, The Netherlands, 1992; Volume 26, pp. 144–145. [Google Scholar]
- Thébaud, G.; Yvon, M.; Alary, R.; Sauvion, N.; Labonne, G. Efficient transmission of ‘Candidatus Phytoplasma prunorum’ is delayed by eight months due to a long latency in its hostalternating vector. Phytopathology 2009, 99, 265–273. [Google Scholar] [CrossRef]
- Mohd Rodzik, F.F.; Sudirman, N.A.; Teh, C.K.; Ong, A.L.; Heng, H.Y.; Yaakop, S.; Mohd-Assaad, N.; Ong-Abdullah, M.; Ata, N.; Amit, S.; et al. Development of nuclear DNA markers for applications in genetic diversity study of oil palm-pollinating weevil populations. Insects 2023, 14, 157. [Google Scholar] [CrossRef]
- Woo, J.; Heo, J.S.; Kim, K.-Y.; Kim, K.-S.; Hwang, H.-J.; Yoon, M.; An, H.; Kang, K.H.; Park, J.S.; Nam, K.-W.; et al. Population genetic analysis of the wild hard-shelled mussel, Mytilus unguiculatus (Valenciennes 1858) in South Korea using a microsatellite multiplex assay. Thalassas 2023, 1–12. [Google Scholar] [CrossRef]
- Charlesworth, D.; Charlesworth, B. Inbreeding depression and its evolutionary consequences. Ann. Rev. Ecol. System. 1987, 18, 237–268. [Google Scholar] [CrossRef]
- Lande, R. Genetic variation and phenotypic evolution during allopatric speciation. Am. Nat. 1980, 116, 463–479. [Google Scholar] [CrossRef]
- Caughley, G. Directions in conservation biology. J. Anim. Ecol. 1994, 63, 215–244. [Google Scholar] [CrossRef]
- Wright, S. Evolution and the Genetic of Population, Variability Within and among Natural Populations; University of Chicago Press: Chicago, IL, USA, 1978; pp. 213–220. [Google Scholar]
- Statistics Korea. Agriculture; Forestry & Fishery Census Report; Statistics Korea: Daejeon, Republic of Korea, 2015; Volume 4. [Google Scholar]
- Jeon, H.; Kim, D.; Cho, M.; Yiem, M.; Chang, Y. Recent status of major fruit tree pest occurrences in Korea. J. Korean Soc. Hortic. Sci. 2000, 41, 607–612. [Google Scholar]
- Rural Development Administration. Map of Pear Plantation Changes; Rural Development Administration: Suwon, Republic of Korea, 2010. [Google Scholar]
- Lee, H.C.; Yang, M.M.; Yeh, W.B. Identification of two invasive Cacopsylla chinensis (Hemiptera: Psyllidae) lineages based on two mitochondrial sequences and restriction fragment length polymorphism of cytochrome oxidase I amplicon. J. Econ. Entomol. 2008, 101, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; Wang, C.L. Integrated control of pear psylla Cacopsylla chinensis and pear decline. In Proceedings of the Symposium on Integrated Management Technology of Insect Vectors and Insect-Borne Diseases, July 2011; Taiwan Agricultural Research Institute: Taichung City, Taiwan, 2011; pp. 91–105. [Google Scholar]
- Cho, G.; Malenovský, I.; Burckhardt, D.; Inoue, H.; Lee, S. DNA barcoding of pear psyllids (Hemiptera: Psylloidea: Psyllidae), a tale of continued misidentifications. Bull. Entomol. Res. 2020, 110, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cen, Y.; He, Y.; Wu, Y.; Huang, S.; Lu, J. The first complete mitochondrial genome sequence of Cacopsylla citrisuga (Yang & Li), a new insect vector of Huanglongbing in Yunnan Province, China. Mitochondrial DNA Part B 2021, 6, 575–577. [Google Scholar] [PubMed]
- Kuznetsova, V.G.; Labina, E.S.; Shapoval, N.A.; Maryańska-Nadachowska, A.N.N.A.; Lukhtanov, V.A. Cacopsylla fraudatrix sp. n.(Hemiptera: Psylloidea) recognised from testis structure and mitochondrial gene COI. Zootaxa 2012, 3547, 55–63. [Google Scholar] [CrossRef]
- Gwiazdowski, R.A.; Foottit, R.G.; Maw, H.E.L.; Hebert, P.D. The Hemiptera (Insecta) of Canada: Constructing a reference library of DNA barcodes. PLoS ONE 2015, 10, e0125635. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).