STICS Soil–Crop Model Performance for Predicting Biomass and Nitrogen Status of Spring Barley Cropped for 31 Years in a Gleysolic Soil from Northeastern Quebec (Canada)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Field Database
2.2. Plant Analysis
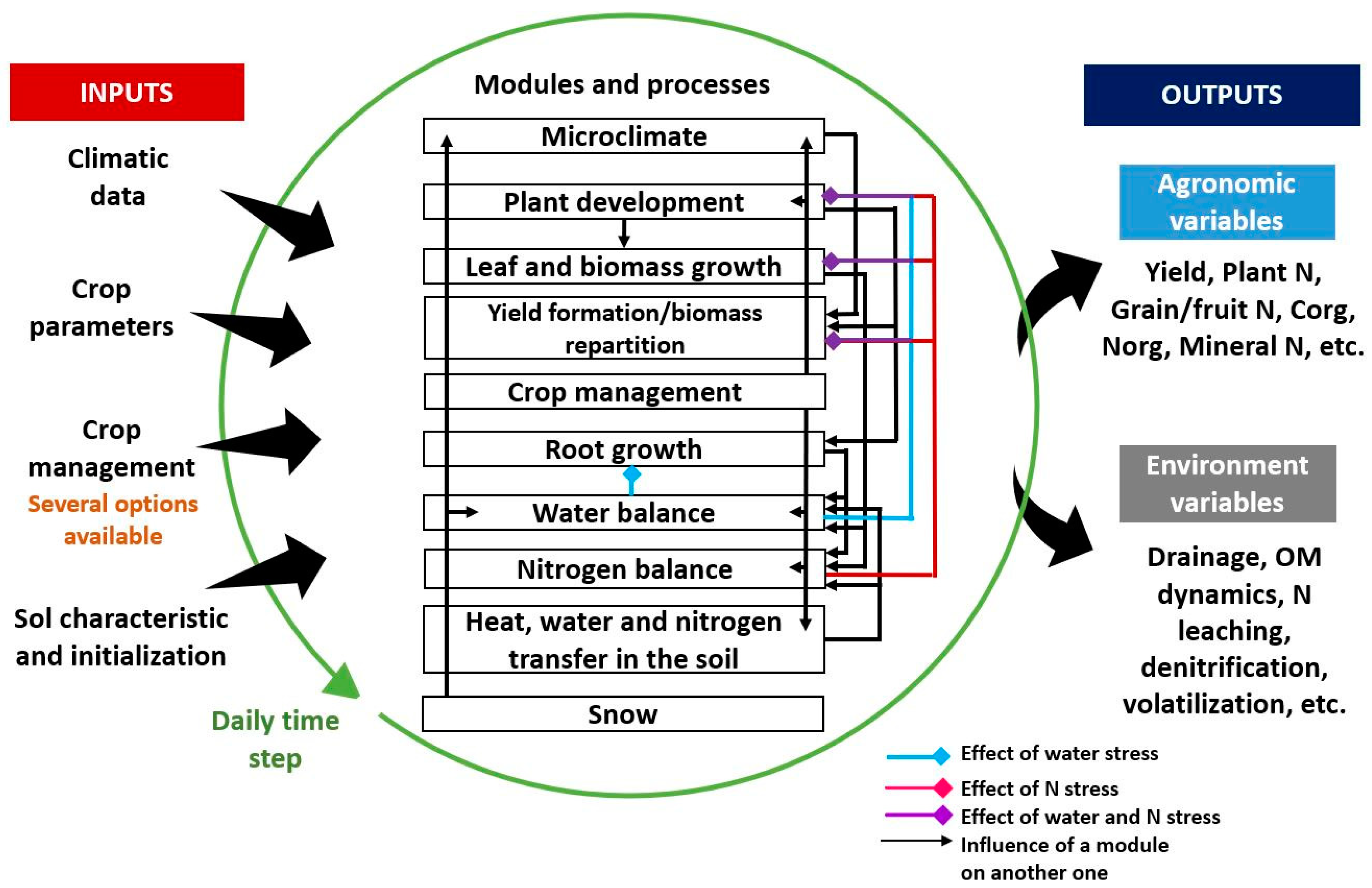
2.3. STICS Soil–Crop Model Overview
2.4. Model Inputs and Simulation Options
2.5. Calibration of Crop Parameters for New Cultivars and STICS Performance Evaluation
2.6. Statistical Analysis and Model Evaluation
3. Results
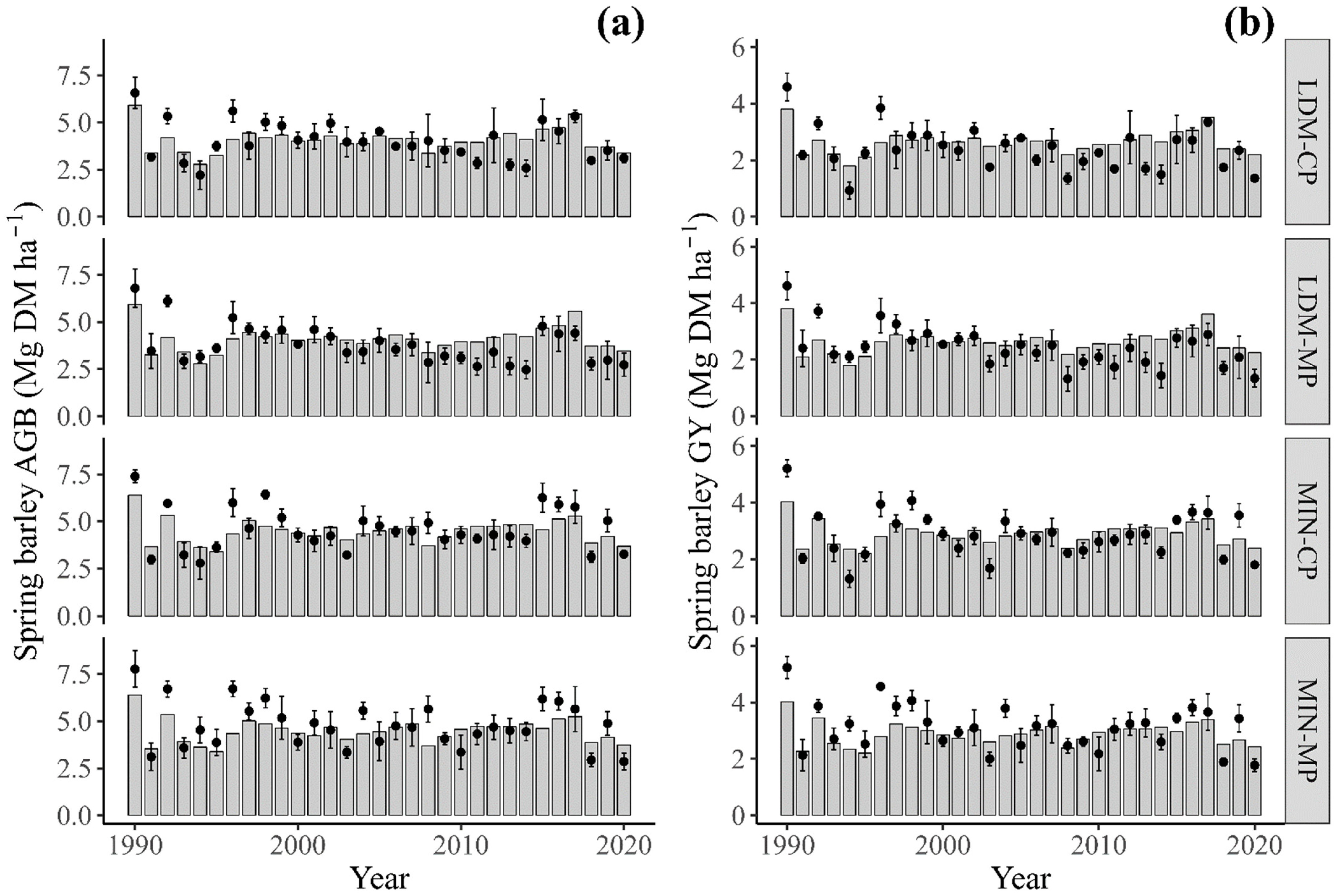
3.1. Statistical Analysis of Field-Observed Data
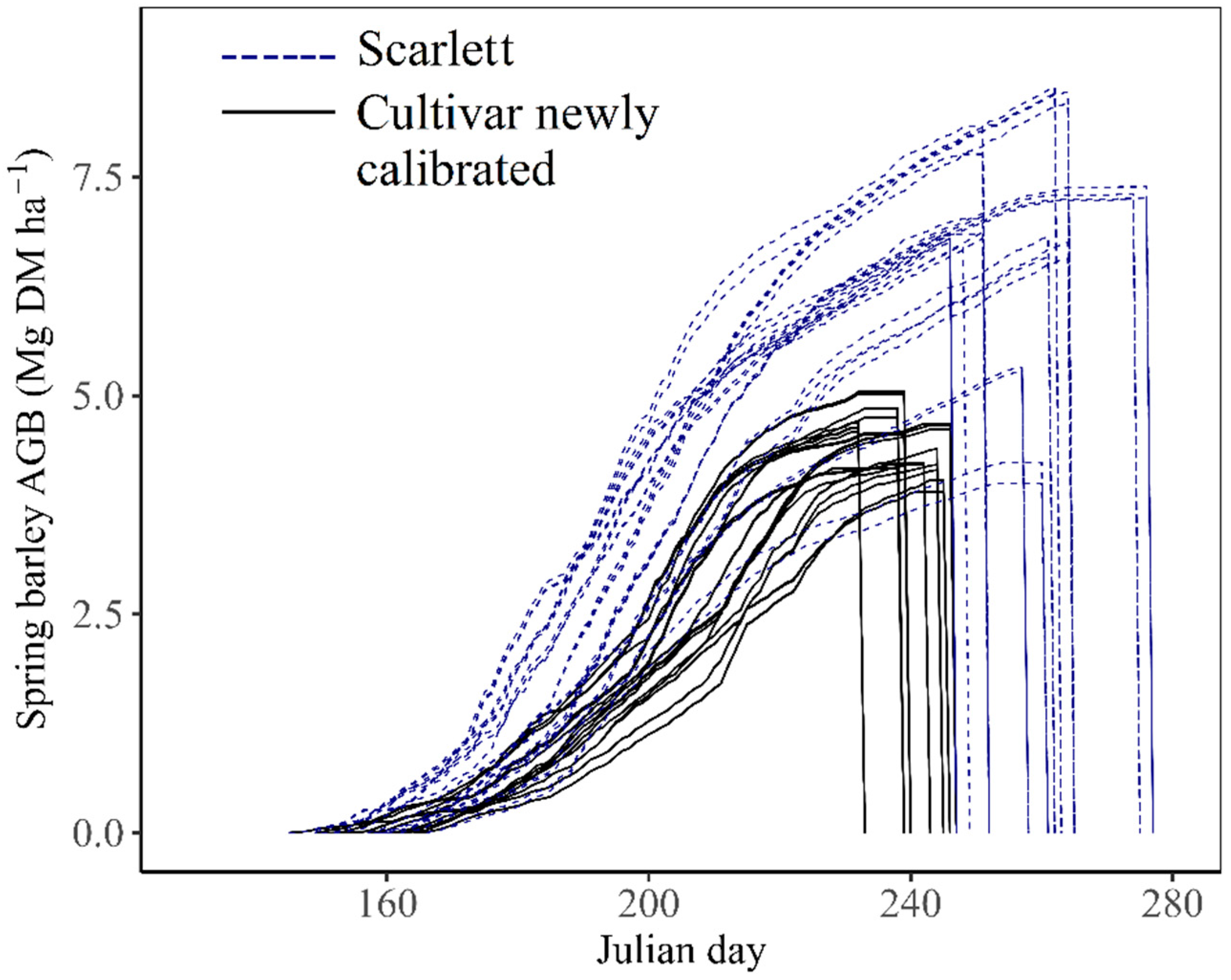
3.2. Calibration to Add New Cultivar Adapted to Northeastern Quebec Conditions in STICS
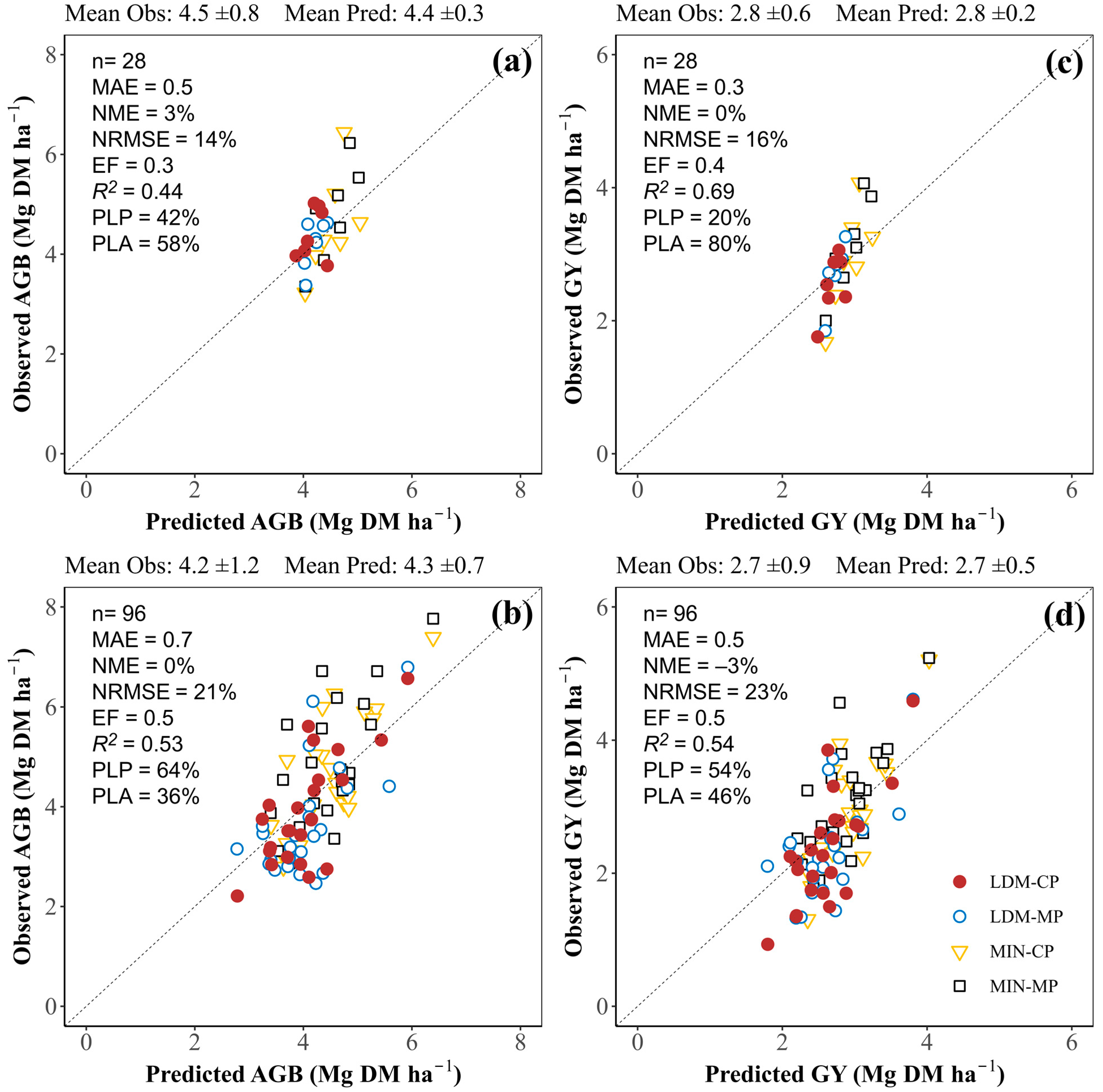
3.3. Comparison between Observed and Predicted Values
3.3.1. Aboveground Biomass and Grain Yield at Harvest
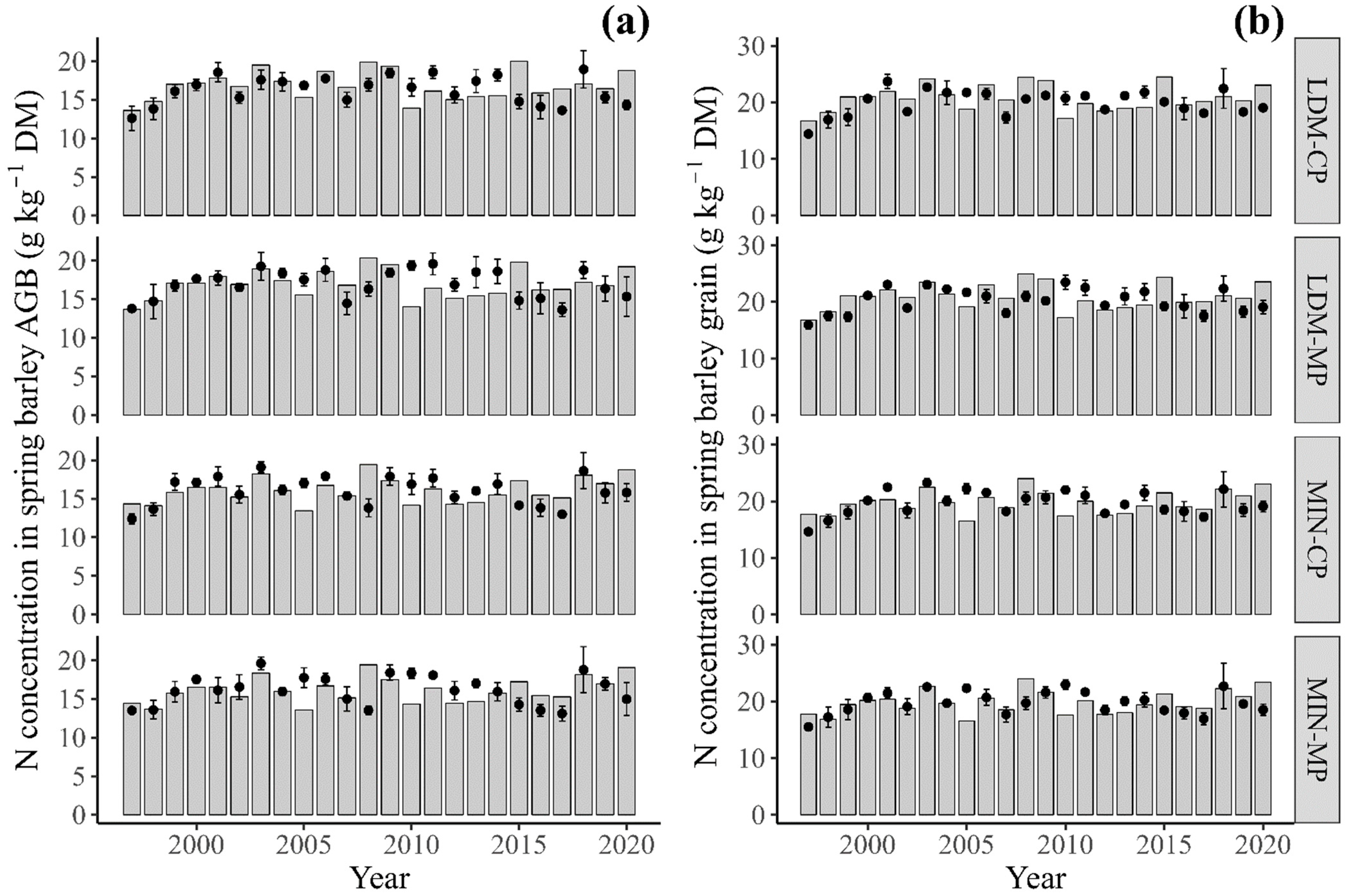
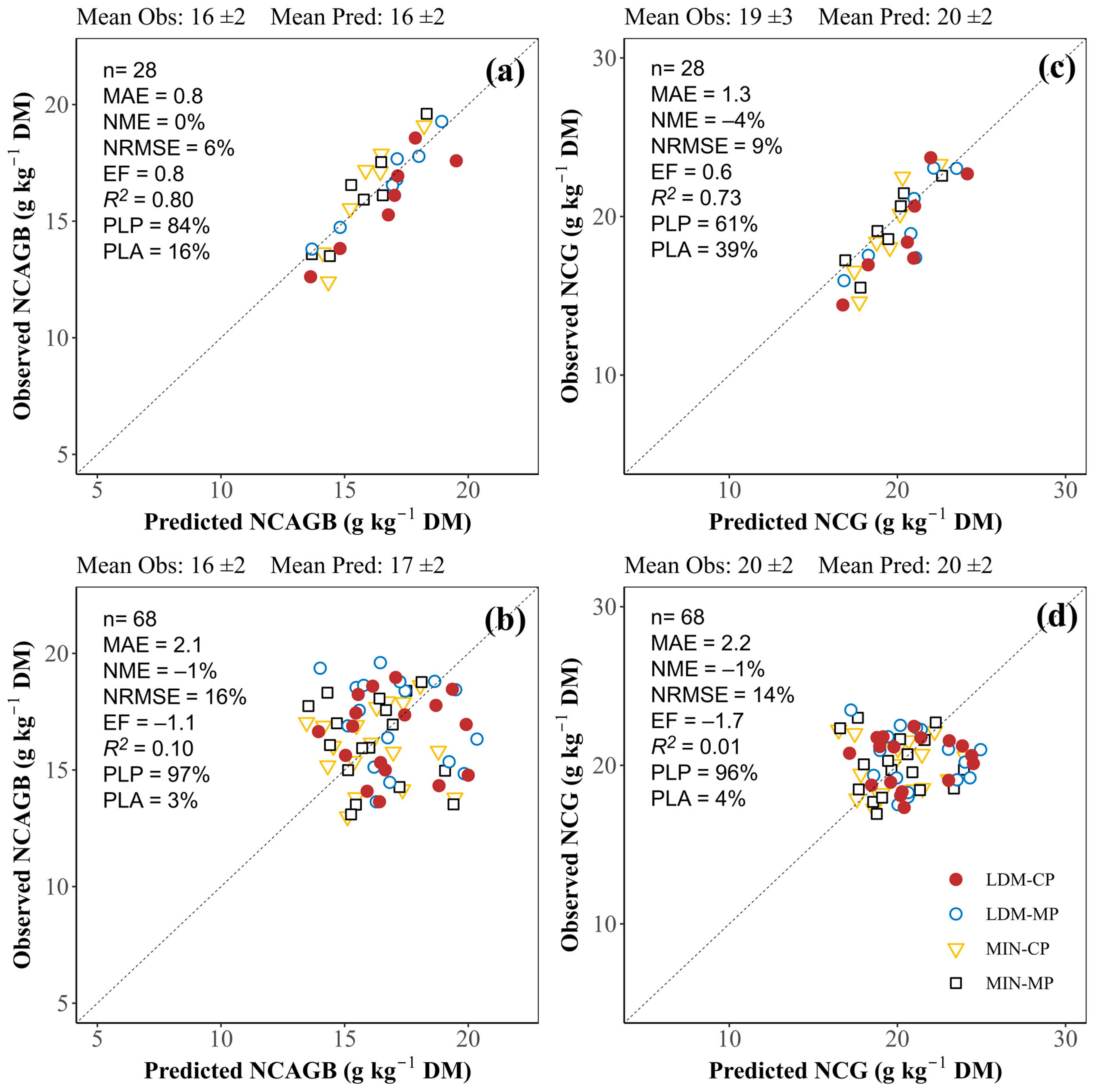
3.3.2. Nitrogen Concentration in Aboveground Biomass and in Grain at Harvest
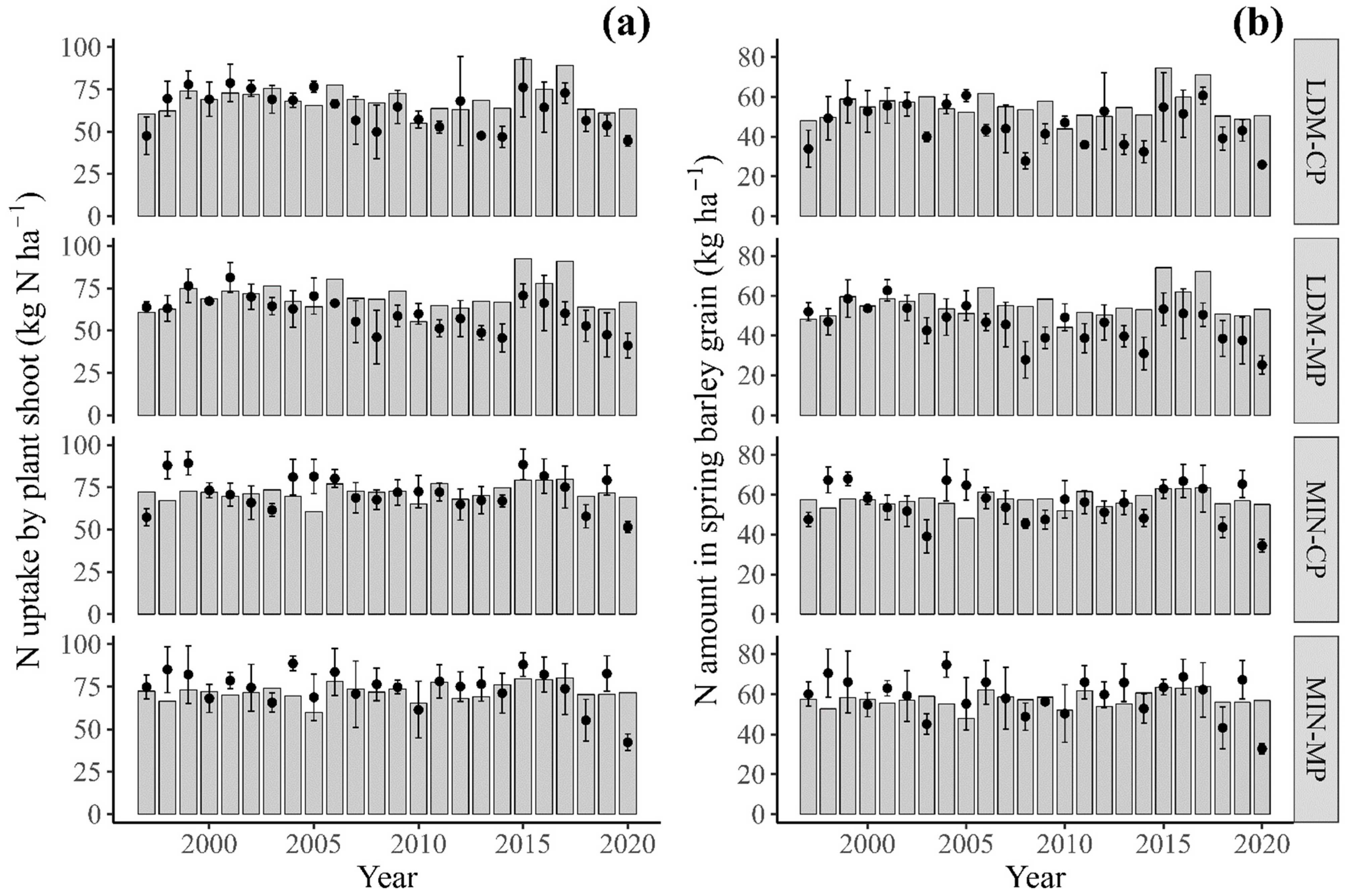
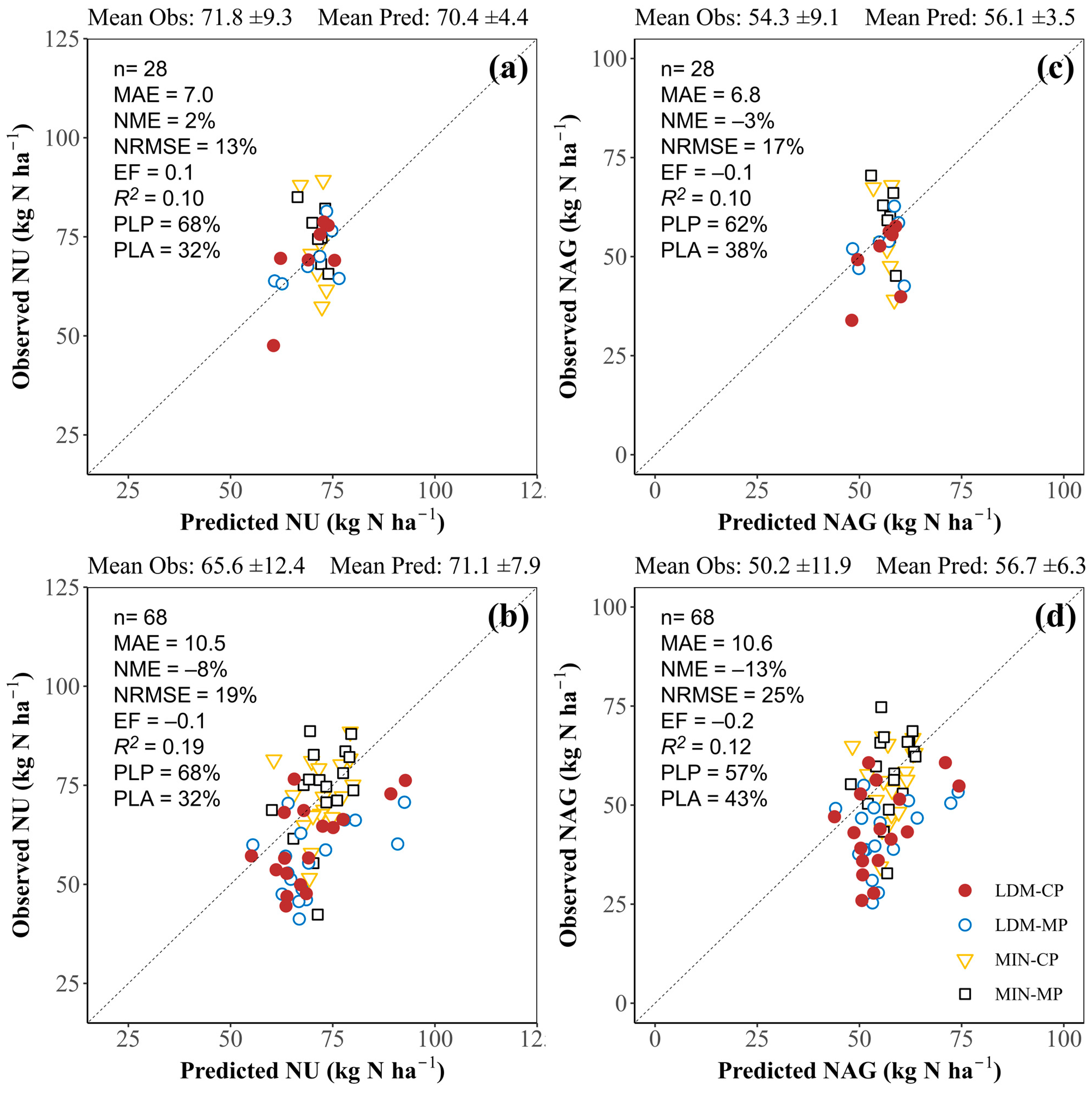
3.3.3. Plant N Uptake and Amount of N in Grain
3.4. STICS Performance in Relation to Climatic Conditions
4. Discussion
4.1. STICS Calibration for Spring Barley Cultivars Adapted to Climatic Conditions of Northeastern Quebec
4.2. STICS Performance
4.3. STICS Process-Based Model vs. Statistical Model
4.4. Suggestions to Improve Model Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec. Sectoral Diagnostic Portrait of the Grain Industry in Quebec; Bibliothèque et Archives Nationales du Québec: Montréal, QC, Canada, 2020; 51p. (In French) [Google Scholar]
- Bulman, P.; Mather, D.E.; Smith, D.L. Genetic Improvement of Spring Barley Cultivars Grown in Eastern Canada from 1910 to 1988. Euphytica 1993, 71, 35–48. [Google Scholar] [CrossRef]
- Holland, J.; Brown, J.L.; MacKenzie, K.; Neilson, R.; Piras, S.; McKenzie, B.M. Over Winter Cover Crops Provide Yield Benefits for Spring Barley and Maintain Soil Health in Northern Europe. Eur. J. Agron. 2021, 130, 126363. [Google Scholar] [CrossRef]
- Statistics Canada. Estimated Area, Yield, Production, Average Farm Price and Total Farm Value of Major Field Crops, in Metric and Imperial Units. 2008. Available online: https://www.pgq.ca/articles/services-dinformation-sur-les-marches/portrait-quebec/production-quebec/ (accessed on 13 January 2023).
- Friedt, W.; Horsley, R.D.; Harvey, B.L.; Poulsen, D.M.; Lance, R.C.; Ceccarelli, S.; Grando, S.; Capettini, F. Barley Breeding History, Progress, Objectives, and Technology. In Barley: Production, Improvement, and USES; Blackwell Publishing: Hoboken, NJ, USA, 2011; pp. 160–220. [Google Scholar]
- Bélanger, G.; Bootsma, A. Impacts of climate change on agriculture in Quebec [Paper presentation]. In Proceedings of the Présentation au 65e congrès de l’Ordre des Agronomes du Québec, Quebec, Sainte-Foy, 7–8 June 2002. 20p. (In French). [Google Scholar]
- Agriculture and Agri-Food Canada. Effective Growing Degree Days—Quebec. 2010. Available online: https://publications.gc.ca/collections/collection_2018/aac-aafc/A59-55-2010-eng.pdf (accessed on 23 February 2023).
- Moore, T. Soils of Quebec. In Digging into Canadian Soils. An Introduction to Soil Science; Canadian Society of Soil Science: Pinawa, MB, Canada, 2021; pp. 401–409. [Google Scholar]
- Setter, T.L.; Burgess, P.; Waters, I.; Kuo, J. Genetic Diversity of Barley and Wheat for Waterlogging Tolerance in Western Australia. In Proceedings of the 9th Australian Barley Technical Symposium, Melbourne, VIC, Australia, 12–16 September 1999; Australian Barley Technical Symposium Inc.: Melbourne, VIC, Australia, 1999; pp. 1–7. [Google Scholar]
- Cao, W.; Moss, D.N. Temperature and Daylength Interaction on Phyllochron in Wheat and Barley. Crop Sci. 1989, 29, 1046–1048. [Google Scholar] [CrossRef]
- Juskiw, P.; Jame, Y.-W.; Kryzanowski, L. Phenological Development of Spring Barley in a Short-Season Growing Area. Agron, J. 2001, 93, 370–379. [Google Scholar] [CrossRef]
- Ma, B.L.; Smith, D.L. Apical Development of Spring Barley under Field Conditions in Northeastern North America. Crop Sci. 1992, 32, 144–149. [Google Scholar] [CrossRef]
- Russell, G. Barley Knowledge Base; Joint Research Centre: Brussels, Belgium, 1990; p. 135. [Google Scholar]
- Oteng-Darko, P.; Yeboah, S.; Addy, S.N.T.; Amponsah, S.; Danquah, E.O. Crop Modeling: A Tool for Agricultural Research—A Review. J. Agric. Res. Develop. 2013, 2, 1–6. [Google Scholar]
- Quintero, D.; Díaz, E. A Comparison of Two Open-Source Crop Simulation Models for a Potato Crop. Agron. Colomb. 2020, 38, 382–387. [Google Scholar] [CrossRef]
- Basso, B.; Cammarano, D.; Troccoli, A.; Chen, D.; Ritchie, J.T. Long-Term Wheat Response to Nitrogen in a Rainfed Mediterranean Environment: Field Data and Simulation Analysis. Eur. J. Agron. 2010, 33, 132–138. [Google Scholar] [CrossRef]
- Salo, T.J.; Palosuo, T.; Kersebaum, K.C.; Nendel, C.; Angulo, C.; Ewert, F.; Bindi, M.; Calanca, P.; Klein, T.; Moriondo, M. Comparing the Performance of 11 Crop Simulation Models in Predicting Yield Response to Nitrogen Fertilization. J. Agric. Sci. 2016, 154, 1218–1240. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, H.; Fan, J.; Xiang, Y.; Liu, X.; Liao, Z.; Abdelghany, A.E.; Zhang, F.; Li, Z. Evaluation of AquaCrop Model for Greenhouse Cherry Tomato with Plastic Film Mulch under Various Water and Nitrogen Supplies. Agric. Water Manag. 2022, 274, 107949. [Google Scholar] [CrossRef]
- Saadi, S.; Pattey, E.; Jégo, G.; Champagne, C. Prediction of Rainfed Corn Evapotranspiration and Soil Moisture Using the STICS Crop Model in Eastern Canada. Field Crops Res. 2022, 287, 108664. [Google Scholar] [CrossRef]
- Lammoglia, S.-K.; Moeys, J.; Barriuso, E.; Larsbo, M.; Marín-Benito, J.-M.; Justes, E.; Alletto, L.; Ubertosi, M.; Nicolardot, B.; Munier-Jolain, N. Sequential Use of the STICS Crop Model and of the MACRO Pesticide Fate Model to Simulate Pesticides Leaching in Cropping Systems. Environ. Sci. Pollut. Res. 2017, 24, 6895–6909. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Beaudoin, N.; Ferchaud, F.; Mary, B.; Strullu, L.; Chlébowski, F.; Clivot, H.; Herre, C.; Duval, J.; Louarn, G. Long-Term Modelling of Soil N Mineralization and N Fate Using STICS in a 34-Year Crop Rotation Experiment. Geoderma 2020, 357, 113956. [Google Scholar] [CrossRef]
- Yin, X.; Kersebaum, K.-C.; Beaudoin, N.; Constantin, J.; Chen, F.; Louarn, G.; Manevski, K.; Hoffmann, M.; Kollas, C.; Armas-Herrera, C.M.; et al. Uncertainties in Simulating N Uptake, Net N Mineralization, Soil Mineral N and N Leaching in European Crop Rotations Using Process-Based Models. Field Crops Res. 2020, 255, 107863. [Google Scholar] [CrossRef]
- Constantin, J.; Beaudoin, N.; Launay, M.; Duval, J.; Mary, B. Long-Term Nitrogen Dynamics in Various Catch Crop Scenarios: Test and Simulations with STICS Model in a Temperate Climate. Agric. Ecosyst. Environ. 2012, 147, 36–46. [Google Scholar] [CrossRef]
- Gardi, M.W.; Memic, E.; Zewdu, E.; Graeff-Hönninger, S. Simulating the Effect of Climate Change on Barley Yield in Ethiopia with the DSSAT-CERES-Barley Model. Agron. J. 2022, 114, 1128–1145. [Google Scholar] [CrossRef]
- Yin, X.; Kersebaum, K.C.; Kollas, C.; Manevski, K.; Baby, S.; Beaudoin, N.; Öztürk, I.; Gaiser, T.; Wu, L.; Hoffmann, M. Performance of Process-Based Models for Simulation of Grain N in Crop Rotations across Europe. Agric. Syst. 2017, 154, 63–77. [Google Scholar] [CrossRef]
- Pasquel, D.; Roux, S.; Richetti, J.; Cammarano, D.; Tisseyre, B.; Taylor, J.A. A Review of Methods to Evaluate Crop Model Performance at Multiple and Changing Spatial Scales. Precis. Agric. 2022, 23, 1489–1513. [Google Scholar] [CrossRef]
- Di Paola, A.; Valentini, R.; Santini, M. An Overview of Available Crop Growth and Yield Models for Studies and Assessments in Agriculture. J. Sci. Food Agric. 2016, 96, 709–714. [Google Scholar] [CrossRef]
- Rötter, R.P.; Palosuo, T.; Kersebaum, K.C.; Angulo, C.; Bindi, M.; Ewert, F.; Ferrise, R.; Hlavinka, P.; Moriondo, M.; Nendel, C. Simulation of Spring Barley Yield in Different Climatic Zones of Northern and Central Europe: A Comparison of Nine Crop Models. Field Crops Res. 2012, 133, 23–36. [Google Scholar] [CrossRef]
- Jame, Y.W.; Cutforth, H.W.; Selles, F.; Campbell, C.A.; Jedel, P.; Kryzanowski, L. Determine the Best Crop Management Option on Canadian Prairies with a Computerized Decision Support System. In Soils and Crops Workshop; University of Saskatchewan: Saskatoon, SK, Canada, 1997; pp. 356–363. [Google Scholar]
- Brisson, N.; Mary, B.; Ripoche, D.; Hélène Jeuffroy, M.; Ruget, F.; Nicoullaud, B.; Gate, P.; Devienne-Barret, F.; Antonioletti, R.; Durr, C.; et al. STICS: A Generic Model for the Simulation of Crops and Their Water and Nitrogen Balances. I. Theory and Parameterization Applied to Wheat and Corn. Agronomie 1998, 18, 311–346. [Google Scholar] [CrossRef]
- Brisson, N.; Gary, C.; Justes, E.; Roche, R.; Mary, B.; Ripoche, D.; Zimmer, D.; Sierra, J.; Bertuzzi, P.; Burger, P. An Overview of the Crop Model STICS. Eur. J. Agron. 2003, 18, 309–332. [Google Scholar] [CrossRef]
- Beaudoin, N.; Launay, M.; Sauboua, E.; Ponsardin, G.; Mary, B. Evaluation of the Soil Crop Model STICS over 8 Years against the “on Farm” Database of Bruyères Catchment. Eur. J. Agron. 2008, 29, 46–57. [Google Scholar] [CrossRef]
- Coucheney, E.; Buis, S.; Launay, M.; Constantin, J.; Mary, B.; de Cortázar-Atauri, I.G.; Ripoche, D.; Beaudoin, N.; Ruget, F.; Andrianarisoa, K.S. Accuracy, Robustness and Behavior of the STICS Soil–Crop Model for Plant, Water and Nitrogen Outputs: Evaluation over a Wide Range of Agro-Environmental Conditions in France. Environ. Model. Softw. 2015, 64, 177–190. [Google Scholar] [CrossRef]
- Lebonvallet, S. Quinoa Establishment and Its Culture Simulation on the Bolivian Altiplano. Ph.D. Thesis, Institut des Sciences et Industries du Vivant et de l’Environnement (Agro Paris Tech), Avignon, France, 2008. Available online: https://theses.hal.science/pastel-00003841/ (accessed on 26 January 2023). (In French, with English Abstract).
- Sierra, J.; Brisson, N.; Ripoche, D.; Noël, C. Application of the STICS Crop Model to Predict Nitrogen Availability and Nitrate Transport in a Tropical Acid Soil Cropped with Maize. Plant Soil 2003, 256, 333–345. [Google Scholar] [CrossRef]
- Corre-Hellou, G.; Faure, M.; Launay, M.; Brisson, N.; Crozat, Y. Adaptation of the STICS Intercrop Model to Simulate Crop Growth and N Accumulation in Pea–Barley Intercrops. Field Crops Res. 2009, 113, 72–81. [Google Scholar] [CrossRef]
- Jégo, G.; Pattey, E.; Bourgeois, G.; Morrison, M.J.; Drury, C.F.; Tremblay, N.; Tremblay, G. Calibration and Performance Evaluation of Soybean and Spring Wheat Cultivars Using the STICS Crop Model in Eastern Canada. Field Crops Res. 2010, 117, 183–196. [Google Scholar] [CrossRef]
- Jégo, G.; Chantigny, M.; Pattey, E.; Bélanger, G.; Rochette, P.; Vanasse, A.; Goyer, C. Improved Snow-Cover Model for Multi-Annual Simulations with the STICS Crop Model under Cold, Humid Continental Climates. Agric. For. Meteorol. 2014, 195–196, 38–51. [Google Scholar] [CrossRef]
- Jégo, G.; Pattey, E.; Bourgeois, G.; Drury, C.F.; Tremblay, N. Evaluation of the STICS Crop Growth Model with Maize Cultivar Parameters Calibrated for Eastern Canada. Agron. Sust. Dev. 2011, 31, 557–570. [Google Scholar] [CrossRef]
- Morissette, R.; Jégo, G.; Bélanger, G.; Cambouris, A.N.; Nyiraneza, J.; Zebarth, B.J. Simulating Potato Growth and Nitrogen Uptake in Eastern Canada with the STICS Model. Agron. J. 2016, 108, 1853–1868. [Google Scholar] [CrossRef]
- Jégo, G.; Bélanger, G.; Tremblay, G.F.; Jing, Q.; Baron, V.S. Calibration and Performance Evaluation of the STICS Crop Model for Simulating Timothy Growth and Nutritive Value. Field Crops Res. 2013, 151, 65–77. [Google Scholar] [CrossRef]
- Ministère de l’agriculture, des pêcheries et de l’alimentation du Québec. Saguenay-Lac-Saint-Jean Agri-Food Portrait 2010; M. de l’agriculture, des pêcheries et de l’alimentation, Ed.; Direction Régionale du Saguenay-Lac-Saint-Jean: Saguenay, QC, Canada, 2014. (In French) [Google Scholar]
- Lapointe, R. Profil 2005 de La Production Agricole de La Région Du Saguenay-Lac-Saint-Jean; M. de l’agriculture, des pêcheries et de l’alimentation, Ed.; Direction Régionale du Saguenay-Lac-Saint-Jean: Saguenay, QC, Canada, 2006. [Google Scholar]
- Agriculture and Agri-Food Canada. The Normandin Research Farm in Quebec Looks to the Future. Government of Canada. Available online: https://agriculture.canada.ca/en/agri-info/normandin-research-farm-quebec-looks-future (accessed on 23 September 2023).
- Lafond, J.; Angers, D.A.; Pageau, D.; Lajeunesse, J. Sustainable Cereal and Forage Production in Dairy-Based Cropping Systems. Can. J. Plant Sci. 2016, 97, 473–485. [Google Scholar] [CrossRef]
- Kong, D.; Choo, T.M.; Narasimhalu, P.; Jui, P.; Ferguson, T.; Therrien, M.C.; Ho, K.M.; May, K.W. Genetic Variation and Adaptation of 76 Canadian Barley Cultivars. Can. J. Plant Sci. 1994, 74, 737–744. [Google Scholar] [CrossRef]
- Isaac, R.A.; Johnson, W.C. Determination of Total Nitrogen in Plant Tissue, Using a Block Digestor. J. Assoc. Off. Anal. Chem. 1976, 59, 98–100. [Google Scholar] [CrossRef]
- Beaudoin, N.; Lecharpentier, P.; Ripoche, D.; Strullu, L.; Mary, B.; Leonard, J.; Launay, M.; Justes, E. STICS Soil-Crop Model. Conceptual Framework, Equations and Uses; Éditions Quæ: Versailles, France, 2022. [Google Scholar]
- Brisson, N. Conceptual Basis, Formalisations and Parameterization of the STICS Crop Model; Éditions Quæ: Versailles, France, 2008. [Google Scholar]
- Lemaire, G.; Jeuffroy, M.-H.; Gastal, F. Diagnosis Tool for Plant and Crop N Status in Vegetative Stage: Theory and Practices for Crop N Management. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar] [CrossRef]
- Zhao, B. Determining of a Critical Dilution Curve for Plant Nitrogen Concentration in Winter Barley. Field Crops Res. 2014, 160, 64–72. [Google Scholar] [CrossRef]
- Nicolardot, B.; Recous, S.; Mary, B. Simulation of C and N Mineralisation during Crop Residue Decomposition: A Simple Dynamic Model Based on the C:N Ratio of the Residues. Plant Soil 2001, 228, 83–103. [Google Scholar] [CrossRef]
- Saxton, K.E.; Rawls, W.J. Soil Water Characteristic Estimates by Texture and Organic Matter for Hydrologic Solutions. Soil Sci. Soc. Am. J. 2006, 70, 1569–1578. [Google Scholar] [CrossRef]
- Malhi, S.S.; Johnston, A.M.; Loeppky, H.; Vera, C.L.; Beckie, H.J.; Bandara, P.M.S. Immediate Effects of Time and Method of Alfalfa Termination on Soil Mineral Nitrogen, Moisture, Weed Control, and Seed Yield, Quality, and Nitrogen Uptake. J. Plant Nutr. 2007, 30, 1059–1081. [Google Scholar] [CrossRef]
- Martel, Y.A.; Lasalle, P. Radiocarbon Dating of Organic Matter from a Cultivated Topsoil in Eastern Canada. Can. J. Soil. Sci. 1977, 57, 375–377. [Google Scholar] [CrossRef]
- Guillaume, S.; Bergez, J.-E.; Wallach, D.; Justes, E. Methodological Comparison of Calibration Procedures for Durum Wheat Parameters in the STICS Model. Eur. J. Agron. 2011, 35, 115–126. [Google Scholar] [CrossRef]
- Hickey, L.T.; Germán, S.E.; Pereyra, S.A.; Diaz, J.E.; Ziems, L.A.; Fowler, R.A.; Platz, G.J.; Franckowiak, J.D.; Dieters, M.J. Speed Breeding for Multiple Disease Resistance in Barley. Euphytica 2017, 213, 64. [Google Scholar] [CrossRef]
- Buis, S.; Wallach, D.; Guillaume, S.; Varella, H.; Lecharpentier, P.; Launay, M.; Guerif, M.; Bergez, J.-E.; Justes, E. The STICS Crop Model and Associated Software for Analysis, Parameterization, and Evaluation. Methods Introd. Syst. Models Agric. Res. 2011, 2, 395–426. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. Available online: https://cran.r-project.org/package=nlme (accessed on 22 September 2023).
- Alfons, A.; cvTools: Cross-Validation Tools for Regression Models. Vignette, R Foundation for Statistical Computing. Available online: https://cran.r-project.org/web/packages/cvTools/cvTools.pdf (accessed on 22 September 2023).
- Correndo, A.A.; Hefley, T.J.; Holzworth, D.P.; Ciampitti, I.A. Revisiting Linear Regression to Test Agreement in Continuous Predicted-Observed Datasets. Agric. Syst. 2021, 192, 103194. [Google Scholar] [CrossRef]
- Falconnier, G.N.; Journet, E.-P.; Bedoussac, L.; Vermue, A.; Chlébowski, F.; Beaudoin, N.; Justes, E. Calibration and Evaluation of the STICS Soil-Crop Model for Faba Bean to Explain Variability in Yield and N2 Fixation. Eur. J. Agron. 2019, 104, 63–77. [Google Scholar] [CrossRef]
- Jamieson, P.D.; Porter, J.R.; Wilson, D.R. A Test of the Computer Simulation Model ARCWHEAT1 on Wheat Crops Grown in New Zealand. Field Crops Res. 1991, 27, 337–350. [Google Scholar] [CrossRef]
- Piñeiro, G.; Perelman, S.; Guerschman, J.P.; Paruelo, J.M. How to Evaluate Models: Observed vs. Predicted or Predicted vs. Observed? Ecol. Model. 2008, 216, 316–322. [Google Scholar] [CrossRef]
- Correndo, A.A.; Hefley, T.; Holzworth, D.P.; Ciampitti, I.A. R-Code Tutorial: Revisiting Linear Regression to Test Agreement in Continuous Predicted-Observed Datasets. Harvard Dataverse 2021. [Google Scholar] [CrossRef]
- Ho, K.M.; Choo, T.M.; Martin, R.A. AC Maple Barley. Can. J. Plant Sci. 2002, 82, 93–94. [Google Scholar] [CrossRef]
- Ho, K.M.; Seaman, W.L.; Choo, T.M.; Martin, R.A.; Rowsell, J.; Guillemette, L.; Dion, Y.; Rioux, S. AC Legend Barley. Can. J. Plant Sci. 2000, 80, 113–115. [Google Scholar] [CrossRef]
- Ho, K.M.; Seaman, W.L.; Choo, T.M.; Martin, R.A. AC Hamilton Barley. Can. J. Plant Sci. 1995, 75, 697–698. [Google Scholar] [CrossRef]
- Spaner, D.; Todd, A.G.; McKenzie, D.B. The Effect of Seeding Rate and Nitrogen Fertilization on Barley Yield and Yield Components in a Cool Maritime Climate. J. Agron. Crop Sci. 2001, 187, 105–110. [Google Scholar] [CrossRef]
- Mapfumo, E.; Chanasyk, D.S.; Puurveen, D.; Elton, S.; Acharya, S. Historic Climate Change Trends and Impacts on Crop Yields in Key Agricultural Areas of the Prairie Provinces in Canada: A Literature Review. Can. J. Plant Sci. 2023, 103, 243–258. [Google Scholar] [CrossRef]
- Calderini, D.F.; Dreccer, M.F.; Slafer, G.A. Consequences of Breeding on Biomass, Radiation Interception and Radiation-Use Efficiency in Wheat. Field Crops Res. 1997, 52, 271–281. [Google Scholar] [CrossRef]
- Gallagher, J.N.; Biscoe, P.V. Radiation Absorption, Growth and Yield of Cereals. J. Agric. Sci. 1978, 91, 47–60. [Google Scholar] [CrossRef]
- Raj Singh, D.S.; Biswas, B.; Mani, J.K. Radiation Interception and Radiation Use Efficiency in Barley. J. Agrometeorol. 2012, 14, 358–362. [Google Scholar]
- Bingham, I.J.; Blake, J.; Foulkes, M.J.; Spink, J. Is Barley Yield in the UK Sink Limited?: I. Post-Anthesis Radiation Interception, Radiation-Use Efficiency and Source–Sink Balance. Field Crops Res. 2007, 101, 198–211. [Google Scholar] [CrossRef]
- Muurinen, S.; Peltonen-Sainio, P. Radiation-Use Efficiency of Modern and Old Spring Cereal Cultivars and Its Response to Nitrogen in Northern Growing Conditions. Field Crops Res. 2006, 96, 363–373. [Google Scholar] [CrossRef]
- Ahmadi, A.; Joudi, M.; Janmohammadi, M. Late Defoliation and Wheat Yield: Little Evidence of Post-Anthesis Source Limitation. Field Crops Res. 2009, 113, 90–93. [Google Scholar] [CrossRef]
- Maydup, M.L.; Antonietta, M.; Guiamet, J.J.; Tambussi, E.A. The Contribution of Green Parts of the Ear to Grain Filling in Old and Modern Cultivars of Bread Wheat (Triticum Aestivum L.): Evidence for Genetic Gains over the Past Century. Field Crops Res. 2012, 134, 208–215. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, Y.; Zhang, Y.; Fischer, T.; Zhao, Z.; Zhou, X.; Wang, Z.; Wang, E. The Contribution of Spike Photosynthesis to Wheat Yield Needs to Be Considered in Process-Based Crop Models. Field Crops Res. 2020, 257, 107931. [Google Scholar] [CrossRef]
- Sansoulet, J.; Pattey, E.; Kröbel, R.; Grant, B.; Smith, W.; Jégo, G.; Desjardins, R.L.; Tremblay, N.; Tremblay, G. Comparing the Performance of the STICS, DNDC, and DayCent Models for Predicting N Uptake and Biomass of Spring Wheat in Eastern Canada. Field Crops Res. 2014, 156, 135–150. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J.; Hastie, T.; Tibshirani, R.; Friedman, J. Model Assessment and Selection. In The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer: Berlin/Heidelberg, Germany, 2009; pp. 219–259. [Google Scholar] [CrossRef]
- Spaner, D.; McKenzie, D.B.; Todd, A.G.; Simms, A.; MacPherson, M.; Woodrow, E.F. Six Years of Adaptive and On-Farm Spring Cereal Research in Newfoundland. Can. J. Plant Sci. 2000, 80, 205–216. [Google Scholar] [CrossRef]
- Maharjan, G.R.; Prescher, A.-K.; Nendel, C.; Ewert, F.; Mboh, C.M.; Gaiser, T.; Seidel, S.J. Approaches to Model the Impact of Tillage Implements on Soil Physical and Nutrient Properties in Different Agro-Ecosystem Models. Soil Tillage Res. 2018, 180, 210–221. [Google Scholar] [CrossRef]
- Maestrini, B.; Mimić, G.; van Oort, P.A.J.; Jindo, K.; Brdar, S.; Athanasiadis, I.N.; van Evert, F.K. Mixing Process-Based and Data-Driven Approaches in Yield Prediction. Eur. J. Agron. 2022, 139, 126569. [Google Scholar] [CrossRef]
- Jing, Q.; Jégo, G.; Bélanger, G.; Chantigny, M.H.; Rochette, P. Simulation of Water and Nitrogen Balances in a Perennial Forage System Using the STICS Model. Field Crops Res. 2017, 201, 10–18. [Google Scholar] [CrossRef]
- Rieux, C.M.; Vanasse, A.; Chantigny, M.H.; Gélinas, P.; Angers, D.A.; Rochette, P.; Royer, I. Yield and Bread-Making Potential of Spring Wheat Under Mineral and Organic Fertilization. Crop Sci. 2013, 53, 1139–1147. [Google Scholar] [CrossRef]
- Rakotovololona, L. Experimental Quantification and Modelling of Production, Water and Nitrogen Flows in Organic Cropping Systems. Ph.D. Thesis, Institut Agronomique, Vétérinaire et Forestier de France, Paris, France, 2018. Available online: http://www.theses.fr/2018iavf0024 (accessed on 23 February 2021). (In French, with English Abstract).
- Bista, D.R.; Heckathorn, S.A.; Jayawardena, D.M.; Mishra, S.; Boldt, J.K. Effects of Drought on Nutrient Uptake and the Levels of Nutrient-Uptake Proteins in Roots of Drought-Sensitive and -Tolerant Grasses. Plants 2018, 7, 28. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar] [CrossRef]
- Kherif, O.; Seghouani, M.; Justes, E.; Plaza-Bonilla, D.; Bouhenache, A.; Zemmouri, B.; Dokukin, P.; Latati, M. The First Calibration and Evaluation of the STICS Soil-Crop Model on Chickpea-Based Intercropping System under Mediterranean Conditions. Eur. J. Agron. 2022, 133, 126449. [Google Scholar] [CrossRef]
- Ziadi, N.; Bélanger, G.; Claessens, A.; Lefebvre, L.; Cambouris, A.N.; Tremblay, N.; Nolin, M.C.; Parent, L.-É. Determination of a Critical Nitrogen Dilution Curve for Spring Wheat. Agron. J. 2010, 102, 241–250. [Google Scholar] [CrossRef]
- Ziadi, N.; Brassard, M.; Bélanger, G.; Cambouris, A.N.; Tremblay, N.; Nolin, M.C.; Claessens, A.; Parent, L.-É. Critical Nitrogen Curve and Nitrogen Nutrition Index for Corn in Eastern Canada. Agron. J. 2008, 100, 271–276. [Google Scholar] [CrossRef]
- Jégo, G.; Sansoulet, J.; Pattey, E.; Beaudoin, N.; Bélanger, G.; Ziadi, N.; Tremblay, N.; Grant, C.; Tremblay, G.; O’Donovan, J.; et al. Determination of Nitrogen Dilution Curves of Corn, Canola, and Spring Wheat in Canada Using Classical and Bayesian Approaches. Eur. J. Agron. 2022, 135, 126481. [Google Scholar] [CrossRef]
| Soil Characteristics | Values |
|---|---|
| Soil texture | Silty clay |
| Soil classification (Food and Agriculture Organization, 2014) | Humic gleysol |
| Clay < 2 µm (g kg−1) at 0–20 cm | 490 |
| Silt (2–50 µm) (g kg−1) | 430 |
| Sand (50–2000 µm) (g kg−1) | 80 |
| Organic N (g kg−1) | 1.7 |
| CaCO3 (%) | <1 |
| pHwater at 0–20 cm | 5.6 |
| Field capacity (% dry-mass soil): | |
| 0–20 cm | 29.0 |
| 20–40 cm | 26.7 |
| 40–100 cm | 25.6 |
| Wilting point (% dry-mass soil): | |
| 0–20 cm | 20.0 |
| 20–40 cm | 19.2 |
| 40–100 cm | 18.6 |
| Bulk density (gsoil cm−3 soil): | |
| 0–20 cm | 1.36 |
| 20–40 cm | 1.50 |
| 40–100 cm | 1.60 |
| Source | AGB (Mg DM ha−1) | GY (Mg DM ha−1) | NCAGB (g kg−1 DM) | NCG (g kg−1 DM) | NU (kg N ha−1) | NAG (kg N ha−1) |
|---|---|---|---|---|---|---|
| p-Value | ||||||
| Year (Y) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| N source type (N) | <0.0001 | <0.0001 | <0.0001 | 0.0008 | <0.0001 | <0.0001 |
| Tillage system (T) | 0.9464 | <0.0001 | <0.0001 | 0.1857 | 0.3647 | 0.0007 |
| Y × N | <0.0001 | <0.0001 | <0.0001 | 0.0535 | <0.0001 | <0.0001 |
| Y × T | <0.0001 | <0.0001 | 0.0002 | 0.1805 | 0.0003 | 0.0002 |
| N × T | 0.0002 | 0.0007 | 0.0328 | 0.4104 | 0.0332 | 0.0199 |
| Y × N × T | 0.6422 | 0.4050 | 0.9160 | 0.9316 | 0.3748 | 0.6536 |
| Mean of field-observed values | ||||||
| N source type | ||||||
| MIN | 4.7 a | 3.0 a | 16.1 b | 19.8 b | 73.2 a | 57.1 a |
| LDM | 3.9 b | 2.4 b | 16.6 a | 20.2 a | 62.0 b | 45.7 b |
| Tillage system | ||||||
| MP | 4.3 a | 2.8 a | 16.6 a | 20.1 a | 67.2 a | 52.2 a |
| CP | 4.3 a | 2.6 b | 16.2 b | 20.0 a | 68.0 a | 50.6 b |
| N source type ∗ Tillage system | ||||||
| MIN-MP | 4.8 a | 3.1 a | 16.2 b | 19.8 b | 74.1 a | 58.8 a |
| MIN-CP | 4.6 b | 2.9 b | 16.0 b | 19.8 b | 72.3 a | 55.4 b |
| LDM-MP | 4.0 c | 2.4 c | 17.0 a | 20.3 a | 60.3 c | 45.6 c |
| LDM-CP | 3.8 d | 2.4 c | 16.3 b | 20.1 ab | 63.7 b | 45.7 c |
| Independent Variable | MAE | RMSE | NRMSE (%) |
|---|---|---|---|
| AGB (Mg DM ha−1) | 0.6 | 0.7 | 17 |
| GY (Mg DM ha−1) | 0.4 | 0.5 | 17 |
| NCAGB (g kg−1 DM) | 1.0 | 1.3 | 8 |
| NCG (g kg−1 DM) | 0.9 | 1.3 | 7 |
| NU (kg N ha−1) | 8.7 | 11.3 | 17 |
| NAG (kg N ha−1) | 7.1 | 9.3 | 18 |
| Parameter Name and Definition | Default Values in STICS | Newly Calibrated Values | Source |
|---|---|---|---|
| Phenological stages | |||
| stlevamf: sum of degree days between the beginning of growth and maximum acceleration of leaf growth (°C d) | 400 | 480 | Optimization |
| stamflax: sum of degree days between the maximum acceleration of leaf growth and the maximum LAI (°C d) | 340 | 420 | Optimization |
| stlevdrp: sum of degree days between the beginning of growth and the beginning of the reproductive stage (°C d) | 940 | 800 | [66,67,68]/ Calculation |
| stdrpmat: sum of degree days between the beginning of grain filling and the maturity (°C d) | 615 | 565 | Calculation |
| Leaves | |||
| dlaimaxbrut: maximum rate of daily increase in LAI (m2 plant−1 °C d−1) | 0.00077 | 0.00028 | Optimization |
| durvief: maximal lifespan of an adult leaf (Q10) | 200 | 180 | Optimization |
| hautmax: maximum height of crop (m) | 1.00 | 0.85 | [66,67,68] |
| Innsen: N stress function active on senescence | −0.17 | −0.18 | Optimization |
| Innturgmin: N stress function active on leaf expansion | −0.65 | −0.73 | Optimization |
| Shoot biomass growth | |||
| teopt: beginning of the thermal optimum plateau for net photosynthesis (°C) | 12 | 16 | Optimization |
| efcroijuv: maximum radiation use efficiency during the juvenile phase (g DM MJ−1) | 2.25 | 1.75 | Optimization |
| efcroiveg: maximum radiation use efficiency during the vegetative phase (g DM MJ−1) | 4.5 | 2.2 | Optimization |
| efcoirepro: maximum radiation use efficiency during the reproductive phase (g DM MJ−1) | 4.5 | 4.1 | Optimization |
| Nitrogen | |||
| INNimin: instantaneous NNI corresponding to INNmin | −0.5 | −0.77 | Optimization |
| Yield formation | |||
| nbgrmax: maximum number of grains per surface area (grain m−2) | 26,000 | 17,500 | [69] |
| pgrainmaxi: maximum weight of one grain (g) | 0.044 | 0.046 | [66,67,68] |
| nbjgrain: number of days used to compute viable grains number (d) | 20 | 30 | Optimization |
| cgrain: slope of relationship between grain number and growth rate | 0.028 | 0.132 | Optimization |
| vitircarb: rate of increase in the C harvested index vs. time (g g−1d−1) | 0.0192 | 0.031 | Optimization |
| vitirazo: rate of increase in the N harvest index vs. time (g g−1d−1) | 0.0308 | 0.038 | Optimization |
| Variables | n | Mean Obs | Mean Pred | NME | NRMSE | EF |
|---|---|---|---|---|---|---|
| 0.06 ≤ exofac < 0.14 | ||||||
| AGB (Mg DM ha−1) | 36 | 4.4(1.4) * | 4.2(0.8) | 5 | 23 | 0.4 |
| GY (Mg DM ha−1) | 36 | 2.7(1.0) | 2.7(0.5) | −1 | 26 | 0.5 |
| NCAGB (g kg−1 DM) | 20 | 15(2) | 17(2) | −14 | 21 | −1.7 |
| NCG (g kg−1 DM) | 20 | 19(2) | 21(2) | −10 | 15 | −2.5 |
| NU (kg N ha−1) | 20 | 62.5(13.9) | 74.3(7.8) | −19 | 25 | −0.3 |
| NAG (kg N ha−1) | 20 | 47.0(14.9) | 59.1(6.2) | −26 | 34 | −0.2 |
| 0 < exofac < 0.06 | ||||||
| AGB (Mg DM ha−1) | 40 | 4.4(1.3) | 4.4(0.7) | −1 | 18 | 0.6 |
| GY (Mg DM ha−1) | 40 | 2.8(0.9) | 2.9(0.4) | −3 | 22 | 0.6 |
| NCAGB (g kg−1 DM) | 36 | 17(2) | 17(1) | −1 | 11 | −0.4 |
| NCG (g kg−1 DM) | 36 | 20(2) | 21(2) | −4 | 10 | −0.6 |
| NU (kg N ha−1) | 36 | 67.3(12.0) | 71.8(7.3) | −7 | 16 | 0.1 |
| NAG (kg N ha−1) | 36 | 51.0(10.6) | 57.2(5.9) | −12 | 23 | −0.3 |
| exofac = 0 | ||||||
| AGB (Mg DM ha−1) | 20 | 3.7(0.6) | 4.0(0.6) | −9 | 20 | −0.5 |
| GY (Mg DM ha−1) | 20 | 2.4(0.4) | 2.6(0.4) | −8 | 19 | −0.5 |
| NCAGB (g kg−1 DM) | 12 | 17(1) | 15(1) | 17 | 18 | −11.5 |
| NCG (g kg−1 DM) | 12 | 22(1) | 18(1) | 17 | 19 | −12.3 |
| NU (kg N ha−1) | 12 | 65.7(10.9) | 63.9(5.1) | 3 | 18 | −0.2 |
| NAG (kg N ha−1) | 12 | 53.1(9.2) | 50.9(4.0) | 4 | 18 | −0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravelojaona, N.; Jégo, G.; Ziadi, N.; Mollier, A.; Lafond, J.; Karam, A.; Morel, C. STICS Soil–Crop Model Performance for Predicting Biomass and Nitrogen Status of Spring Barley Cropped for 31 Years in a Gleysolic Soil from Northeastern Quebec (Canada). Agronomy 2023, 13, 2540. https://doi.org/10.3390/agronomy13102540
Ravelojaona N, Jégo G, Ziadi N, Mollier A, Lafond J, Karam A, Morel C. STICS Soil–Crop Model Performance for Predicting Biomass and Nitrogen Status of Spring Barley Cropped for 31 Years in a Gleysolic Soil from Northeastern Quebec (Canada). Agronomy. 2023; 13(10):2540. https://doi.org/10.3390/agronomy13102540
Chicago/Turabian StyleRavelojaona, Nomena, Guillaume Jégo, Noura Ziadi, Alain Mollier, Jean Lafond, Antoine Karam, and Christian Morel. 2023. "STICS Soil–Crop Model Performance for Predicting Biomass and Nitrogen Status of Spring Barley Cropped for 31 Years in a Gleysolic Soil from Northeastern Quebec (Canada)" Agronomy 13, no. 10: 2540. https://doi.org/10.3390/agronomy13102540
APA StyleRavelojaona, N., Jégo, G., Ziadi, N., Mollier, A., Lafond, J., Karam, A., & Morel, C. (2023). STICS Soil–Crop Model Performance for Predicting Biomass and Nitrogen Status of Spring Barley Cropped for 31 Years in a Gleysolic Soil from Northeastern Quebec (Canada). Agronomy, 13(10), 2540. https://doi.org/10.3390/agronomy13102540








