Developmental, Phytochemical and Enzymatic Changes in Pot Marigold (Calendula officinalis L.) cvs. Hybrid and French with Salicylic Acid (SA) and Polyamine Spermidine (SP) Foliar Spray
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments Preparation
2.2. Vegetative Growth and Floral Examination
2.3. Antioxidant Activity
2.4. Total Phenolic Compound
2.5. Total Carotenoids Content
2.6. Total Flavonoids Content
2.7. Qualitative Analysis of the Phytochemicals
- Test for alkaloids
- Test for saponins
- Test for phytosterols
- Test for tannins
- Test for flavonoids
- Test for proteins and amino acids
- Test for diterpenes
2.8. Antioxidant Enzyme Activities
- Superoxide dismutase
- Peroxidase
- Catalase
2.9. Statistical Analysis
3. Results
3.1. Vegetative Parameters
3.1.1. Plant Height
3.1.2. Leaf Length
3.1.3. Number of Leaves per Plant
3.1.4. Stem Thickness
3.2. Reproductive Parameters
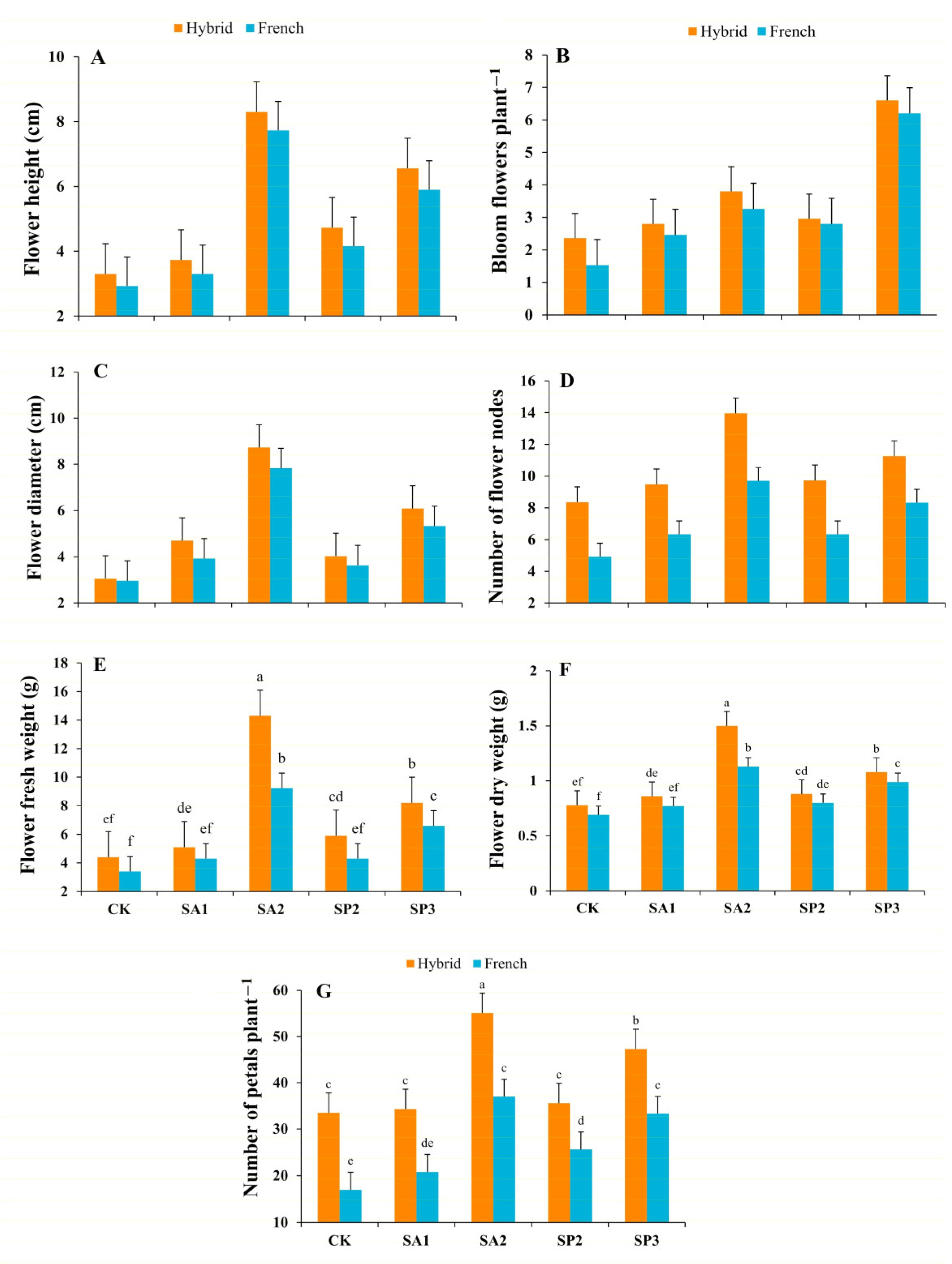
3.2.1. Flower Height
3.2.2. Bloom Flowers per Plant
3.2.3. Flower Diameter
3.2.4. Nodes of Flower per Plant
3.2.5. Fresh Weight of Flowers
3.2.6. Dry Weight of Flower
3.2.7. Number of Petals per Flower
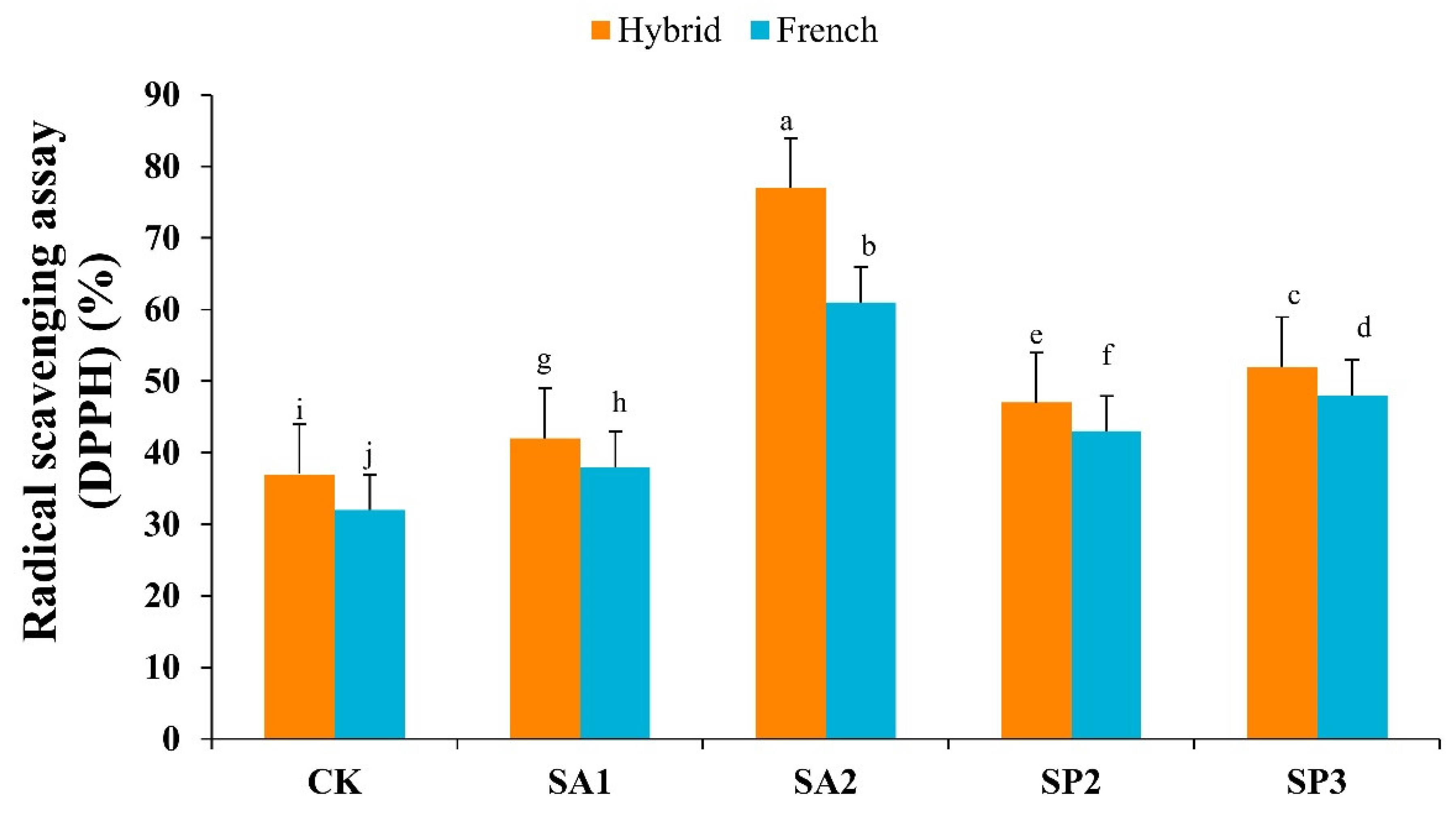
3.3. Antioxidant Activity
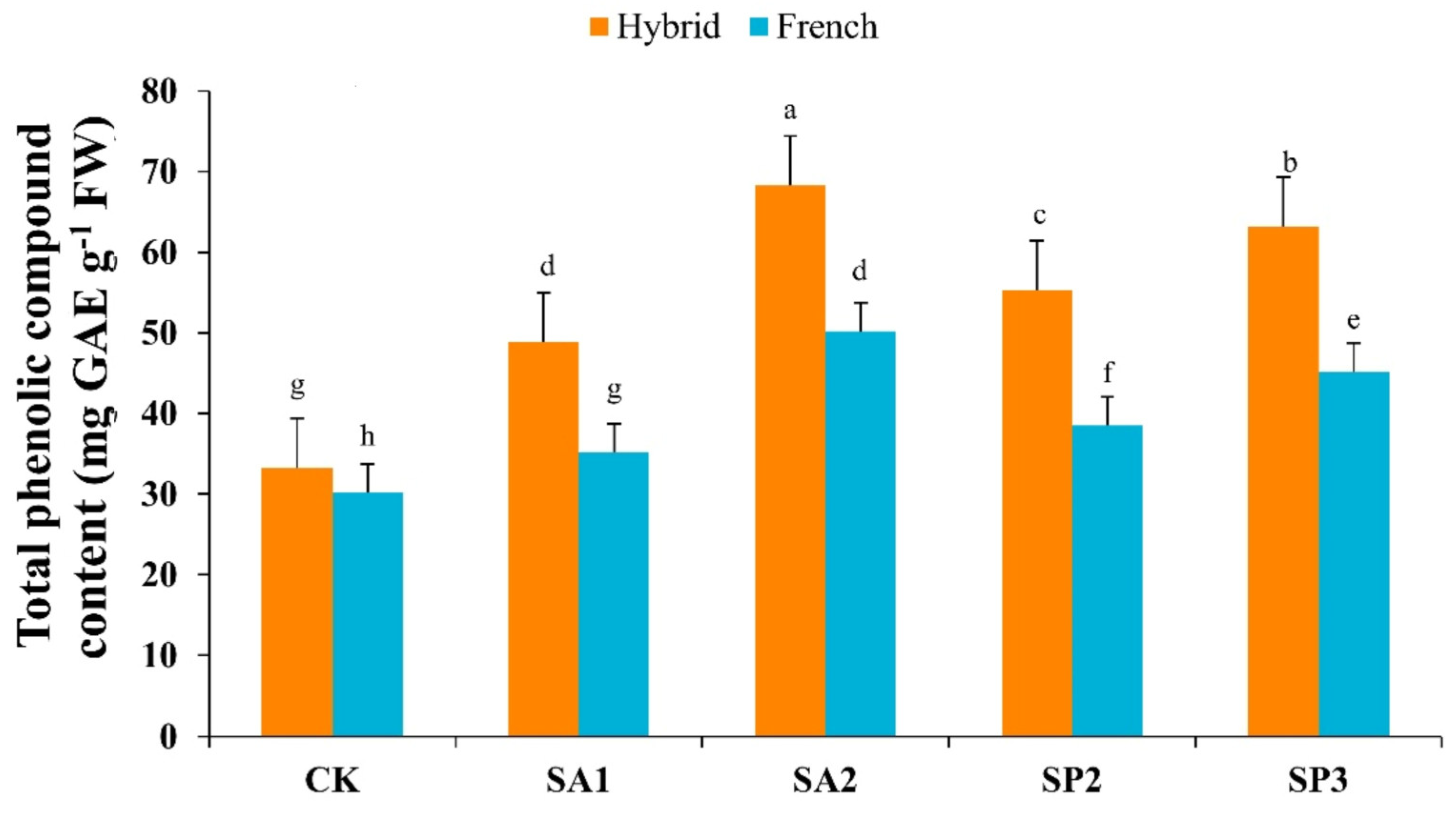
3.4. Total Phenolic Compounds
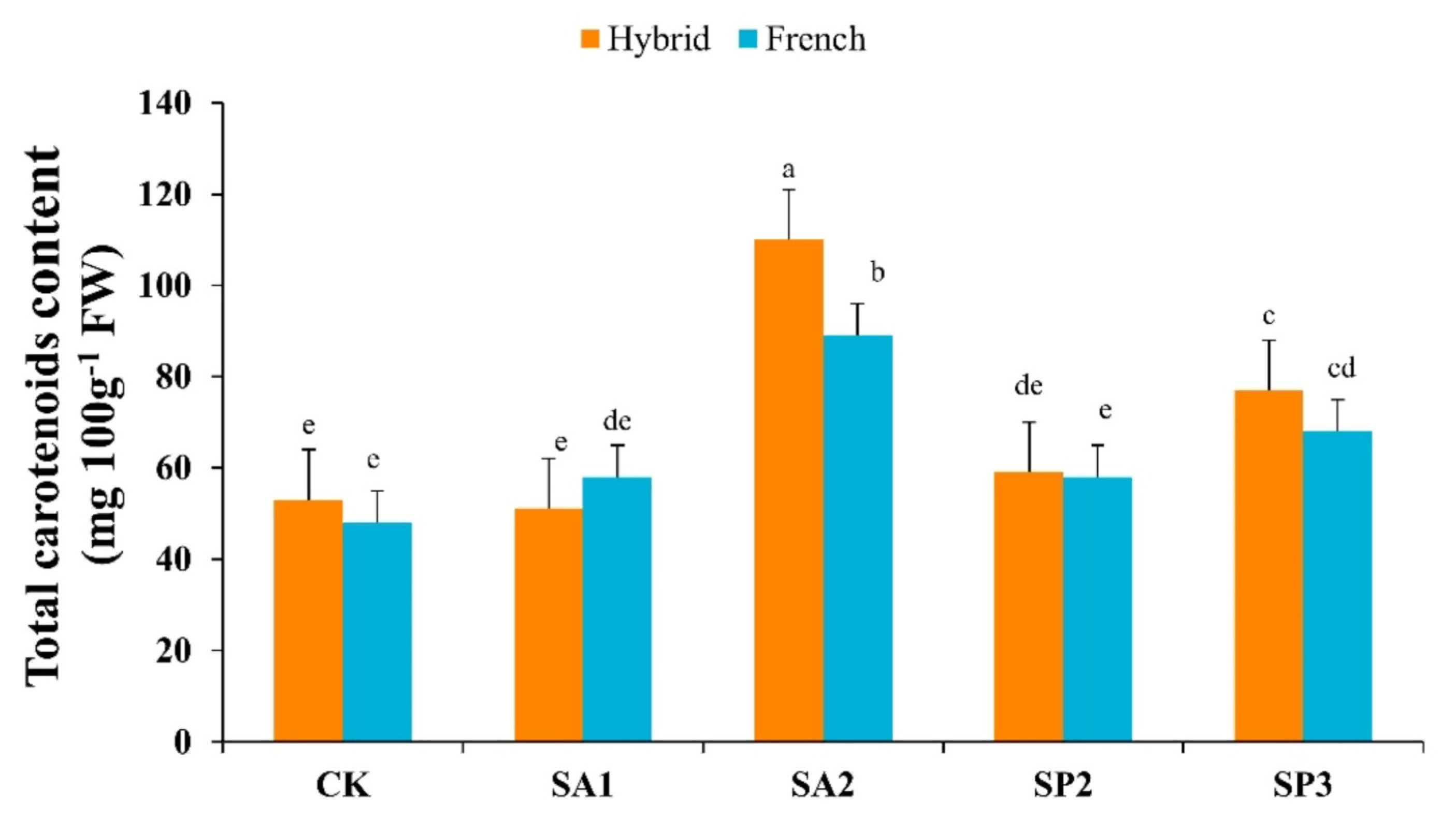
3.5. Total Carotenoids
3.6. Total Flavonoids
3.7. Qualitative Phytochemical Screening
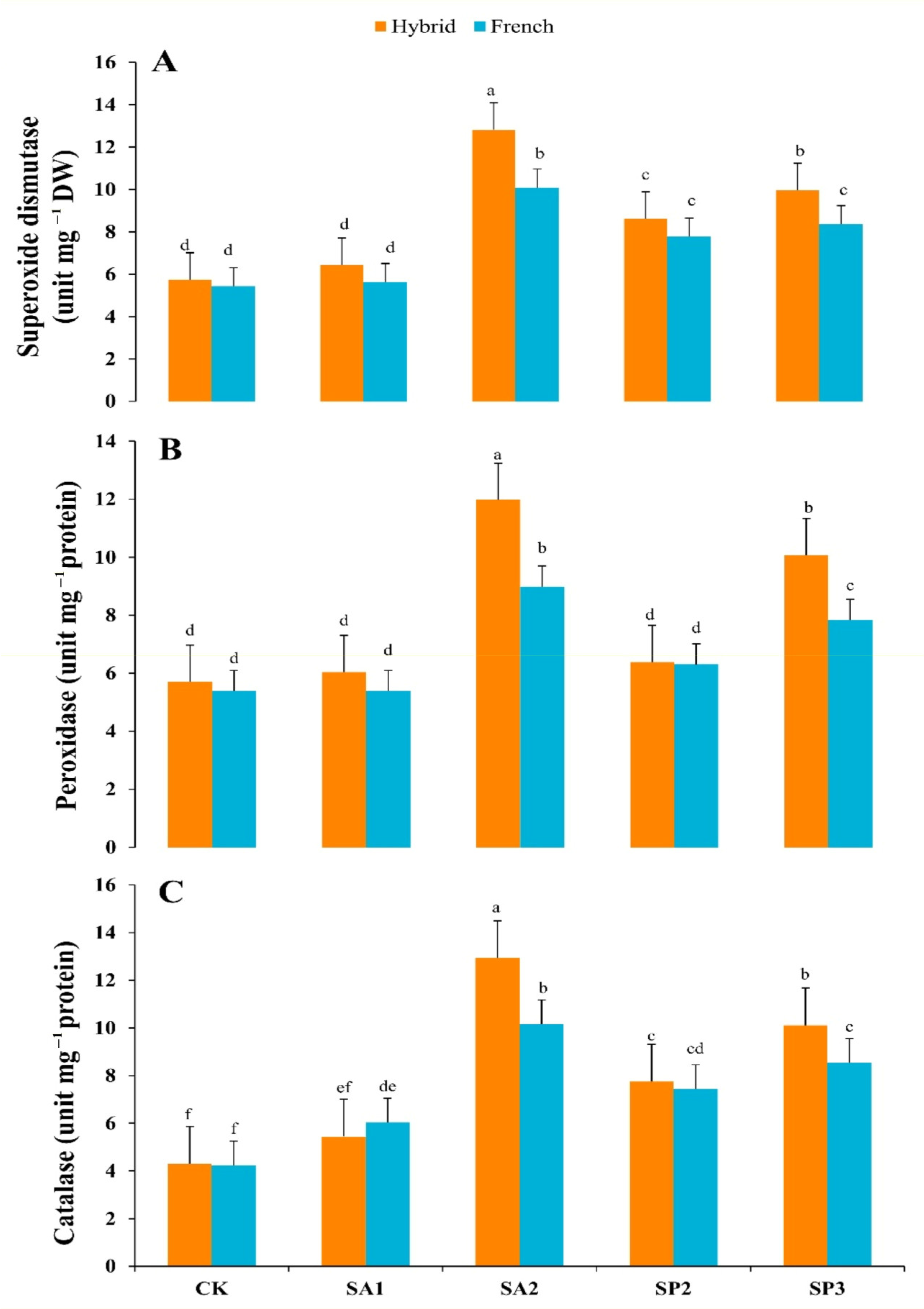
3.8. Antioxidant Enzymes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sobati-Nasab, Z.; Alirezalu, A.; Noruzi, P. Effect of foliar application of nickel on physiological and phytochemical characteristics of pot marigold (Calendula officinalis). J. Agric. Food Res. 2021, 3, 100108. [Google Scholar] [CrossRef]
- Manivannan, A.; Narasegowda, S.; Prakash, T. Comparative study on color coordinates, phenolics, flavonoids, carotenoids, and antioxidant potential of marigold (Tagetes sp.) with diverse colored petals. J. Food Meas. Charact. 2021, 15, 4343–4353. [Google Scholar] [CrossRef]
- Basit, A.; Shah, K.; Rahman, M.U.; Xing, L.; Zuo, X.; Han, M.; Alam, N.; Khan, F.; Ahme, I.; Khalid, M.A. 15. Salicylic acid an emerging growth and flower inducing hormone in marigold (Tagetes sp. L.). Pure Appl. Biol. 2018, 7, 1301–1308. [Google Scholar] [CrossRef]
- Nwafor, I.; Nwafor, C.; Manduna, I. Constraints to cultivation of medicinal plants by smallholder farmers in South Africa. Horticulturae 2021, 7, 531. [Google Scholar] [CrossRef]
- Gazim, Z.C.; Rezende, C.M.; Fraga, S.R.; Svidzinski, T.I.E.; Cortez, D.A.G. Antifungal activity of the essential oil from Calendula officinalis L. (Asteraceae) growing in Brazil. Braz. J. Microbiol. 2008, 39, 61–63. [Google Scholar] [CrossRef]
- Sadique, S.; Ali, M.M.; Usman, M.; Hasan, M.U.; Yousef, A.F.; Adnan, M.; Gull, S.; Nicola, S. Effect of foliar supplied PGRs on flower growth and antioxidant activity of African marigold (Tagetes erecta L.). Horticulturae 2021, 7, 378. [Google Scholar] [CrossRef]
- Jowkar, M.M.; Salehi, H. Effects of different preservative solutions on the vase life of cut tuberose flowers at usual home conditions. In Proceedings of the VIII International Symposium on Postharvest Physiology of Ornamental Plants, Wageningen, The Netherlands, 10–14 August 2003; Volume 669, pp. 411–416. [Google Scholar]
- Pawar, A.; Saraladevi, D.; Kannan, M.; SabirAhamed, A. Effect of micronutrients and plant growth regulators on growth, flowering and yield attributes of marigold (Tagetes erecta L.). Madras Agric. J. 2019, 106, 1. [Google Scholar] [CrossRef]
- Ali, M.M.; Anwar, R.; Malik, A.U.; Khan, A.S.; Ahmad, S.; Hussain, Z.; Hasan, M.U.; Nasir, M.; Chen, F. Plant growth and fruit quality response of strawberry is improved after exogenous application of 24-epibrassinolide. J. Plant Growth Regul. 2022, 41, 1786–1799. [Google Scholar] [CrossRef]
- Bauters, L.; Stojilković, B.; Gheysen, G. Pathogens pulling the strings: Effectors manipulating salicylic acid and phenylpropanoid biosynthesis in plants. Mol. Plant Pathol. 2021, 22, 1436–1448. [Google Scholar] [CrossRef]
- Qidwai, T.; Shreeya, T.; Saha, S.; Sharma, M. Functional Defense Signals in Plants. In Microbial Metatranscriptomics Belowground; Springer: Berlin/Heidelberg, Germany, 2021; pp. 543–556. [Google Scholar]
- Janda, T.; Gondor, K.O.; Pál, M.; Szalai, G. Salicylic Acid Signalling under Stress Conditions in Plants. In Jasmonates and Salicylates Signaling in Plants; Springer: Berlin/Heidelberg, Germany, 2021; pp. 255–264. [Google Scholar]
- Arif, Y.; Sami, F.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salicylic acid in relation to other phytohormones in plant: A study towards physiology and signal transduction under challenging environment. Environ. Exp. Bot. 2020, 175, 104040. [Google Scholar] [CrossRef]
- Martínez, C.; Pons, E.; Prats, G.; León, J. Salicylic acid regulates flowering time and links defence responses and reproductive development. Plant J. 2004, 37, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Nandy, S.; Hazra, A.K.; Pandey, D.K.; Ray, P.; Dey, A. Elicitation of industrially promising vanillin type aromatic compound 2-hydroxy 4-methoxy benzaldehyde (MBAlD) yield in the in-vitro raised medicinal crop Hemidesmus indicus (L.) R. Br. by methyl jasmonate and salicylic acid. Ind. Crops Prod. 2021, 164, 113375. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Ullah, M.A.; Nadeem, M.; Tungmunnithum, D.; Hano, C. Exogenous application of salicylic acid and gibberellic acid on biomass accumulation, antioxidant and anti-inflammatory secondary metabolites production in multiple shoot culture of Ajuga integrifolia Buch. Ham. ex D. Don. Ind. Crops Prod. 2020, 145, 112098. [Google Scholar] [CrossRef]
- Sood, S.; Nagar, P. Post-harvest alterations in polyamines and ethylene in two diverse rose species. Acta Physiol. Plant 2008, 30, 243–248. [Google Scholar] [CrossRef]
- Yousefi, F.; Jabbarzadeh, Z.; Amiri, J.; Rasouli-Sadaghiani, M.H. Response of roses (Rosa hybrida L.‘Herbert Stevens’) to foliar application of polyamines on root development, flowering, photosynthetic pigments, antioxidant enzymes activity and NPK. Sci. Rep. 2019, 9, 16025. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Tiburcio, A.F.; Alcazar, R. Potential applications of polyamines in agriculture and plant biotechnology. In Polyamines; Humana Press: New York, NY, USA, 2018; pp. 489–508. [Google Scholar]
- Amira, E.A.; Behija, S.E.; Beligh, M.; Lamia, L.; Manel, I.; Mohamed, H.; Lotfi, A. Effects of the ripening stage on phenolic profile, phytochemical composition and antioxidant activity of date palm fruit. J. Agric. Food Chem. 2012, 60, 10896–10902. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rosenberg, M.S.; Wang, X. Meta-analysis: The past, present and future. New Phytol. 2007, 176, 742–745. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Edeoga, H.O.; Okwu, D.; Mbaebie, B. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Orech, F.O.; Akenga, T.; Ochora, J.; Friis, H.; Aagaard-Hansen, J. Potential toxicity of some traditional leafy vegetables consumed in Nyang’oma Division, Western Kenya. Afr. J. Food Agric. Nutr. Dev. 2005, 5, 1. [Google Scholar] [CrossRef]
- Soforowa, E. Medicinal Plants and Traditional Medicine in Africa; John Willey & Sons: New York, NY, USA, 1982. [Google Scholar]
- Makkar, H.P.; Blümmel, M.; Borowy, N.K.; Becker, K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J. Sci. Food Agric. 1993, 61, 161–165. [Google Scholar] [CrossRef]
- Fernandez, C.; San Miguel, E.; Fernandez-Briera, A. Superoxide dismutase and catalase: Tissue activities and relation with age in the long-lived species Margaritifera margaritifera. Biol. Res. 2009, 42, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Berger, R. Methods of enzymatic analysis. Volume III “Enzyme 1: Oxidoreductases, Transferases”. Weinheim/Deerfield Beach, Florida/Basel: Verlag Chemie 1983., 605 S., 18 Abb., 43 Table 224 DM (wenn alle Bände), 258 DM (Einzelband). Engineering 1984, 4, 346. [Google Scholar] [CrossRef]
- Clairbone, A. Catalase activity. In Handbook of Methods for Oxygen Radical Research; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Chen, T.; White, J.F.; Li, C.; Nan, Z. Exogenous spermidine enhances Epichloë endophyte-induced tolerance to NaCl stress in wild barley (Hordeum brevisubulatum). Plant Soil 2021, 468, 77–95. [Google Scholar] [CrossRef]
- Gorni, P.H.; Pacheco, A.C.; Moro, A.L.; Silva, J.F.A.; Moreli, R.R.; de Miranda, G.R.; Pelegrini, J.M.; Spera, K.D.; Junior, J.L.B.; da Silva, R.M.G. Salicylic acid foliar application increases biomass, nutrient assimilation, primary metabolites and essential oil content in Achillea millefolium L. Sci. Hortic. 2020, 270, 109436. [Google Scholar] [CrossRef]
- Youssef, S.; Abd Elhady, S.A.E.; Abu El-Azm, N.A.I.; El-Shinawy, M.Z. Foliar application of salicylic acid and calcium chloride enhances growth and productivity of lettuce (Lactuca sativa). Egypt. J. Hort. 2017, 44, 1–16. [Google Scholar] [CrossRef]
- Soltani, Y.; Saffari, V.R.; Maghsoudi Moud, A.A. Response of growth, flowering and some biochemical constituents of Calendula officinalis L. to foliar application of salicylic acid, ascorbic acid and thiamine. J. Ethno-Pharm. Prod. 2014, 1, 37–44. [Google Scholar]
- Janda, T.; Szalai, G.; Pál, M. Salicylic acid signalling in plants. Int. J. Mol. Sci. 2020, 21, 2655. [Google Scholar] [CrossRef]
- Bayat, H.; Alirezaie, M.; Neamati, H. Impact of exogenous salicylic acid on growth and ornamental characteristics of calendula (Calendula officinalis L.) under salinity stress. J. Stress Physiol. Biochem. 2012, 8, 258–267. [Google Scholar]
- Narute, T.; Parulekar, Y.; Narute, T. Effect of plant growth regulators on yield and yield attributing character of marigold cv. Calcutta Marigold under Konkan conditions. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 3998–4005. [Google Scholar] [CrossRef]
- Saeed, T.; Hassan, I.; Abbasi, N.A.; Jilani, G. Antioxidative activities and qualitative changes in gladiolus cut flowers in response to salicylic acid application. Sci. Hortic. 2016, 210, 236–241. [Google Scholar] [CrossRef]
- Alaey, M.; Babalar, M.; Naderi, R.; Kafi, M. Effect of pre-and postharvest salicylic acid treatment on physio-chemical attributes in relation to vase-life of rose cut flowers. Postharvest Biol. Technol. 2011, 61, 91–94. [Google Scholar] [CrossRef]
- Mubarik, M.S.; Khan, S.H.; Sajjad, M.; Raza, A.; Hafeez, M.B.; Yasmeen, T.; Rizwan, M.; Ali, S.; Arif, M.S. A manipulative interplay between positive and negative regulators of phytohormones: A way forward for improving drought tolerance in plants. Physiol. Plant 2021, 172, 1269–1290. [Google Scholar] [CrossRef]
- Shimoji, Y.; Tamura, Y.; Nakamura, Y.; Nanda, K.; Nishidai, S.; Nishikawa, Y.; Ishihara, N.; Uenakai, K.; Ohigashi, H. Isolation and identification of DPPH radical scavenging compounds in Kurosu (Japanese unpolished rice vinegar). J. Agric. Food Chem. 2002, 50, 6501–6503. [Google Scholar] [CrossRef] [PubMed]
- Akshaya, H.; Namita, K.P.S.; Saha, S.; Panwar, S.; Bharadwaj, C. Determination and correlation of carotenoid pigments and their antioxidant activities in marigold (Tagetes sp.) flowers. Indian J. Agric. Sci. 2017, 87, 390–396. [Google Scholar]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Gong, Y.; Hou, Z.; Gao, Y.; Xue, Y.; Liu, X.; Liu, G. Optimization of extraction parameters of bioactive components from defatted marigold (Tagetes erecta L.) residue using response surface methodology. Food Bioprod. Process. 2012, 90, 9–16. [Google Scholar] [CrossRef]
- Oueslati, A.; Rigane, G.; Ghazghazi, H.; Salem, R.B.; Hamdi, S.; Hannachi, M.; Jouili, H.; L’ammari, Y. Phenolic content, antioxidant and antimicrobial activities of Trachyspermum ammi aerial parts growing wild in the north of Tunisia. J. New Sci. 2016, 25, 1151–1160. [Google Scholar]
- Baenas, N.; Ferreres, F.; García-Viguera, C.; Moreno, D.A. Radish sprouts—Characterization and elicitation of novel varieties rich in anthocyanins. Food Res. Int. 2015, 69, 305–312. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Hamayun, M.; Khan, A.L.; Na, C.I.; Kang, S.M.; Han, H.H.; Lee, I. Exogenous application of plant growth regulators increased the total flavonoid content in Taraxacum officinale Wigg. Afr. J. Biotechnol. 2009, 8, 5727–5732. [Google Scholar] [CrossRef]
- Osama, S.; El Sherei, M.; Al-Mahdy, D.A.; Bishr, M.; Salama, O. Effect of salicylic acid foliar spraying on growth parameters, γ-pyrones, phenolic content and radical scavenging activity of drought stressed Ammi visnaga L. plant. Ind. Crops Prod. 2019, 134, 1–10. [Google Scholar] [CrossRef]
- Dong, J.Z.; Wang, Y.; Wang, S.H.; Yin, L.P.; Xu, G.J.; Zheng, C.; Lei, C.; Zhang, M.Z. Selenium increases chlorogenic acid, chlorophyll and carotenoids of Lycium chinense leaves. J. Sci. Food Agric. 2013, 93, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Júnior, G.S.C.; de Lima, R.d.L.S.; Gheyi, H.R.; Carvalho, J.M.F.C.; Soares, M.R.A.; Sofiatti, V. Physiological aspects of castor bean cv. BRS Energia in response to foliar application of gibberellic and salicylic acid. Aust. J. Crop Sci. 2016, 10, 193–198. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Xin, P.; Shuang-Lin, Z.; Jun-Yao, H.; Li, D. Influence of rare earth elements on metabolism and related enzyme activity and isozyme expression in Tetrastigma hemsleyanum cell suspension cultures. Biol. Trace Elem. Res. 2013, 152, 82–90. [Google Scholar] [CrossRef]
- Mallahi, T.; Saharkhiz, M.J.; Javanmardi, J. Salicylic acid changes morpho-physiological attributes of feverfew (Tanacetum parthenium L.) under salinity stress. Acta Ecol. Sin. 2018, 38, 351–355. [Google Scholar] [CrossRef]
- El-Lakany, A. medicinal plants ‘stress factors: Effects on metabolites and novel perspectives for tolerance. BAU J.-Health Wellbeing 2022, 5, 6. [Google Scholar]
- Ncube, B.; Finnie, J.; Van Staden, J. Quality from the field: The impact of environmental factors as quality determinants in medicinal plants. S. Afr. J. Bot. 2012, 82, 11–20. [Google Scholar] [CrossRef]
- Marini, M.; Ni’mah, T.; Mahdalena, V.; Komariah, R.H.; Sitorus, H. Potensi Daya Tolak Ekstrak Daun Marigold (Tagetes erecta L.) terhadap Nyamuk Aedes aegypti. Balaba J. Litbang Pengendali. Penyakit Bersumber Binatang Banjarnegara 2018, 14, 53–62. [Google Scholar] [CrossRef]
- Devika, R.; Koilpillai, J. Phytochemical screening studies of bioactive compounds of Tagetes erecta. Int. J. Pharm. Bio. Sci. 2012, 3, 596–602. [Google Scholar]
- Thorat, S.S.; Shirote, P. Isolation, Phytochemical Screening and Pharmacological Evaluation of Tagetes erecta Leaves Extract. Int. Res. J. Pharm. Med. Sci. 2019, 2, 57–59. [Google Scholar]
- Ali, S.; Ali, K.; Hussain, Z.; Khan, M.S.; Khan, W.M.; Wali, S.; Shuaib, M. Phytochemical screening and antimicrobial activity of selected medicinal plant species. Pure Appl. Biol. 2017, 6, 418–425. [Google Scholar] [CrossRef]
- Ali, B. Salicylic acid: An efficient elicitor of secondary metabolite production in plants. Biocatal. Agric. Biotechnol. 2021, 31, 101884. [Google Scholar] [CrossRef]


| Treatment | Tannins | Flavonoids | Diterpenes | Saponins | Phytosterols | Alkaloids | Proteins |
|---|---|---|---|---|---|---|---|
| Hybrid marigold | |||||||
| CK | − | ++ | − | − | ++ | − | ++ |
| SA1 | ++ | ++ | ++ | ++ | ++ | − | +++ |
| SA2 | − | +++ | − | − | +++ | − | ++ |
| SP2 | ++ | ++ | ++ | ++ | ++ | ++ | +++ |
| SP3 | − | ++ | ++ | − | − | ++ | ++ |
| French marigold | |||||||
| CK | − | − | − | ++ | − | − | − |
| SA1 | ++ | +++ | − | ++ | ++ | ++ | ++ |
| SA2 | +++ | − | − | − | − | − | ++ |
| SP2 | ++ | ++ | ++ | ++ | +++ | ++ | ++ |
| SP3 | ++ | ++ | ++ | ++ | − | ++ | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, S.; Ahmed, W.; Mihoub, A.; Jamal, A.; Farhan Saeed, M.; Masood, N.; Radicetti, E.; Fawad, M.; Nicola, S. Developmental, Phytochemical and Enzymatic Changes in Pot Marigold (Calendula officinalis L.) cvs. Hybrid and French with Salicylic Acid (SA) and Polyamine Spermidine (SP) Foliar Spray. Agronomy 2023, 13, 191. https://doi.org/10.3390/agronomy13010191
Ahmad S, Ahmed W, Mihoub A, Jamal A, Farhan Saeed M, Masood N, Radicetti E, Fawad M, Nicola S. Developmental, Phytochemical and Enzymatic Changes in Pot Marigold (Calendula officinalis L.) cvs. Hybrid and French with Salicylic Acid (SA) and Polyamine Spermidine (SP) Foliar Spray. Agronomy. 2023; 13(1):191. https://doi.org/10.3390/agronomy13010191
Chicago/Turabian StyleAhmad, Sohail, Waseem Ahmed, Adil Mihoub, Aftab Jamal, Muhammad Farhan Saeed, Nasir Masood, Emanuele Radicetti, Muhammad Fawad, and Silvana Nicola. 2023. "Developmental, Phytochemical and Enzymatic Changes in Pot Marigold (Calendula officinalis L.) cvs. Hybrid and French with Salicylic Acid (SA) and Polyamine Spermidine (SP) Foliar Spray" Agronomy 13, no. 1: 191. https://doi.org/10.3390/agronomy13010191
APA StyleAhmad, S., Ahmed, W., Mihoub, A., Jamal, A., Farhan Saeed, M., Masood, N., Radicetti, E., Fawad, M., & Nicola, S. (2023). Developmental, Phytochemical and Enzymatic Changes in Pot Marigold (Calendula officinalis L.) cvs. Hybrid and French with Salicylic Acid (SA) and Polyamine Spermidine (SP) Foliar Spray. Agronomy, 13(1), 191. https://doi.org/10.3390/agronomy13010191












