Optimization of In Vitro Propagation of Pear (Pyrus communis L.) ‘Pyrodwarf®(S)’ Rootstock
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Explant Disinfection
2.3. Establishment of In Vitro Culture
2.4. Shoot Multiplication and Root Induction
2.5. Acclimatization Process

2.6. Measured Traits and Data Analysis
3. Results
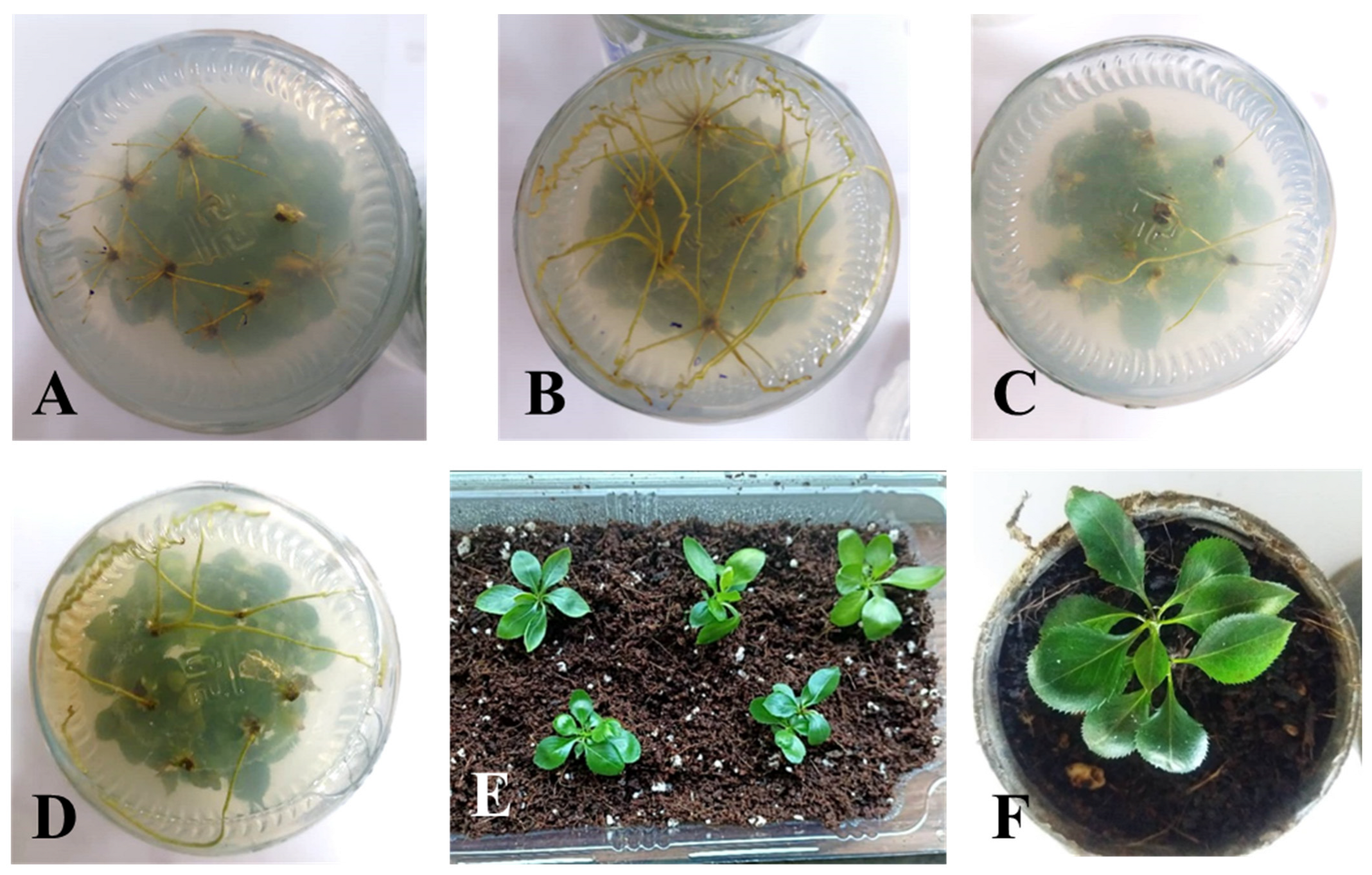
3.1. Microshoot Establishment
3.2. Shoot Proliferation
3.3. Root Induction and Acclimatization
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thakur, A.; Dalal, R.P.S.; Navjot, G. Micropropagation of pear (Pyrus spp.): A review. Agric. Rev. 2008, 29, 260–270. [Google Scholar]
- Dongxia, W.; Pauliina, P.; Iiris, L.; Sanna, F.; Tuuli, H.; Jaana, L.; Tapani, R. Rehardening capacity in the shoots and buds of three European pears (Pyrus communis [L.]) cultivars following a warm spell in midwinter. Sci. Hort. 2020, 273, 109638. [Google Scholar] [CrossRef]
- Claveria, E.; Asin, L.; Iglesias, I.; Vilardell, P.; Bonany, J.; Simard, M.H.; Dolcet-Sanjuan, R. In vitro screening for tolerance to iron chlorosis as a reliable selection tool in a pear rootstocks breeding program. Acta Hortic. 2010, 935, 199–206. [Google Scholar] [CrossRef]
- Aygun, A.; Dumanoglu, H. In vitro shoot proliferation and in vitro and ex vitro root formation of Pyrus elaeagrifolia Pallas. Front. Plant Sci. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, S.; Yadollahi, A.; Arab, M.M.; Soltani, M.; Eftekhari, M.; Sabzalipoor, H.; Sheikhi, A.; Shiri, J. Combining gene expression programming and genetic algorithm as a powerful hybrid modeling approach for pear rootstocks tissue culture media formulation. Plant Met. 2019, 15, 136. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, A.; Khadem, A.; Moradian, M.; Kharrazi, M. Optimization of in vitro culture of pear rootstock (Pyrodwarf). J. Hortic. Sci. 2018, 32, 301–310. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–479. [Google Scholar] [CrossRef]
- Quoirin, M.; Lepoivre, P. Etude de milieux adaptes aux cultures in vitro de Prunus. Acta Hort. 1977, 78, 437–442. [Google Scholar] [CrossRef]
- Driver, J.A.; Kuniyuki, H. In vitro propagation of Paradox walnut rootstock. HortScience 1984, 19, 507–509. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B.H. Woody plant medium (WPM)—A mineral nutrient formation for microculture for woody plant species. HortScience 1981, 16, 453. [Google Scholar]
- Bommineni, V.R.; Mathews, H.; Samuel, S.B.; Kramer, M.; Wagner, D.R. A new method for rapid in vitro propagation of apple and pear. HortScience 2001, 36, 1102–1106. [Google Scholar] [CrossRef]
- Bell, R.L.; Reed, B.M. In vitro tissue culture of pear: Advances in techniques for micropropagation and germplasm preservation. Proc. 8th IS Pear. Acta Hort. 2002, 596, 412–418. [Google Scholar] [CrossRef]
- Anirudh, T.; Kanwar, J.S. Micropropagation of ‘Wild pear’ Pyrus pyrifolia (Burm F.) Nakai. II. Induction of rooting. Not. Bot. Hort. Agrobot. Cluj. 2008, 36, 104–111. [Google Scholar] [CrossRef]
- Ružić, D.; Vujović, T.; Milenković, S.; Cerović, R.; Miletić, R. The influence of imidazole fungicides on multiplication in vitro of pyrodwarf pear rootstock. Aus. J. Crop Sci. 2008, 1, 63–68. [Google Scholar] [CrossRef]
- Ružić, D.; Vujović, T.; Nikolić, D.; Cerović, R. In vitro growth responses of the ‘Pyrodwarf’ pear rootstock to cytokinin types. Rom. Biotechnol. Lett. 2011, 16, 6630–6637. [Google Scholar]
- Chevreau, E.; Thibault, B.; Arnaud, Y. Micropropagation of Pear (Pyrus communis L.). In Hightech and Micropropagation II; Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin, Germany, 1992; Volume 18, pp. 244–261. [Google Scholar]
- Reed, B.M.; DeNoma, J.; Wada, S.; Postman, J. Micropropagation of pear (Pyrus sp.). In Methods in Molecular Biology; Humana Press: Clifton, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Shen, X.S.; Mullins, M.G. Propagation in vitro of pear, Pyrus communis L., cultivars “William’s Bon Chretien”, “Packham’s Triumph” and “Beurre Bosc”. Sci. Hort. 1984, 23, 51–57. [Google Scholar] [CrossRef]
- Dolcet-Sanjuan, R.; Mok, D.W.S.; Mok, M.C. Micropropagation of Pyrus and Cydonia and their responses to Fe-limiting conditions. Plant Cell Tiss. Org. Cult. 1990, 21, 191–199. [Google Scholar] [CrossRef]
- Rodriguez, R.; Diaz-Sala, C.; Cuozzo, L.; Ancora, G. Pear in vitro propagation using a double-phase culture system. HortScience 1991, 26, 62–64. [Google Scholar] [CrossRef]
- Al-Maarri, K.; Arnaud, Y.; Miginiac, E. Micropropagation of Pyrus communis cultivar “Passe Crassane” seedlings and cultivar “Williams”: Factors affecting root formation in vitro and ex vitro. Sci. Hort. 1994, 58, 207–214. [Google Scholar] [CrossRef]
- Iglesias, I.; Vilardell, P.; Bonany, J.; Claveria, E.; Dolcet-Sanjuan, R. Micropropagation and field evaluation of the pear (Pyrus communis L.) “IGE 2002”, a new selection of the cultivar Dr. Jules Guyot. J. Am. Soc. Hort. Sci. 2004, 129, 389–393. [Google Scholar] [CrossRef]
- Berardi, G.; Rodrigo, I.; Davide, N. Micropropagation of Pyrus calleryana Dcn. from seedlings. Sci. Hort. 1993, 53, 157–165. [Google Scholar] [CrossRef]
- Yeo, D.Y.; Reed, B.M. Micropropagation of three Pyrus rootstocks. HortScience 1995, 30, 620–623. [Google Scholar] [CrossRef]
- Damiano, C.; Liberali, M.; Avanzato, D.; Preka, P. Micropropagazione di Pyrus communis var. Pyraster. Macfrut Agro. Bio. Frut. Cesena 1996, 10–11, 132–133. [Google Scholar]
- Shibli, R.A.; Ajlouni, M.M.; Jaradat, A.; Aljanabi, S.; Shatnawi, M. Micropropagation in wild pear (Pyrus syrica). Sci. Hort. 1997, 68, 237–242. [Google Scholar] [CrossRef]
- Chevreau, E.; Bell, R. Pyrus spp. pear and Cydonia spp. quince. In Biotechnology of Fruit and Nut Crops; Litz, R.E., Ed.; CABI Publishing: Trowbridge, UK, 2005; pp. 543–565. [Google Scholar] [CrossRef]
- Ruzić, Đ.; Vujović, T.; Cerović, R. In vitro multiplication of semi-dwarfing pear rootstock ‘Pyrodwarf’ in relation to cytokinin types. Acta Hortic. 2016, 1139, 279–284. [Google Scholar] [CrossRef]
- Jamshidi, S.; Yadollahi, A.; Ahmadi, H.; Arab, M.M.; Soltani, M.; Eftekhari, M.; Sabzalipoor, H.; Sheikhi, A.; Shiri, J. Predicting in vitro culture medium macro-nutrients composition for pear rootstocks using regression analysis and neural network models. Front. Plant Sci. 2016, 7, 274. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute. SAS/STAT Software, Version 9.1 for Windows; SAS Institute: Cary, NC, USA, 2003. [Google Scholar]
- Ahmadi Aghdam, K.; Motallebi-Azar, A.R.; Zaare Nahandi, F.; Gohari, G. Interaction effect of indole butyric acid and putrescin on in vitro root regeneration of Pyrodwarf rootstock (Pyrus communis L.). Iran. J. Hortic. Sci. 2021, 52, 721–730. [Google Scholar] [CrossRef]
- Rossi, V.; Paoli, G.D.; Pozzo, F.D. Propagation of Pyrus calleryana sel. D6 by in vitro culture. Acta Hort. 1991, 300, 145–148. [Google Scholar] [CrossRef]
- Moretti, C.; Scozzoli, A.; Pasini, D.; Paganelli, F. In vitro propagation of pear cultivars. Acta Hort. 1992, 300, 115–118. [Google Scholar] [CrossRef]
- Hu, C.Y.; Wang, P.J. Handbook of Plant Cell Culture; Evans, D.A., Sharp, W.R., Ammirato, P.V., Yamada, Y., Eds.; MacMillan: New York, NY, USA, 1983; Volume I, pp. 177–227. [Google Scholar]
- Dwivedi, S.K.; Bist, L.D. In vitro micropropagation of mehal pear. Indian J. Hort. 1997, 54, 223–228. [Google Scholar]
- Predieri, S.; Govoni, M. In vitro propagation of compact pear clones. Acta Hort. 1998, 475, 127–132. [Google Scholar] [CrossRef]
- Kaviani, B.; Negahdar, N. Propagation, micropropagation and cryopreservation of Buxus hyrcana Pojark., an endangered ornamental shrub. S. Afr. J. Bot. 2017, 111, 326–335. [Google Scholar] [CrossRef]
- Dong, C.; Li, X.; Xi, Y. Micropropagation of Pyracantha coccinea. HortScience 2017, 52, 271–273. [Google Scholar] [CrossRef]
- Adibi Baladeh, D.; Kaviani, B. Micropropagation of medlar (Mespilus germanica L.), a Mediterranean fruit tree. Int. J. Fruit Sci. 2021, 21, 242–254. [Google Scholar] [CrossRef]
- Bell, R.L.; Scorza, R.; Srinivasan, C.H.; Weeb, K. Transformation of Beurre Bosc pear with the rolc gene. J. Am. Soc. Hortic. Sci. 1999, 124, 570–574. [Google Scholar] [CrossRef]
- Kaviani, B. Some useful information about micropropagation. J. Ornamen. Plants 2015, 5, 29–40. [Google Scholar]
- Hassanen, S.A.; Gabr, M.F. In vitro propagation of pear Pyrus betulaefolia rootstock. Am.-Eur. J. Agric. Environ. Sci. 2012, 12, 484–489. [Google Scholar]
- Hausman, J.F. Changes in peroxidase activity, auxin level and ethylene production during root formation by poplar shoots raised in vitro. Plant Growth Regul. 2003, 13, 263–268. [Google Scholar] [CrossRef]
- Baviera, J.A.; Garcia, J.L.; Ibarra, M. Commercial in vitro micropropagation of pear cv. conference. Acta Hortic. 1989, 256, 63–68. [Google Scholar] [CrossRef]
- Rehman, H.U.; Gill, M.I.S.; Sidhu, G.S.; Dhaliwal, H.S. Micropropagation of Kainth (Pyrus pashia)—An important rootstock of pear in northern subtropical region of India. J. Exp. Biol. Agric. Sci. 2014, 2, 188–196. [Google Scholar]
- Reed, B.M. Screening Pyrus germplasm for in vitro rooting response. HortScience 1995, 30, 1292–1294. [Google Scholar] [CrossRef]
- Thimann, K.V. Hormone Action in the Whole Life of Plants; University of Massachusetts Press: Amherts, MA, USA, 1977; p. 510. [Google Scholar] [CrossRef]
- Hazarika, B.N. Acclimatization of tissue cultured plants. Curr. Sci. 2003, 85, 1704–1712. Available online: https://www.jstor.org/stable/24109975 (accessed on 9 January 2023).
- Sahari Moghaddam, A.; Kaviani, B.; Mohammadi Torkashvand, A.; Abdousi, V.; Eslami, A.R. Micropropagation of English yew, an ornamental-medicinal tree. J. Ornamen. Plants 2022, 12, 1–9. [Google Scholar]
- Chugh, S.; Guha, S.; Rao, I.U. Micropropagation of orchids: A review on the potential of different explants. Sci. Hortic. 2009, 122, 507–520. [Google Scholar] [CrossRef]
- Jeon, M.W.; Ali, M.B.; Hahn, E.J.; Paek, K.Y. Effects of photon flux density on the morphology, photosynthesis and growth of a CAM orchid Doritaenopsis during post-micropropagation acclimatization. Plant Growth Regul. 2005, 45, 139–147. [Google Scholar] [CrossRef]
- Hamdeni, I.; Louhaichi, M.; Slim, S.; Boulila, A.; Bettaieb, T. Incorporation of organic growth additives to enhance in vitro tissue culture for producing genetically stable plants. Plants 2022, 11, 3087. [Google Scholar] [CrossRef]
- Martinelli, F.; Busconi, M.; Fogher, C.; Sebastiani, L. Development of an efficient regeneration protocol for pear rootstock Pyrodwarf and assessment of SSR variability in regenerating shoots. Caryologia 2009, 62, 62–68. [Google Scholar] [CrossRef]

| Treatment | Shoot Number | Shoot Length | Leaf Number | ||
|---|---|---|---|---|---|
| Culture Medium | BA (mg·L−1) | Kin (mg·L−1) | (cm) | ||
| MS | 0 | 0 | 1.025 ef ± 0.124 | 1.190 j ± 0.313 | 2.535 ij ± 0.197 |
| 1 | 0 | 1.668 de ± 0.203 | 2.903 d–f ± 0.762 | 7.345 c ± 0.573 | |
| 2 | 0 | 1.327 d–f ± 0.160 | 1.589 hi ± 0.419 | 3.769 h ± 0.292 | |
| 0 | 0.5 | 1.296 d–f ± 0.158 | 1.457 h–j ± 0.382 | 2.325 jk ± 0.181 | |
| 1 | 0.5 | 1.549 d–f ± 0.187 | 2.184 g ± 0.575 | 4.220 g ± 0.327 | |
| 2 | 0.5 | 3.017 bc ± 0.364 | 3.314 bc ± 0.870 | 8.348 b ± 0.651 | |
| 0 | 1 | 1.127 ef ± 0.135 | 1.355 ij ± 0.354 | 2.625 i ± 0.205 | |
| 1 | 1 | 2.905 c ± 0.353 | 2.267 g ± 0.596 | 5.122 f ± 0.397 | |
| 2 | 1 | 4.352 a ± 1.716 | 3.182 cd ± 0.834 | 10.02 a ± 0.780 | |
| WPM | 0 | 0 | 0.955 f ± 0.114 | 1.702 h ± 0.448 | 1.655 l ± 0.131 |
| 1 | 0 | 1.337 d–f ± 0.159 | 4.045 a ± 1.060 | 7.015 d ± 0.547 | |
| 2 | 0 | 1.657 de ± 0.200 | 2.955 de ± 0.772 | 2.215 k ± 0.172 | |
| 0 | 0.5 | 1.237 d–f ± 0.151 | 2.277 g ± 0.596 | 2.135 k ± 0.163 | |
| 1 | 0.5 | 1.357 d–f ± 0.168 | 2.607 f ± 0.686 | 4.130 g ± 0.319 | |
| 2 | 0.5 | 2.675 c ± 0.327 | 3.592 b ± 0.941 | 7.345 c ± 0.572 | |
| 0 | 1 | 1.127 ef ± 0.135 | 2.177 g ± 0.571 | 2.557 ij ± 0.200 | |
| 1 | 1 | 1.88 d ± 0.229 | 2.832 ef ± 0.744 | 3.790 h ± 0.294 | |
| 2 | 1 | 3.64 b ± 0.928 | 3.077 c–e ± 0.809 | 6.675 e ± 0.523 | |
| Treatment (mg·L−1) | Rooting (%) | Root Number | Root Length (cm) | Acclimatization (%) |
|---|---|---|---|---|
| 0 IAA | 0 d | 0 d | 0 d | 0 d |
| 0.5 IAA | 50 b ± 2.87 | 3.50 b ± 0.14 | 1.18 c ± 0.12 | 40 b ± 3.02 |
| 1 IAA | 95 a ± 5.13 | 5.50 a ± 0.31 | 2.39 a ± 0.08 | 90 a ± 5.35 |
| 0 IBA | 0 d | 0 d | 0 d | 0 d |
| 0.5 IBA | 20 c ± 2.22 | 2.00 c ± 0.082 | 1.75 b ± 0.046 | 15 cd ± 1.22 |
| 1 IBA | 45 b ± 3.65 | 1.50 cd ± 0.036 | 2.68 a ± 0.20 | 20 c ± 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaviani, B.; Barandan, A.; Tymoszuk, A.; Kulus, D. Optimization of In Vitro Propagation of Pear (Pyrus communis L.) ‘Pyrodwarf®(S)’ Rootstock. Agronomy 2023, 13, 268. https://doi.org/10.3390/agronomy13010268
Kaviani B, Barandan A, Tymoszuk A, Kulus D. Optimization of In Vitro Propagation of Pear (Pyrus communis L.) ‘Pyrodwarf®(S)’ Rootstock. Agronomy. 2023; 13(1):268. https://doi.org/10.3390/agronomy13010268
Chicago/Turabian StyleKaviani, Behzad, Azam Barandan, Alicja Tymoszuk, and Dariusz Kulus. 2023. "Optimization of In Vitro Propagation of Pear (Pyrus communis L.) ‘Pyrodwarf®(S)’ Rootstock" Agronomy 13, no. 1: 268. https://doi.org/10.3390/agronomy13010268
APA StyleKaviani, B., Barandan, A., Tymoszuk, A., & Kulus, D. (2023). Optimization of In Vitro Propagation of Pear (Pyrus communis L.) ‘Pyrodwarf®(S)’ Rootstock. Agronomy, 13(1), 268. https://doi.org/10.3390/agronomy13010268









