Response of Strawberry to the Substitution of Blue Light by Green Light in an Indoor Vertical Farming System
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Cultural Conditions
2.2. Indoor Vertical Farming System and Growing Conditions
2.3. Green and Blue Light Treatments
2.4. Growth Parameters
2.5. Leaf Nutrient Status
2.6. Gas Exchange Parameters
2.7. Fruit Firmness and Selected Ion Content
2.8. Fruit Organoleptic and Bioactive Compounds
2.8.1. Total and Reducing Sugars
2.8.2. Anthocyanins
2.8.3. Antioxidant Activity by DPPH
2.8.4. Antioxidant Activity by FRAP
2.8.5. Antioxidant Activity by ABTS
2.8.6. Total Phenols and Flavonoids
2.8.7. Total Soluble Solids and Titratable Acidity
2.9. Experimental Design and Statistical Analysis
3. Results
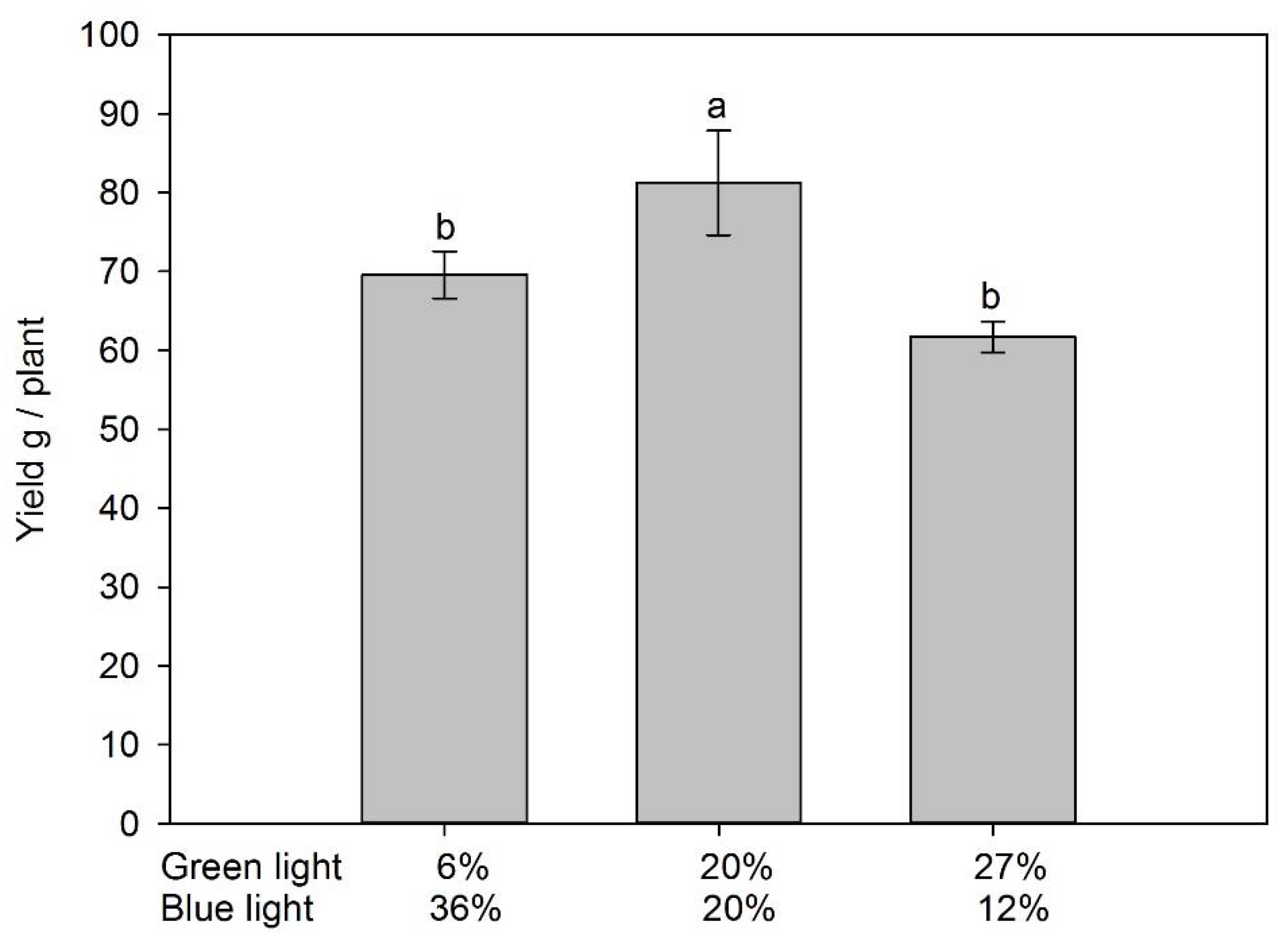
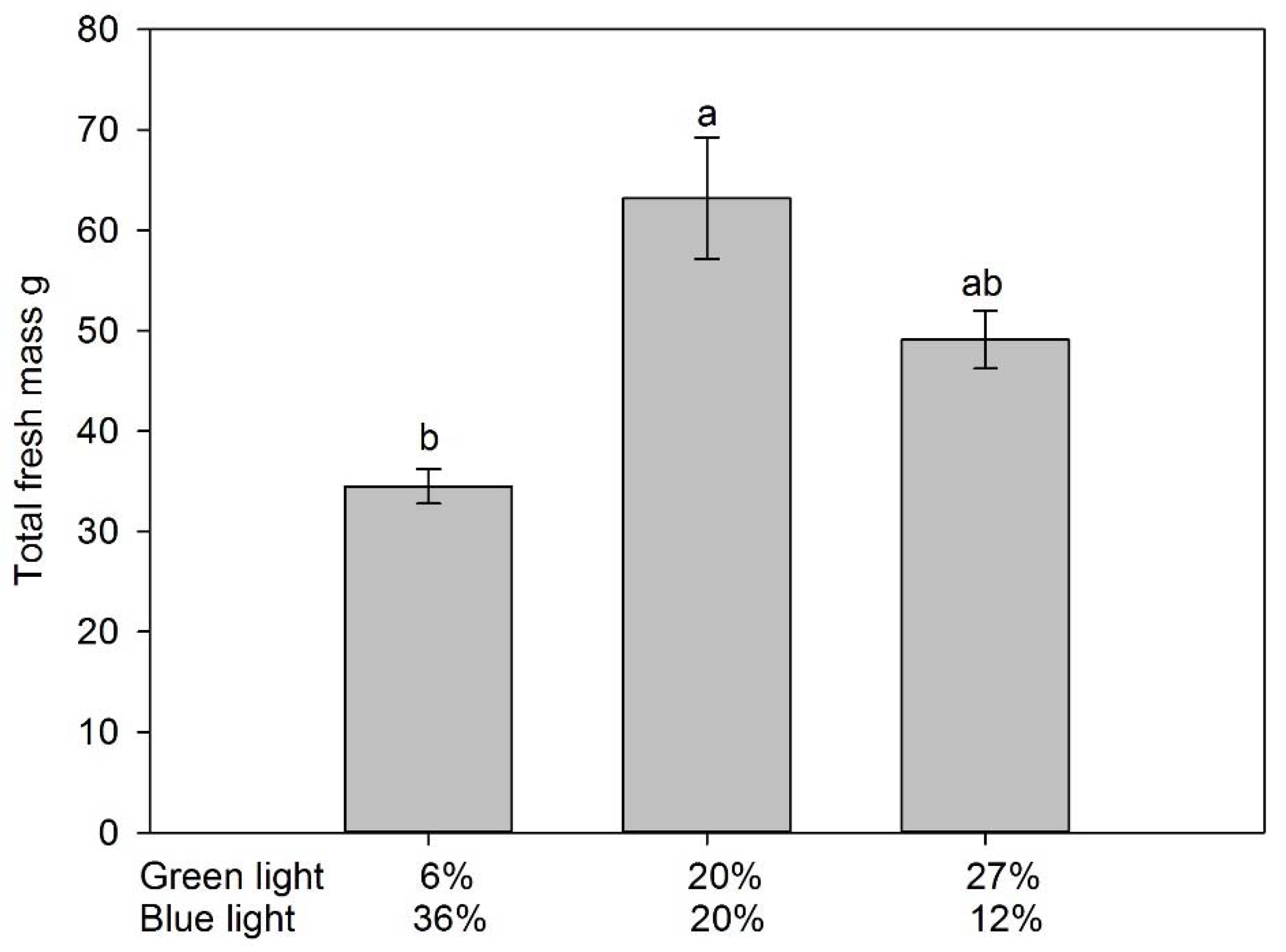
3.1. Fruit Yield and Vegetative Growth
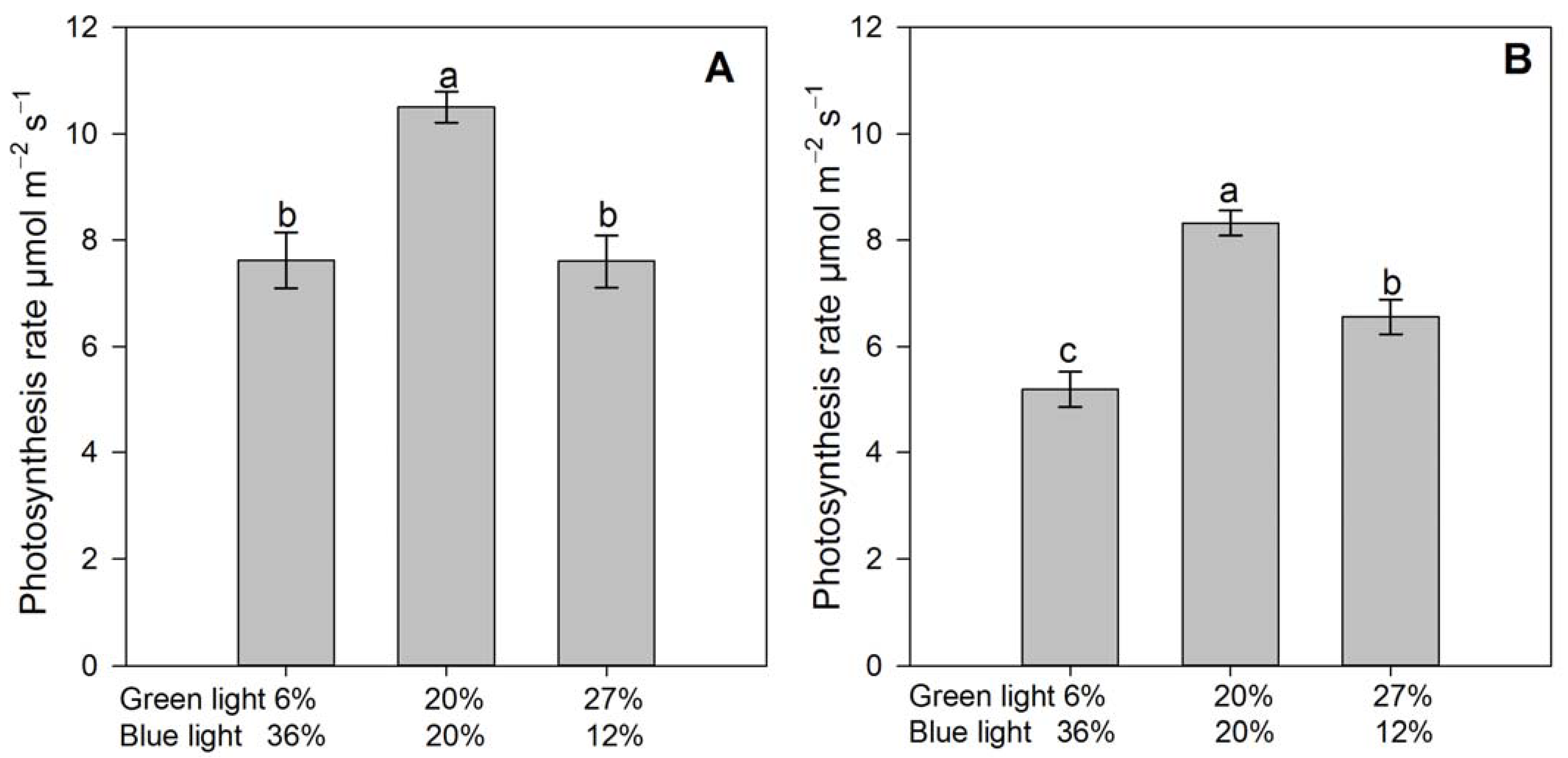
3.2. Gas Exhange Parameters
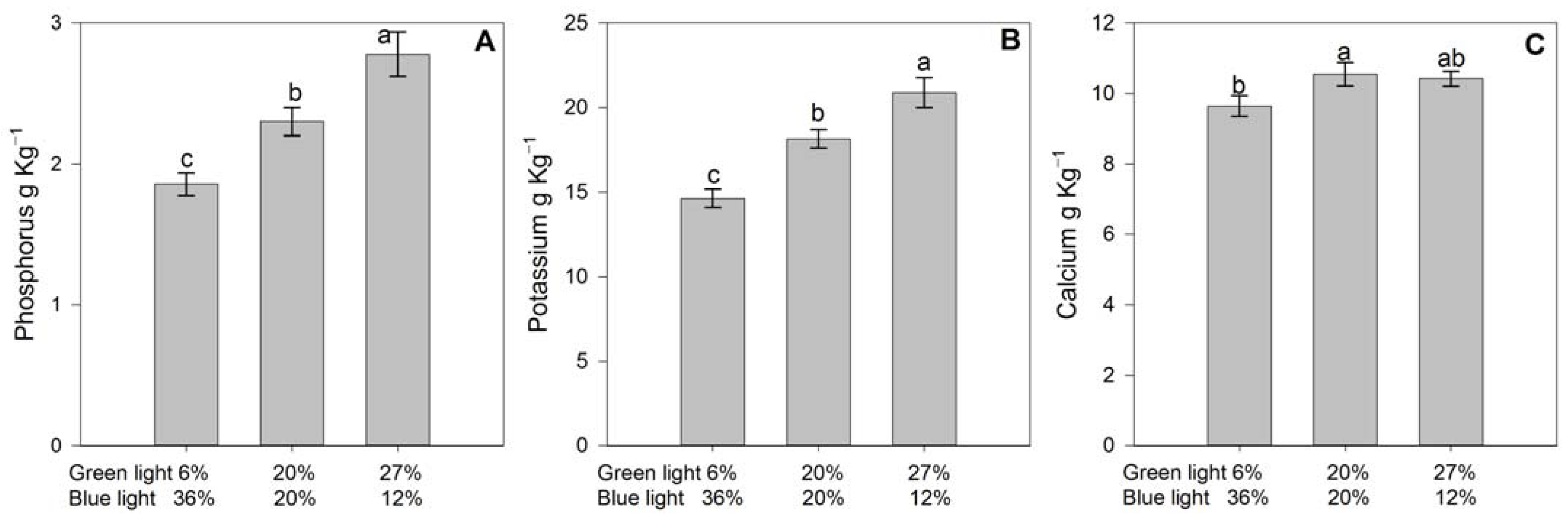
3.3. Leaf Nutrient Status
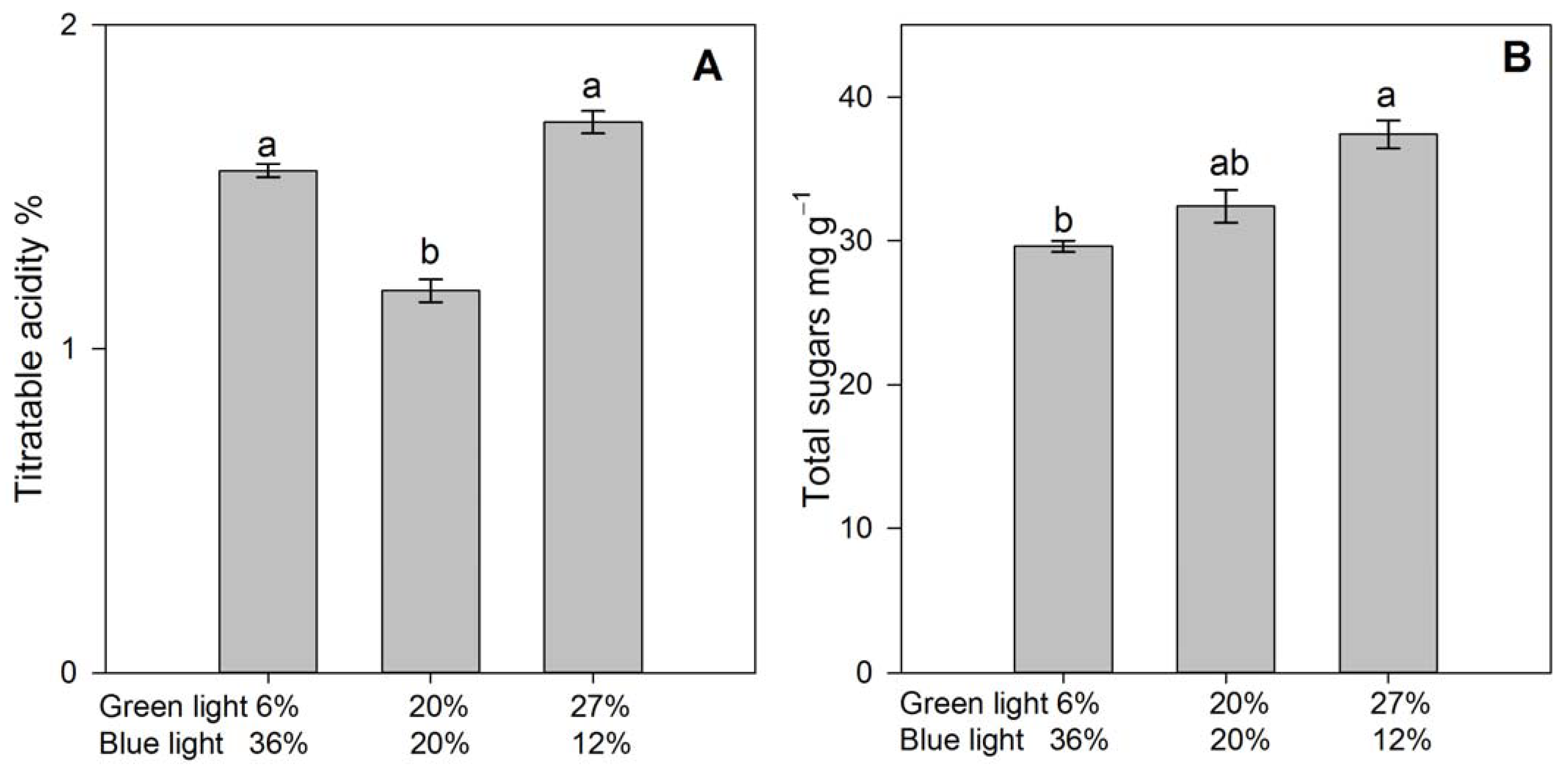
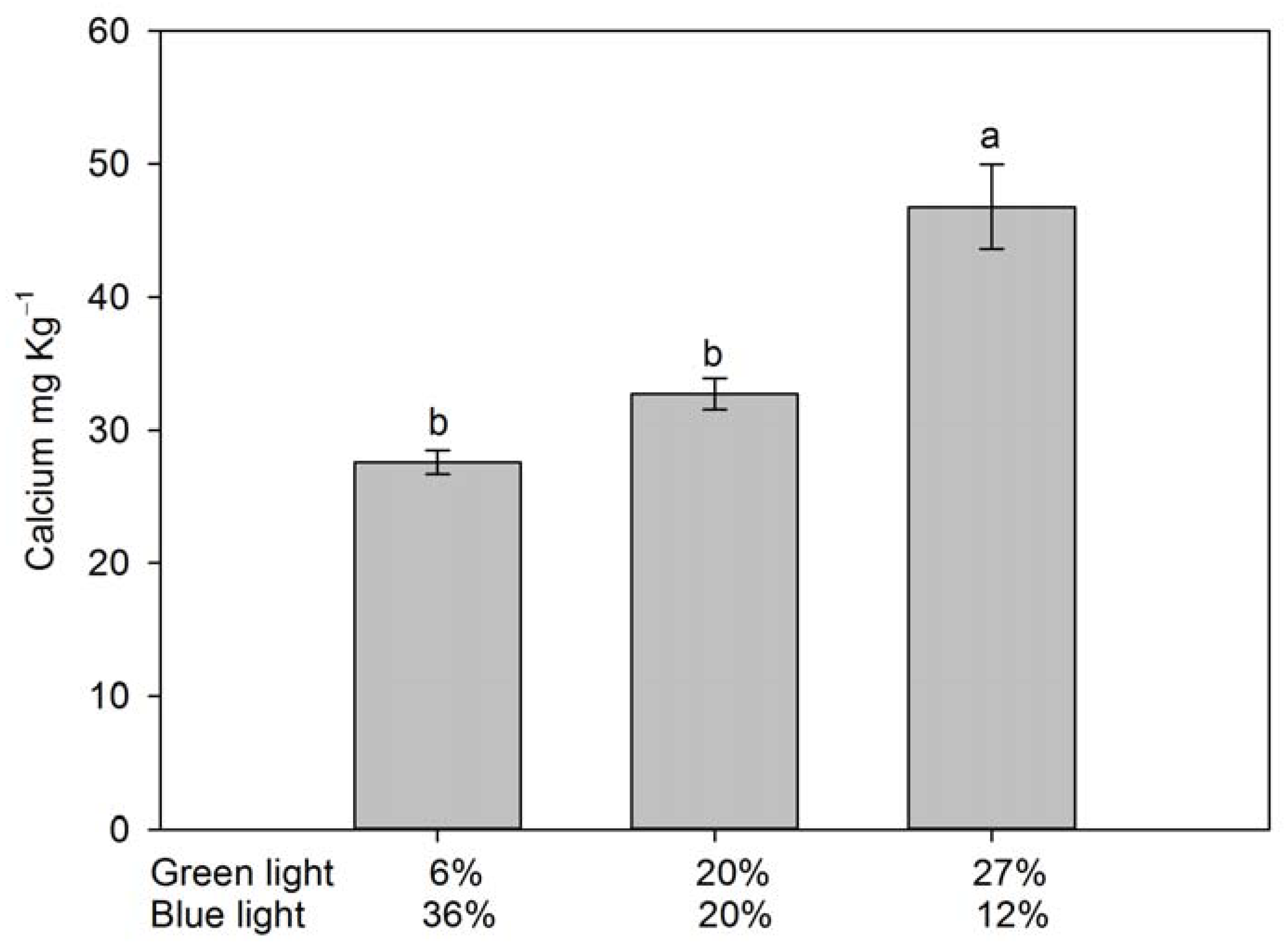
3.4. Fruit Quality and Selected Ion Concentrations in Fruit Extract
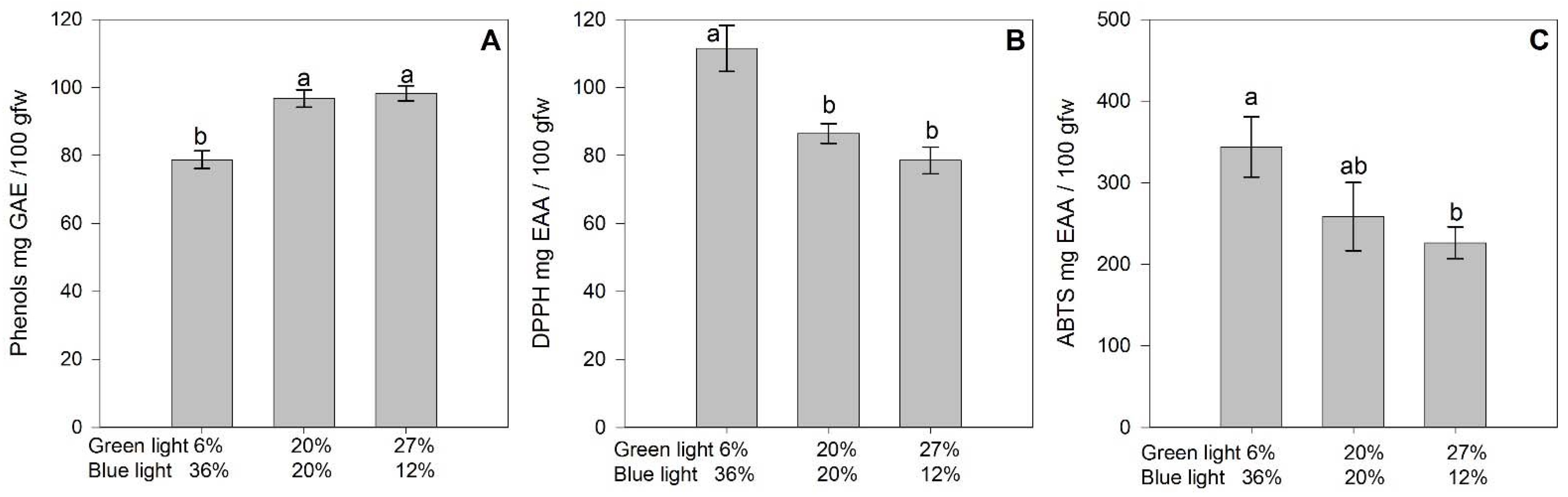
3.5. Fruit Bioactive Compounds
4. Discussion
4.1. Plant Growth and Yield
4.2. Gas Exchange Parameters
4.3. Fruit Quality Atributes
4.4. Fruit Antioxidant Capacity
4.5. Leaf and Fruit Nutrient Status
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kosai, T.; Niu, G.; Takagaki, M. Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Associated Press: London, UK, 2019; p. 405. [Google Scholar]
- Yoshida, H.; Mizuta, D.; Fukuda, N.; Hikosaka, S.; Goto, E. Effects of varying light quality from single-peak blue and red light-emitting diodes during nursery period on flowering, photosynthesis, growth, and fruit yield of everbearing strawberry. Plant Biotechnol. 2016, 33, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, K.; Ito, E.; Sago, Y.; Yasutake, D.; Miyoshi, Y.; Kitano, M.; Miyauchi, K.; Okimura, M.; Imai, S. High yields of strawberry by applying vertically-moving beds on the basis of leaf photosynthesis. Environ. Control. Biol. 2012, 50, 143–152. [Google Scholar] [CrossRef][Green Version]
- Hidaka, K.; Dan, K.; Imamura, H.; Miyoshi, Y.; Takayama, T.; Sameshima, K.; Okimura, M. Effect of supplemental lighting from different light sources on growth and yield of strawberry. Environ. Control. Biol. 2013, 51, 41–47. [Google Scholar] [CrossRef]
- Maeda, K.; Ito, Y. Effect of different PPFDs and photoperiods on growth and yield of everbearing strawberry ‘Elan’ in plant factory with white LED lighting. Environ. Control. Biol. 2020, 58, 99–104. [Google Scholar] [CrossRef]
- Razzak, M.A.; Asaduzzaman, M.; Tanaka, H.; Asao, T. Effects of supplementing green light to red and blue light on the growth and yield of lettuce in plant factories. Sci. Hortic. 2022, 305, 111429. [Google Scholar] [CrossRef]
- Bian, Z.; Yang, Q.; Li, T.; Cheng, R.; Barnett, Y.; Lu, C. Study of the beneficial effects of green light on lettuce grown under short-term continuous red and blue light-emitting diodes. Physiol. Plant. 2018, 164, 226–240. [Google Scholar] [CrossRef]
- Bian, Z.; Wang, Y.; Zhang, X.; Grundy, S.; Hardy, K.; Yang, Q.; Lu, C. A transcriptome analysis revealing the new insight of green light on tomato plant growth and drought stress tolerance. Front. Plant Sci. 2021, 12, 2325. [Google Scholar] [CrossRef]
- Claypool, N.B.; Lieth, J.H. Green light improves photosystem stoichiometry in cucumber seedlings (Cucumis sativus) compared to monochromatic red light. Plants 2021, 10, 824. [Google Scholar] [CrossRef]
- Soltanpour, P.N.; Johnson, G.W.; Workman, S.M.; Jones, J.B.; Miller, R.O. Inductively coupled plasma emission spectrometry and inductively coupled plasma mass spectrometry. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of North America: Madison, WI, USA, 1996; pp. 91–139. [Google Scholar]
- Bremner, J.M. Total nitrogen. In Methods of Soil Analysis. Part II. Chemical Methods; Sparks, D.L., Ed.; Springer Soil Science Society of America: Madison, WI, USA, 1996; pp. 1085–1086. [Google Scholar]
- Somogy, A.; Rosta, K.; Puszyai, P.; Tulassay, Z.; Nagy, G. Antioxidant measurements. Physiol. Meas. 2007, 8, 41–55. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Culiver, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. In Lebensmittel Wissenschaft and Technologie. Food Science Technology; Springer: Amsterdam, The Netherlands, 1995; Volume 28, pp. 25–30. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical action decolonization assay free. Radic. Biol. Med. 1999, 26, 1231–1337. [Google Scholar] [CrossRef] [PubMed]
- Margraf, T.; Karnopp, A.R.; Rosso, N.D.; Granato, D. Comparison between Folin-Ciocalteu and Prussian blue assays to estimate the total phenolic content of juices and teas using 96 well microplates. J. Food Sci. 2015, 80, 2397–2403. [Google Scholar] [CrossRef] [PubMed]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Aplicultural Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemist: Arlington, VA, USA, 1990; p. 1298. [Google Scholar]
- Guiamba, H.; Zhang, X.; Sierka, E.; Lin, K.; Ali, M.M.; Ali, W.M.; Lamlon, S.F.; Kalaji, H.M.; Telesinski, A.; Yousef, A.F.; et al. Enhancement of photosynthesis efficiency and yield of strawberry (Fragaria ananassa Duch.) plants via LED systems. Front. Plant Sci. 2022, 13, 918038. [Google Scholar] [CrossRef]
- Trojak, M.; Skowron, E.; Sobala, T.; Kocurek, M.; Pałyga, J. Effects of partial replacement of red by green light in the growth spectrum on photomorphogenesis and photosynthesis in tomato plants. Photosynth. Res. 2022, 151, 295–312. [Google Scholar] [CrossRef]
- Nguyen, T.P.D.; Jang, D.C.; Tran, T.T.H.; Nguyen, Q.T.; Kim, I.S.; Hoang, T.L.H.; Vu, N.T. Influence of green light added with red and blue LEDs on the growth, leaf microstructure and quality of spinach (Spinacia oleracea L.). Agronomy 2021, 11, 1724. [Google Scholar] [CrossRef]
- Torres-Quezada, E.A.; Zotarelli, L.; Whitaker, V.M.; Santos, B.M.; Hernandez-Ochoa, I. Initial crown diameter of strawberry bare-root transplants affects early and total fruit yield. HortTechnology 2015, 25, 203–208. [Google Scholar] [CrossRef]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef]
- Liu, J.; Van Iersel, M.W. Photosynthetic physiology of blue, green, and red light: Light intensity effects and underlying mechanisms. Front. Plant Sci. 2021, 12, 619987. [Google Scholar] [CrossRef]
- Inoue, S.I.; Kinoshita, T. Blue light regulation of stomatal opening and the plasma membrane H+-ATPase. Plant Physiol. 2017, 174, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Vialet-Chabrand, S.; Matthews, J.S.; Lawson, T. Light, power, action! Interaction of respiratory energy-and blue light-induced stomatal movements. New Phytol. 2021, 231, 2231–2246. [Google Scholar] [PubMed]
- Díaz-Galián, M.V.; Torres, M.; Sanchez-Pagán, J.D.; Navarro, P.J.; Weiss, J.; Egea-Cortines, M. Enhancement of strawberry production and fruit quality by blue and red LED lights in research and commercial greenhouses. S. Afr. J. Bot. 2021, 140, 269–275. [Google Scholar] [CrossRef]
- Pašalić, B.; Todorović, V.; Koleška, I.; Bosančić, B.; Đekić, N. Effects of salinity on color changes, sugar and acid concentration in tomato fruit. Agriclturae Conspec. Sci. 2017, 81, 137–142. [Google Scholar]
- Iglesias, M.J.; García-López, J.; Collados-Luján, J.F.; López-Ortiz, F.; Díaz, M.; Toresano, F.; Camacho, F. Differential response to environmental and nutritional factors of high-quality tomato varieties. Food Chem. 2015, 176, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.G.; Kwon, J.K.; Moon, B.Y.; Kang, N.J.; Park, K.S.; Cho, M.W.; Kim, Y.C. Effect of different light emitting diode (LED) lights on the growth characteristics and the phytochemical production of strawberry fruits during cultivation. Korean J. Hortic. Sci. Technol. 2013, 31, 56–64. [Google Scholar] [CrossRef][Green Version]
- Choi, H.G.; Moon, B.Y.; Kang, N.J. Effects of LED light on the production of strawberry during cultivation in a plastic greenhouse and in a growth chamber. Sci. Hortic. 2015, 189, 22–31. [Google Scholar] [CrossRef]
- Ouzounis, T.; Razi Parjikolaei, B.; Fretté, X.; Rosenqvist, E.; Ottosen, C.O. Predawn and high intensity application of supplemental blue light decreases the quantum yield of PSII and enhances the amount of phenolic acids, flavonoids, and pigments in Lactuca sativa. Front. Plant Sci. 2015, 6, 19. [Google Scholar] [CrossRef]
- Bian, Z.H.; Cheng, R.F.; Yang, Q.C.; Wang, J.; Lu, C. Continuous light from red, blue, and green light-emitting diodes reduces nitrate content and enhances phytochemical concentrations and antioxidant capacity in lettuce. J. Am. Soc. Hortic. Sci. 2016, 141, 186–195. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: London, UK, 2012; pp. 135–189. [Google Scholar]
- Ma, Y.; Hu, L.; Wu, Y.; Tang, Z.; Xiao, X.; Lyu, J.; Xie, J.; Yu, J. Green light partial replacement of red and blue light improved drought tolerance by regulating water use efficiency in cucumber seedlings. Front. Plant Sci. 2022, 13, 878932. [Google Scholar] [CrossRef]
- Wang, Z.; Yamaji, N.; Huang, S.; Zhang, X.; Shi, M.; Fu, S.; Guangzhe, Y.; Jian, F.M.; Xia, J. OsCASP1 is required for Casparian strip formation at endodermal cells of rice roots for selective uptake of mineral elements. Plant Cell 2019, 31, 2636–2648. [Google Scholar] [CrossRef] [PubMed]

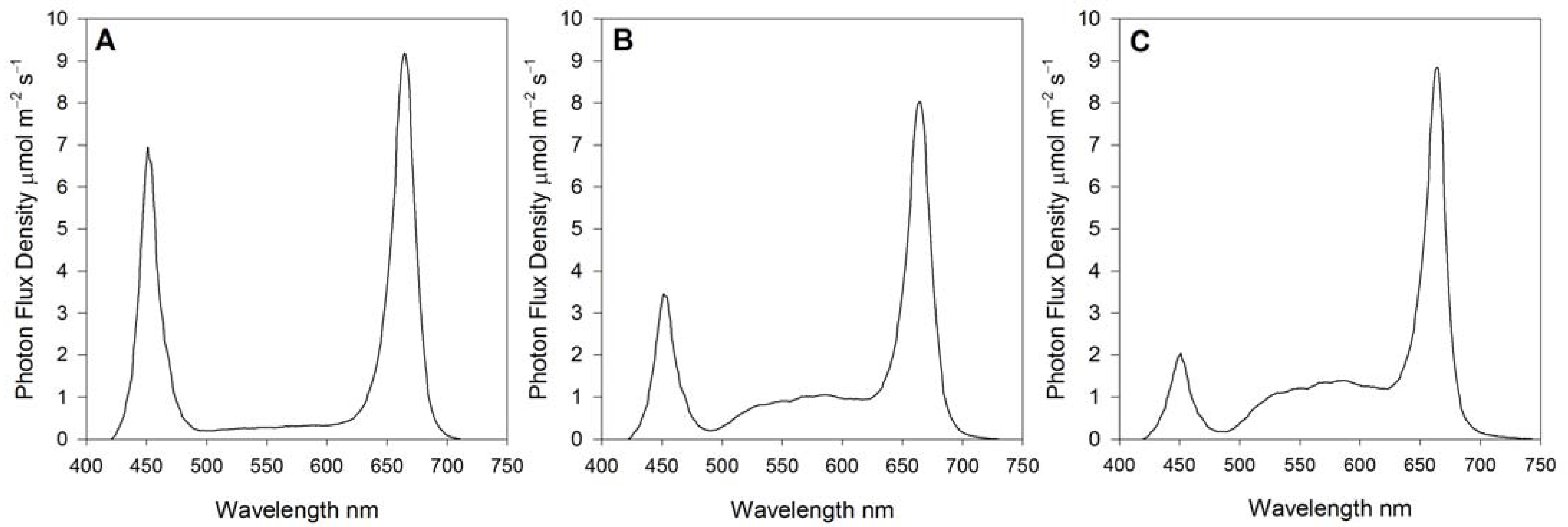
| Blue Light 400–500 nm | Green Light 500–600 nm | Red Light 600–700 nm | Photosynthetic Photon Flux Density | Blue Light 400–500 nm | Green Light 500–600 nm | Red Light 600–700 nm |
|---|---|---|---|---|---|---|
| µmol m−2 s−1 | % | |||||
| 155.6 | 27.7 | 251.6 | 434.9 | 36 | 6 | 58 |
| 84.5 | 84.1 | 252.0 | 420.6 | 20 | 20 | 60 |
| 52.8 | 112.9 | 261.9 | 427.5 | 12 | 27 | 61 |
| Green/Blue Light | Fruit Number | Leaf Number * | Number of Crowns | Fresh Mass (g) | ||
|---|---|---|---|---|---|---|
| Root | Leaves | Crowns | ||||
| 6%/36% | 5.75 ± 0.44 | 20.7 ± 2.94b | 1.63 ± 0.33b | 12.4 ± 1.97b | 15.9 ± 1.40 | 9.28 ± 1.23 |
| 20%/20% | 7.67 ± 1.45 | 25.8 ± 3.22ab | 1.80 ± 0.27b | 34.3 ± 8.49a | 22.1 ± 2.66 | 11.3 ± 1.22 |
| 27%/12% | 5.40 ± 0.30 | 33.9 ± 5.84a | 3.47 ± 0.21a | 24.7 ± 3.10ab | 21.5 ± 3.22 | 12.6 ± 0.34 |
| ANOVA | p = 0.207 | p = 0.018 | p < 0.001 | p = 0.018 | p = 0.119 | p = 0.278 |
| Green/Blue Light | Dry Mass (g) | |||
|---|---|---|---|---|
| Total * | Root | Leaves | Crowns | |
| 6%/36% | 12.9 ± 1.13b | 4.00 ± 0.46b | 5.88 ± 0.34 | 3.08 ± 0.60 |
| 20%/20% | 19.5 ± 6.47a | 10.2 ± 1.84a | 6.85 ± 0.40 | 4.11 ± 0.52 |
| 27%/12% | 15.8 ± 2.03ab | 8.39 ± 0.47a | 6.19 ± 0.76 | 4.12 ± 0.16 |
| ANOVA | p = 0.021 | p = 0.013 | p = 0.527 | p = 0.312 |
| Green/Blue Light | Transpiration Rate mmol H2O m−2 s−1 * | CO2 Internal Concentration ppm | Stomatic Conductance mmol H2O m−2 s−1 |
|---|---|---|---|
| 6%/36% | 3.26 ± 0.30a | 281.2 ± 8.92a | 0.138 ± 0.02a |
| 20%/20% | 3.23 ± 0.29a | 250.8 ± 5.49b | 0.147 ± 0.02a |
| 27%/12% | 2.21 ± 0.19b | 262.1 ± 8.03b | 0.108 ± 0.01b |
| ANOVA | p < 0.001 | p = 0.002 | p = 0.048 |
| Green/Blue Light | Transpiration Rate mmol H2O m−2 s−1 * | CO2 Internal Concentration ppm | Stomatic Conductance mmol H2O m−2 s−1 |
|---|---|---|---|
| 6%/36% | 1.76 ± 0.23ab | 267.9 ± 10.18a | 0.0783 ± 0.012b |
| 20%/20% | 2.00 ± 0.18a | 239.5 ± 6.93b | 0.1008 ± 0.010a |
| 27%/12% | 1.71 ± 0.21b | 262.9 ± 7.97a | 0.0921 ± 0.014ab |
| ANOVA | p = 0.049 | p < 0.001 | p = 0.036 |
| Green/Blue Light | N g Kg−1 | Mg g Kg−1 | Firmness N | Total Soluble Solids * °Brix | Reducing Sugars mg g−1 |
|---|---|---|---|---|---|
| 6%/36% | 21.6 ± 1.71 | 3.27 ± 0.19 | 0.54 ± 0.04 | 9.08 ± 0.28b | 3.66 ± 0.16 |
| 20%/20% | 23.3 ± 1.56 | 3.39 ± 0.17 | 0.49 ± 0.14 | 8.25 ± 0.29b | 3.45 ± 0.23 |
| 27%/12% | 23.9 ± 2.24 | 3.15 ± 0.13 | 0.50 ± 0.02 | 12.79 ± 1.23a | 3.41 ± 0.17 |
| ANOVA | p = 0.186 | p = 0.129 | p = 0.282 | p < 0.001 | p = 0.627 |
| Green/Blue Light | NO3− mg kg−1 | K mg kg−1 | Flavonoids mg EQ/100 gfw | Anthocyanins mg g−1 | FRAPS mg EAA/100 gfw |
|---|---|---|---|---|---|
| 6%/36% | 157 ± 14.05 | 5290 ± 281.28 | 11.63 ± 0.88 | 25.11 ± 3.09 | 32.48 ± 5.60 |
| 20%/20% | 158 ± 10.84 | 5497 ± 276.01 | 12.96 ± 0.23 | 23.75 ± 2.65 | 21.67 ± 3.10 |
| 27%/12% | 173 ± 10.90 | 6077 ± 257.74 | 12.62 ± 1.21 | 23.50 ± 1.29 | 22.97 ± 4.14 |
| ANOVA | p = 0.617 | p = 0.170 | p = 0.448 | p = 0.852 | p = 0.244 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avendaño-Abarca, V.H.; Alvarado-Camarillo, D.; Valdez-Aguilar, L.A.; Sánchez-Ortíz, E.A.; González-Fuentes, J.A.; Cartmill, A.D. Response of Strawberry to the Substitution of Blue Light by Green Light in an Indoor Vertical Farming System. Agronomy 2023, 13, 99. https://doi.org/10.3390/agronomy13010099
Avendaño-Abarca VH, Alvarado-Camarillo D, Valdez-Aguilar LA, Sánchez-Ortíz EA, González-Fuentes JA, Cartmill AD. Response of Strawberry to the Substitution of Blue Light by Green Light in an Indoor Vertical Farming System. Agronomy. 2023; 13(1):99. https://doi.org/10.3390/agronomy13010099
Chicago/Turabian StyleAvendaño-Abarca, Víctor H., Daniela Alvarado-Camarillo, Luis Alonso Valdez-Aguilar, Estanislado A. Sánchez-Ortíz, José A. González-Fuentes, and Andrew D. Cartmill. 2023. "Response of Strawberry to the Substitution of Blue Light by Green Light in an Indoor Vertical Farming System" Agronomy 13, no. 1: 99. https://doi.org/10.3390/agronomy13010099
APA StyleAvendaño-Abarca, V. H., Alvarado-Camarillo, D., Valdez-Aguilar, L. A., Sánchez-Ortíz, E. A., González-Fuentes, J. A., & Cartmill, A. D. (2023). Response of Strawberry to the Substitution of Blue Light by Green Light in an Indoor Vertical Farming System. Agronomy, 13(1), 99. https://doi.org/10.3390/agronomy13010099







