Abstract
Root and tuber crops (RTCs) are the second-most important carbohydrate commodity after cereals. Many species of the RTCs are vegetatively propagated, making their shoot tips the preferred material to be conserved for future uses. Shoot tip cryopreservation provides an important tool to support the long-term conservation of plant genetic resources. Over the past four decades, significant efforts have been undertaken to move shoot tip cryopreservation of RTCs from research projects to full-scale implementation in cryobanks. This comprehensive review focuses on the history of cryopreservation protocols developed in RTCs. The encapsulation and vitrification solution-based cryopreservation techniques followed by ultra-rapid freezing and thawing have been highly successful. Additionally, different strategies for improving the cryotolerance of shoot tips have been introduced to further increase post-cryopreservation recovery. Finally, the research conducted to explain the mechanism underlying cryoprotection and differential cryotolerance including the use of histological studies are highlighted.
Keywords:
cassava; cryopreservation; long-term storage; potato; root and tuber crop; shoot tips; sweet potato; taro 1. Introduction
1.1. Importance of Root and Tuber Crops to Humans
Root and tuber crops (RTCs) are plants that produce underground storage structures such as tubers (e.g., potato), tuberous roots (e.g., cassava, sweet potato), taproots (e.g., yam, carrot), a corm which is a thickened underground stem (e.g., taro) and many other forms [1]. Despite the diverse morphologies of the storage organs, RTCs have the high carbohydrate content in common, which makes starchy RTCs the second-most important commodity after cereals in terms of carbohydrate consumption [1,2]. In addition, RTCs also contain different levels of proteins, vitamins, antioxidants, and other phytochemical constituents that provide nutritional and health benefits to humans [2,3,4,5,6,7].
Worldwide, potato (Solanum tuberosum L.), cassava (Manihot esculenta Crantz), and sweet potato [Ipomoea batatas (L.) Lam.] are consumed by billions of people. In 2020, the global production of potato, cassava, and sweet potato reached to approx. 359, 303, and 89 million tons, respectively [8]. Potato is the world’s fourth-most important food crop after maize, rice, and wheat [9], and is the staple food of 1.3 billion people [10]. Potato contributes to food security on a global scale, due to its wide adaptability to various climates and soils [3,10,11,12], its yield capacity [3], and as a processed product with high commercial value [13]. Moreover, promoting potato as a staple crop has potentially reduced the carbon-land-water uses and environmental impacts of crops production in China [14]. Cassava is among the most important tropical RTCs, contributing to food security and income generation [15]. Attributes such as rusticity, tolerance to drought, harvest flexibility and low input requirements make cassava an important crop for food and nutritional security [15,16,17]. Sweet potato is another important food crop grown in the wet tropical and subtropical regions [4,8,18]. Sweet potato is rich in protein, fibers, vitamins, minerals, and antioxidants [2,4], and, like cassava, has a wide ecological adaptation and harvest flexibility [6,19]. In addition to their edible starchy tuberous roots, the leaves of sweet potato and cassava are widely used as a source of gross energy, protein, and mineral elements for animal feeds, and can also be exploited as bioenergy crops [4,15,20,21,22].
The global production of other RTCs such as yam (Dioscorea spp.) and taro [Colocasia esculenta (L.) Schott] are smaller compared to potato, sweet potato, and cassava but serve as a staple crop in resource-limited communities of the Asia-Pacific region, Africa, and Latin America [23,24]. Classically, most of these crops are selected from landraces by farmers, and only recently progress has been made in breeding programs [25,26]. Therefore, the values in these crops are expected to increase in the near future so they would not only contribute to food security, but also to rural development and poverty alleviation [27,28,29,30]. According to FAO/OECD, the world production and utilization of RTCs is projected to increase by about 18% in 2020s [9]. Nevertheless, continuous efforts regarding the conservation, breeding, propagation, and production of RTCs are needed to ensure global agrobiodiversity and to feed a growing population [31,32].
1.2. Conserving RTCs as the Filed, Greenhouse, and In Vitro Collections
The availability of and easy access to plant genetic resources are essential for future breeding program advances. Conserving the genetic diversity of RTCs is therefore pivotal to ensure food security challenged by the climate change and increasing world population [33,34]. Most RTCs are of tropical and subtropical origins. These regions contain the most abundant diversity of RTCs and, are therefore priority locations for establishing the conservation programs [23]. While potato is commonly grown and consumed in temperate countries, the wild potatoes are found in the Andean highlands from 10 to 20° S in South America [11,23]. Home to the well-known potato, the Andean altiplano is also the center of genetic origin of many other RTCs such as oca (Oxalis tuberosa Molina), mashua (Tropaeolum tuberosum Ruiz & Pav.), ulluco (Ullucus tuberosus Caldas) and maca (Lepidium meyenii Walp) [35,36]. Therefore, the genebank of the International Potato Center (CIP) was based in Lima, Peru, and started its operations in 1971. CIP manages and facilitates access to highly diverse germplasm of potato, sweet potato, and other Andean RTCs [37]. Currently, the CIP genebank holds the most diverse collection of cultivated potato and sweet potato, as well as their wild relatives [38].
The wild RTC species are naturally preserved in their indigenous habitats but are increasingly threatened by diseases and pests, ageing of the plants, expansion of agriculture, urbanization, and climate change [39,40]. Many wild species of RTCs can produce orthodox seeds and thus are amenable to be stored dry at the conventional conditions of a seed bank (−18 ± 3 °C; 15 ± 3% relative humidity) or under cryogenic storage (<−70 °C) for prolonged periods of time [23,34,41]. However, when conservation of particular gene combinations is required, clonal propagation is better suited over seeds. Since almost all RTCs are propagated vegetatively, the simplest strategy is conserving the RTCs as whole plants in field and greenhouse collections. These collections allow the germplasm to be observed throughout the year and to be readily available for characterization, exchange, and other uses [34,42]. However, the maintenance and management of such field collections are expensive and are at risk of losses from attacks by pests, diseases, and environmental disasters [23,43]. Thus, over time, farming communities have lost unique accessions of RTCs due to susceptibility to these biotic and abiotic stresses [23,44]. Thanks to the strong partnership between CIP and local communities in the Peruvian Andes, the lost potato landraces were successfully reintroduced. This collaboration allows for a dynamic exchange between the ex situ and in situ conservations. Healthy and valuable landraces are returned to local communities, and in exchange landraces identified from these communities are safely stored in the CIP genebank [44].
Using plant tissue culture technologies, in vitro genebanks have been established for many vegetatively propagated crops, including RTCs [43,45,46]. In vitro genebanks provide an alternative to field collections for the short- and medium-term storage of plant genetic resources [34]. Additionally, plant materials kept in vitro are free of most pests and diseases and can be delivered on a year-round basis [23]. In vitro plant collections are often maintained under slow growth conditions to minimize labor input, as well as to reduce the risks of microbial contamination, human errors such as mislabeling, and somaclonal variation [23,41]. Slow-growth storage is achieved by modifying environmental conditions, including the culture medium composition, temperature, and light intensity [47]. In vitro culture collections also come with some limitations as their maintenance is labor-intensive, has a high cost of infrastructure and requires skilled and trained technicians [23,48]. Moreover, it is well known that genetic instability due to somaclonal variation may increase with time in culture, resulting in the loss of genetic integrity of the stored accessions [48,49,50].
1.3. Cryopreservation of RTCs and Its Challenges
If possible, long-term storage of RTC genetic resources should be through cryopreservation, with in vitro and/or field collections maintained for active use [51]. Cryopreservation is the storage of biological materials in liquid nitrogen (LN, −196 °C) or in its vapor phase (LNV, −150 to −196 °C). Under these conditions, propagules are preserved in a state in which metabolic and biological processes are halted [43,52,53]. Cryopreservation procedures have already been established and implemented for many vegetatively propagated crops, including most RTCs [38]. Cryopreservation techniques for RTCs have been developed using pollen, seeds, shoot tips, and cell suspensions as a plant source [54,55,56,57,58]. The propagule choice to be cryopreserved depends on whether the seed is orthodox, whether in vitro/cryopreservation protocols are already developed, and on whether the genes or the specific genetic combination of elite germplasm are the program conservation targets [59]. For clonal conservation, shoot tip cryopreservation is preferred over seeds or pollen, as they could readily developed into whole plants with high level of genetic fidelity [59,60]. To ensure the survival and regeneration capacity of cryopreserved samples, freezable water content needs to be reduced prior to LN exposure. As such, the cell solution will vitrify and not form lethal ice crystals that irreversibly damage membranous structures [53,61].
Following the first successful tissue culture reports of potato in the 1970s [62], Grout & Henshaw [63] were the first scientists to successfully apply shoot tip cryopreservation for potato. Since then, shoot tip cryopreservation has been successfully applied to many other important crops, including RTCs. At present, the most successful shoot tip cryopreservation procedures make use of encapsulation-dehydration and vitrification solution-based methods [43,52,60,64,65,66]. To date, successful cryobanks for potato and sweet potato have been established at several institutes, such as CIP, Leibniz Institute of Plant Genetics (IPK), the New Zealand Institute for Plant and Food Research (PFR), and US Department of Agriculture-Agricultural Research Service (USDA-ARS) [43,67,68]. The International Center for Tropical Agriculture (CIAT) [69] and the International Institute of Tropical Agriculture (IITA) have established cryo-collections for cassava and yam, respectively [70].
The process of cryopreservation has a high initial cost of initiating materials in LN and facility costs; however, once material is in LN, maintenance cost can be kept to a minimum as compared with other conservation methods [43,70,71]. Cryopreservation protocols consist of several steps, each often requiring optimization in case a new species is dealt with [59]. Although great success has been achieved in cryopreservation of potato [55,56], some genotypes of cassava [72], sweet potato [73], yam [70,74], and minor RTCs such as oca [75] are still recalcitrant to the newly developed cryogenic protocols. These “cryopreservation-recalcitrant” RTCs will therefore require method modifications and optimization.
Since the first report on RTCs shoot tip cryopreservation was published in 1978 [63], much research has been carried out with methods developed and optimized to achieve regrowth levels that satisfy the genebank standards for cryopreserved RTC collections. The aims and scope of this review is therefore to provide updated and comprehensive information on the recent development and progress of shoot tip cryopreservation in RTCs. In addition, we will discuss fundamental studies performed in RTCs on cryoprotection and tolerance to cryopreservation including histological studies.
2. Overview of Protocols to Cryopreserve Shoot Tip of RTCs
2.1. From Two-Step (Programmed) Cooling to Ultra-Rapid Freezing
2.1.1. Two-Step (Programmed) Cooling
Bajaj [76] was the first to report a two-step cooling protocol for RTC shoot tip cryopreservation. In this method, tissues containing apical meristem were treated with cryoprotectant solutions containing sucrose and glycerol before the two-step cooling. The first step of slow cooling was performed in LNV, thus reducing the cellular water content of tissue through freeze-induced dehydration, and was followed by a direct immersion in LN [76]. While the survival percentage of post-thawed tissues ranged from 7 to 18%, no shoot regrowth was reported [76]. Noticeably, in the study of Kartha et al. [77], an innovative approach to cryopreserve cassava shoot tips was included. In this method, shoot tips were cryoprotected with 15% dimethyl sulphoxide (DMSO) and 3% sucrose for 15 min at room temperate, and then transferred into 2–3 μL droplets of the same cryoprotectant solution placed on aluminum foil strips before slow cooling [77]. The use of aluminum foil facilitated the further development of rapid-freezing methods that are still widely applied to date [52,60].
2.1.2. Ultra-Rapid Freezing
Grout & Henshaw [63] further improved the two-step cooling protocol to the direct freezing of shoot tips in LN. In their study, potato (S. goniocalyx) shoot tips consisting of the apical dome and 2–4 leaf primordia were incubated on Murashige and Skoog [78] medium (MS) supplemented with 3% (w/v) sucrose and 1.0 mg L−1 benzyladenine (BA) for 72 h, then treated with a cryoprotectant solution containing 10% of DMSO for 1 h before immersion in LN. The cryopreserved shoot tips were thawed in liquid MS at 35 °C for 1 min and subsequently transferred to recovery medium. This is the first cryopreservation protocol that led to shoot tip regrowth (11%). Both ultra-fast cooling and warming proved the essential requirements to avoid the lethal intracellular ice crystals formation [79,80]. Improved shoot regrowth levels were further obtained in potato by combining DMSO cryoprotection with the use of aluminum foil strip to facilitate ultra-rapid freezing [81,82,83,84]. For example, an average plant regeneration rate of 39% was obtained for 125 tested potato varieties after applying the DMSO droplet freezing method [82].
Although the initial two-step freezing protocols and DMSO droplet freezing method achieved shoot tip regrowth in potato [85,86,87] and cassava [77,88], and showed improved survival of embryogenic tissues of sweet potato [89], many RTCs are not amenable to these protocols [88,90]. Moreover, the development and adaptation of these protocols to high-throughput cryopreservation of diverse RTCs needed further refinement and simplification [91]. Additionally, most classical two-step cooling procedures require the use of expensive programmable freezing devices to achieve precise freezing parameters, thus reducing the broad application of this method. Therefore, this review only provides basic information regarding the two-step cooling and the rapid-freezing methods applying DMSO, due to its limited use in current shoot tip cryopreservation.
2.2. Development of Air-Drying Based Methods
2.2.1. Encapsulation-Dehydration Method (En-De)
This method was first reported by Fabre and Dereuddre [92] for cryopreserving potato (S. phureja) shoot tips, based on the technology for producing artificial seeds. In this method, shoot tips were suspended in 3% (w/v) sodium alginate solution, and then individual explants in alginate solution were dropped into a 100 mM calcium chloride solution by pipette for encapsulation. The encapsulated shoot tips were precultured with 0.5 M sucrose and dehydrated under sterile air flow for 4 h, prior to the two-step freezing and slow thawing. This first En-de attempt resulted in low shoot regrowth of 9.3% [92]. Working with S. phureja and S. tuberosum, Bouafia et al. [93] further improved the En-de protocol by optimizing the sucrose concentration in the preculture medium (0.75 M for 2 days), dehydrating the tissues with silica gel to a water content of 0.20–0.22 g g−1 (dry weight), and performing a rapid freezing technique. With these modifications, the shoot tip regrowth rates ranged from 50 to 78% [93]. Grospietsch et al. [94] improved the dehydration tolerance of shoot tips by a stepwise preculture protocol, i.e., increasing the sucrose concentrations to 2.0 M for 5 days for the donor plants, followed by preculturing the isolated shoot tips with 0.7 M sucrose for 1 day. With this optimized preculture procedure, 78.8% survival level was obtained for the potato (S. tuberosum) cv. ‘Desirée’ [94]. Preculturing the beads induces desiccation tolerance to the En-de protocols, and the progressive increase in sucrose concentration can avoids the deleterious effects of a direct exposure to high sucrose levels [95,96].
The En-de method also proved to be successful for cassava [97]. In this method, encapsulated shoot tips were pretreated in liquid MS medium supplemented with 0.75 M sucrose for 1 day or with 0.5 M sucrose for 3 days prior to 6 h of desiccation. The moisture content of beads was then dropped to approx. 30% fresh weight basis before LN storage. This procedure resulted in 60% shoot tip survival on a hormone-free recovery medium without an intermediate callus phase [97]. In general, the common steps in the En-de method are (1) encapsulation of shoot tips in calcium alginate beads; (2) preculturing the shoot tips with sucrose-enriched medium before and/or after encapsulation at either fixed or increasing concentrations; (3) partial dehydration of beads by air drying in a laminar flow hood or in the presence of silica gel; and (4) rapid freezing and thawing [53,64,97].
While the dehydration of the En-de may take hours, the encapsulated shoot tips are easily handled in the dehydration process, which is also straightforward and less time sensitive than procedures making use of toxic cryoprotectants [64,66]. To date, En-de has been applied to well over 70 different plant species, including a wide range of RTCs [64,98], such as sweet potato [99], yam [100,101], and taro [102]. Examples of RTCs that were successfully cryopreserved with the En-de method are listed in Table 1.

Table 1.
The use of encapsulation with air drying and (ultra-) rapid freezing for cryopreservation of root and tuber crops (RTCs).
2.2.2. Dehydration Cryo-Plate Method (D Cryo-Plate)
D cryo-plate method combines calcium-alginate encapsulation on a cryoplate with dehydration [104]. In En-de protocols, dehydrated beads are transferred to cryovials before a rapid plunging into LN for cooling and in a water bath for warming [64,97,99]. The cooling and warming rates achieved using En-de are usually about 200 °C min−1 and 80–140 °C min−1, respectively [105,106]. The D cryo-plate method achieves much higher cooling (4000–5000 °C min−1) and warming rates (3000–4500 °C min−1), because shoot tips adhered to the wells of cryo-plates are directly exposed to LN for cooling and in unloading solution (ULS) for warming, resulting in high shoot tip regrowth [106,107,108,109]. Niino et al. [104] were the first to successfully apply the D cryo-plate to 20 genotypes of mat rush (Juncus decipiens). Since then, D cryo-plate has been applied for the successful cryopreservation of many species, with regrowth levels that satisfy the genebank standards for cryopreserved collections [60,110].
Yamamoto et al. [56] achieved 80–100% of shoot tip regrowth across the 16 varieties of potato cryopreserved using D cryo-plate method. In their study, shoot tips (2.0 mm) were precultured overnight in MS medium supplemented with 0.3 M sucrose, and transferred individually into each well of the aluminum cryo-plate with 5 μL of alginate solution (2% alginate in a calcium-free medium supplemented with 0.4 M sucrose). Then, calcium chloride solution (0.1 M calcium chloride in medium supplemented with 0.4 M sucrose) was added dropwise to the cryo-plate for polymerization. Cryo-plates with shoot tips were placed in a loading solution (LS; 2 M glycerol and 1 M sucrose in MS medium) for 30 min at 25 °C, followed by air drying dehydration in a laminar flow hood for 2.0 h at 25 °C with 40–50% of air humidity. Thereafter, cryo-plates with shoot tips were transferred into uncapped 2-mL cryovials, followed by direct immersion in LN. For warming, cryo-plates were removed from the cryovials and placed in ULS (1 M sucrose in MS medium) for 15 min at room temperature. The encapsulated meristems were then detached from the cryo-plates and transferred into the recovery medium [56]. Using protocols described by Yamamoto et al. [56], Valle Arizaga et al. [103] achieved regrowth levels ranged from 73 to 97% for shoot tip cryopreservation of 11 ulluco lines.
Valle Arizaga et al. [111] further optimized the D cryo-plate protocol by addition of a paper mounting step to the cryo-plate with alginate gel and shoot tips. In the paper mounting step, a sheet of BEMCOT paper (7 × 30 mm) was used to cover the cryo-plates adhered with shoot tips to avoid losing the samples during the cooling and warming steps [111]. With this optimized protocol, the shoot tip regrowth levels ranged from 70 to 93% across 13 potato genotypes, comparable to the original D cryo-plate protocol [111]. The high shoot tip regrowth levels in D cryo-plate method have suggested that it is an efficient cryopreservation methodology for preserving valuable plant genetic resources. Moreover, the D cryo-plate uses air dehydration and eliminates the risks of chemical stress that could be caused by exposure to highly concentrated vitrification solutions [60,104]. Additional studies are required to determine the applicability of this method to other major RTCs.
Recently, a new adaptation of the D cryo-plate method has been developed for storage of garlic shoot tips at −80 °C (and not at the usual −196 °C), due to the high glass transition temperatures (Tg) of air-desiccated tissues (−44.7 to −39.4 °C after 90–120 min of dehydration) [112]. Practically, it is advocated to maintain the cryopreserved material well below the Tg temperature, as the extremely low (almost nil) molecular mobility of the vitrified matrix impedes the formation and growth of lethal ice crystals while inhibiting most ageing reactions. In their protocol, shoot tips were precultured on 1/2 MS medium containing 0.3 M sucrose for 2 days at 25 °C, embedded in alginate drops on cryo-plates, treated by LS with 1.0 M sucrose and 2.0 M glycerol for 30 min at 25 °C, and dried in a laminar flow cabinet for 120 min. Then, the cryo-plates were transferred in 2 mL-cryovial and cooled directly into a deep freezer at −80 °C. For warming, cryo-plates were removed from the cryovials, and placed in ULS (1 M sucrose in 1/2 MS medium) for 30 min at 25 °C. Alginate beads were then detached from the cryo-plates and transferred into the recovery medium. With this protocol, the shoot tip regrowth levels ranged from 91.7 to 100% and averaged at 95.3% across 7 garlic genotypes [112]. The main advantage of −80 °C storage compared to conventional cryopreservation methods are that it proved possible to use relatively cheap freezers for storage and it is free from the regular supply of LN [112]. However, more research in the −80 °C storage is needed before this method can apply as an alternative means for the cryopreservation of RTCs [112].
2.3. Development of Vitrification Solution-Based Methods
2.3.1. Vitrification and Vitrification Solution Method (Vi)
Vitrification refers to the solidification of an aqueous solution into an amorphous state (a glass) without the formation of a crystal during the rapid cooling process [52,61]. Therefore, vitrification is an indispensable status to avoid freezing injuries associated with ice crystal formation in biological materials during cryopreservation. As a consequence of the vitrification process, molecular mobility is also highly restricted, and plant tissues can remain viable during long-term storage at −196 °C [113]. Successful vitrification requires dehydration of cells prior to exposure to LN temperatures by exposing plant tissues to high concentrations of cryoprotectants (both penetrating and nonpenetrating) to increase the viscosity of the cell solution [52,61]. To enhance tolerance to the dehydration process, donor plants are often exposed to cold or osmotic acclimation before excised tissues are precultured on medium with high sucrose concentration and subsequently transferred to a glycerol-sucrose solution [59,114]. During preculture on sucrose-enriched medium, concentrations of sugar, starch, and proline are greatly increased in the shoot tips and may enhance the stability of membranes under conditions of severe dehydration [115,116]. DMSO has been widely used as a cryoprotectant to induce the vitreous status in shoot tip cryopreservation of RTCs in 1980–1990s, but only showed limited success [85,88,117,118].
Significant success in shoot tip cryopreservation was obtained following the application of plant vitrification solution 2 (PVS2), which consists of 15% (w/v) DMSO, 15% (w/v) ethylene glycol, 30% (w/v) glycerol, and 0.4 M of sucrose [119]. Since the first report on the development of PVS2 published in 1990 on navel orange callus, much research has been carried out and PVS2 has made an outstanding contribution to the plant cryopreservation research and plant germplasm conservation worldwide [120,121].
Towill and Jarret [122] were the first to report the PVS2-based vitrification (Vi) methods for sweet potato shoot tip cryopreservation. In this study, shoot tips (0.5–0.7 mm containing 3 to 4 leaf primordia) were precultured for 2 days in MS medium supplemented with 3% ethylene glycol, followed by stepwise dehydration with PVS2 treatment. PVS2-treated shoot tips were enclosed to a paper strip soaked with 80% PVS2 (the optimized final concentration), and rapidly immersed in LN. Following thawing in MS medium containing 1.2 M sucrose, cryopreserved shoot tips were post-thaw cultured on recovery medium [122]. While the use of paper strip as the carrier facilitates rapid cooling and thawing rates, most surviving shoot tips developed callus, and a variable shoot regrowth percentage (64 ± 37%) was obtained [122].
Sarkar and Naik [123] later established the first Vi protocol for shoot tip of four S. tuberosum cultivars using a stepwise PVS2 treatment, as previously used by Towill & Jarret [122] for sweet potato. They reported that the highest regrowth (56%) occurred when potato shoot tips (0.5–0.7 mm) were precultured on 1/2 MS medium supplemented with 0.3 M sucrose and 0.2 M mannitol for two days, prior to treatment with 20% PVS2 (30 min, 24 °C), 60% PVS2 (15 min, ice bath) and 100% ice cold PVS2 (5 min) [123]. The survival rates improved when cryopreserved shoot tips were incubated on post-thaw culture medium containing high sucrose (0.2 M) under diffused light for the first week, before being transferred to standard recovery medium (0.09 M sucrose) [123]. Kryszczuk et al. [118] further optimized the Vi protocol by using a one-step PVS2 treatment for cryopreserving potato shoot tips. In their study, shoot tips (1.0 mm) harvested from one-week-old cultures were incubated overnight in basal MS medium with 30 g L−1 sucrose, and then osmoprotected with LS (2 M glycerol + 0.4 M sucrose in MS medium) for 20 min followed by full-strength PVS2 exposure for 30 min (at 0 °C). The shoot tips were then placed in cryovials containing 1.5 mL of fresh PVS2 and directly plunged into LN for cryopreservation. For thawing, the cryovials were rapidly warmed in a water bath at 38 °C for two minutes, and PVS2 was drained and shoot tips were washed three times with ULS (1.2 M sucrose in liquid MS), before being transferred to recovery medium. This cryoprotocol resulted in 42–88% of shoot tip regrowth in the tested genotypes [118]. This study showed that potato shoot tips could tolerate the full-strength PVS2 treatment without a stepwise PVS2 treatment. The Vi method was also successfully tested on nine potato cultivars in China, and shoot regrowth levels were ranged from 11.1–45.0% [124].
Charoensub et al. [125] were the first to report a Vi protocol for cassava resulting in 75% shoot regrowth of cryopreserved shoot tips. In this study, shoot tips were precultured on MS medium supplemented with 0.3 M sucrose for 16 h, treated with LS (2 M glycerol + 0.4 M sucrose) for 20 min, and followed by an exposure to PVS2 for 45 min at 25 °C before the LN exposure [125]. This protocol was further tested in 10 cassava cultivars and showed an average of 70% post-thaw recovery [125].
While the Vi method was shown to achieve satisfactory shoot tip regrowth in yam [74,117,126] and taro [127], a further improved shoot tip regrowth was achieved using other vitrification solution-based methods [108,124,128]. Studies making use of Vi protocol for potato cryopreservation were compared with other Vi-based methods in Figure 1.
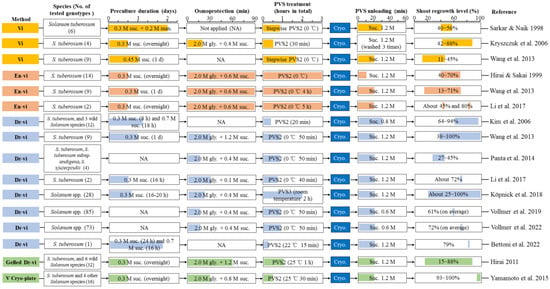
Figure 1.
Comparison of major steps of various vitrification solution-based methods for shoot tip cryopreservation in potato [56,68,107,108,118,123,124,129,130,131,132,133,134]. Abbreviations: gly, glycerol; man, mannitol; PVS, Plant vitrification solution; suc, sucrose.
2.3.2. Encapsulation-Vitrification Method (En-vi)
Based on the success of En-de and the development of PVS2 for plant cryopreservation, an En-vi method was developed for cryopreservation of carnation (Diathus caryophyllus L.) shoot tips [135]. This method was first tested in potato by Hirai & Sakai [107], combining the advantages of the easy manipulation of encapsulated explants and dehydration by vitrification solutions. In their study, axillary shoot tips (1 mm) were excised from cold-hardened (4 °C for three weeks) segments and precultured on MS medium supplemented with 0.3 M sucrose for 16 h. After encapsulation, shoot tips were osmoprotected with LS (2 M glycerol + 0.6 M sucrose in MS medium) at 25 °C for 90 min, followed by PVS2 treatment at 0 °C for 3 h. The beads were then placed in cryovials containing 1.0 mL of fresh PVS2 and directly plunged into LN for cryopreservation. For thawing, the cryovials were rapidly warmed in a water bath at 38 °C water bath, PVS2 was drained and shoot tips were washed in 1.2 M sucrose solution for 10 min to remove the cryoprotectants before being cultured in recovery medium. This En-vi protocol resulted in 40–70% post-cryopreservation recovery for 14 potato cultivars, much higher compared to the En-de method [107]. Further studies using En-vi protocol also achieved satisfactory shoot tip regrowth in nine cultivars of China’s potato (13–71%) [124] and two purple-fleshed potato cultivars (45–80%) [129].
Following a similar En-vi protocol proposed by Hirai & Sakai [107] for potato shoot tips, shoot tips of four cassava cultivars were successfully cryopreserved with survival levels ranging from 58 to 85% [136]. A high average regrowth rate (80%) was reported for three sweet potato cultivars that were cryopreserved by En-vi [137]. In this protocol, encapsulated shoot tips were precultured in MS medium supplemented with 0.3 M sucrose for 16 h, and osmoprotected with 2 M glycerol and 1.6 M sucrose, before being dehydrated with PVS2 for 1 h at 25 °C [137]. Although the En-vi has been successfully applied to many plant species, this method is less studied compared to other cryopreservation methods for RTCs. Even though this technique has been used with a limited number of RTCs, it possesses great potential both in terms of efficiency and practicality [52,53,138].
2.3.3. Droplet-Vitrification Method (Dr-vi)
Dr-vi is derived from the DMSO droplet freezing methods developed by Kartha et al. [77] and Schäfer-Menuhr et al. [81,82,83] for cryopreserving cassava and potato shoot tips, respectively. In the Dr-vi method, shoot tips are placed into a droplet of vitrification solution on foil strips [79], similar to the DMSO droplet freezing method. Dr-vi allows samples to obtain the ultra-fast cooling and warming rates, which are much faster compared to the methods using capped vials, due to the direct contact of explants with LN and the ULS [79,139,140]. Following the PVS2-based Dr-vi method, sweet potato shoot tips were firstly cryopreserved by Pennycooke & Towill [141] with 62% recovery reported. In this method, shoot tips (0.5–1 mm) were excised from 4- to 8-week-old in vitro stock cultures and then incubated in a liquid MS medium containing 2% sucrose at 25 °C for 1 day. Shoot tips were precultured in liquid MS medium supplemented with 0.3 M sucrose for one day at 25 °C, then osmoprotected with LS (2 M glycerol + 0.4 M sucrose in MS medium) for 1 h min at 22 °C. Osmoprotected shoot tips were exposed to PVS2 for 16 min at 22 °C and then transferred to PVS2 droplets of about 10 μL, placed on sterile aluminum foil strips, and directly immersed in partially solidified nitrogen (about −208 °C). For warming, the aluminum foils containing the shoot tips were immersed, for 20 min, in ULS (1.2 M sucrose in MS medium) at 22 °C, and transferred to recovery medium [141]. Shoot formation without intermediate callus was observed in all surviving shoot tips [141], showing a significant progress as compared to the traditional PVS2- Vi method, in which almost all the surviving sweet potato shoot tips formed callus after cryopreservation [122]. Although considerable effort has been made to establish the Dr-vi method for the cryopreservation of sweet potato collections [128,142], accessions react very differently to the cryopreservation protocol, i.e., some of the cultivars achieved a regeneration rate of 66% while others barely reached 2% [142]. A recent report by Wilms et al. [73] shows that Dr-vi can overcome the genotype-specific responses in sweet potato species, resulting in satisfactory post-thaw regrowth rates of more than 40% in seven out of ten cultivars tested. Briefly, in their study, shoot tips were harvested from 3- to 9-week-old in vitro plants and treated with LS (2 M glycerol and 0.4 M sucrose in MS medium) for 20 min at room temperature. Osmoprotected shoot tips were exposed to chilled PVS2 for 30 min, transferred to a thin layer of PVS2 droplets on sterile aluminum foil strips, and directly immersed in LN. For warming, the aluminum foil strips containing the shoot tips were immersed in ULS (1.2 M sucrose in MS medium) for 15 min at room temperature and incubated in post-thaw culture medium enriched with 0.3 M sucrose for 24 h, before being transferred to recovery medium in darkness for 1 week, followed by a transfer to light conditions [73]. Given that this protocol resulted in high recovery levels, it seems to be a practical and promising sweet potato cryopreservation methodology.
For potato, Dr-vi was first tested by Halmagyi et al. [143] and resulted in shoot regrowth levels that ranged from 46 to 55% across the three tested cultivars. Kim et al. [108] later compared the effects of different PVS2 exposure times (20 and 60 min) with various freezing techniques on the efficiency of cryopreservation of potato shoot tips. They found that the highest survival was achieved when shoot tips were treated with PVS2 for 20 min following Dr-vi. In the Dr-vi protocol optimized by Kim et al. [108], shoot tips (1.5 mm) were stepwise precultured with sucrose at 0.3 M for 8 h and 0.7 M for 18 h without loading treatment prior to the PVS2 incubation, as suggested by Yoon et al. [144]. The highest post-thaw survival after cryopreservation was obtained when cryopreserved shoot tips were warmed in pre-heated ULS (0.8 M sucrose in liquid MS medium) at 40 °C for 30 s, followed by treatment in pre-cooled ULS for 30 min [108]. With this optimized protocol, the shoot tip survival levels ranged from 64 to 94.4% across 12 potato cultivars [108].
Although the loading step was ignored in the Dr-vi protocols proposed by Kim et al. [108] and Yong et al. [144], it was proved to be a necessary step when the preculture was performed with a lower sucrose level (e.g., 0.3 M) [124] or when was not applied at all [130]. In the protocol optimized by Wang et al. [124], an LS (2 M glycerol with 0.2 to 1.0 M sucrose in liquid MS medium) incubation for 30 min was necessary to enhance potato shoot tip tolerance to dehydration and freezing [124]. This method resulted in an average of 71% post-cryopreservation recovery in nine potato cultivars [124]. In addition, they found that the Dr-vi method resulted in a higher shoot tip recovery compared to En-vi (38%) and Vi (28%) [124]. In the Dr-vi protocol proposed by Panta et al. [130] for cryopreservation of CIP potato accessions, apical shoot tips (2.0 mm) excised from 3-week-old cultures were directly exposed to LS without preculture. Osmoprotected shoot tips were then treated with PVS2 for 50 min on ice, placed into a droplet of PVS2 on an aluminum foil strip and quickly plunged into LN. Cryopreserved shoot tips were post-thaw cultured on MS medium with daily culturing onto fresh medium with decreased sucrose levels (daily transfers following 0.3 M, 0.2 M, 0.1 M and finally in 0.07 M) in darkness for 1 week, before being transferred to normal light conditions [130]. In comparison with CIP’s PVS2-based Vi and IPK’s DMSO-based droplet methods that had been applied in potato cryobanks, the optimized Dr-vi protocol proposed by Panta et al. [130] resulted in significantly higher recovery rates [130]. Panta et al. [145] further improved the PVS2-based Dr-vi protocol by including a cold-hardening culture phase before shoot tip excision. The cold-hardening pretreatment for three weeks at 6 °C significantly increased the post-cryo recovery in drought and frost tolerant potato cultivars, resulting in high recovery rate (40–100%) in 63% of the 755 assessed accessions [145].
In cassava, Dumet et al. [146] were the first to report the efficacy of Dr-vi for shoot tip cryopreservation, based on the protocol successfully established for Musaceae [79]. In this protocol, shoot tips were excised from 3–4-week-old in vitro cultures and directly treated with LS (2 M glycerol and 0.4 M sucrose in basal medium) for 20 min at 24 °C. Thereafter, shoot tips were exposed to PVS2 for 30 min on ice, and then transferred to PVS2 droplet on aluminum foil strips before immersion in LN. For warming, cryotubes were taken out from the LN and the aluminum foil strips holding shoot tips were quickly placed in an ULS (1.2 M glycerol and 0.4 M sucrose in liquid basal medium) for 15 min. Cryopreserved shoot tips were post-thaw cultured on MS medium supplemented with 0.3 M sucrose for 24 h, before being cultured in recovery medium [146]. This Dr-vi protocol resulted in shoot tip regrowth ranging from 38–48%, which was higher compared to the conventional En-de method (7–14%) [146]. This Dr-vi protocol was further tested to cryopreserve yam shoot tips, resulting in shoot recovery levels ranging from 0 to 60% [70]. To overcome the recalcitrance of cassava to cryopreservation in CIAT, Dr-vi was tested in 100 clones which previously showed low shoot regrowth levels (>30%) after a En-de [72]. About 70–75% of the clones tested achieved post-thaw regrowth levels that accomplish the genebank standards for the implementation of cryopreserved cassava collections [72].
Dr-vi has been applied to many important plant species from different climatic environments and the number of species successfully cryopreserved using this method is continuously increasing [59,60,66,139]. Dr-vi has been actively tested for other minor RTCs and resulted in variable shoot regrowth levels: 0–50% [147] and 21–51% [74] in yam, 73–100% [148] in taro, and over 66% in yacon [Smallanthus sonchifolius (Poepp.) H.Rob.] [149]. Dr-vi is currently the most widely applied cryoprotocol for cryopreserving plant germplasm within genebanks, including those for potato [66,69,150,151], cassava [69], sweet potato [151] and yam [70]. Detailed procedures of applying Dr-vi for shoot tip cryopreservation of potato and other RTCs are presented in Figure 1 and Table 2, respectively.
2.3.4. Vitrification Cryo-Plate Method (V Cryo-Plate)
To facilitate the easy handling of explants at different stages of Dr-vi method, Hirai [134] proposed a Gelled Dr-vi protocol for cryopreservation of potato shoot tips. In this study, 10–15 precultured shoot tips were transferred into a droplet of approximate 15 μL of sodium-alginate solution, which was previously placed on an aluminum foil strip. Then, calcium chloride solution (0.1 M calcium chloride) was added dropwise to aluminum foil strip for polymerization. Aluminum foil strips with shoot tips were then osmoprotected, PVS2-dehydrated, and directly immersed into LN. This protocol resulted in 70% shoot tip regrowth rate in 26 potato cultivars and six wild potatoes, which was similar to the regrowth achieved by Dr-vi (about 70%) and much higher than the conventional Vi and En-vi methods (about 40%) [134]. The Gelled Dr-vi was soon improved and standardized by the development of aluminum cryo-plates [104,109]. The V cryo-plate method is based on PVS2-vitrification dehydration and was first tested in shoot tip cryopreservation of Dalmatian chrysanthemum (Tanacetum cinerariifolium) [109]. General procedures are similar for both the V cryo-plate and D cryo-plate methods, with the main difference that shoot tips are air-dried in D cryo-plate instead of using vitrification solution in V cryo-plate. The V cryo-plate was later tested and compared with D cryo-plate by Yamamoto et al. [56] and obtained similarly high post-thaw regrowth rates (>90%) in 13 varieties and four lines of potato. In ulluco, the V cryo-plate produced lower (43%) shoot regrowth compared to D cryo-plate (>73%) [103]. The V cryo-plate and gelled Dr-vi protocols can be easily adapted from the available vitrification solution-based protocols, with ultra-rapid freezing and thawing benefits comparable to the Dr-vi method [110,134]. By adhering shoot tips to aluminum foils or plates, these methods simplify the handling of shoot tips at different stages of cryopreservation, allowing precise control of the treatment duration, and reducing the risk of mechanical injury to the shoot tips [109,110,152]. While V cryo-plate has been actively tested and implemented for routine use in cryobanks [60,110,153], more efforts are still needed to test the efficiency of this method for cryopreserving other minor RTCs in genebank collections.

Table 2.
The use of Droplet-vitrification for shoot tip cryopreservation of root and tuber crops (RTCs) except potato.
Table 2.
The use of Droplet-vitrification for shoot tip cryopreservation of root and tuber crops (RTCs) except potato.
| Plant Species (No. of Tested Genotypes) | Pretreatment of Donor Plants and Preculture of Explants | Osmoprotection | PVS Dehydration and Unloading | Post-Thaw Culture (PTC) | Shoot Regrowth (%) | Ref. |
|---|---|---|---|---|---|---|
| Cassava [Manihot esculenta (48)] | Meristems (0.7 mm) excised from in vitro seedlings that are 3 to 4 weeks old, without preculture | 2 M gly with 0.4 M suc for 20 min at 24 °C | PVS2 for 30 min on ice; 2 M gly with 0.4 M suc for 15 min for unloading | With 0.3 M suc, 1.07 μM NAA, 0.23 μM GA3 and 0.66 μM BA for the first day and the same medium with 0.1 M suc for the rest of the recovery. The first 8 days of PTC was in darkness | 6–86 | [146] |
| M. esculenta (9) | Meristems (0.5 mm) excised from cultures that are 3 weeks old, without preculture | 2 M gly with 0.4 M suc for 20–60 min | PVS2 for 30 min on ice; 1.2 M gly with 0.4 M suc for unloading | With 0.3 M suc, 1 mg L−1 ascorbic acid and then transfer to standard medium for final recovery. The first 7 days of PTC was in darkness | About 60–98 | [70] |
| M. esculenta (100) which showed recalcitrance to En-de (potst-thaw regrowth rate <30%) | Shoot tips (2 mm) excised from cultures that are 3 months old, without preculture | 2 M gly with 0.4 M suc for 2 h | PVS2 for 30 min on ice; 1.2 M suc for unloading | With 0.3 M suc and 0.2% active charcoal for 2 days in darkness, and then transfer to basal medium with 2.32 μM Kinetin, 0.72 μM GA3 and vitamins | Higher than 30% for 70–75% tested accessions | [72] |
| Sweet potato [Ipomoea batatas (1)] | Apical shoot tips (0.5–1.0 mm) excised from cultures that are 4 to 8 weeks old, cultured on liquid basal medium with 2% suc for 1 day, followed by preculture with 0.3 M suc for another day | 2 M gly with 0.4 M suc for 2 h | PVS2 for 16 min at 22 °C; 1.2 M suc for unloading | With 1 μM NAA, 0.5 μM BA, 0.1 μM Kinetin for 2 days in the dark, under dim light for 3 days, before being transferred to normal conditions | 62 | [141] |
| I. betatas (1) | Shoot tips (length of 2.5 and a width of 1.5 mm) excised from cultures that are 5 to 7 weeks old and stepwise precultured with 0.3 and 0.5 M suc for 31 and 17 h, respectively | 2 M gly with 0.5 M suc for 50 min | PVS3 for 1 h at room temperature; 0.9 M suc for unloading | With 1 g L−1 casein hydrolysate, 1 mg L−1 GA3, 0.5 mg L−1 BA for 1 week in the dark, and then cultured with 1 g L−1 casein hydrolysate, 0.5 mg L−1 GA3 for further recovery | 19 | [128] |
| I. betatas (30) | Apical shoot tips 1.0–1.2 mm excised from 4–8-week-old cultures and precultured 1 d with 0.35 M suc | 2 M gly with 0.4 M suc for 20 min | PVS2 for 30 min on ice; 1.2 M suc for unloading | First 9 days of PTC in darkness with 10 mg L−1 GA3, 10 mL coconut water, and 0.3, 0.1, 0.09 M stepwise with decreased suc level, each level for 3 days, and then transferred to fresh medium with 0.09 M suc under diffuse light for 4 days before being moved to the normal conditions | 2–66 | [142] |
| I. betatas (10) | Axillary meristems (1 mm) excised from cultures that are 3 to 9 weeks old, without preculture | 2 M gly with 0.4 M suc for 20 min | PVS2 for 30 min on ice; 1.2 M suc for unloading | With 0.3 M suc overnight in the dark, and then moved to regeneration medium with 2.22 μM BA for 7 days in the dark, before being transferred to the normal conditions | 10–84 | [73] |
| Yam [Dioscorea bulbifera, D. alata, D. cayenensis, D. polystachya (4)] | Apical shoot tips (2–4 mm) excised from shoots after 3 weeks of cold-hardening and precultured with 0.3 or 0.44 M sucrose for 3 days | 2 M gly with 0.4 M suc for 20 min | PVS2 for 20 min at 23 °C; thawing was performed with 0.09 M suc for 3 min followed by 5 min unloading with 1.17 M suc | Recovered on MS with 2 mg L−1 BA and 0.1 mg L−1 NAA | 0 for D. alata and 27–47 for the other species | [147] |
| D. alata, D. rotundata (21) | Meristems (0.5 mm) excised from cultures that are 3 weeks old, without preculture | 2 M gly with 0.4 M suc for 20–60 min | PVS2 for 30 min on ice; 1.2 M gly with 0.4 M suc for unloading | With 0.3 M suc and 1 mg L−1 ascorbic acid, and then transfer to standard meristem medium for final recovery. The first 7 days of PTC was in darkness | 0–60 | [70] |
| D. deltoidei (15) | Shoot tips (2 mm) excised from cultures that are 4 weeks old and precultured with 0.3 M sucrose for 16 h | 2 M gly with 0.4 M suc for 20 min | PVS2 for 90 min at 0 °C; 1.2 M suc for unloading | With 1.5 mg L−1 BA, 0.2 mg L−1 NAA, 0.2 mg L−1 GA3 for 10 days, and then transferred to medium with 0.5 mg L−1 zeatin. The first 5 days of PTC was in darkness | 21–51 | [74] |
| Oca [Oxalis tuberosa (4)] | Shoot tips (2 mm) excised from cultures that are 3 weeks old, without preculture | 2 M gly with 0.4 M suc for 20 min | PVS2 for 1 h on ice; 1.2 M suc for unloading | With 0.04 mg L−1 kinetin, 0.1 mg L−1 GA3 and 0.3 M suc for the first 2 days in the dark, and then with 0.1 M suc for another 2 days in the dark, and finally plated with 0.07 M suc and 2 mg L−1 calcium pantothenate for final recovery | 7–15 | [75] |
| D. deltoidei (15) | Shoot tips (2 mm) excised from cultures that are 4 weeks old and precultured with 0.3 M sucrose for 16 h | 2 M gly with 0.4 M suc for 20 min | PVS2 for 90 min at 0 °C; 1.2 M suc for unloading | With 1.5 mg L−1 BA, 0.2 mg L−1 NAA, 0.2 mg L−1 GA3 for 10 days, and then transferred to medium with 0.5 mg L−1 zeatin. The first 5 days of PTC was in darkness | 21–51 | [74] |
| Oca [Oxalis tuberosa (4)] | Shoot tips (2 mm) excised from cultures that are 3 weeks old, without preculture | 2 M gly with 0.4 M suc for 20 min | PVS2 for 1 h on ice; 1.2 M suc for unloading | With 0.04 mg L−1 kinetin, 0.1 mg L−1 GA3 and 0.3 M suc for the first 2 days in the dark, and then with 0.1 M suc for another 2 days in the dark, and finally plated with 0.07 M suc and 2 mg L−1 calcium pantothenate for final recovery | 7–15 | [75] |
| Taro [Colocasia esculenta (18)] | Apical hoot tips (0.8–1 mm) excised from in vitro shoots cultured on MS with 0.26 M suc for 4–8 weeks | 2 M gly with 0.4 M suc for 20 min | PVS2 for 20–40 min on ice; 1.2 M suc for unloading | With 0.3 M suc overnight in the dark, and then with 0.1 M suc for another 5 days in the dark and 10 days under dim light | 73–100 | [148] |
| C. esculenta (1) | Apical shoot tips (0.8–1.0 mm) excised from cultures that are 3 months old and precultured with 0.3 M sucrose overnight | 2 M gly with 0.4 M suc and 5% DMSO for 20 min | PVS3 for 10 min; 1.2 M suc for unloading | With 0.3 M suc for 2 days in the dark, and then transferred to basal medium with 0.1 M for final recovery for the next 13 days | 78 (survival) | [154] |
| Ulluco [Ullucus tuberosus (1)] | Apical shoot tips excised from the lateral shoots of in vitro nodal sections cultured on MS with 2 M suc for 5 days and precultured with 0.7 M suc for 12 h | Not specified | PVS3 for 1.5 h; thawing was performed with cryovials by immersing into a 40 °C water bath for 30 min. Unloading of PVS3 was exempted | PTC was performed with 0.5 mg L−1 kinetin and IAA, and 0.2 mg L−1 GA3 | 52.5 | [155] |
| U. tuberosus (4) | Shoot tips (2 mm) excised from cultures that are 3 weeks old, without preculture | 2 M gly with 0.4 M suc for 20 min | PVS2 for 1 h on ice; 1.2 M suc for unloading | Similar to the PTC protocol of Oca [Sánchez et al. 2011] | 11–35 | [75] |
| Yacon [Smallanthus sonchifolius (5)] | Apical shoot tips (2–3 mm) excised from cultures that are 2–3 weeks old and precultured with 0.3 M sucrose in darkness overnight | 2 M gly with 0.4 M suc for 20 min | PVS2 (0 °C) or PVS3 (22 °C) for 60 min; 1.2 M and 0.3 M suc were used for unloading PVS2 and PVS3, respectively | For the shoot tips treated with PVS2, PTC begins with 0.3 M suc for 1 day in darkness followed by basal MS medium for another 6 days in the dark. For the shoot tips treated with PVS3, PTC was performed with basal MS medium for the first 7 days in the dark | 66–75 | [149] |
| Callerya speciosa (Champ.) Schot | Axillary shoot tips (1.5 mm) excised from cultures that are 40 days old | 2 M gly with 0.4 M suc for 4 h on a shaker at 150 rpm | PVS2 (0 °C) for 60 min on a shaker at 150 rpm; 1.2 M suc for unloading | PTC begins with 0.3 M suc for 2 days, and were then transferred to MS with 2.2 μM BA, 0.6 μM IAA and 0.1 Μm GA3 for final recovery, first 6 days of PTC were in the dark | 60 | [156] |
Abbreviations: BA, 6-benzyl adenine; GA3, gibberellic acid; gly, glycerol; IAA, Indole-3-acetic acid; MS, Murashige and Skoog (1962) medium; NAA, 1-Naphthaleneacetic acid; PTC, post-thaw culture; PVS2, plant vitrification solution 2; PVS3 plant vitrification solution 3; suc, sucrose.
3. Key Strategies for Improving the Shoot Regrowth of RTCS after Cryopreservation
While great success has been achieved in cryobanking of potato, species- and genotype-specific responses to cryopreservation still exist in other RTCs, limiting the widespread use of cryopreservation procedures [68,72,73]. At present, most shoot tip cryopreservation procedures for vegetatively propagated crops make use of air-drying and vitrification solution-based methods, and thus do not require programmable freezers [60,66,153,157]. Often, several parameters should be considered to develop a successful cryopreservation including the quality and type of materials, pretreatment of donor plants, preculture and osmoprotection conditions, alleviation of osmotic stress, dehydration methods, cooling and thawing conditions, and post-thaw requirements [59]. It is recommended to assess the shoot regrowth levels and not solely survival to determine cryopreservation efficacies. On some cases, only shoot tip survival after 2 weeks was reported [108,144,158]. Longer evaluation periods (up to two months) are usually required to determine the value of a cryopreservation procedure [130,132].
3.1. Pretreatment and Type of Plant Materials
Pretreating the donor plants using cold acclimation or exposure to high sugar levels enhances the physiological tolerance of tissues to stresses associated with cryopreservation [52,53]. In potato, cold hardening of the donor plants prior to shoot tip excision has been routinely applied such as 4–5 °C for 3 weeks preceding the application of Vi, En-vi and Dr-vi methods [107,124,129] and 6–8 °C for 3 weeks in Dr-vi method [132]. Tropical species generally do not tolerate cold acclimation; therefore, osmotic agents were applied to induce the optimal physiological state of shoot tip donor cultures [94]. Similarly, cold preculture also proved undesirable for S. tuberosum whereas 0.3 M sucrose preculture enhanced plant regeneration of this species after cryopreservation [159]. Using an En-de method for cryopreservation of potato shoot tips, Grospietsch et al. [94] showed that incubation of donor plants on a medium supplemented with 2 M sucrose for 5 days followed by shoot tip preculture on 0.7 M sucrose for 1 day increased the shoot regrowth levels after cryopreservation. Noticeably, successful cryopreservation was also obtained using potato shoot tips excised from non-hardened cultures following En-de [93], Dr-vi [108], gelled Dr-vi [134] and V/D cryo-plate [56] methods.
In a Dr-vi method for cryopreserving of yam shoot tips, preconditioning treatment using an alternating temperature regime of 5 °C during the night and 28 °C during the day for 3 weeks resulted in 30 to 50% shoot recovery [147]. For red bud taro, preconditioning of donor plants with 0.29 M sucrose for 8 weeks prior to the shoot tip excision resulted in a recovery rate of about 60%, which was significantly higher than the recovery obtained from shoots cultured at normal sucrose level (0.09 M) (40%) [102]. In sweet potato, preconditioning the donor plants with 0.4 M sucrose for three days improved the regrowth of cryopreserved shoot tips using Vi method, resulting in 50–86% shoot regrowth for three accessions [160].
In addition to cold acclimation and the use of osmotic agents, Yoon et al. [144] found that culture conditions such as high light intensity (130 μmol m−2 s−1), ventilation of culture vessels, and low planting density led an increase in post-thaw survival in potato. Edesi et al. [161] investigated the effect of light spectral conditions and found that potato shoot tip donor plants maintained under blue LEDs were short and tiny but achieved increased survival from 26 to 66%, 4 to 31%, and 16 to 48% for cultivars Agrie Dzeltenie, Anti, and Désirée, respectively, compared to illumination by red LEDs [161]. In cassava, incubating donor cultures at a temperature of 21–23 °C and light intensity of 75 μmol m−2 s−1 increased the shoot recovery rate after freezing [97].
Although apical meristems were mostly applied in shoot tip cryopreservation of RTCs [108,146,147], the use of axillary shoot tips resulted in higher shoot regrowth in sweet potato as compared with the apical counterpart [73]. While, for potato, Halmagyi et al. [143] found higher shoot regrowth levels in three cultivars when apical shoot tips instead of axillary shoot tips were used. In contrast, for potato shoot tips cryopreserved with En-vi, Hirai and Sakai [107] found minimal or no effects in post-thaw difference between axillary and apical shoot tips. The size of excised shoot tips also affects the optimal pretreatment and cryopreservation conditions [152]. Shoot tip measuring 1–2 mm in length, depending upon the species, are often the preferred explant for cryopreservation procedures [132,133,162], but larger shoot tips (2.0–2.5 mm) proved suitable for cryopreservation of potato shoot tips by the D cryo-plate method [56]. The excision of bigger shoot tips requires less manipulations, and similarly to the excision of axillary meristem, only the removal of one leaf with its petiole is required prior to the final cut. Few operations may result in less mechanical damage that would support the viable post-thaw regrowth. Apart from shoot tips, potato microtubers (≤2.0 mm in diameter) induced from in vitro segments were successfully cryopreserved following air drying and rapid freezing technique [163]. This method is free from shoot tip excision and may also benefited from the least mechanical damage to the donor tissues.
While both the apical and the axillary shoot tips can be used as explants, the uniformity of shoot tips is another factor that may influence recovery after cryopreservation [59]. Charoensub et al. [162] combined a simple and effective micropropagation protocol to obtain uniform cassava shoot tips for cryopreservation. In their study, young apices were excised from 12-day-old plantlets derived from mononodal microcuttings, which were obtained from 2-month-old in vitro cultures [162]. Likewise, high post-thaw recovery levels following Dr-vi protocol (79%) [133] and V/D cryo-plate protocols (>80%) [56] were obtained in potato shoot tips were excised from micro-cuttings cultured for 1 or 2 weeks before shoot tip isolation, respectively. The use of micro-cuttings ensures the production of a large number of relatively homogeneous apical shoot tips, and increases the chances of a positive and uniform response to subsequent cryogenic treatments [59,164].
3.2. Pathogen-Free Status
Many RTCs are vegetatively propagated and are particularly susceptible to various intracellular pathogens such as virus, viroid, and phytoplasma [165,166,167,168]. Vegetative propagation of RTCs can result in virus transmission from generation to generation, with virus titers accumulating as a result of repeated propagation and infection events [169,170]. Moreover, the presence of plant pathogens limits the safe movement of plant materials across borders [171]. Besides eradication, there are no effective measures for controlling theses pathogens once plants are infected. Viral diseases have long been known to affect the physiological status and the vegetative growth of in vitro cultures [133,172,173,174]. For example, in potato, the co-infection of potato leafroll virus (PLRV) and potato virus Y (PVY) results in altered physiological metabolism, and significantly reduced vegetative growth and microtuber production [173]. Li et al. [175] further found that co-stress imposed by virus infection and salt significantly reduced growth and microtube production and caused severely oxidative damage to the in vitro potato plantlets. Therefore, application of osmotic dehydration in cryoprocedures may result in excessive damage to the plants stressed with virus infection, thus resulting in lowered post-thaw shoot recovery. While the influence of intracellular pathogen on the recovery of RTCs after shoot tip cryopreservation has not yet been investigated thoroughly, several studies have already indicated that shoot tip donor plants should preferably be free from viral pathogens [93,176]. Therefore, a regime implementing pathogen eradication prior to shoot tip cryopreservation would improve post-thaw shoot recovery of plant species that showed recalcitrant to cryopreservation. These virus-infected cultures can be candidates for, among other eradication techniques, cryotherapy, in which the virus is eradicated due to exposure of meristems to LN [177,178].
In addition, the growth of previously undetected endogenous bacteria may arise in cryopreserved shoot tips during post-thaw recovery, therefore screening of in vitro materials for the absence of endophytic bacteria on bacterial growth medium prior to cryopreservation is recommended [179].
3.3. Preculture and Osmoprotection Conditions
Optimizing sucrose concentrations and the duration of preculture are important factors for enhancing tolerance to dehydration and subsequent freezing process [47,52,59,180]. Preculture plays an indispensable role in all cryopreservation procedures applying air-desiccation, as it reduces freezable water from cells through osmosis and improves the tolerance of plant tissues to the subsequent dehydration [64,97]. Sucrose is the most frequently used osmotic agent and has been tested for a wide range of RTCs (Table 2). Commonly, the one-step (direct) preculture with 0.3–1.0 M sucrose was applied in shoot tip cryopreservation of RTCs [56,100,101,163,181]. Using En-de for cryopreservation of potato shoot tips, Bouafia et al. [93] tested either stepwise increase (0.3 to 1.0 M sucrose, 12 h each concentration) or direct preculture (0.75 M sucrose for 2 days). They found that the highest shoot regrowth was achieved when encapsulated shoot tips were precultured with 0.75 M sucrose for 2 days, with an average shoot regrowth level of 65% across the five cultivars [93]. In general, for the cryopreservation of potato shoot tips by D cryo-plate, the precultured shoot tips were often osmoprotected as for Vi-based protocols [56]. Although En-de has proven to be a less effective method for cryopreservation of RTCs as compared to the Dr-vi [72,107,134], it may overcome problems associated with the sensitivity to the toxic vitrification solutions, because sucrose is the only osmotic agent for the En-de and D cryo-plate method.
In vitrification solution-based methods, RTCs shoot tips were often precultured in medium supplemented with 0.3 M sucrose prior to osmoprotection, which usually applies 2 M glycerol with 0.4 M sucrose to induce tolerance to the subsequent PVS treatment (Figure 1, Table 2). However, in some species, such as sweet potato and potato, shoot tips were successfully cryopreserved without preculture [73,182] or without the need of osmoprotection [133]. For example, using the Dr-vi method for potato “Dunluce”, Bettoni et al. [133] found that shoot tips stepwise precultured with sucrose at 0.3 M for 24 h and 0.7 M for 16 h prior to PVS2 treatment (15 min at 22 °C) resulted in high shoot regrowth level (79%) after LN exposure, even without osmoprotection step. Similarly, in white yam, LS treatment did not influence the post-thaw survival of shoot tips using Vi method [183].
In another example of using the V cryo-plate for cryopreserving potato, Yamamoto et al. [56] found that shoot tips precultured at 0.3 M sucrose overnight and then osmoprotected in LS with sucrose ranging from 0.8 to 1.6 M produced similar shoot regrowth levels (97–100%). Likewise, LS containing 2 M glycerol and varying sucrose levels (0.2–1.0 M) were applied potato shoot tips using a Dr-vi protocol [124]. Interestingly, when the optimized Gelled Dr-vi protocol was applied for cryopreserving 25 potato cultivars, 10 of them showed unsatisfactorily levels of regrowth after cryoexposure. However, the addition of different sucrose concentration (1.0–1.8 M) to the LS and variation of treatment duration (45–105 min) produced significantly higher regrowth levels [134], which indicates that the recalcitrance to cryopreservation can be minimized by adjusting the osmoprotection conditions.
3.4. Dehydration Methods
A suitable dehydration by either air drying or using exposure to PVSs is one of the main factors to ensure success in shoot tip cryopreservation procedures [53]. For successful cryopreservation, most if not all freezable water must be removed from shoot tip cells, prior to the LN exposure [131,184,185]. The accurate control of dehydration and the prevention of chemical toxicity injury or excessive osmotic stress during dehydration are indispensable for successful cryopreservation [110,186].
The success of the En-de protocol depends upon the extent of dehydration and the residual moisture content of the encapsulated beads before freezing [59,64,112,150]. Beads are usually dehydrated to a moisture content of approx. 20% fresh weight basis before LN storage (Table 1). Although the two dehydration methods can produce similar shoot recovery results [187], the air-drying method may be more difficult to control, due to the variations in the physical environment (humidity, temperature, and air flow velocity); therefore, desiccation using silica gel may be more reproducible than air drying dehydration and is thus highly recommended [64,180,188].
Vitrification procedures often make use of PVS2 or PVS3 (50% w/v sucrose and 50% w/v glycerol) [189]. PVS2 is the most frequently used vitrification solution and has been tested for a wide range of plant genera, including RTCs (Figure 1 and Table 1). In Dr-vi protocols, shoot tips were generally exposed to PVS2 on ice for 30 to 60 min [73,75,142,146] or sometimes treated at room temperature with shorter incubation times [133,141,147]. To reduce the harmful effects of DMSO in PVS2, 0 °C exposures is often recommended [52,190]. However, not all RTCs are amenable to full-strength PVS2, even at 0 °C. An adjustment can therefore be performed by either optimizing the concentrations of the different PVS2 components [191], or by testing other PVSs [108,117,128]. For example, the shoot regrowth of some South African sweet potato accessions was significantly improved after cryopreservation by reducing the DMSO content in PVS2 from 15 to 5% [191]. In other studies, various PVSs were compared for cryopreserving RTCs shoot tips. In an early study applying Vi and Dr-vi protocols in yam, Leunufna & Keller [117] compared the efficacy of PVS2, PVS3, and PVS4 [192]. They found that PVS2 or PVS4 incubations at 23 °C for 15–30 min achieved satisfactory shoot regrowth, while PVS3 was impractical for Dr-vi due to its weak adhesion to the aluminum foil [117]. In contrast, the PVS3 dehydration was later optimized and applied successfully in Dr-vi for shoot tip cryopreservation of potato [131], yacon [149], and ulluco [155]. Additionally, PVS3 has been routinely applied for cryopreserving potato shoot tips at the gene bank of Gatersleben, Germany [131]. These results provide alternative options for species that showed recalcitrance to PVS2 dehydration.
3.5. Alleviation of Oxidative Stress and Freezing Injury
The cryopreservation protocols can impose dramatic stresses to the plant tissues, resulting in burst of reactive oxygen species (ROS) [193,194]. These stresses arise from shoot tip excision, osmotic injury, physical and chemical desiccations, and changes in temperature [193,195,196,197,198]. The excessive accumulation of ROS is toxic to cell systems and cause damage to DNA, proteins, and lipids, resulting in a low shoot tip regrowth after cryopreservation [194,199,200] and genetic instability of cryo-derived regenerants [201]. Several pretreatments such as cold acclimation as well as the preculture of shoot tip donor plants or shoot tips with high level of sucrose have improved the antioxidant activity in cell systems, leading to alleviated osmotic stress and improved shoot tip recovery after cryopreservation [202,203]. In addition to strengthen the plasticity of intrinsic antioxidant system, the exogenous addition of various enzymatic and non-enzymatic antioxidants in shoot tip pretreatment and preculture media have improved regrowth and quality of plantlets in cryopreserved shoot tips [65,152,164,194,204].
The adverse effects of oxidation during cryopreservation of RTCs have been minimized by incorporating plant hormones that also act as antioxidants, and elicitors of defense proteins in cryoprotection solutions or in shoot tip pretreatment. The addition of melatonin at 0.05–0.1 μM in the osmoprotection step enhanced the tolerance of yam shoot tip to physical desiccation over dry silica gel and led to increased shoot regeneration from 15 to 35% after the En-de cryopreservation [205]. Following a D cryo-plate procedure for eradication of the potato virus S from potato, Ruiz-Sáenz et al. [158] found that the pretreatment of donor plants with salicylic acid (SA) at 10−6 M for 28 days before shoot tip isolation resulted in survival percentages of 28–70% across the two potato cultivars that showed strong recalcitrance to shoot tip cryopreservation (0% shoot regrowth). In addition, other non-enzymatic antioxidants such as ascorbic acid, proline, gold nanoparticles, and vitamin E, and enzymatic antioxidants such as catalase have been applied to improve the shoot tip cryopreservation in other crops [194]. Noticeably, protocols that include antioxidants during the cryopreservation process resulted in reduced oxidation and increased shoot tip regrowth after freezing [65,152,164,193,203,204,206,207,208,209,210].
To alleviate freezing injuries in shoot tip cryopreservation of potato, Seo et al. [211] tested the influence of anti-freezing-protein type III (AFP III) on shoot regrowth after Vi cryopreservation. In this study, AFP III at 0–2000 ng mL−1 was added either to the LS or to the PVS2. They found that the supplementing 500 ng mL−1 AFP III to PVS2 or 1500 ng mL−1 AFP III to LS produced the highest shoot tip recovery, supporting the use of AFP III as a potent cryoprotectant component for the improvement of shoot tip regrowth in some difficult-to-cryopreserve RTCs [211].
3.6. Improved Thawing and Post-Thaw Culture
3.6.1. The Level of Sucrose in the Thawing Process
Thawing is a critical factor that affects shoot regrowth after LN exposure. Shoot tip thawing procedures that achieve fast warming rates are suggested to ensure the speedy transition from glass to liquid without ice recrystallization within plant cells [79,132,212]. In ultra-rapid freezing methods such as Dr-vi and V/D cryo-plate, cryopreserved shoot tips were rapidly warmed in ULS with high level of sucrose (0.8–1.2 M) [73,108,124,148,149] at room temperature [104,109] or pre-warmed to 35–40 °C for 30 s, then transferred to room temperature and held for 15–20 min prior to plating onto appropriate recovery media [133,144]. The use of 1.2 M sucrose in ULS is used as a standard concentration for many species, including RCTs. However, the ULS containing reduced sucrose concentration were also effective for shoot tip cryopreservation of some RTCs. For potato shoot tips cryopreserved using Dr-vi method, Kim et al. [108] found the highest survival (64–94%) when warming was performed using 0.8 M sucrose for 30 min, as compared to 0.3 and 1.2 M sucrose. Recently, Vollmer et al. [132] presented a detailed study accessing five different sucrose concentrations ranging between 0.0–1.2 M on the recovery rate of 16 potato landraces and 85 potato cultivars cryopreserved with the PVS2-Dr-vi method [132]. They found that sucrose concentrations of 0.3–0.9 M resulted in significantly higher recovery rates compared to the routinely used 1.2 M sucrose concentration, and highest recovery was observed with a sucrose concentration of 0.6 M across the 101 potato accessions (nine taxa) [132]. Interestingly, the potato accessions with high shoot regrowth levels (81–87%) responded equally to ULS sucrose concentration from 0.3 to 1.2 M [132]. These results suggest that the sucrose concentration in ULS also requires optimization, particularly for recalcitrant species.
3.6.2. The Level of Sucrose and Ammonium in Post-Thaw Culture
In Vi-based methods applying ultra-rapid thawing, rehydration occurs once cryopreserved shoot tips are transferred to the medium containing lower levels of sucrose after the unloading process. In potato, post-thawed shoot tips were normally transferred from 0.8–1.2 M sucrose of the thawing process to the basal levels (≤0.09 M) of the post-thaw recovery medium [56,108,129]. In case of sensitive and recalcitrant species, a post-thaw culture with progressively decreased sucrose levels might help the recovery process. For example, rewarmed shoot tips in ULS at 1.2 M sucrose were post-thaw cultured on recovery medium with progressively decreased sucrose levels from 0.3, to 0.2, to 0.1 M (daily transfers) and maintained on 0.07 M [130]. In Dr-Vi method, following rewarming with ULS at 1.2 M sucrose, potato shoot tips were transferred to an intermediate recovery medium containing 0.6 M sucrose overnight, before being transferred to the normal sucrose medium [133]. When applying the Dr-vi for cryopreserving cassava shoot tips, following ULS treatment with 1.2 M sucrose, shoot tips were cultured on recovery medium with 0.3 M sucrose overnight and then transferred to standard sucrose level [146]. The use of 0.3 M sucrose has been routinely applied in the first step of post-thaw recovery of cassava [72], yam [70], sweet potato [73,142], taro [148], and yacon [149].
In a recent study published by Vollmer et al. [68], the effect of sucrose concentration of the recovery medium was assessed in a large-scale experiment with 73 diverse potato genotypes. Placing cryopreserved shoot tips directly on MS culture medium with a normal sucrose concentration of 0.07 M (9 days in darkness, 4 days diffuse light, then normal light conditions) resulted in increased recovery rate (71.5%) compared to a stepwise decrease of the sucrose concentration in the recovery medium from 0.3 M to 0.07 M (routine protocol; 59.5% of shoot tip regrowth). This protocol was further implemented during routine cryopreservation of a wide range of potato accessions and resulted in increased regrowth levels from 57.9% (3067 accessions; old routine protocol) to 73.2% (1019 accessions; stable sucrose concentration of 0.07 M). Based on these results, the long-held dogma that a high concentration of sucrose in the recovery medium was necessary to reduce the osmotic shock (PVS2) is not true in the case of potato and may not be necessary in other species as well. It is important to highlight that these results were obtained with a very high sample size and diversified genotypes (potato landraces belonging to nine taxa and coming from 38 countries) [68].
Ammonium (NH4+) and nitrate (NO3−) are the major sources of inorganic nitrogen for plants, which promote the plant growth at low external supplies, but cause toxicity at high levels [213,214]. Nitrogen can regulate genes involved in metabolic processes, as well as in the production and scavenging of ROS, thus mediating plant responses to external stress [215]. In vitro cultures of RTCs have been mostly maintained in MS-based medium with a total nitrogen concentration of 60 mM and a ratio of nitrate to ammonium 2:1, which might be far above the amount required for some species [216]. Media requirements during the micropropagation process may be different from those required for shoot tip regrowth and therefore it is important that post-thaw conditions be favorable for direct shoot tip regrowth [186]. Following the freeze-thaw cycle, the cryopreserved shoot tips are more sensitive to ammonium levels in the recovery medium and improvements in shoot regrowth have been achieved by reducing or eliminating ammonium from the recovery medium during the first few days of post-thaw incubation [99,164].
The use of reduced ammonium in recovery medium has long been applied in shoot tip cryopreservation of many plant species, including RTCs [99,141,217]. For example, improved recovery in sweet potato was achieved when in vitro cultures were maintained and multiplied on MS medium with half-strength ammonium nitrate and potassium nitrate prior to shoot tip excision [141]. A later study by the same team demonstrated that the shoot tip regrowth of sweet potato cryopreserved by Dr-vi increased three-fold when the ammonium-free MS medium was used for the first 5 days of post-thaw culture [99]. The improved recovery of sweet potato shoot tips with reduced ammonium has also been reported using Dr-vi [128], but satisfactory post-thaw regrowth was achieved in shoot tips recovered with standard strength of ammonium following En-vi [137] and Dr-vi [73] protocols. For other RTCs, the ammonium-free recovery medium was not applied as often as for sweet potato. For potato, following a Vi cryopreservation, shoot tips were incubated in recovery medium without ammonium for three days before being transferred to full-strength MS medium [206]. For yam, a Vi procedure resulted in 39–58% shoot regrowth for two species when shoot tips were post-thaw cultured on MS medium containing 1/5 ammonium nitrate [126]. Based on these findings, it can be suggested that the reduced ammonium in recovery media can be tested for those RTCs relatively recalcitrant to cryopreservation.
3.6.3. The Influence of Plant Growth Regulators on Post-Thaw Recovery
In cryopreserved shoot tips, more vacuolated and differentiated cells are damaged, allowing only the highly cytoplasmic meristematic cells to regenerate [47,52,59,180]. Therefore, it is important to have optimized culture medium composition and plant growth regulators (PGRs) to encourage direct shoot regrowth from the meristem, without an intermediate callus phase [72,124,126]. This helps avoid the production of somaclonal variants [186,201,218]. To avoid callus formation in potato shoot tips cryopreserved by En-de, post-thaw culture was first performed for one week with 0.01 mg L−1 6-Benzylaminopurine (6-BA), 1 mg L−1 naphthaleneacetic acid (NAA) and 5 mg L−1 gibberellic acid (GA3), before being transferred to regeneration medium supplemented with GA3 at 0.1 μg L−1 for final recovery [93]. Similarly, basal medium containing GA3 at 0.5 mg L−1 was used in an En-vi protocol for potato shoot tip cryopreservation [107]. In a comparison of three vitrification solution-based methods for cryopreserving potato shoot tips, Wang et al. [124] found the use of GA3 at 0.05 mg L−1 led to highest shoot regrowth after Vi and Dr-vi. Furthermore, for the En-vi method, higher shoot regrowth was achieved in shoot tips post-cultured in medium supplemented with zeatin riboside (ZR) at 0.8 mg L−1 and GA3 at 2 mg L−1 as compared with those grown with GA3 at 0.05 mg L−1 [124]. For recalcitrant yam species, a post-thaw culture of shoot tips without PGRs produced no shoot regrowth following a Vi protocol, but shoot tip regrowth was achieved when shoot tips were post-thaw cultured with a combination of BA, NAA, and GA3 [126]. In cassava, the shoot regrowth after cryopreservation was affected by the type of cytokinin used and its concentration. Kinetin at 0.5 mg L−1 was more efficient than 2-isopentenyladenine (2iP), 6-BA, thidiazuron (TDZ) and adenine [219]. Interestingly, Yamamoto et al. [56] showed that the shoot tips of 16 potato accessions could be regenerated on basal MS medium without PGRs following cryopreservation by V and D cryo-plate resulting in high recovery percentages of 96.7 and 93.3%, respectively [56]. Therefore, the response of cryopreserved shoot tips to post-thaw cultures may be species-dependent or even protocol-dependent, particularly for the recalcitrant species or with non-optimized cryopreservation protocols.
Noticeably, in post-thaw recovery under high levels of cytokinin, hyperhydricity may occur in the surviving tissues that negatively affects the shoot regrowth after cryopreservation [59,164,220,221]. Hyperhydricity was observed in cassava shoot tip post-thaw cultured with 2.32 μM kinetin after a Dr-vi protocol [72]. It was ameliorated by transferring the shoot tips to a basic recovery medium containing 20 g L−1 sucrose and 0.2% active charcoal [72]. Furthermore, increasing the agar concentration in the recovery medium could reduce hyperhydricity after cryoexposure of cassava shoot tips [219].
3.7. Light Conditions
To reduce oxidation in post-thaw cultures, cryopreserved shoot tips were mostly maintained in the dark for the first 2–9 days before being transferred to normal light conditions [124,132] (Table 2). In some cases, a transitional culture under a dim light was performed before exposure to normal culture conditions [141,142,148]. Additionally, it is worth mentioning that in the V cryo-plate protocol tested for potato, high regrowth levels were achieved when cryopreserved shoot tips were directly post-thaw cultured under normal light conditions [56]. Therefore, a variation in the light regimes can be considered depending on the genotype response to cryogenic procedures. Edesi et al. [222] investigated the effect of light spectral qualities on survival and regeneration of potato shoot tips cryopreserved by the DMSO-droplet method. Following a direct exposure to the various light conditions, significantly higher regrowth was achieved when cryopreserved shoot tips were incubated in a combination of red light with 10% of blue, which doubled the regrowth levels in all four cultivars tested [222]. Based on these findings, a gradual transition of light intensity with altered light spectrum may be an important requirement for successful cryopreservation, particularly in plant species recalcitrant to cryopreservation. Additionally, adjustment of growth conditions of shoot tip donor plants can also influence shoot recovery after cryoexposure. In cassava, higher illumination (75 μE m−2 s−1) for pretreating donor cultures increased the shoot tip recovery to a level of 50–60% after cryopreservation [219]. A similarly positive impact of different pre- and post-freeze light regimes in shoot regrowth was observed in cryopreserved shoot tips of potato [87]. Therefore, the optimization of the light conditions before shoot tip excision can also influence the shoot tip recovery after cryopreservation.
4. Exploring the Scientific Basis behind Shoot Tip Recovery after Cryopreservation
4.1. Metabolic Responses to the Cold and Osmotic Treatments
In order to achieve satisfactory regrowth levels after cryoexposure, cold-hardening or (and) osmotic treatments are required to induce the optimal physiological state for successful dehydration and cryopreservation for some crops [150,181]. Therefore, understanding the basis behind is of great importance to overcome the challenges in shoot tip cryopreservation. Kaczmarczyk et al. [223] detected higher concentrations of soluble sugars (glucose, fructose, and sucrose) in all potato accessions tested after an alternating temperature pretreatment (22/8 °C day/night temperature) of donor plants for seven days before shoot tip isolation. Similarly, increased levels of carbohydrates and polyols were also revealed in potato shoot tips harvested from shoots previously treated with 0.11 M sorbitol for 21 days [224]. In addition, a proteome analysis showed different protein patterns between the osmotically hardened shoots and non-hardened controls [224]. Based on these observations, the detailed evaluation of carbohydrates was combined with proteomic studies to generate a new knowledge and insights behind the pretreatment-induced tolerance to cryopreservation [159,182,225].
Following the cold acclimation (6 °C) and osmotic (0.3 M sucrose or 0.21 M sorbitol) pretreatments for 2 weeks, osmotically active compounds (sugar) accumulated in potato cultivar Désirée (S. tuberosum) and frost-tolerant S. commersonii [225]. These results suggested an important role of sucrose, fructose, and glucose in acclimation to cold, since there is a larger carbohydrate accumulation in Désirée compared to S. commersonii when submitted to osmotic stress [225]. In addition, 94 differentially abundant proteins were found between the two cultivars depending on the pretreatment, and were classified with functions in carbohydrate, protein and lipid metabolism, transcription and translation, oxidative homeostasis, and in response to stress [225]. Folgado et al. [182] further correlated the changes of sugar content and proteome with post-thaw recovery in cultivated potato cultivar Désirée and its frost-resistant relative S. commersonii cryopreserved by Dr-Vi protocol. In their study, shoot tip donor plants were submitted to osmotic stress (0.3 M sucrose) and chilling (6 °C) treatment for 15 days before shoot tip isolation. Significantly improved regrowth was found in potato S. commersonii pretreated with both chilling and osmotic treatments, whereas for the cultivar Désirée only osmotic treatment resulted in improved shoot regrowth after cryopreservation [182]. The improved shoot regrowth and tolerance to cryopreservation stress were associated with the accumulation of oxidative homeostasis-related proteins and sugars, which were shown to be genotype-specific [182]. However, this study cannot point out a single compound or metabolic pathway highly linked to the ability of shoot tips to survive cryopreservation [182]. The same sugar analysis and proteomics were further expanded to four Solanum species after the same sucrose preculture and cold acclimation as described by Folgado et al. [182], revealing the concentration of sucrose and raffinose family of oligosaccharides (FOS) as primary indicators of tolerance to cryopreservation [159]. In addition, it has been confirmed that the carbon metabolism-related proteins, together with stress-response and oxidative-homeostasis related proteins were the main class of proteins that changed in abundance after the pretreatments in shoot tip donor plants [159].
While similar strategies and pretreatments were applied, results obtained from these studies by Folgado et al. [159,182,225] progressively increased the understanding of the changing carbohydrates and proteome underlying the genotypic responses during osmotic and cold pretreatments, which have been routinely applied to improve the shoot tip regrowth of RTCs after cryopreservation [94,102,129,132,160]. The accumulated sugars and proteins in response to the cold/osmotic pretreatments may also ease the subsequent vitrification process within shoot tips.
As PVS3 was also suitable for shoot tip cryopreservation of many plant species [226,227], including potato [131,227], Köpnick et al. [131] presented a detailed study to analyze the soluble sugars and adenosine triphosphate (ATP) content in potato shoot tips following the DMSO droplet freezing and PVS3 Dr-vi methods. Sucrose, glucose, and fructose levels significantly increased following preculture in combination with cryoprocedures and LN treatment and were reduced during regeneration. In contrast, ATP reached its minimum level after cryoprotection and cryopreservation, and was followed by a rapid recovery following the post-thaw culture [131]. The reduced ATP levels are an indicator of inhibited ATP synthase caused, in particular, by inactivation of mitochondrial functions [228], due to the plant response to osmotically cryoprotective stresses that also contribute to the overproduction of ROS concomitantly [197,198]. Therefore, elimination of excess ROS as well as repair of osmotic damage on cellular components are necessary to achieve satisfactory shoot tip regrowth after cryopreservation. All these requirements depend on the recovered mitochondrial activity, as well as the increased ATP synthesis [228]. Therefore, the high level of ATP measured may be linked to the viability of post-thaw cultured shoot tips. However, only a low correlation between the ATP content and the shoot tip regrowth was observed by Köpnick et al. [131]. Therefore, as suggested by the authors, further analysis on the measurements of different time points during regeneration is needed before considering ATP level as an indicator of regrowth capacity in shoot tip cryopreservation [131].
4.2. Anti-Oxidative/Freezing Responses behind Shoot Tip Cryopreservation
Seo et al. [211] observed significantly higher expressions of CRTDRE-binding factor 1 (CBF1) and Dehydrin 1 (DHN1) when potato leaves were exposed to 4 °C for three days. The expression of the genes CBF1 and DHN1 with stress-responsive plasma membrane H+-ATPase (PMHA) and proline-rich cell wall protein (PRCWP) were therefore measured in post-thaw cultured shoot tips after addition of various concentrations of AFP III to the LS and PVS2 treatment [206]. The upregulation of these genes was strongly associated with the improved shoot regrowth obtained from the AFP III treatment to either LS or PVS2, supporting the use of AFP III to protect cell membranes from cold-induced stress [206].
It has also been noted that for potato cultivars recalcitrant to cryopreservation, the addition of SA (10−6 M) to the pretreatment medium was effective to increase shoot recovery after LN exposure [158]. To understand the mechanism of SA-supported shoot tip cryopreservation, Ruiz-Sáenz et al. [229] measured the antioxidant activities such as catalase (CAT) and peroxidase (POX), as well as the accumulation of hydrogen peroxide (H2O2) and proline in shoot tip donor plants after SA treatment. They found that the SA treatment reduced CAT enzymatic activity and increased accumulation of H2O2 and proline, as well as enhanced water potential prior to cryopreservation, while the increased CAT and POX activities and decreased production of H2O2 were observed after cryopreservation and were associated with higher survival levels after LN exposure [229].
4.3. Understanding the Responses of Cell and Tissue to Cryogenic Treatment under Microscope
Histological studies were performed to observe cell survival patterns in longitudinal sections of potato shoot tip following a two-step cooling protocol using DMSO as cryoprotectant and rapid cooling and warming techniques [63,230]. The use of scanning electron microscopy (SEM) confirmed various surviving patterns in cryopreserved potato shoot tips, and the presence of a number of freeze-damaged cells in a surviving shoot tip did not prevent its subsequent organized growth into a healthy plant without an intervening callus phase [230]. In addition, transmission electron microscopy (TEM) and light microscopy (LM) were applied and revealed that most of the surviving cells were located in the apical dome and leaf primordia, indicating that the cells from these areas are more likely to survive after cryopreservation [230]. However, a portion of these surviving cells exhibited numerous small and empty vesicles in the cytoplasm, as well as damages to the mitochondria, plasmalemma, and nuclear membrane, which were not observed in the non-frozen control material [229]. These results indicate that, although healthy shoots were later obtained, significant damages may still occur in survived meristems during the initial post-thaw recovery.
Following the Vi protocol, Golmirzaie et al. [231] applied TEM for histological observation in apices of four potato genotypes after LN exposure. Similar symptoms of damages to the shoot tip tissues were detected as previously noted by Grout & Henshaw [230]. In another study, TEM and LM were applied to potato shoot tips cryopreserved by a DMSO droplet method [232]. After an incubation with DMSO for 2 h, cells contained numerous small vesicles, enlarged mitochondria and chloroplasts, as well as irregularly shaped vacuoles. Following cryopreservation, surviving shoots remained green during the first two days of post-thaw recovery, while the green color disappeared in those that failed to recover. Moreover, the viable cells were mainly found in the leaf primordia, which support the regeneration of cryo-derived shoots started with the regrowth of young leaves followed by shoot formation from these viable tissues [232].
Driven by the success of Vi-based methods, Wang et al. [233] used LM to compare the cryo-injuries of potato shoot tip cells following Vi, En-vi and Dr-vi. The Vi protocol resulted in the most severe injury to cells of apical meristem, which was linked to its low regrowth levels and long regeneration time as compared to those cryopreserved using En-vi and Dr-vi methods [129,233]. Following cryopreservation, only apical shoot tips survived after En-vi, while both apical and axillary shoot tips revived after Dr-vi [129].
To date, studies on the histological observation in shoot tip cryopreservation of other RTCs are quite limited as compared with potato. Ultrastructural studies for other RTC crops would lead to a better understanding of their cellular response to cryoprotective procedures.
5. Conclusions and Future Prospects
RTCs serve as an important source of energy and nutrients in human diet. The conservation of the genetic diversity of cultivated RTCs and their wild relatives forms the basis of elite crop cultivars to tackle future demanding challenges. Cryopreservation is considered an efficient strategy that facilitates the safe, cost-effective, and long-term preservation of plant genetic resources. Over the past four decades, significant progress has been achieved in shoot tip cryopreservation of a wide range of plant genera. Many RTCs are vegetatively propagated and shoot tip cryopreservation is particularly of high value because they can maintain a high level of genetic stability.
Since the first report on potato shoot tip cryopreservation was published in 1977, much research has been carried out, with methods optimized to satisfy the genebank standards for the implementation of cryopreserved potato, cassava, and sweet potato collections. The encapsulation technique combined with sucrose preculture prior to air desiccation achieved shoot regrowth in all major RTCs. The development of PVS2 vitrification solution-based methods contributed considerably to the application of cryopreservation in many institutes around the world. Among the vitrification solution-based methods, Dr-vi and V cryo-plate facilitate ultra-rapid freezing and thawing and have resulted in the highest shoot regrowth levels in most RTCs. The D cryo-plate also proved applicable in shoot tip cryopreservation of potato and ulluco. Cryopreservation is now successfully implemented for conserving large collections of potato shoot tips. For other RTCs such as cassava, sweet potato, and yam, pilot cryobanks have been established, but further research is urgently needed to overcome the recalcitrance of some species/genotypes to cryopreservation.
To further improve the efficiency of shoot tip cryopreservation in recalcitrant species, the Vi- and En-de-based procedures followed by ultra-rapid freezing (Figure 2a) are recommended. However, each step within the cryoprocedure must be careful optimized (Figure 3). Various parameters can be considered for protocol improvement, including (1) pretreatment of donor plants with cold acclimatization or with high level of sucrose or other osmotic agents under optimized light conditions prior to the shoot tip excision; (2) improvement of the shoot tip quality and uniformity through optimization of in vitro culture systems; (3) addition of antioxidants and/or elicitors of defense-related proteins in plants to the preculture and pretreatment media; (4) optimization of the component concentrations, duration, and culture conditions in preculture and osmoprotection steps; (5) comparison of various PVSs or their modifications, as well as the extent and conditions during dehydration, or optimization the water content of the encapsulated shoot tips prior LN exposure; (6) incorporation of anti-freeze proteins or antioxidants to alleviate osmotic stress and freezing injuries and increase cold tolerance; (7) optimization of sugar concentration and ULS temperature during the thawing process for Vi-based methods; and (8) post-thawing with stepwise decreased sucrose, reduced ammonium and optimized concentration and type of PGRs under adequate light conditions (Figure 3). To date, most of the cryopreservation studies were focusing solely on the optimization of the pre-LN steps and only a few studies have explored the effect of post-thaw conditions (light, medium composition, and growth regulators). However, at least for some RTCs recalcitrant to cryopreservation, these factors may have a major impact on the regeneration of healthy plants.
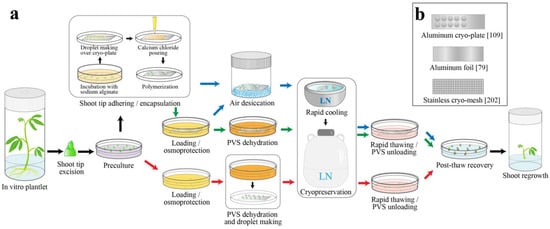
Figure 2.
(a) Comparison of major steps of Droplet-vitrification (Dr-vi), Vitrification cryo-plate (V cryo-plate) and Dehydration cryo-plate (D cryo-plate) for shoot tip cryopreservation. Blue, green, and red arrows indicate procedures applied in D cryo-plate, V cryo-plate, and Dr-vi, respectively. (b) Illustrations of aluminum cryo-plate, aluminum foil, and stainless cryo-mesh that can be applied in the V/D cryo-plate protocols, while the aluminum foil is used in Dr-vi.
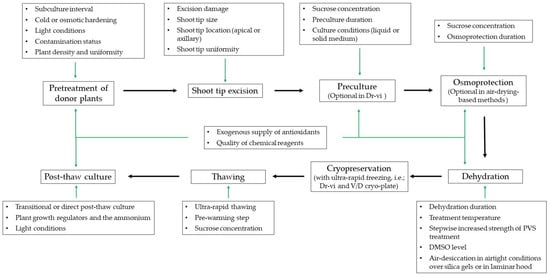
Figure 3.
Factor that can be considered during the development of a shoot tip cryopreservation protocol. Black arrows point to the major steps following the shoot tip cryopreservation, while green arrows indicate strategies that could be considered for each step. Abbreviations: DMSO, dimethyl sulfoxide; Dr-vi, Droplet-vitrification; PVS, plant vitrification solution; V/D cryo-plate, Vitrification/Dehydration cryo-plate.
To obtain better understanding of the mechanism underlying the improved tolerance to cryoprotective procedures, potato was often used as a “model plant”, and evaluations of carbohydrates, the proteome and ATP have been made following the different cryopreservation steps. A high level of sugar accumulated during the preculture has been reported to be important in improving the survival of cryopreserved shoot tip cells. The ATP level was significantly lowered in cryoprotective procedures but improved following the post-thaw culture. Following cold and osmotic pretreatments, the proteome reveals altered expression of genes associated with carbon metabolism, stress-response, and oxidative-homeostasis. The stress-responsive genes were also drastically up-regulated following an exogenous supply of anti-freeze protein to the LS or PVS treatment. In addition, the pretreatment of shoot tip donor plants with SA resulted in reduced CAT enzymatic activity and increased accumulation of H2O2 and proline, which may facilitate the induction of tolerance to cryoprotection. In potato, histological observations were also implemented with SEM and LM to better understand the response of shoot tips to cryopreservation at both cellular and tissues levels. While much attention has been given to potato, the efforts made in the cryobiology study of other RTCs such as cassava and sweet potato were not proportional to the importance of these crops. Therefore, further studies are needed to better understand the cryotolerance of these RTC species. Nevertheless, the achievements highlighted in RTCs would also benefit research and application of shoot tip cryopreservation in other crops as well as the use of cryotherapy methods in the production of pathogen-free plants. The improved shoot tip regrowth after cryopreservation would also ensure the genetic integrity of cryo-derived regenerants.
Author Contributions
A.-L.Z. and M.-R.W.; data collection, figure preparation and original draft preparation, Z.L. and L.X.; manuscript revisions and funding acquisition, B.P., J.C.B. and R.V.; data collection and revisions; Q.-C.W.; manuscript proposal and manuscript revision. All authors have read and agreed to the published version of the manuscript.
Funding
M.-R.W., L.X. and Z.-Y.L. acknowledge National Key R&D Program of China (No. 2021YFC2600603); Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences: 1630032022010; Ministry of Agriculture Tropical Species Resource Protection Project, No. 19211045, and Agricultural Variety Improvement and Upgrading Project: RZJP202001. B.P. acknowledge the CGIAR Research Programme Root Tuber and Bananas (CRP-RTB) and the Directorate-General Development Cooperation and Humanitarian Aid, Belgium (DGD) for the financial support of the project ‘Safeguarding vegetatively-propagated crop diversity to nourish people now and in the future’.
Data Availability Statement
Not applicable.
Acknowledgments
M.-R.W. would like to acknowledge the supports from other students studying and working at Qiao-Chun Wang’s lab from 2012 to 2022, and feel grateful for being at Northwest A&F University from 2009 as a bachelor student to 2022 as a post-doc. Thanks to the Wei River and grand Qin Mountains which perpetually flows and stands to the south of the campus for bringing fun and relaxation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zierer, W.; Rüscher, D.; Sonnewald, U.; Sonnewald, S. Tuber and tuberous root development. Annu. Rev. Plant Biol. 2021, 72, 551–580. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, A.; Kumar, T.J. Roots and tuber crops as functional foods: A review on phytochemical constituents and their potential health benefits. Int. J. Food Sci. 2016, 2016, 3631647. [Google Scholar] [CrossRef]
- Bradshaw, J.E.; Bonierbale, M. Potatoes. In Root and Tuber Crops; Bradshaw, J.E., Ed.; Springer: New York, NY, USA, 2010; pp. 1–52. [Google Scholar] [CrossRef]
- Lebot, V. Sweet Potato. In Root and Tuber Crops; Bradshw, J.E., Ed.; Springer: New York, NY, USA, 2010; pp. 97–125. [Google Scholar]
- Epping, J.; Laibach, N. An underutilized orphan tuber crop—Chinese yam: A review. Planta 2020, 252, 58. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.K. A comprehensive review of sweet potato (Ipomoea batatas [L.] Lam): Revisiting the associated health benefits. Trends Food Sci. Technol. 2021, 115, 512–529. [Google Scholar] [CrossRef]
- Leidi, E.O.; Altamirano, A.M.; Mercado, G.; Rodriguez, J.P.; Ramos, A.; Alandia, G.; Sørensen, M.; Jacobsen, S.-E. Andean roots and tubers crops as sources of functional foods. J. Funct. Foods 2018, 51, 86–93. [Google Scholar] [CrossRef]
- FAOSTAT. 2020. Available online: https://www.fao.org/faostat/en/#data/QC (accessed on 23 June 2022).
- OECD/FAO. OECD-FAO Agricultural Outlook 2021–2030; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- Devaux, A.; Goffart, J.P.; Kromann, P.; Andrade-Piedra, J.; Polar, V.; Hareau, G. The potato of the future: Opportunities and challenges in sustainable Agri-food systems. Potato Res. 2021, 64, 681–720. [Google Scholar] [CrossRef]
- Machida-Hirano, R. Diversity of potato genetic resources. Breed Sci. 2015, 65, 26–40. [Google Scholar] [CrossRef]
- Devaux, A.; Goffart, J.-P.; Petsakos, A.; Kromann, P.; Gatto, M.; Okello, J.; Suarez, V.; Hareau, G. Global Food Security, Contributions from Sustainable Potato Agri-Food Systems. In The Potato Crop; Campos, H., Ortiz, O., Eds.; Springer: Cham, Switzerland, 2020; pp. 3–36. [Google Scholar] [CrossRef]
- Zaheer, K.; Akhtar, M.H. Potato Production, Usage, and Nutrition—A Review. Cri. Rev. Food Sci. Nutr. 2016, 56, 711–721. [Google Scholar] [CrossRef]
- Liu, B.; Gu, W.; Yang, Y.; Lu, B.; Wang, F.; Zhang, B.; Bi, J. Promoting potato as staple food can reduce the carbon-land-water impacts of crops in China. Nat. Food 2021, 2, 570–577. [Google Scholar] [CrossRef]
- Ceballos, H.; Okogbenin, E.; Pérez, J.C.; Augusto, L.; López-Valle, B.; Debouck, D. Cassava. In Root and Tuber Crops; Bradshaw, J.E., Ed.; Springer: New York, NY, USA, 2010; pp. 53–96. [Google Scholar] [CrossRef]
- Muiruri, S.K.; Ntui, V.O.; Tripathi, L.; Tripathi, J.N. Mechanisms and approaches towards enhanced drought tolerance in cassava (Manihot esculenta). Curr. Plant Biol. 2021, 28, 100227. [Google Scholar] [CrossRef]
- Amelework, A.B.; Bairu, M.W.; Maema, O.; Vender, S.L.; Laing, M. Adoption and Promotion of Resilient Crops for Climate Risk Mitigation and Import Substitution: A Case Analysis of Cassava for South African Agriculture. Front. Sustain. Food Syst. 2021, 5, 617783. [Google Scholar] [CrossRef]
- Tang, C.; Lu, Y.; Jiang, B.; Chen, J.; Mo, X.; Yang, Y.; Wang, Z. Energy, Economic, and Environmental Assessment of Sweet Potato Production on Plantations of Various Sizes in South China. Agronomy 2022, 12, 1290. [Google Scholar] [CrossRef]
- Motsa, N.M.; Modi, A.T.; Mabhaudhi, T. Sweet potato (Ipomoea batatas L.) as a drought tolerant and food security crop. S. Afr. J. Sci. 2015, 111, 1–8. [Google Scholar] [CrossRef]
- Nimoh, F.; Asare, G.O.; Twumasi, I.; Anaman, R. Consumers’ Willingness to Consume Cassava Leaves as a Leafy Vegetable in the Kumasi Metropolis, Ghana. Int. J. Food Stud. 2018, 7, 38–50. [Google Scholar] [CrossRef]
- Li, M.; Zi, X.; Tang, J.; Xu, T.; Gu, L.; Zhou, H. Effects of cassava foliage on feed digestion, meat quality, and antioxidative status of geese. Poult. Sci. 2020, 99, 423–429. [Google Scholar] [CrossRef]
- Fathima, A.A.; Sanitha, M.; Tripathi, L.; Muiruri, S. Cassava (Manihot esculenta) dual use for food and bioenergy: A review. Food Energy Secur. 2022, 00, e380. [Google Scholar] [CrossRef]
- Tay, D. Tropical and Subtropical Root and Tuber Crops. In Conservation of Tropical Plant Species; Normah, M.N., Chin, H.F., Reed, B.M., Eds.; Springer: New York, NY, USA, 2013; pp. 249–298. [Google Scholar] [CrossRef]
- Lebot, V. Aroids, Cassava. In Tropical Root and Tuber Crops: Cassava, Sweet Potato, Yams and Aroids, 2nd ed.; Stubbs, R., Ed.; CABI: Oxfordshire, UK, 2020; pp. 40–42. [Google Scholar]
- Arnau, G.; Abraham, K.; Sheela, M.N.; Chair, H.; Sartie, A.; Asiedu, R. Yams. In Root and Tuber Crops; Bradshaw, J.E., Ed.; Springer New York: London, UK, 2010; pp. 127–148. [Google Scholar] [CrossRef]
- Quero-Garcia, J.; Ivancic, A.; Lebot, V. Taro and Cocoyam. In Root and Tuber Crops; Bradshaw, J.E., Ed.; Springer: New York, NY, USA, 2010; pp. 149–172. [Google Scholar] [CrossRef]
- Li Pun, H.H.; Mares, V.; Quiroz, R.; León Velarde, C.U.; Valdivia, R.; Reinoso, J. Pursuing the Millennium Development Goals in the Andean Altiplano. Mt. Res. Dev. 2006, 26, 15–19. [Google Scholar] [CrossRef]
- Olusegun, O.V.; Obi-Egbedi, O.; Adeniran, L.O. Root and tuber expansion programme and poverty reduction among farmers in Southwest Nigeria. J. Dev. Agric. Econ. 2015, 7, 332–343. [Google Scholar] [CrossRef]
- Aboajah, F.N.; Ejechi, M.E.; Viashima, S.S.; Adeyongu, S.K.; Muogbo, P.C. Sweet Potato Production for Poverty Alleviation in Nasarawa State, Nigeria. Int. J. Environ. Agric. Biotech. 2018, 3, 380–387. [Google Scholar] [CrossRef]
- Agbarevo, M.N.B.; Okringbo, J.I. Effect of Technologies of National Root Crops Research Institute, Umudike on Poverty Reduction among Farmers in Umuahia Agricultural Zone, Abia State. J. Community Commun. Res. 2020, 5, 83–90. [Google Scholar]
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef]
- van Ittersuma, M.K.; van Bussela, L.G.J.; Wolfa, J.; Grassinib, P.; van Wartb, J.; Guilpartb, N.; Claessensc, L.; de Grootd, H.; Wiebee, K.; Mason-D’Croz, D.; et al. Can sub-Saharan Africa feed itself? Proc. Natl. Acad. Sci. USA 2016, 13, 14964–14969. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, G.; López Noriega, I. Conservation and Use of Genetic Resources of Underutilized Crops in the Americas—A Continental Analysis. Sustainability 2014, 6, 980–1017. [Google Scholar] [CrossRef]
- Ellis, D.; Salas, A.; Chavez, O.; Gomez, R.; Anglin, N. Ex Situ Conservation of Potato [Solanum Section Petota (Solanaceae)] Genetic Resources in Genebanks. In The Potato Crop; Campos, H., Ortiz, O., Eds.; Springer: Cham, Switzerland, 2020; pp. 109–138. [Google Scholar]
- Izquierdo, J.; Roca, W. Under-ultilized Andean Food Crops: Status and Prospects of Plant Biotechnology for The conservation and Sustainable Agricultural Use of Genetic Resources. Acta Hortic. 1998, 457, 157–172. [Google Scholar] [CrossRef]
- Flores, H.E.; Walker, T.S.; Guimarães, R.L.; Bais, H.P.; Vivanco, J.M. Andean Root and Tuber Crops: Underground Rainbows. HortScience 2003, 38, 161–167. [Google Scholar] [CrossRef]
- CIPotato. 2022. Available online: https://cipotato.org/potato/wild-potato-species/ (accessed on 16 July 2022).
- CIP Genebanks, CGIAR Genebank Platform. 2022. Available online: https://www.genebanks.org/genebanks/international-potato-centre/ (accessed on 1 October 2022).
- Jarvis, A.; Lane, A.; Hijmans, R.J. The effect of climate change on crop wild relatives. Agric. Ecosyst. Environ. 2008, 126, 13–23. [Google Scholar] [CrossRef]
- Brummitt, N.A.; Bachman, S.T.; Griffiths-Lee, J.; Lutz, M.; Moat, J.F.; Farjon, A.; Donaldson, J.S.; Hilton-Taylor, C.; Meagher, T.R.; Albuquerque, S.; et al. Green Plants in the Red: A Baseline Global Assessment for the IUCN Sampled Red List Index for Plants. PLoS ONE 2015, 10, e0135152. [Google Scholar] [CrossRef]
- FAO. Genebank Standards for Plant Genetic Resources for Food and Agriculture. 2014. Available online: https://www.fao.org/3/i3704e/i3704e.pdf (accessed on 2 January 2023).
- Lebot, V. Aroids, Origin and History. In Tropical Root and Tuber Crops: Cassava, Sweet Potato, Yams and Aroids, 2nd ed.; Stubbs, R., Ed.; CABI: Oxfordshire, UK, 2020; pp. 329–334. [Google Scholar]
- Panis, B.; Nagel, M.; Van den houwe, I. Challenges and Prospects for the Conservation of Crop Genetic Resources in Field Genebanks, in In Vitro Collections and/or in Liquid Nitrogen. Plants 2020, 9, 1634. [Google Scholar] [CrossRef]
- Lüttringhaus, S.; Pradel, W.; Suarez, V.; Manrique-Carpintero, N.C.; Anglin, N.L.; Ellis, D.; Hareau, G.; Jamora, N.; Smale, M.; Gómez, R. Dynamic guardianship of potato landraces by Andean communities and the genebank of the International Potato Center. CABI Agric. Biosci. 2021, 2, 1–16. [Google Scholar] [CrossRef]
- Reed, B.M.; Gupta, S.; Uchendu, E.E. In Vitro Genebanks for Preserving Tropical Biodiversity. In Conservation of Tropical Plant Species; Normah, M.N., Chin, H.F., Reed, B.M., Eds.; Springer: New York, NY, USA, 2013; pp. 77–106. [Google Scholar] [CrossRef]
- Bamberg, J.B.; Martin, M.W.; Abad, J.; Jenderek, M.M.; Tanner, J.; Donnelly, D.J.; Nassar, A.M.K.; Veilleux, R.E.; Novy, R.G. In vitro technology at the US Potato Genebank. In Vitro Cell. Dev. Biol.-Plant 2016, 52, 213–225. [Google Scholar] [CrossRef]
- Engelmann, F. In vitro conservation methods. In Biotechnology and Plant Genetic Resources: Conservation and Use; Ford-Lloyd, B.V., Newbury, J.H., Callow, J.A., Eds.; CABI International: Wallingford, UK, 1997; pp. 119–162. [Google Scholar]
- Ruta, C.; Lambardi, M.; Ozudogru, E.A. Biobanking of vegetable genetic resources by in vitro conservation and cryopreservation. Biodivers. Conserv. 2020, 29, 3495–3532. [Google Scholar] [CrossRef]
- Bairu, M.W.; Aremu, A.O.; van Staden, J. Somaclonal variation in plants: Causes and detection methods. Plant Growth Regul. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Van den houwe, I.; Chase, R.; Sardos, J.; Ruas, M.; Kempenaers, E.; Guignon, V.; Massart, S.; Carpentier, S.; Panis, B.; Rouard, M.; et al. Safeguarding and using global banana diversity: A holistic approach. CABI Agric. Biosci. 2020, 1, 15. [Google Scholar] [CrossRef]
- Reed, B.M.; Engelmann, F.; Dulloo, M.E.; Engels, J.M.M. Technical guidelines for the management of field and in vitro germplasm collections. In IPGRI Handbooks for Genebanks No. 7; International Plant Genetic Resources Institute: Rome, Italy, 2004; p. 106. [Google Scholar]
- Sakai, A.; Engelmann, F. Vitrification, Encapsulation-vitrification and Droplet-Vitrification: A review. Cryo-Lett. 2007, 28, 151–172. [Google Scholar]
- Benson, E.E.; Harding, K. Cryopreservation of Shoot Tips and Meristems: An Overview of Contemporary Methodologies. In Plant Cell Culture Protocols, Methods in Molecular Biology; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Humana: Totowa, NJ, USA, 2012; Volume 877, pp. 191–226. [Google Scholar] [CrossRef]
- Sadia, B.; Anthony, P.; Lowe, C.K.; Power, J.B.; Davey, M.R. Culture treatments for enhancing post-thaw recovery of cryopreserved suspension cells of potato cv. Desiree. Cell. Mol. Biol. Lett. 2003, 8, 979–989. [Google Scholar] [PubMed]
- Kaczmarczyk, A.; Rokka, V.-M.; Keller, E.R.J. Potato Shoot Tip Cryopreservation. A Review. Potato Res. 2011, 54, 45–79. [Google Scholar] [CrossRef]
- Yamamoto, S.; Wunna; Rafique, T.; Valle Arizaga, M.; Fukui, K.; Cruz Cutierrez, E.J.; Castillo Martinez, C.R.; Watanabe, K.; Niino, T. The Alumium Cryo-plate Increases Efficiency of Cryopreservation Protocols for Potato Shoot tips. Am. J. Potato Res. 2015, 92, 250–257. [Google Scholar] [CrossRef]
- Smyda-Dajmund, P. Cryopreservation of shoot tips and pollen of potato. Plant Breed. Seed Sci. 2017, 76, 75–80. [Google Scholar] [CrossRef]
- Vollmer, R.; Espirilla, J.; Villagaray, R.; Cárdenas, J.; Castro, M.; Sánchez, J.C.; Manrique-Carpintero, N.; Ellis, D.; Anglin, N.L. Cryopreservation of Potato Shoot Tips for Long-Term Storage. Methods Mol. Biol. 2021, 2354, 21–54. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Volk, G.M. Challenges in implementing plant shoot tip cryopreservation technologies. Plant Cell Tissue Organ Cult. 2021, 144, 21–34. [Google Scholar] [CrossRef]
- Wang, M.-R.; Lambardi, M.; Engelmann, F.; Pathirana, R.; Panis, B.; Volk, G.M.; Wang, Q.-C. Advances in cryopreservation of in vitro-derived propagules: Technologies and explant sources. Plant Cell Tissue Organ Cult. 2021, 144, 7–20. [Google Scholar] [CrossRef]
- Zámečník, J.; Faltus, M.; Bilavcik, A. Vitrification Solutions for Plant Cryopreservation: Modification and Properties. Plants 2021, 10, 2623. [Google Scholar] [CrossRef] [PubMed]
- Westcott, R.J.; Henshaw, G.G.; Roca, W.M. Tissue culture storage of potato germplasm: Culture initiation and plant regeneration. Plant Sci. Lett. 1977, 9, 309–315. [Google Scholar] [CrossRef]
- Grout, B.W.W.; Henshaw, G.G. Freeze Preservation of Potato Shoot-tip Cultures. Ann. Bot. 1978, 42, 1227–1229. [Google Scholar] [CrossRef]
- Engelmann, F.; Arnao, M.T.G.; Wu, Y.; Escobar, R. Development of Encapsulation Dehydration. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 59–75. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Kretzschmar, A.A.; Bonnart, R.; Shepherd, A.; Volk, G.M. Cryopreservation of 12 Vitis species using apical shoot tips derived from plants grown in vitro. HortScience 2019, 54, 976–981. [Google Scholar] [CrossRef]
- Panis, B. Sixty years of plant cryopreservation: From freezing hardy mulberry twigs to establishing reference crop collections for future generations. Acta Hortic. 2019, 1234, 1–7. [Google Scholar] [CrossRef]
- Jenderek, M.M.; Reed, B.M. Cryopreserved storage of clonal germplasm in the USDA National Plant Germplasm System. In Vitro Cell. Dev. Biol.-Plant 2017, 53, 299–308. [Google Scholar] [CrossRef]
- Vollmer, R.; Villagaray, R.; Castro, M.; Cárdenas, J.; Pineda, S.; Espirilla, J.; Anglin, N.; Ellis, D.; Azevedo, V. The world’s largest potato cryobank at the International Potato Center (CIP)—Status quo, protocol improvement through large-scale experiments and long-term viability monitoring. Front. Plant Sci. 2022, 13, 1059817. [Google Scholar] [CrossRef] [PubMed]
- Niino, T.; Arizaga, M.V. Cryopreservation for preservation of potato genetic resources. Breed Sci. 2015, 65, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Dumet, D.; Diebiru, E.; Adeyemi, A.; Akinyemi, O.; Gueye, B.; Franco, J. Cryopreservation for the ‘In Perpetuity’ Conservation of Yam and Cassava Genetic Resources. Cryo-Lett. 2013, 34, 107–118. [Google Scholar]
- Acker, J.P.; Adkins, S.; Alves, A.; Horna, D.; Toll, J. Feasibility Study for a Safety Back-Up Cropreservation Facility. Independent Expert Report; Bioversity International: Rome, Italy, 2017. [Google Scholar]
- Escobar, R.H.; Muñoz, L.; Rios, A.; Núñez, A.; Tohme, J. Using a Droplet-Vitrification Method to Partially Overcome the Recalcitrance of Cassava to Cryostorage. Acta Hortic. 2014, 1039, 227–232. [Google Scholar] [CrossRef]
- Wilms, H.; Sleziak, N.F.; Van der Auweraer, M.; Brands, M.; Verleije, M.; Hardeman, D.; Andre, E.; Panis, B. Development of a fast and user-friendly cryopreservation protocol for sweet potato genetic resources. Sci. Rep. 2020, 10, 14674. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Malhotra, E.V.; Chandra, R.; Gowthami, R.; Sultan, S.M.; Bansal, S.; Shankar, M.; Agrawal, A. Cryopreservation and genetic stability assessment of regenerants of the critically endangered medicinal plant Dioscorea deltoidea Wall. ex Griseb. for cryobanking of germplasm. In Vitro Cell. Dev. Biol.-Plant 2022, 58, 521–529. [Google Scholar] [CrossRef]
- Sánchez, D.F.; Panta, A.; Tay, D.; Roca, W. Cryopreservation of Ulluco (Ullucus tuberosus Cal.) and Oca (Oxalis tuberosa Mol.) Shoot Tips Using the PVS2 Droplet-Vitrification Method. Acta Hortic. 2011, 908, 339–346. [Google Scholar] [CrossRef]
- Bajaj, Y.P.S. Initiation of shoots and callus from potato-tuber sprouts and axillary buds frozen at −196 °C. Crop Improv. 1977, 4, 48–53. [Google Scholar]
- Kartha, K.K.; Leung, N.L.; Mroginski, L.A. In vitro Growth Responses and Plant Regeneration from Cryopreserved Meristems of Cassava (Manihot esculenta Crantz). Z. Pflanzenphysiol. 1982, 107, 133–140. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Panis, B.; Piette, B.; Swennen, R. Droplet vitrification of apical meristems: A cryopreservation protocol applicable to all Musaceae. Plant Sci. 2005, 168, 45–55. [Google Scholar] [CrossRef]
- Elliott, G.D.; Wang, S.; Fuller, B.J. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 2017, 76, 74–91. [Google Scholar] [CrossRef]
- Schäfer-Menuhr, A.; Schumacher, H.M.; Mix-Wagner, G. Langzeitlagerung alter Kartoffelsorten durch Kryokonservierung der Meristeme. Landbauforsch. Völkenrode 1994, 44, 301–313. [Google Scholar]
- Schäfer-Menuhr, A.; Müller, E.; Mix-Wagner, G. Cryopreservation: An alternative for the long-term storage of old potato cultivars. Potato Res. 1996, 39, 507–513. [Google Scholar] [CrossRef]
- Schäfer-Menuhr, A.; Schumacher, H.M.; Mix-Wagner, G. Cryopreservation of potato cultivars-design of a method for routine application in genebanks. Acta Hortic. 1997, 447, 447–482. [Google Scholar] [CrossRef]
- Mix-Wagner, G.; Schumacher, H.M.; Cross, R.J. Recovery of Potato Apices After Several Years of Storage in Liquid Nitrogen. Cryo-Lett. 2002, 24, 33–41. [Google Scholar]
- Towill, L.E. Improved Survival after Cryogenic Exposure of Shoot Tips Derived from In Vitro Plantlet Cultures of Potato. Cryobiology 1983, 20, 567–573. [Google Scholar] [CrossRef]
- Towill, L.E. Survival at ultra-low temperatures of shoot tips from Solanum tuberosum groups Andigena, Phureja, Stenotomum, Tuberosum, and other tuber-bearing Solanum species. Cryo-Lett. 1984, 5, 319–326. [Google Scholar]
- Benson, E.E.; Harding, K.; Smith, H. Variation in recovery of cryopreserved shoot-tips of Solanum tuberosum exposed to different pre- and post-freeze light regimes. Cryo-Lett. 1989, 10, 323–344. [Google Scholar]
- Escobar, R.H.; Mafla, G.; Roca, W.M. A methodology for recovering cassava plants from shoot tips maintained in liquid nitrogen. Plant Cell Rep. 1997, 16, 474–478. [Google Scholar] [CrossRef]
- Bhatti, M.H.; Percival, T.; Davey, C.D.M.; Henshaw, G.G.; Blakesley, D. Cryopreservation of embryogenic tissue of a range of genotypes of sweet potato (Ipomoea Batatas [L.] Lam.) using an encapsulation protocol. Plant Cell Rep. 1997, 16, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-A.; Dhital, S.P.; Fang, Y.-L.; Khu, D.-M.; Song, Y.-S.; Park, E.-J.; Kang, C.-W.; Lim, H.-T. Application of Slow-Freezing Cryopreservation Method for the Conservation of Diverse Potato (Solanum tuberosum L.) Genotypes. J. Plant Biotechnol. 2005, 7, 183–186. [Google Scholar]
- Vollmer, R.; Villagaray, R.; Cárdenas, J.; Castro, M.; Chávez, O.; Anglin, N.L.; Ellis, D. A large-scale viability assessment of the potato cryobank at the International Potato Center (CIP). In Vitro Cell. Dev. Biol.-Plant 2017, 53, 309–317. [Google Scholar] [CrossRef]
- Fabre, J.; Dereuddre, J. Encapsulation dehydration a new approach to cryopreservation of Solanum shoot-tips. Cryo-Lett. 1990, 11, 413–426. [Google Scholar]
- Bouafia, S.; Jelti, N.; Lairy, G.; Blanc, A.; Bonnel, E.; Dereuddre, J. Cryopreservation of potato shoot tips by encapsulation-dehydration. Potato Res. 1996, 39, 69–78. [Google Scholar] [CrossRef]
- Grospietsch, M.; Stodulková, E.; Zámecnik, J. Effect of osmotic stress on the dehydration tolerance of Solanum tuberosum shoot tips. Cryo-Lett. 1999, 20, 339–346. [Google Scholar]
- Matsumoto, T.; Sakai, A. An approach to enhance dehydration tolerance of alginate-coated dried meristems cooled to −196 °C. Cryo-Lett. 1995, 16, 299–306. [Google Scholar]
- Takagi, H. Recent developments in cryopreservation of shoot tips of tropical species. In Cryopreservation of Tropical Plant Germplasm; Engelmann, F., Takagi, H., Eds.; International Plant Genetics Research Institute: Rome, Italy, 2000; pp. 178–193. [Google Scholar]
- Engelmann, F.; Benson, E.E.; Chabrillange, N.; Gonzalez Arnao, M.T.; Mari, S.; Michaux-Ferriere, N.; Paulet, F.; Glaszmann, J.C.; Charrier, A. Cryopreservation of Several Tropical Plant Species using Encapsulation/Dehydration of Apices. In Current Issues in Plant Molecular and Cellular Biology. Current Plant Science and Biotechnology in Agriculture; Terzi, M., Cella, R., Falavigna, A., Eds.; Springer: Dordrecht, The Netherlands, 1995; Volume 22, pp. 315–320. [Google Scholar] [CrossRef]
- Benson, E.E.; Harding, K.; Ryan, M.; Petrenko, A.; Petrenko, Y.; Fuller, B. Alginate Encapsulation to Enhance Biopreservation Scope and Success: A Multidisciplinary Review of Current Ideas and Applications in Cryopreservation and Non-freezing Storage. Cryo-Lett. 2018, 39, 14–38. [Google Scholar]
- Pennycooke, J.C.; Towill, L.E. Medium alternations improve regrowth of sweet potato (Ipomoea batatas [L.] Lam.) shoot tips cryopreserved by vitrification and encapsulation-dehydration. Cryo-Lett. 2001, 22, 381–389. [Google Scholar]
- Mandal, B.B.; Chandel, K.P.S.; Dwivedi, S. Cryopreservation of Yam (Dioscorea spp.) Shoot Apices by Encapsulation-Dehydration. Cryo-Lett. 1996, 17, 165–174. [Google Scholar]
- Malaurie, B.; Trouslot, M.F.; Engelmann, F.; Chabrillange, N. Effect of Pretreatment Conditions on the Cryopreservation of in vitro-cultured Yam (Dioscorea alata ‘Brazo Fuerte’ and D. bulbifera ‘Nouméa Imboro’) Shoot Apices by Encapsulation-Dehydration. Cryo-Lett. 1998, 19, 15–26. [Google Scholar]
- Hong, S.-R.; Yin, M.-H. A simple cryopreservation protocol for in vitro-grown shoot tips of Chinese genuine red bud taro (Colocasia esculenta L. Schott Var. Cormosus CV. Hongyayu) by encapsulation-dehydration. Sci. Hortic. 2013, 162, 226–233. [Google Scholar] [CrossRef]
- Valle Arizaga, M.; Yamamoto, S.I.; Tanaka, D.; Fukui, K.; Nohara, N.; Nishikawa, T.; Kazuo, W.; Niino, T. Cryopreservation of in vitro shoot tips of ulluco (Ullucus tuberosus Cal.) using D cryo-plate method. Cryo-Lett. 2017, 38, 419–427. [Google Scholar]
- Niino, T.; Yamamoto, S.; Fukui, K.; Martínez, C.R.C.; Arizaga, M.V.; Matsumoto, T.; Engelmann, F. Dehydration Improves Cryopreservation of Mat Rush (Juncus decipiens Nakai) Basal Stem Buds on Cryo-plates. Cryo-Lett. 2013, 34, 549–560. [Google Scholar]
- Scottez, C.; Chevreau, E.; Godard, N.; Arnaud, Y.; Duron, M.; Dereuddre, J. Cryopreservation of Cold-Acclimated Shoot Tips of Pear In Vitro Cultures after Encapsulation-Dehydration. Cryobiology 1992, 29, 691–700. [Google Scholar] [CrossRef]
- Niino, T.; Sakai, A. Cryopreservation of alginate coated in vitro-grown shoot tips of apple, pear and mulberry. Plant Sci. 1992, 87, 199–206. [Google Scholar] [CrossRef]
- Hirai, D.; Sakai, A. Cryopreservation of in vitro-grown meristems of potato (Solanum tuberosum L.) by encapsulation-vitrification. Potato Res. 1999, 42, 153–160. [Google Scholar] [CrossRef]
- Kim, H.-H.; Yoon, J.-W.; Park, Y.-E.; Cho, E.-G.; Sohn, J.-K.; Kim, T.-S.; Engelmann, F. Cryopreservation of Potato Cultivated Varieties and Wild Species: Critical Factors in Droplet Vitrification. Cryo-Lett. 2006, 27, 223–234. [Google Scholar]
- Yamamoto, S.-i.; Rafique, T.; Priyantha, W.S.; Fukui, K.; Matsumoto, T.; Niino, T. Development of a cryopreservation procedure using aluminum cryo-plates. Cryo-Lett. 2011, 32, 256–265. [Google Scholar]
- Niino, T.; Yamamoto, S.; Matsumoto, T.; Engelmann, F.; Arizaga, M.V.; Tanaka, D. Development of V and D cryo-plate methods as effective protocols for cryobanking. Acta Hortic. 2019, 1234, 249–262. [Google Scholar] [CrossRef]
- Valle Arizaga, M.; Navarro, O.F.V.; Martinez, C.R.C.; Gutiérrez, E.J.C.; Delgado, H.A.L.; Yamamoto, S.; Watanabe, K.; Niino, T. Improvement to the D Cryo-plate Protocol Applied to Practical Cryopreservation of In Vitro Grown Potato Shoot Tips. Hort. J. 2017, 86, 222–228. [Google Scholar] [CrossRef]
- Tanaka, D.; Sakuma, Y.; Yamamoto, S.; Valle Arizaga, M.; Niino, T.; Masumoto, T. Development of −80 °C storage for Allium shoot tips using D cryo-plate method. Plant Cell Tissue Organ Cult. 2021, 144, 115–122. [Google Scholar] [CrossRef]
- Faltus, M.; Bilavcik, A.; Zamecnik, J. Vitrification Ability of Combined and Single Cryoprotective Agents. Plants 2021, 10, 2392. [Google Scholar] [CrossRef]
- Matsumoto, T.; Sakai, A.; Yamada, K. Cryopreservation of in vitro-grown apical meristems of wasabi (Wasabia japonica) by vitrification and subsequent high plant regeneration. Plant Cell Rep. 1994, 13, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Sakai, A. Cryopreservation of axillary shoot tips of in vitro-grown grape (Vitis) by a two-step vitrification protocol. Euphytica 2003, 131, 299–304. [Google Scholar] [CrossRef]
- Zhu, G.-Y.; Geuns, J.M.C.; Dussert, S.; Swennen, R.; Panis, B. Change in sugar, sterol and fatty acid composition in banana meristems caused by sucrose-induced acclimation and its effects on cryopreservation. Physiol. Plant. 2006, 128, 80–94. [Google Scholar] [CrossRef]
- Leunufna, S.; Keller, E.R.J. Investigating a new cryopreservation protocol for yams (Dioscorea spp.). Plant Cell Rep. 2003, 21, 1159–1166. [Google Scholar] [CrossRef]
- Kryszczuk, A.; Keller, J.; Grübe, M.; Zimnoch-Guzowska, E. Cryopreservation of potato (Solanum tuberosum L.) shoot tips using vitrification and droplet method. J. Food. Agric. Environ. 2006, 4, 196–200. [Google Scholar]
- Sakai, A.; Kobayashi, S.; Oiyama, I. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep. 1990, 9, 30–33. [Google Scholar] [CrossRef]
- Matsumoto, T.; Niino, T. Manual of Cryopreservation Methods Using Cryo-Plate: V and D Cryo-Plate Procedure as an Effective Protocol for Cryobanks; Niino, T., Matsumoto, T., Yamamoto, S.-I., Maki, S., Tanata, D., Engelmann, F., Eds.; Plant Tissue Culture and Cryopreservation Group (PTCCcryoG): Tsukuba, Japan, 2017; pp. 8–15. [Google Scholar]
- Matsumoto, T.; Niino, T. The development of plant vitrification solution 2 and recent PVS2-based vitrification protocols. Acta Hortic. 2014, 1039, 21–28. [Google Scholar] [CrossRef]
- Towill, L.E.; Jarrett, R.L. Cryopreservation of sweet potato (Ipomoea batatas [L.] Lam.) shoot tips by vitrification. Plant Cell Rep. 1992, 11, 175–178. [Google Scholar] [CrossRef]
- Sarkar, D.; Naik, P.S. Cryopreservation of shoot tips of tetraploid potato (Solanum tuberosum L.) clones by vitrification. Ann. Bot. 1998, 82, 455–461. [Google Scholar] [CrossRef]
- Wang, B.; Wang, R.; Li, J.; Ma, Y.; Sheng, W.; Li, M.; Wang, Q. Development of three vitrification-based cryopreservation procedures for shoot tips of China’s potato. Cryo-Lett. 2013, 34, 369–380. [Google Scholar]
- Charoensub, R.; Phansiri, S.; Sakai, A.; Yongmanitchai, W. Cryopreservation of cassava in vitro-grown shoot tips collet to −196 °C by vitrification. Cryo-Lett. 1999, 20, 89–94. [Google Scholar]
- Mukherjee, P.; Mandal, B.B.; Bhat, K.V.; Biswas, A.K. Cryopreservation of Asian Dioscorea bulbifera L. and D. alta L. by Vitrification: Importance of Plant Growth Regulators. Cryo-Lett. 2009, 20, 100–111. [Google Scholar]
- Takagi, H.; Thinh, N.T.; Islam, O.M.; Senboku, T.; Sakai, A. Cryopreservation of in vitro-grown shoot tips of taro (Colocasia esculenta (L.) Schott) by vitrification. 1. Investigation of basic conditions of the vitrification procedure. Plant Cell Rep. 1997, 16, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-U.; Kim, H.-H. Cryopreservation of Sweet Potato Shoot Tips Using a Droplet-Vitrification Procedure. Cryo-Lett. 2015, 36, 344–352. [Google Scholar]
- Li, J.-W.; Chen, H.-Y.; Li, X.-Y.; Zhang, Z.; Blystad, D.-R.; Wang, Q.-C. Cryopreservation and evaluations of vegetative growth, microtuber production and genetic stability in regenerants of purple-fleshed potato. Plant Cell Tissue Organ Cult. 2017, 128, 641–653. [Google Scholar] [CrossRef]
- Panta, A.; Panis, B.; Ynouye, C.; Swennen, R.; Roca, W. Development of a PVS2 Droplet Vitrification Method for Potato Cryopreservation. Cryo-Lett. 2014, 35, 255–266. [Google Scholar]
- Köpnick, C.; Grübe, M.; Stock, J.; Senula, A.; Mock, H.-P.; Nagel, M. Changes of soluble sugars and ATP content during DMSO droplet freezing and PVS3 droplet vitrification of potato shoot tips. Cryobiology 2018, 85, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, R.; Villagaray, R.; Castro, M.; Anglin, N.L.; Ellis, D. Cryopreserved potato shoot tips showed genotype-specific response to sucrose concentration in rewarming solution (RS). Plant Cell Tissue Organ Cult. 2019, 136, 353–363. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Mathew, L.; Pathirana, R.; Wiedow, C.; Hunter, D.A.; McLachlan, A.; Khan, S.; Tang, J.; Nadarajan, J. Eradication of Potato Virus S, Potato Virus A, and Potato Virus M from Infected in vitro Grown Potato Shoots Using in vitro Therapies. Front. Plant Sci. 2022, 13, 878733. [Google Scholar] [CrossRef]
- Hirai, D. Gelled Droplet Vitrification Improves Recovery of Cryopreserved Potato Germplasm. Cryo-Lett. 2011, 32, 287–296. [Google Scholar]
- Tannoury, M.; Ralambosoa, J.; Kaminski, M.; Dereuddre, J. Cryopreservation by vitrification of coated shoot tips of carnation (Diathus caryophyllus L.) cultured in vitro. C. R. Acad. Sci. Paris Ser. III 1991, 313, 633–638. [Google Scholar]
- Charoensub, R.; Hirai, D.; Sakai, A. Cryopreservation of in vitro-grown shoot tips of cassava by encapsulation-vitrification method. Cryo-Lett. 2004, 25, 51–58. [Google Scholar]
- Hirai, D.; Sakai, A. Simplified cryopreservation of sweet potato [Ipomoea batatas (L.) Lam.] by optimizing conditions for osmoprotection. Plant Cell Rep. 2003, 21, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.Y.; Lee, G.A.; Lee, Y.Y.; Gwag, J.G.; Son, E.H.; Park, H.J. Cryopreservation of in Vitro Grown Shoot Tips of Sweet Potato (Ipomoea batatas L.) by the Encapsulation-Vitrification Method. Korean J. Plant Res. 2016, 29, 635–641. [Google Scholar] [CrossRef]
- Panis, B.; Piette, B.; André, E.; Van den Houwe, I.; Swennen, R. Droplet vitrification: The first generic cryopreservation protocol for organized plant tissues? Acta Hortic. 2011, 908, 157–163. [Google Scholar] [CrossRef]
- Matsumoto, T. Cryopreservation of plant genetic resources: Conventional and new methods. Rev. Agric. Sci. 2017, 5, 13–20. [Google Scholar] [CrossRef]
- Pennycooke, J.C.; Towill, L.E. Cryopreservation of shoot tips from in vitro plants of sweet potato [Ipomoea batatas (L.) Lam.] by vitrification. Plant Cell Rep. 2000, 19, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, R.; Panta, A.; Tay, D.; Roca, W.; Ellis, D. Effect of Sucrose Preculture and PVS2 Exposure on the Cryopreservation of Sweet Potato Shoot Tips [Ipomea batatas (L.) Lam.] Using the PVS2 Droplet Vitrification. Acta Hortic. 2014, 1039, 265–271. [Google Scholar] [CrossRef]
- Halmagyi, A.; Deliu, C.; Coste, A. Plant regrowth from potato shoot tips cryopreserved by a combined vitrification-droplet method. Cryo-Lett. 2005, 26, 313–322. [Google Scholar]
- Yoon, J.-W.; Kim, H.-H.; Ko, H.-C.; Hwang, H.-S.; Hong, E.-S.; Cho, E.-G.; Engelmann, F. Cryopreservation of Cultivated and Wild Potato Varieties by Droplet Vitrification: Effect of Subculture of Mother-Plants and of Preculture of Shoot Tips. Cryo-Lett. 2006, 27, 211–222. [Google Scholar]
- Panta, A.; Panis, B.; Ynouye, C.; Swennen, R.; Roca, W.; Tay, D.; Ellis, D. Improved cryopreservation method for the long-term conservation of the world potato germplasm collection. Plant Cell Tissue Organ Cult. 2015, 120, 117–125. [Google Scholar] [CrossRef]
- Dumet, D.; Korie, S.; Adeyemi, A. Cryobanking Cassava Germplasm at IITA. Acta Hortic. 2009, 908, 439–446. [Google Scholar] [CrossRef]
- Leunufna, S.; Keller, E.R.J. Cryopreservation of Yam Using Vitrification Modified by Including Droplet Method: Effects of Cold Acclimation and Sucrose. Cryo-Lett. 2005, 26, 93–102. [Google Scholar]
- Sant, R.; Panis, B.; Taylor, M.; Tyagi, A. Cryopreservation of shoot tips by droplet vitrification applicable to taro (Colocasia esculenta var. esculenta) accessions. Plant Cell Tissue Organ Cult. 2008, 92, 107–111. [Google Scholar] [CrossRef]
- Hammond, S.D.H.; Viehmannova, I.; Zámečník, J.; Panis, B.; Faltus, M. Droplet-vitrification methods for apical bud cryopreservation of yacon [Smallanthus sonchifolius (Poepp. and Endl.) H. Rob.]. Plant Cell Tissue Organ Cult. 2021, 147, 197–208. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Marković, Z.; Bi, W.; Volk, G.M.; Matsumoto, T.; Wang, Q.-C. Grapevine Shoot Tip Cryopreservation and Cryotherapy: Secure Storage of Disease-Free Plants. Plants 2021, 10, 2190. [Google Scholar] [CrossRef]
- CIP. Cryopreservation. Available online: https://cipotato.org/genebankcip/process/cryopreservation/ (accessed on 31 August 2022).
- Bettoni, J.C.; Bonnart, R.; Shepherd, A.; Kretzschmar, A.A.; Volk, G.M. Modifications to a Vitis shoot tip cryopreservation procedure: Effect of shoot tip size and use of cryoplates. Cryo-Lett. 2019, 40, 103–112. [Google Scholar]
- Agrawal, A.; Sharma, N.; Gupta, S.; Bansal, S.; Srivastava, V.; Malhotra, E.V.; Chander, S.; Gowthami, R.; Singh, K. Biotechnological applications for plant germplasm conservation at ICAR-National Bureau of Plant Genetic Resources, India—Recent achievements. Acta Hortic. 2022, 1339, 29–41. [Google Scholar] [CrossRef]
- Noor Camellia, N.A.; Abdul Muhaimin, A.K.; Umikalsum, M.B.; Rozlaily, Z. Cryopreservation of In vitro Grown Taro (Colocasia esculenta) Apical Meristem using a Droplet-vitrification PVS3 Technique. Tran. Malays. Soc. Plant Physiol. 2021, 28, 240–245. [Google Scholar]
- Zamecnikova, J.; Fernandez, E.; Viehmannova, I.; Zamecnik, J.; Faltus, M. Preparation of Shoot Tips by Sucrose and Dehydration Pre-Treatment of Ullucus tuberosus Cal. for Cryopreservation. Acta Hortic. 2011, 908, 331–338. [Google Scholar] [CrossRef]
- Li, Z.; Li, T.; Xu, L.; Panis, B. Cryopreservation of Callerya speciosa (Champ.) Schot Through Droplet-Vitrification. Propag. Ornam. Plants 2013, 13, 189–195. [Google Scholar]
- Agrawal, A.; Gowthami, R.; Chander, S.; Srivastava, V. Sustainability of In Vitro Genebanks and Cryogenebanks. Indian J. Genet. Plant Breed. 2022, 35, 180–184. [Google Scholar] [CrossRef]
- Ruiz-Sáenz, D.R.; Ayala-Hernández, D.D.; Niino, T.; Cruz-Gutiérrez, E.J.; Aquino-Martínez, J.G.; López-Delgado, H.A. Salicylic Acid-Cryotherapy Treatment for Elimination of Potato Virus S from Solanum Tuberosum. Am. J. Potato Res. 2019, 96, 225–234. [Google Scholar] [CrossRef]
- Folgado, R.; Panis, B.; Sergeant, K.; Renaut, J.; Swennen, R.; Hausman, J.-F. Unravelling the effect of sucrose and cold pretreatment on cryopreservation of potato through sugar analysis and proteomics. Cryobiology 2015, 71, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-H.; Tsai, S.-F.; Liaw, S.-L. Cryopreservation of Sweet Potato Using Vitrification Technique: Effects of the Vitrification Procedure and Sucrose Pretreatments. Propag. Ornam. Plants 2021, 21, 123–130. [Google Scholar]
- Edesi, J.; Pirttilä, A.M.; Häggman, H. Modified light spectral conditions prior to cryopreservation alter growth characteristics and cryopreservation success of potato (Solanum tuberosum L.) shoot tips in vitro. Plant Cell Tissue Organ Cult. 2017, 128, 409–421. [Google Scholar] [CrossRef]
- Charoensub, R.; Phansiri, S.; Yongmanitchai, W.; Sakai, A. Routine cryopreservation of in vitro-grown axillary apices of cassava (Manihot esculenta Crantz) by vitrification: Importance of a simple mononodal culture. Sci. Hortic. 2003, 98, 485–492. [Google Scholar] [CrossRef]
- Uchendu, E.E.; Shukla, M.; Saxena, P.K.; Keller, J.E.R. Cryopreservation of potato microtubers: The critical roles of sucrose and desiccation. Plant Cell Tissue Organ Cult. 2016, 124, 649–656. [Google Scholar] [CrossRef]
- Volk, G.M.; Shepherd, A.N.; Bonnart, R. Successful cryopreservation of Vitis shoot tips: Novel pre-treatment combinations applied to nine species. Cryo-Lett. 2018, 39, 322–330. [Google Scholar]
- Wang, B.; Ma, Y.-L.; Zhang, Z.-B.; Wu, Z.-M.; Wu, Y.-F.; Wang, Q.-C.; Li, M.-F. Potato viruses in China. Crop Prot. 2011, 30, 1117–1123. [Google Scholar] [CrossRef]
- Legg, J.P.; Kumar, P.L.; Makeshkumar, T.; Tripathi, L.; Ferguson, M.; Kanju, E.; Ntawuruhunga, P.; Cuellar, W. Chapter Four—Cassava Virus Diseases: Biology, Epidemiology, and Management. Adv. Virus Res. 2015, 91, 85–142. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.P. Sweet Potato: Ipomoea batatas. In Plant Protection in Tropical Root and Tuber Crops; Springer: New Delhi, India, 2015; pp. 119–134. [Google Scholar]
- Luo, G.F.; Podolyan, A.; Kidanemariam, D.B.; Pilotti, C.; Houliston, G.; Sukal, A.C. A Review of Viruses Infecting Yam (Dioscorea spp.). Viruses 2022, 14, 662. [Google Scholar] [CrossRef] [PubMed]
- Priegnitz, U.; Lommen, W.J.M.; van der Vlugt, R.A.A.; Struik, P.C. Potato yield and yield components as affected by positive selection during several generations of seed multiplication in southwestern Uganda. Potato Res. 2000, 63, 507–543. [Google Scholar] [CrossRef]
- Thomas-Sharma, S.; Abdurahman, A.; Ali, S.; Andrade-Piedra, J.L.; Bao, S.; Charkowski, A.O.; Crook, D.; Kadian, M.; Kromann, P.; Struik, P.C.; et al. Seed degeneration in potato: The need for an integrated seed health strategy to mitigate the problem in developing countries. Plant Pathol. 2015, 65, 3–16. [Google Scholar] [CrossRef]
- Hadidi, A.; Barba, M. Economic impact of pome and stone fruit viruses and viroids. In Virus and Virus-Like Diseases of Pome and Stone Fruits; Hadidi, A., Barba, M., Candresse, T.H., Jelkmann, W., Eds.; American Phytopathological Society: St. Paul, MN, USA, 2011; pp. 1–7. [Google Scholar] [CrossRef]
- Anžlovar, S.; Kovac, M.; Ravnikar, M. Photosynthetic pigments in healthy and virus-infected potato plantlets (Solanum tuberosum L.) grown in vitro. Phyton 1996, 36, 221–230. [Google Scholar]
- Li, J.-W.; Wang, B.; Song, X.-M.; Wang, R.-R.; Chen, L.; Zhang, H.; Zhang, Z.-B.; Wang, Q.-C. Potato leafroll virus (PLRV) and Potato virus Y (PVY) influence vegetative growth, physiological metabolism, and microtuber production of in vitro-grown shoots of potato (Solanum tuberosum L.). Plant Cell Tissue Organ Cult. 2013, 114, 313–324. [Google Scholar] [CrossRef]
- Chen, J.; Tang, H.-H.; Li, L.; Qin, S.-J.; Wang, G.-P.; Hong, N. Effects of virus infection on plant growth, root development and phytohormone levels in in vitro-cultured pear plants. Plant Cell Tissue Organ Cult. 2017, 131, 359–368. [Google Scholar] [CrossRef]
- Li, J.-W.; Chen, H.-Y.; Li, J.; Zhang, Z.; Blystad, D.-R.; Wang, Q.-C. Growth, microtuber production and physiological metabolism in virus-free and virus-infected potato in vitro plantlets grown under NaCl-induced salt stress. Eur. J. Plant Pathol. 2018, 152, 417–432. [Google Scholar] [CrossRef]
- Wang, M.R.; Hao, X.Y.; Zhao, L.; Cui, Z.H.; Volk, G.M.; Wang, Q.C. Virus infection reduces shoot proliferation of in vitro stock cultures and ability of cryopreserved shoot tips to regenerate into normal shoots in ‘Gala’ apple (Malus × domestica). Cryobiology 2018, 82, 52–58. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, M.R.; Li, J.W.; Cui, Z.H.; Volk, G.M.; Wang, Q.C. Cryobiotechnology: A double-edged sword for obligate plant pathogens. Plant Dis. 2019, 103, 1058–1067. [Google Scholar] [CrossRef]
- Wang, M.R.; Bi, W.-L.; Bettoni, J.C.; Zhang, D.; Volk, G.M.; Wang, Q.-C. Shoot tip cryotherapy for plant pathogen eradication. Plant Pathol. 2022, 71, 1241–1254. [Google Scholar] [CrossRef]
- Panis, B. Cryopreservation of Musa germplasm. In Technical Guidelines of Bioversity International; Benson, E., Engelmann, F., Eds.; Bioversity International: Rome, Italy, 2009; pp. 8–9. [Google Scholar]
- Benson, E.E. Cryopreservation of phytodiversity: A critical appraisal of theory & practice. Crit. Rev. Plant. Sci. 2008, 27, 141–219. [Google Scholar] [CrossRef]
- Engelmann, F. Plant cryopreservation: Progress and prospects. In Vitro Cell. Dev. Biol.-Plant 2004, 40, 427–433. [Google Scholar] [CrossRef]
- Folgado, R.; Sergeant, K.; Renaut, J.; Swennen, R.; Hausman, J.-F.; Panis, B. Changes in sugar content and proteome of potato in response to cold and dehydration stress and their implications for cryopreservation. J. Proteom. 2014, 98, 99–111. [Google Scholar] [CrossRef]
- Kyesmu, P.M.; Takagi, H.; Yashima, S. Cryopreservation of white yam (Dioscorea rotundata) shoot apices by vitrification. In Proceedings of the Annual Meeting of Japan Molecular Biology, Kumamoto, Japan, 20–12 July 1997; p. 162. [Google Scholar]
- Sherlock, G.; Block, W.; Benson, E.E. Thermal Analysis of the Plant Encapsulation-Dehydration Cryopreservation Protocol Using Silica Gel as the Desiccant. Cryo-Lett. 2005, 26, 45–54. [Google Scholar]
- Teixeira, A.S.; Faltus, M.; Zámečcník, J.; González-Benito, M.E.; Molina-García, A.D. Glass transition and heat capacity behaviors of plant vitrification solutions. Thermochim. Acta 2014, 593, 43–49. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Costa, M.D.; Gardin, J.P.P.; Kretzschmar, A.A.; Pathirana, R. Cryotherapy: A new technique to obtain grapevine plants free of viruses. Rev. Bras. Frutic. 2016, 38, e-833. [Google Scholar] [CrossRef]
- Wang, Q.; Tanne, E.; Arav, A.; Gafny, R. Cryopreservation of in vitro-grow shoot tips of grapevine by encapsulation-dehydration. Plant Cell Tissue Organ. Cult. 2000, 63, 41–46. [Google Scholar] [CrossRef]
- Carra, A.; Carimi, F.; Bettoni, J.C.; Pathirana, R. Progress and Challenges in the Application of Synthetic Seed Technology for Ex Situ Germplasm Conservation in Grapevine (Vitis spp.). In Synthetic Seeds; Faisal, A., Alatar, A.A., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 439–467. [Google Scholar] [CrossRef]
- Nishizawa, S.; Sakai, A.; Amano, Y.; Matsuzawa, T. Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci. 1993, 91, 67–73. [Google Scholar] [CrossRef]
- Yamada, T.; Sakai, A.; Matsumura, T.; Higuchi, S. Cryopreservation of apical meristems of white clover (Trifolium repens L.) by vitrification. Plant Sci. 1991, 78, 81–87. [Google Scholar] [CrossRef]
- Manamela, M.T.; Mycock, D.J. Responses of the Buds of Three South African Sweet Potato (Ipomoea Batatas) Accessions to Different Cryoprotectants. Cryo-Lett. 2019, 40, 357–366. [Google Scholar]
- Sakai, A. Development of cryopreservation techniques. In Cryopresevation of Tropical Plant Germplasm, Current Progress and Application; Engelmann, F., Takagi, H., Eds.; IPGRI: Rome, Italy, 2000; pp. 1–7. [Google Scholar]
- Uchendu, E.; Muminova, M.; Gupta, S.; Reed, B.M. Antioxidant and anti-stress compounds improve regrowth of cryopreserved Rubus shoot tips. In Vitro Cell. Dev. Biol.-Plant 2010, 46, 386–393. [Google Scholar] [CrossRef]
- Ren, L.; Wang, M.R.; Wang, Q.-C. ROS-induced oxidative stress in plant cryopreservation: Occurrence and alleviation. Planta 2021, 254, 124. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, D.; Jiang, X.; Gai, Y.; Wang, W.; Reed, B.M.; Shen, X. Peroxidation due to cryoprotectant step is a vital factor for cell survival in Arabidopsis cryopreservation. Plant Sci. 2013, 212, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, D.; Jiang, X.-N.; Gai, Y.; Wang, W.-M.; Reed, B.M.; Shen, X.-H. Transcriptomic profiling revealed the regulatory mechanism of Arabidopsis seedlings response to oxidative stress from cryopreservation. Plant Cell Rep. 2015, 34, 2161–2178. [Google Scholar] [CrossRef]
- Prudente, D.O.; Paiva, R.; Nery, F.C.; Paiva, P.D.O.; Alves, J.D.; Máximo, W.P.F.; Silva, L.C. Compatible solutes improve regrowth, ameliorate enzymatic antioxidant systems, and reduce lipid peroxidation of cryopreserved Hancornia speciosa Gomes lateral buds. In Vitro Cell. Dev. Biol.-Plant 2017, 53, 352–362. [Google Scholar] [CrossRef]
- Vianna, M.G.; Garcia, R.O.; Mansur, E.; Engelmann, F.; Pacheco, G. Oxidative stress during the cryopreservation of Passiflora suberosa L. shoot tips using the V-Cryo-plate technique: Determination of the critical stages of the protocol. Plant Cell Tissue Organ Cult. 2019, 139, 369–379. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trend Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Wang, M.-R.; Bi, W.; Shukla, M.R.; Ren, L.; Hamborg, Z.; Blystad, D.-R.; Saxena, P.K.; Wang, Q.-C. Epigenetic and Genetic Integrity, Metabolic Stability, and Field Performance of Cryopreserved Plants. Plants 2021, 10, 1889. [Google Scholar] [CrossRef]
- Funnekotter, B.; Bunn, E.; Mancera, R.L. Cryo-mesh: A Simple Alternative Cryopreservation Protocol. Cryo-Lett. 2017, 38, 155–159. [Google Scholar]
- Mathew, L.; Burritt, D.J.; McLachlan, A.; Pathirana, R. Combined pre-treatments enhance antioxidant metabolism and improve survival of cryopreserved kiwifruit shoot tips. Plant Cell Tissue Organ Cult. 2019, 138, 193–205. [Google Scholar] [CrossRef]
- Bi, W.L.; Hao, X.Y.; Cui, Z.H.; Volk, G.M.; Wang, Q. Droplet-vitrification cryopreservation of in vitro-grown shoot tips of grapevine (Vitis spp.). In Vitro Cell. Dev. Biol.-Plant 2018, 54, 590–599. [Google Scholar] [CrossRef]
- Uchendu, E.E.; Keller, E.R.J. Melatonin-loaded alginate beads improve cryopreservation of yam (Dioscorea alata and D. cayenensis). Cryo-Lett. 2016, 37, 77–87. [Google Scholar]
- Wang, Z.C.; Deng, X.X. Cryopreservation of shoot-tips of citrus using vitrification: Effect of reduced form of glutathione. Cryo-Lett. 2004, 25, 43–50. [Google Scholar]
- Wang, Q.C.; Valkonen, J.P.T. Improved recovery of cryotherapy- treated shoot tips following thermotherapy of in vitro grown stock shoots of raspberry (Rubus idaeus L.). Cryo-Lett. 2009, 30, 171–182. [Google Scholar]
- Reed, B.M. Antioxidants and cryopreservation, the new normal? Acta Hortic. 2014, 1039, 41–48. [Google Scholar] [CrossRef]
- Chen, G.Q.; Ren, L.; Zhang, J.; Reed, B.M.; Zhang, D.; Shen, X.H. Cryopreservation affects ROS-induced oxidative stress and antioxidant response in Arabidopsis seedlings. Cryobiology 2015, 70, 38–47. [Google Scholar] [CrossRef]
- Chen, G.Q.; Ren, L.; Zhang, D.; Shen, X.H. Glutathione improves survival of cryopreserved embryogenic calli of Agapanthus praecox subsp. orientalis. Acta Physiol. Plant 2016, 38, 250. [Google Scholar] [CrossRef]
- Seo, J.H.; Naing, A.H.; Jeon, S.M.; Kim, C.K. Anti-freezing-protein type III strongly influences the expression of relevant genes in cryopreserved potato shoot tips. Plant Mol. Biol. 2018, 97, 347–355. [Google Scholar] [CrossRef]
- Reed, B.M.; Uchendu, E. Controlled Rate Cooling. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 77–92. [Google Scholar]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Liu, Y.; von Wirén, N. Ammonium as a signal for physiological and morphological responses in plants. J. Exp. Bot. 2017, 68, 2581–2592. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.; Cakmak, T.; Cooper, A.; Lager, I.; Rasmusson, A.G.; Escobar, M.A. Distinct signaling pathways and transcriptome response signatures differentiate ammonium- and nitrate-supplied plants. Plant Cell Environ. 2010, 33, 1486–1501. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.J. (Eds.) The components of plant tissue culture media I: Macro-and micro-nutrients. In Plant Propagation by Tissue Culture; Springer: Dordrecht, The Netherlands, 2008; pp. 65–113. [Google Scholar] [CrossRef]
- Feng, C.; Yin, Z.; Ma, Y.; Zhang, Z.; Chen, L.; Wang, B.; Li, B.; Huang, Y.; Wang, Q. Cryopreservation of sweetpotato (Ipomoea batatas) and its pathogen eradication by cryotherapy. Biotechnol. Adv. 2011, 29, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, F. Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell. Dev. Biol.-Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Escobar, R.H.; Debouck, D.; Roca, W.M. Development of cassava cryopreservation. In Cryopreservation of Tropical Plant Germplasm; Engelmann, F., Takagi, H., Eds.; JIRCAS: Tsukuba, Japan; IPGRI: Rome, Italy, 2000; pp. 222–226. [Google Scholar]
- Macro-Medina, A.; Casas, J.L.; Swennen, R.; Panis, B. Cryopreservation of Thymus moroderi by Droplet Vitrification. Cryo-Lett. 2010, 31, 14–23. [Google Scholar]
- Vollmer, R.; Villagaray, R.; Egúsquiza, V.; Espirilla, J.; García, M.; Torres, A.; Rojas, E.; Panta, A.; Barkley, N.A.; Ellis, D. The Potato Cryobank at the International Potato Center (CIP): A Model for Long Term Conservation of Clonal Plant Genetic Resources Collections of the Future. Cryo-Lett. 2016, 37, 318–329. [Google Scholar]
- Edesi, J.; Kotkas, K.; Pirttilä, A.M.; Häggman, H. Does light spectral quality affect survival and regeneration of potato (Solanum tuberosum L.) shoot tips after cryopreservation? Plant Cell Tissue Organ Cult. 2014, 119, 599–607. [Google Scholar] [CrossRef]
- Kaczmarczyk, A.; Shvachko, N.; Lupysheva, Y.; Hajirezaei, M.-R.; Keller, E.R.J. Influence of alternating temperature preculture on cryopreservation results for potato shoot tips. Plant Cell Rep. 2008, 27, 1551–1558. [Google Scholar] [CrossRef]
- Criel, B.; Hausman, F.; Oufir, M.; Swennen, R.; Panis, B.; Renaut, J. Proteome and sugar analysis of abiotic stress underlying cryopreservation in potato. Commun. Agric. Appl. Biol. Sci. 2006, 71, 3–6. [Google Scholar]
- Folgado, R.; Panis, B.; Sergeant, K.; Renaut, J.; Swennen, R.; Hausman, J.-F. Differential Protein Expression in Response to Abiotic Stress in Two Potato Species: Solanum commersonii Dun and Solanum tuberosum L. Int. J. Mol. Sci. 2013, 14, 4912–4933. [Google Scholar] [CrossRef]
- Wang, M.-R.; Zhang, Z.; Zámečník, J.; Bilavčík, A.; Blystad, D.-R.; Haugslien, S.; Wang, Q.-C. Droplet-vitrification for shoot tip cryopreservation of shallot (Allium cepa var. aggregatum): Effects of PVS3 and PVS2 on shoot regrowth. Plant Cell Tissue Organ Cult. 2020, 140, 185–195. [Google Scholar] [CrossRef]
- Senula, A.; Nagel, M. Cryopreservation of Plant Shoot Tips of Potato, Mint, Garlic, and Shallot Using Plant Vitrification Solution 3. Methods Mol. Biol. 2021, 2180, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Whelehan, L.M.; Funnekotter, B.; Bunn, E.; Mancera, R.L. Review: The case for studying mitochondrial function during plant cryopreservation. Plant Sci. 2022, 315, 111134. [Google Scholar] [CrossRef]
- Ruiz-Sáenz, D.R.; López-Delgado, H.A.; Hernández, D.D.A.; Trejo, C.; Mora-Herrera, M.E.; Mortera, E.U. Induction of tolerance to cryogenic protocols in Solanum tuberosum by salicylic acid is mediated by enzymatic antioxidant activity and hydrogen peroxide. J. Hortic. Sci. Biotechnol. 2022, 97, 86–95. [Google Scholar] [CrossRef]
- Grout, B.W.W.; Henshaw, G.G. Structural Observations on the Growth of Potato Shoot-tip Cultures after Thawing from Liquid Nitrogen. Ann. Bot. 1980, 46, 243–248. [Google Scholar] [CrossRef]
- Golmirzaie, A.M.; Panta, A.; Delgado, C. Structural observations on potato shoot-tips after thawing from liquid nitrogen. In Cryopreservation of Tropical Plant Germplasm. Current Research Progress and Application; Engelmann, F., Takagi, H., Eds.; Japan International Research Center for Agricultural Sciences: Tsukuba, Japan, 2000; pp. 388–389. [Google Scholar]
- Kaczmarczyk, A.; Rutten, T.; Melzer, M.; Keller, E.R.J. Ultrastructural Changes Associated with Cryopreservation of Potato (Solanum tuberosum L.). Cryo-Lett. 2008, 29, 145–156. [Google Scholar]
- Wang, B.; Li, J.-W.; Zhang, Z.-B.; Wang, R.-R.; Ma, Y.-L.; Blystad, D.-R.; Keller, E.R.J.; Wang, Q.-C. Three vitrification-based cryopreservation procedures cause different cryo-injuries to potato shoot tips while all maintain genetic integrity in regenerants. J. Biotech. 2014, 184, 47–55. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).